Abstract
Background
Adverse perinatal outcomes are common with pregnancy-related mild glucose intolerance. The perinatal impact of improving this population’s health, instead of individual health, has not been quantified.
Methods
We estimated this impact among women with mild glucose intolerance, delivered at The University of North Carolina Women’s Hospital from April 1996 to May 2010. We compared observed with predicted risks of perinatal outcomes after simulating a cohort with a one standard deviation decrease in each glucose value. We estimated absolute and adjusted risks, relative risks, and risk differences with Poisson regression and bootstrapped 95% confidence intervals [CI].
Results
Among 3217 women, mean (SD) 1-h screening result was 157 (16) mg/dL; 3-h diagnostic results were 81 (10), 154 (28), 130 (25), and 104 (26) mg/dL for fasting, 1-h, 2-h, and 3-h, respectively. Compared with observed, predicted risks decreased for preeclampsia (9.1% vs. 6.6%, risk ratio [RR] 0.73 [95% CI 0.60, 0.88]), caesarean delivery (30.1% vs. 26.4%, RR 0.88 [95% CI 0.81, 0.96]), preterm birth (13.0% vs. 9.8%, RR 0.75 [95% CI 0.64, 0.87]), birthweight >4000 g (13.4% vs. 10.5%, RR 0.78 [95% CI 0.67, 0.90]), and shoulder dystocia (3.5% vs. 2.2%, RR 0.61 [95% CI 0.46, 0.83]).
Conclusions
Modestly improved population distribution of glucose tolerance in pregnancies affected by mild glucose intolerance translated to meaningful improvements in perinatal outcomes.
Keywords: adverse perinatal outcomes, gestational diabetes, glycaemic profile, mild glucose intolerance, population health
Glucose intolerance of pregnancy is a continuum in which short- and long-term adverse outcomes increase in prevalence with worsening hyperglycaemia.1 Gestational diabetes (GDM) carries the greatest risk. Women without GDM but with lesser degrees of hyperglycaemia, however, still have a higher risk of poor pregnancy outcomes than normoglycaemic women.1 Evidence suggests that treating individuals with mild glucose intolerance lowers perinatal risk.2–4 Despite adoption of more inclusive criteria for GDM diagnosis, women with mild glucose intolerance who do not meet diagnostic criteria for GDM typically go without potentially beneficial treatment.1,5 In one accepted two-step GDM screening and testing method, women who screen positive but are not diagnosed with GDM may comprise almost 10% of the population.6 Testing continues to vary in research populations and clinical practice. Generally accepted alternate cut-offs for screening and for diagnosis in two-step testing, and the diagnostic criteria recently proposed for one-step testing, may be more inclusive and diagnose additional women with GDM.
Routine clinical management of GDM includes multiple daily self-glucose monitoring, diet and nutrition counselling, and medical management when goal glucose thresholds are not met.7 While treatment may decrease adverse outcomes among women with mild glucose intolerance, standard GDM management is cumbersome and costly to patients, providers, and the health care system. Whether less cost- and resource-intensive treatments or even earlier pregnancy or pre-pregnancy prevention of glucose intolerance could improve glycaemic profile and perinatal outcomes is unknown. Before limited health care resources are directed toward this population, evidence is needed to define what measureable changes in health status are associated with a clinically meaningful difference.
Our emphasis in the current analysis is on the population, instead of the individual, the more traditional approach of clinical trials. We followed the lead of Geoffrey Rose, an epidemiologist known best for transforming the approach to health improvement strategies. In his 1985 seminal paper, Rose contrasted the consequences of a focus on sick individuals vs. sick populations, and the importance of distinguishing the two.8,9 The latter, a ‘sick population’ of women with mild glucose intolerance during pregnancy, is of interest here.8,9 Specifically, we evaluated the risks of adverse pregnancy outcomes in a prospective observational cohort with mild glucose intolerance receiving routine prenatal care. We compared observed outcomes in the cohort with the predicted number of outcomes in the same cohort after shifting the entire population distribution of glucose measures during pregnancy. We sought to quantify the magnitude of improvement in pregnancy outcomes that might be accomplished by potential interventions to improve glucose tolerance before pregnancy or during early pregnancy.
Methods
We identified a prospective observational cohort of all women eligible for GDM screening and delivered at a single tertiary care university hospital between April 1, 1996 and May 31, 2010. We excluded women who delivered prior to 24 weeks’ gestation, with a diagnosis of pre-GDM, or without documented GDM screening or diagnostic test results. For multiple gestations, we used neonatal data for the firstborn. University Institutional Review Board approval was obtained for this study.
