Abstract
Background
HIV-infected persons are at increased cardiovascular disease (CVD) risk, but traditional CVD therapies are understudied in this population. Telmisartan is an angiotensin receptor blocker and PPAR-γ agonist that improves endothelial function and cardiovascular mortality in HIV-uninfected populations. We assessed the effects of telmisartan on endothelial function in older HIV-infected persons at risk for CVD in a small pilot study.
Methods
HIV-infected individuals ≥50 years old on suppressive antiretroviral therapy (ART) with ≥1 traditional CVD risk factor received open label telmisartan 80 mg daily for six weeks. Brachial artery flow-mediated dilation (FMD) measured endothelial function. The primary endpoint was six-week change in maximum relative FMD.
Results
Seventeen participants enrolled; 16 completed all evaluations (88% men, 65% non-White, median age 60 years, CD4+ T lymphocyte count 625 cells/mm3). ART included 71% PI, 29% NNRTI, 29% integrase inhibitor, 65% tenofovir and 29% abacavir. CVD risk factor prevalence included 76% hyperlipidemia, 65% hypertension, 18% smoking and 12% diabetes mellitus. After six weeks, statistically significant blood pressure changes were observed (systolic −16.0 mmHg, diastolic −6.0 mmHg) without significant changes in FMD. In subset analyses, FMD increased more among abacavir-treated, PI-treated and non-smoking participants.
Conclusions
No significant FMD changes were observed after six weeks of telmisartan therapy; however, abacavir- and PI-treated participants and non-smokers showed greater FMD increases. Additional studies are needed to explore the effects of telmisartan on endothelial function among HIV-infected individuals with traditional CVD and/or ART-specific risk factors.
Keywords: HIV, endothelial function, cardiovascular risk
INTRODUCTION
HIV-infected persons are at increased risk of cardiovascular disease (CVD),1–5 and both HIV and antiretroviral therapy (ART) may contribute to this risk.1,6–8 Additionally, as the HIV-infected population continues to age, traditional CVD risk factors may play an increasing role in CVD development.
Endothelial dysfunction is an early and reversible step in the development of atherosclerosis9,10 that promotes chronic inflammatory remodeling of the vascular endothelium.11–13 Arterial flow-mediated dilation (FMD) can be determined using ultrasound as a measure of endothelial function and vascular reactivity.14,15 Brachial artery FMD is reduced in individuals with CVD risk factors,16,17 peripheral artery disease18 and coronary artery disease.19 Brachial artery FMD and coronary artery FMD are closely correlated.20,21
In HIV-infected persons, ART initiation can improve arterial FMD.22,23 However, compared to HIV-uninfected individuals, HIV-infected persons on suppressive ART have persistent endothelial dysfunction24 that may be mediated through HIV-associated chronic inflammation and immune activation and could contribute to the higher CVD risk observed in this population. To date, interventions to improve endothelial dysfunction in HIV-infected adults have had mixed effectiveness, with statins but not ART optimization demonstrating some benefit.25,26 Thus, targeted interventions to improve endothelial dysfunction are needed in aging, HIV-infected individuals.
Telmisartan is a selective antagonist for the angiotensin II type 1 receptor (AT1R) and a partial agonist for the peroxisome proliferator-activated receptor-γ (PPAR-γ) that is approved for the treatment of essential hypertension. AT1R blockade inhibits vasoconstriction and angiotensin II-induced aldosterone and pro-inflammatory cytokine secretion. PPAR-γ agonism leads to nitric oxide release (which mediates vasodilation, inhibits leukocyte-endothelial cell adhesion and prevents platelet aggregation27,28) and may enhance the anti-inflammatory effects of AT1R blockade.29 In HIV-uninfected individuals, telmisartan decreases vascular inflammation and improves FMD.30–33
In HIV-infected persons, reduced circulating inflammatory biomarker levels with telmisartan therapy suggest a potential beneficial effect on endothelial function34,35 that may be independent of its blood pressure-lowering effects; however, the effect of telmisartan on brachial artery FMD in HIV-infected individuals has not yet been assessed. We conducted a pilot study to assess the impact of telmisartan on brachial artery FMD, biomarkers associated with chronic inflammation, CVD and mortality in HIV infection, and levels of immune activation in older HIV-infected adults with traditional CVD risk factors.
METHODS
Study population
Participants were enrolled into this six-week, prospective, open-label, interventional pilot study between October 2012 and July 2013 at the University of California, Los Angeles (UCLA) Center for Clinical AIDS Research and Education. Inclusion criteria included: HIV infection, age ≥50 years, HIV-1 RNA <50 copies/mL at screening and for the twelve weeks prior to entry, stable ART for twelve weeks prior to entry, systolic blood pressure (SBP) >110 mmHg and one or more traditional CVD risk factor (smoking, hypertension, hyperlipidemia, diabetes mellitus). Individuals who reported smoking every day or some days within the past week at study entry were considered to be current smokers.36 Family history of CVD alone was not sufficient for entry. Exclusion criteria included: uncontrolled hypertension (defined as SBP >140 mmHg or diastolic blood pressure [DBP] >90 mmHg); current use of any other angiotensin receptor blocker (ARB), nelfinavir or etravirine; untreated renal artery stenosis; unstable heart disease; active, untreated opportunistic and/or AIDS-defining illness; absolute neutrophil count <750 cells/mm3; hemoglobin <10 g/dL; creatinine clearance <30 mL/min; aspartate transaminase (AST) or alanine transaminase (ALT) greater than three times the upper limit of normal; need for ongoing potassium supplementation; and history of intolerance to any member of the ARB class of agents. Participants on angiotensin converting enzyme inhibitors, lipid-lowering agents, thiazolidinediones and/or insulin-sensitizing agents for at least twelve weeks prior to entry were permitted to enroll, but asked not to titrate these medications during the study period.