GDM screening was performed as part of routine prenatal care between 24 and 28 weeks’ gestation using a 50-g, 1-h oral glucose load test, with plasma glucose values ≥140 mg/dL considered screen-positive. For screen-positive women, diagnostic testing included a 100-g, 3-h oral glucose tolerance test (OGTT). National Diabetes Data Group criteria were used for diagnosis at our institution during the study period.10 Screen-positive women not diagnosed with GDM received routine prenatal care and comprised our study sample. Women diagnosed with and treated for GDM were not the focus of this analysis and thus were excluded.
We abstracted maternal demographic data and pregnancy diagnoses from the University’s Department of Obstetrics and Gynecology Perinatal Database. The Perinatal Database includes prospectively collected data, and details of data abstraction are described elsewhere.6 Self-reported race/ethnicity was abstracted from the database and had been recorded during routine prenatal care as Caucasian, African-American, Hispanic, Asian, or other/not reported.
We included perinatal outcomes shown to improve with treatment of impaired glucose tolerance in other studies.3,4 Abstracted maternal outcomes included: gestational hypertension, preeclampsia [composite of mild, severe eclampsia, and/or HELLP syndrome (haemolysis, elevated liver enzymes, and low platelets)], caesarean delivery, and third or fourth degree perineal laceration. Neonatal outcomes included: preterm birth <37 weeks, macrosomia >4000 g and >4500 g, low birthweight <2500 g, shoulder dystocia (abstracted from provider notation in perinatal record), and neonatal intensive care unit (NICU) stay >24 h.
Descriptive statistics for the study population were calculated as per cent (%) for categorical variables and as the mean and standard deviation (SD) for continuous variables including each glucose measure. We fit separate Poisson models for each outcome, including as independent predictors the 1-h screening glucose load test and each component (fasting; 1-h, 2-h, and 3-h) of the 3-h OGTT. To determine the independent effect of each glucose test result, we included parameters for the effects of maternal characteristics on the outcome of interest (maternal age at delivery, multiparae, race/ethnicity, chronic hypertension, history of caesarean delivery, history of gestational diabetes, history of preeclampsia, or multiple gestation). Maternal age was centered at 30 years and modelled with linear and quadratic terms to allow nonlinear relationships with the outcomes.
To estimate the joint effects of a one SD decrease across all five glucose measures, we subtracted the SD of the observed distribution of each glucose measure from each woman’s glucose test results. Using the parameter estimates from the regression models, we calculated the predicted probability that each individual would experience the outcome of interest given the full set of glucose results that were set to one SD below the patient’s measured values. The sum of the predicted probabilities was used to estimate the number of events that would have been observed in the study population. We calculated the risk difference (RD) per 100 as predicted minus observed risk, number needed to treat (NNT) as 1/RD, and risk ratio (RR) from the observed and predicted outcomes. To obtain 95% confidence intervals [CI], we bootstrapped 2000 complete samples with replacement and conducted the complete analysis in each iteration.11 We report the median RD, NNT, and RR from 2000 iterations, and the empirical 2.5th and 97.5th percentiles of the distribution.
To determine whether the findings were primarily driven by a subgroup of women who were most glucose intolerant and could have been diagnosed with GDM if more inclusive criteria were used, we conducted a sensitivity analysis. We limited the cohort to women who had no elevated results for the OGTT or had a single elevated result on the 1-h (≥180 mg/dL), 2-h (≥155 mg/dL), or 3-h (≥140 mg/dL) results, but with a fasting glucose <95 mg/dL. Based on the criteria used by Landon et al.3 that defined a group of women with mild gestational diabetes, women in our sensitivity analysis would not have met criteria for treatment.
Results
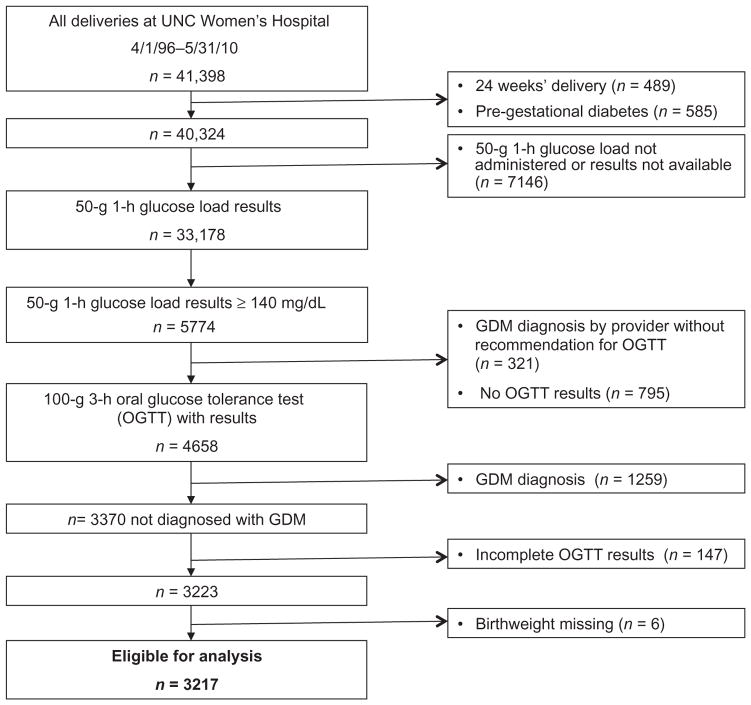
From April 1, 1996, to May 31, 2010, 41 398 women delivered at the university hospital, and 33 179 women met initial study inclusion criteria and were screened for GDM with a 50 g 1-h oral glucose load. A total of 10.2% (3370/33 178) screened positive but were not diagnosed with GDM by testing criteria. As all four components of the OGTT were required for this analysis, 4.4% (147/3370) with one or more missing values were excluded. After excluding six women who were missing birthweight data, 3217 comprised the sample for further analysis, as shown in Figure 1. Mean (SD) 1-h screening result was 157(16) mg/dL; 3-h glucose values were 81 (10), 154 (28), 130 (25), and 104 (26) mg/dL for fasting, 1-h, 2-h, and 3-h, respectively. Other demographic and clinical characteristics of the study population are shown in Table 1.
Figure 1.
Study cohort composition.
Table 1.
Baseline characteristics of pregnant women in study cohort (n = 3217)
| Maternal characteristics and laboratory values | Na | % | Mean | (SD) |
|---|---|---|---|---|
| Maternal age at delivery, years | 29.5 | (5.8) | ||
| Maternal age ≥35 years at delivery | 583 | 18.5 | ||
| Multiparae | 1944 | 60 | ||
| Race/ethnicity | ||||
| Caucasian | 1242 | 38.6 | ||
| African-American | 363 | 11.3 | ||
| Hispanic | 1393 | 43.3 | ||
| Asian | 174 | 5.4 | ||
| Unreported/other | 45 | 1.4 | ||
| Chronic hypertension | 149 | 4.6 | ||
| History of caesarean delivery | 533 | 17.0 | ||
| History of gestational diabetes | 44 | 1.4 | ||
| History of preeclampsia | 104 | 3.2 | ||
| Multiple gestation | 102 | 3.2 | ||
| 50-g 1-h oral glucose load, mmol/L | 8.7 | (0.9) | ||
| 100-g 3-h oral glucose tolerance test, mmol/L | ||||
| Fasting | 4.5 | (0.6) | ||
| 1 h | 8.6 | (1.6) | ||
| 2 h | 7.2 | (1.4) | ||
| 3 h | 5.8 | (1.4) | ||
Numbers may not sum to 100% as a result of rounding.
SD, standard deviation.
Across the observed as well as the predicted cohorts, poor pregnancy outcomes were common. A total of 43% in the observed cohort experienced at least one poor maternal outcome. With an improved glycaemic profile, the predicted prevalence would decrease to 37%. Similarly, 48% of neonates in the observed cohort experienced at least one poor outcome. The predicted prevalence would decrease to 44% with an improved glycaemic profile.
When compared with observed prevalence of adverse maternal outcomes, a population of women with GDM testing values one SD lower would have a lower predicted risk of preeclampsia and caesarean delivery, as shown in Table 2. The relative reduction in preeclampsia (9.1%) would be 27% (RR 0.73 [95% CI 0.60, 0.88]) with an NNT of 42 [95% CI 28, 91]. In the context of this analysis, the NNT of 42 represents how many women would need to present with a lower glycaemic profile to prevent one case of preeclampsia. The relative reduction in caesarean delivery (30.1%) would be 12% (RR 0.88 [95% CI 0.81, 0.96]), and if 28 women had an improved glycaemic profile by one SD, one caesarean may be prevented (NNT 28 [95% CI 18, 78]). Observed and predicted risks of gestational hypertension and perineal laceration did not differ on the relative or absolute scales.
Table 2.
Observed and predicted risk, risk difference (RD), number needed to treat (NNT), relative risk (RR) and 95% confidence intervals [CI] of maternal and neonatal outcomesa (n = 3217)
| Observed
|
Predictedb,c
|
RD per 100c | 95% CI | NNTc | 95% CI | RR | 95% CI | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Risk per 100 | 95% CI | Risk per 100 | 95% CI | |||||||
| Maternal | ||||||||||
| Gestational hypertension | 4.8 | 4.1, 5.6 | 4.1 | 3.0, 5.6 | 0.7 | −0.6, 1.6 | 162 | 63, −163 | 0.86 | 0.67, 1.13 |
| Preeclampsia | 9.1 | 8.1, 10.1 | 6.6 | 5.3, 8.2 | 2.4 | 1.1, 3.6 | 42 | 28, 91 | 0.73 | 0.60, 0.88 |
| Caesarean delivery | 30.1 | 28.5, 31.7 | 26.4 | 23.7, 29.1 | 3.6 | 1.3, 5.7 | 28 | 18, 78 | 0.88 | 0.81, 0.96 |
| Third/fourth degree perineal laceration | 3.8 | 3.1, 4.4 | 4.0 | 2.8, 5.4 | −0.2 | −1.5, 0.8 | NA d | 1.06 | 0.80, 1.39 | |
| Neonatal | ||||||||||
| Preterm birth <37 weeks | 13.0 | 11.8, 14.2 | 9.8 | 8.2, 11.5 | 3.2 | 1.7, 4.7 | 32 | 22, 59 | 0.75 | 0.64, 0.87 |
| Macrosomia >4000 g | 13.4 | 12.2, 14.6 | 10.5 | 8.8, 12.4 | 3.0 | 1.3, 4.4 | 35 | 23, 77 | 0.78 | 0.67, 0.90 |
| Macrosomia >4500 g | 2.4 | 1.8, 2.9 | 1.5 | 1.0, 2.3 | 0.9 | 0.2, 1.4 | 119 | 72, 487 | 0.63 | 0.43, 0.91 |
| Low birthweight <2500 g | 10.0 | 8.8, 11.0 | 10.4 | 8.5, 12.5 | −0.4 | −2.2, 1.2 | NA | 1.04 | 0.88, 1.23 | |
| Shoulder dystocia | 3.5 | 2.9, 4.2 | 2.2 | 1.5, 3 | 1.4 | 0.6, 2 | 75 | 51, 174 | 0.61 | 0.46, 0.83 |
| NICU admission | 19.0 | 18.5, 21.3 | 19.2 | 16.8, 21.9 | 0.8 | −1.6, 2.7 | 138 | 37, −64 | 0.96 | 0.86, 1.08 |
Median and empirical 95% confidence intervals from 2000 bootstrapped resamples.
Predicted risk with a one standard deviation decrease in the 1-h screening glucose and fasting 3-h oral glucose tolerance test results.
All estimates based on models for the effect of glucose levels were also adjusted for maternal age, multiparae, race/ethnicity, chronic hypertension, history of caesarean delivery, history of gestational diabetes, history of preeclampsia, and multiple gestation.
NA represents not applicable because of non-protective estimated effect of lowering glucose values on the outcome.
NICU, neonatal intensive care unit.
When compared with observed prevalence of adverse neonatal outcomes, a population of women with GDM testing values one SD lower would have a lower predicted prevalence of preterm birth <37 weeks, birthweight >4000 g, and shoulder dystocia, as shown in Table 2. The relative reduction in preterm birth <37 weeks (13.0%) would be 25% (RR 0.75 [95% CI 0.64, 0.87]) with an NNT of 32 [95% CI 22, 59]. The relative reduction in birthweight >4000 g (13.4%) and birthweight >4500 g (2.4%), respectively, would be 22% (RR 0.78 [95% CI 0.67, 0.90]) with an NNT of 35 [95% CI 23, 77], and 37% (RR 0.63 [95% CI 0.43, 0.91]) with an NNT of 119 [95% CI 72, 487]. The relative reduction in shoulder dystocia (3.5%) would be 39% (RR 0.61 [95% CI 0.46, 0.83]) with an NNT of 75 [95% CI 51, 174]. Thus, one preterm birth, macrosomic neonate >4000 g, or shoulder dystocia may be prevented for every 32, 35, 75 representative women, respectively, in our population of over 3000 who had an improved glycaemic profile. Observed and predicted risks of low birthweight <2500 g, and NICU admission >24 h did not differ on the relative (RR) or absolute (RD) scales.
To address potential confounding introduced by inclusion of 102 multiple pregnancies, we excluded them and performed a sensitivity analysis of the 3115 women with singleton pregnancies. Among outcomes significant in the full analysis, the prevalence of outcomes and magnitude of the effects of a one SD decrease across all glucose levels was similar to those estimated in the original cohort. No non-significant differences became significant after exclusion of multiples.
Also in a sensitivity analysis, we identified 2724/3217 women (84.7%) in the original cohort who had, at most, a single elevated glucose value from the OGTT and a normal fasting glucose. The magnitude of the effects of a one SD decrease across all glucose levels was very similar to those estimated in the original cohort. Among outcomes significant in the full analysis, the RD for caesarean delivery (−5.0 per 100), preterm birth (−4.1 per 100), macrosomia >4000 g (−3.9 per 100), shoulder dystocia (−1.5 per 100), were slightly larger than those estimated in the full sample, but well within the CI. The RD for preeclampsia (−2.2 per 100) and NICU admission (−0.4 per 100) were smaller but also well within the original CI. No non-significant differences became significant after exclusion of multiples.
Comment
We found that, among women with mild glucose intolerance and their neonates, a measureable improvement in the cohort’s glucose tolerance at time of routine GDM testing would be associated with a clinically meaningful decrease in preeclampsia, caesarean delivery, preterm birth, macrosomia, and shoulder dystocia. As close to half of women and neonates experienced at least one of our measured outcomes, these findings have important public health implications. Existing data describes adverse events and treatment effects for individuals with mild glucose intolerance. Our analysis builds on those data but approaches the morbidity of mild glucose intolerance as a substantial public health challenge.
Strengths of our study include a large, unselected sample size, racial/ethnic diversity, and consistent GDM screening and diagnostic criteria over 14 years. Our reported numbers needed to treat offer a clinical context in which to interpret the impact of population-level health improvements. A relatively small number of women would need to be healthier to avoid one caesarean delivery, macrosomic infant >4000 g, or shoulder dystocia (28, 35, 75, respectively), and these values are in line with those estimated in the recent decision analysis evaluating mild GDM treatment.12 While significant differences were also noted for birthweight >4500 g, the risk for this outcome is overall small with a much larger NNT of 119. A larger sample size may better delineate the potential to decrease this clinically important outcome.
Study findings should be interpreted in the context of some limitations. Women in our cohort may have been diagnosed with GDM using more inclusive thresholds for the 50-g, 1-h oral glucose load or Carpenter-Coustan criteria for the 3-h OGTT. While we cannot overcome that limitation of a retrospective analysis, our sensitivity analysis of a lower risk subset showed the same magnitude of RD between the subset and its modelled cohort. Height and pre-pregnancy weight to calculate body mass index were not available and are an important factor in early pregnancy health and later outcomes.13,14 It is reasonable, however, to assume that mild glucose intolerance is positively correlated with maternal body mass index and would not negate our findings focused on absolute and relative adverse pregnancy risks. In fact, targeting maternal overweight and obesity may be a promising strategy towards improving glycaemic profile.
For this analysis, we assumed that each glucose test result would change by a similar proportion. We recognise that the individual components of screening and diagnostic testing may not change uniformly. However, because these values are typically highly correlated, it is reasonable to expect an improvement in early or pre-pregnancy health to be reflected in each value to some degree. Our models did take into account the joint, independent effects of these measures on adverse perinatal outcomes. We acknowledge that there is not yet an identified intervention known to achieve this improved health status. Existing research has focused on individual health, essential to support the biologic plausibility of our findings.
Our findings are in line with recent prospective trials by Landon et al. and Crowther et al. that demonstrated improved perinatal outcomes among women treated for mild glucose intolerance (two elevated glucose measures) compared with women randomised to standard prenatal care.3,4 Our data extend these findings and show that a measureable improvement in glycaemic profile at a population level can also improve perinatal outcomes. The association between glucose tolerance and adverse perinatal outcomes was evident even among women in our sensitivity analysis who would be considered ‘false positives’ given their normal fasting glucose values and a maximum of one elevated result on the OGTT. The proportion of the population who stand to benefit from improved health is substantial.
Our population of women with mild glucose intolerance, not offered the same intensive treatment as women with GDM in standard practice, may be a group who could benefit from preventive strategies to achieve this quantifiable improvement in health status by the time of GDM screening. Concern exists over whether limited health care resources should be directed to these presumably lower risk women. Recent cost assessment and decision analysis work suggest GDM prevention instead of treatment, and treatment of mild GDM, may be cost-effective.15 For women with mild glucose intolerance, preventing associated pregnancy outcomes may cost less than traditional GDM treatment and impact an even greater proportion of the population.
Thus far, small trials for prevention of GDM, including exercise or nutritional interventions, have not been consistently cost-effective.16 Clinical benefits for maternal glucose profile or neonatal birthweight have also been inconsistent.16–21 We speculate that the primary challenge to effective intervention studies has been a lack of goal-oriented outcomes. Further, perhaps the goal-oriented outcomes should extend beyond the ‘sick individual’, as contrasted by Rose to the ‘sick population’.8
In this analysis, we have shown that a substantial proportion of the population of women with mild glucose intolerance have poor maternal or neonatal outcomes associated with hyperglycaemia. Following the example set by Rose, we have not only shifted from a treatment to a prevention approach, we also have specifically targeted the impact of a ‘population strategy’ that demonstrates even this cohort of women who are now considered ‘false positives’ and given a clean bill of health may benefit from intervention.8 It is not enough to counsel an obese woman to avoid excess weight gain at her first prenatal visit. More effective counselling with goal-oriented strategies could define exactly how much weight gain would change an obese woman’s – or even better, a group of women’s –trajectory towards glucose intolerance. Harnessing the strengths of individual and group behaviour research may improve our ability to evaluate perhaps less cost- and resource-intensive preventive efforts. Moving forward, this should be a public health priority for the substantial numbers of women with mild glucose intolerance who will not meet GDM criteria by any diagnostic criteria but have much to gain from modest improvements in their health status.
References
- 1.Metzger BE, Lowe LP, Dyer AR, Trimble ER, Chaovarindr U, Coustan DR, et al. Hyperglycemia and adverse pregnancy outcomes. New England Journal of Medicine. 2008;358:1991–2002. doi: 10.1056/NEJMoa0707943. [DOI] [PubMed] [Google Scholar]
- 2.Di Cianni G, Seghieri G, Lencioni C, Cuccuru I, Anichini R, De Bellis A, et al. Normal glucose tolerance and gestational diabetes mellitus: what is in between? Diabetes Care. 2007;30:1783–1788. doi: 10.2337/dc07-0119. [DOI] [PubMed] [Google Scholar]
- 3.Landon MB, Spong CY, Thom E, Carpenter MW, Ramin SM, Casey B, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. New England Journal of Medicine. 2009;361:1339–1348. doi: 10.1056/NEJMoa0902430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crowther CA, Hiller JE, Moss JR, McPhee AJ, Jeffries WS, Robinson JS. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. New England Journal of Medicine. 2005;352:2477–2486. doi: 10.1056/NEJMoa042973. [DOI] [PubMed] [Google Scholar]
- 5.Yee LM, Cheng YW, Liddell J, Block-Kurbisch I, Caughey AB. 50-gram glucose challenge test: is it indicative of outcomes in women without gestational diabetes mellitus? Journal of Maternal Fetal and Neonatal Medicine. 2011;24:1102–1106. doi: 10.3109/14767058.2010.546450. [DOI] [PubMed] [Google Scholar]
- 6.Berggren EK, Boggess KA, Stuebe AM, Jonsson Funk M. National Diabetes Data Group vs. Carpenter-Coustan criteria to diagnose gestational Diabetes. American Journal of Obstetetrics and Gynecology. 2011;205:253.e251–253.e257. doi: 10.1016/j.ajog.2011.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.American Congress of Obstetricians and Gynecologists. ACOG Practice Bulletin. Clinical Management Guidelines for Obstetrician-Gynecologists. Number 30, September 2001 (replaces Technical Bulletin Number 200, December 1994) Gestational diabetes. Obstetrics and Gynecology. 2001;98:525–538. [PubMed] [Google Scholar]
- 8.Rose G. Sick individuals and sick populations. International Journal of Epidemiology. 1985;14:32–38. doi: 10.1093/ije/14.1.32. [DOI] [PubMed] [Google Scholar]
- 9.Hunt K, Emslie C. Commentary: the prevention paradox in lay epidemiology – Rose revisited. International Journal of Epidemiology. 2001;30:442–446. doi: 10.1093/ije/30.3.442. [DOI] [PubMed] [Google Scholar]
- 10.Classification and diagnosis of diabetes mellitus and other categories of glucose intolerance. National Diabetes Data Group. Diabetes. 1979;28:1039–1057. doi: 10.2337/diab.28.12.1039. [DOI] [PubMed] [Google Scholar]
- 11.Efron B, Tibshirani R. Bootstrap methods for standard errors, confidence intervals, and other measures of statistical accuracy. Statistical Science. 1986;1:54–77. [Google Scholar]
- 12.Ohno MS, Sparks TN, Cheng YW, Caughey AB. Treating mild gestational diabetes mellitus: a cost-effectiveness analysis. American Journal of Obstetrics and Gynecology. 2011;205:e281–e287. doi: 10.1016/j.ajog.2011.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stuebe AM, Landon MB, Lai Y, Spong CY, Carpenter MW, Ramin SM, et al. Maternal BMI, glucose tolerance, and adverse pregnancy outcomes. American Journal of Obstetrics and Gynecology. 2012;207:e61–e67. doi: 10.1016/j.ajog.2012.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Catalano PM, McIntyre HD, Cruickshank JK, McCance DR, Dyer AR, Metzger BE, et al. The hyperglycemia and adverse pregnancy outcome study: associations of GDM and obesity with pregnancy outcomes. Diabetes Care. 2012;35:780–786. doi: 10.2337/dc11-1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gillespie P, Cullinan J, O’Neill C, Dunne F ATLANTIC DIP Collaborators. Modeling the independent effects of gestational diabetes mellitus on maternity care and costs. Diabetes Care. 2012;36:1111–1116. doi: 10.2337/dc12-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oostdam N, Bosmans J, Wouters MG, Eekhoff EM, van Mechelen W, van Poppel MN. Cost-effectiveness of an exercise program during pregnancy to prevent gestational diabetes: results of an economic evaluation alongside a randomised controlled trial. BMC Pregnancy and Childbirth. 2012;12:64. doi: 10.1186/1471-2393-12-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oostdam N, van Poppel MN, Wouters MG, Eekhoff EM, Bekedam DJ, Kuchenbecker WK, et al. No effect of the FitFor2 exercise programme on blood glucose, insulin sensitivity, and birthweight in pregnant women who were overweight and at risk for gestational diabetes: results of a randomised controlled trial. British Journal of Obstetrics and Gynecology. 2012;119:1098–1107. doi: 10.1111/j.1471-0528.2012.03366.x. [DOI] [PubMed] [Google Scholar]
- 18.Barakat R, Cordero Y, Coteron J, Luaces M, Montejo R. Exercise during pregnancy improves maternal glucose screen at 24–28 weeks: a randomised controlled trial. British Journal of Sports Medicine. 2012;46:656–661. doi: 10.1136/bjsports-2011-090009. [DOI] [PubMed] [Google Scholar]
- 19.Callaway LK, Colditz PB, Byrne NM, Lingwood BE, Rowlands IJ, Foxcroft K, et al. Prevention of gestational diabetes: feasibility issues for an exercise intervention in obese pregnant women. Diabetes Care. 2010;33:1457–1459. doi: 10.2337/dc09-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hopkins SA, Baldi JC, Cutfield WS, McCowan L, Hofman PL. Exercise training in pregnancy reduces offspring size without changes in maternal insulin sensitivity. Journal of Clinical Endocrinology and Metabolism. 2010;95:2080–2088. doi: 10.1210/jc.2009-2255. [DOI] [PubMed] [Google Scholar]
- 21.Stafne SN, Salvesen KA, Romundstad PR, Eggebo TM, Carlsen SM, Morkved S. Regular exercise during pregnancy to prevent gestational diabetes: a randomized controlled trial. Obstetrics and Gynecology. 2012;119:29–36. doi: 10.1097/AOG.0b013e3182393f86. [DOI] [PubMed] [Google Scholar]