Intervention
Enrolled participants received open-label telmisartan 80 mg by mouth daily for six weeks. The primary endpoint was six-week change in brachial artery FMD. Secondary objectives included assessment of six-week changes in blood pressure, lipid and glucose parameters, circulating inflammatory biomarker levels and lymphocyte and monocyte immuno-phenotyping. All assessments occurred in the fasting (nothing to eat or drink except water or medications for at least eight hours) state at weeks 0 and 6. All study documents and procedures were approved by the UCLA institutional review board and in accordance with the Declaration of Helsinki. All participants provided written informed consent prior to initiation of study procedures.
FMD measurement
Endothelial function was assessed by ultrasound brachial artery FMD measurement using a standardized imaging protocol to optimize accuracy and reproducibility.37 FMD ultrasound studies were performed at the University of Southern California Atherosclerosis Research Unit using a standardized protocol 23 by certified sonographers. All scans were performed in the morning, and participants were required to be fasting, abstain from exercise and not smoke tobacco for at least eight hours prior to FMD measurement, as previously described.38 After resting for ten minutes in a temperature-controlled (70–76°F) room, the diameter of the right brachial artery and baseline blood flow were measured. Increased forearm blood flow was induced by placing a pneumatic blood pressure tourniquet around the widest part of the forearm and inflating it to 250 mmHg for five minutes followed by deflation. Repeat brachial artery diameter and blood flow scans were obtained immediately after deflation. Digital images were transmitted via a secure server (Access Point Web software, Freeland Systems, Westminster, Colorado, USA) for interpretation by a single, experienced, centralized reader at the University of Wisconsin Atherosclerosis Imaging Research Program core lab. FMD was defined as the maximum ratio of the brachial artery diameter at 60 and 90 seconds after cuff release to the baseline (pre-occlusion) brachial artery diameter. In a multicenter study conducted using the same techniques in the same lab, blinded, paired readings of 25 FMD studies showed a median (interquartile range, IQR) difference of 0.20% (−0.47–0.49%).39
Immunologic and inflammatory biomarker testing
Concentrations of serum interleukin-6 (IL-6, sensitivity 0.5 pg/mL), adiponectin (sensitivity 4.8 ng/mL), soluble CD14 (sCD14, sensitivity 50.0 ng/mL) and soluble CD163 (sCD163, sensitivity 15.0 ng/mL) were measured by R&D Systems ELISAs; insulin via Roche Elecsys ELISA (sensitivity 0.2 μU/mL); and hyaluronic acid via Corgenix ELISA (sensitivity 50.0 ng/mL) at the University of Vermont Laboratory for Clinical Biochemistry Research under the direction of Dr. Russell Tracy. The homeostasis model assessment of insulin resistance (HOMA-IR) value was calculated as (glucose × insulin)/22.5. Plasma total receptor activator of nuclear factor kappa-B ligand (RANKL, sensitivity 0.5 pmol/L) was measured by Biovendor, LLC ELISA and osteoprotegerin (OPG, sensitivity 25.0 pg/mL) was measured by ALPCO Diagnostics ELISA. Blood for isolation and processing of peripheral blood mononuclear cells was collected, processed and stored according to AIDS Clinical Trials Group standards (https://www.hanc.info/labs/labresources/procedures/Pages/pbmcSop.aspx). Cellular immuno-phenotyping was performed for HLA-DR and CD38 expression on CD4+ and CD8+ T lymphocytes, and CD14 and CD16 expression on monocytes. RANKL, OPG and cellular immune-phenotyping were performed at UCLA.
Outcomes and adverse events
The primary endpoint was the median, within-person, six-week change in maximum relative FMD (%). Brachial artery diameter (absolute value in mm) was measured, as above. Secondary endpoints were median, within-person, six-week changes in: SBP, DBP, total cholesterol, low-density lipoprotein (LDL) cholesterol, high-density lipoprotein (HDL) cholesterol, triglycerides, glucose, circulating inflammatory markers and T lymphocyte and monocyte immuno-phenotypes, as enumerated above. Safety endpoints included reporting of all Grade ≥3 clinical events and Grade ≥2 lab abnormalities as adverse events. Grades were determined using the Division of AIDS Table for Grading the Severity of Adult and Pediatric Adverse Events (Version 1.0, December 2004). FMD values and inflammatory markers were compared between participants treated with protease inhibitor- (PI) vs. non-PI-based ART, abacavir vs. tenofovir use and by smoking status.
Statistical analyses
Sample size and power
Based on calculations by Benndorf et al,30 a 35% change in FMD with telmisartan therapy was expected to be clinically significant. Published improvements in FMD with telmisartan therapy in HIV-uninfected persons range from 18%–99% over 6–48 weeks.30–33 In the majority of studies, telmisartan was administered for 6–12 weeks and effect sizes were skewed towards the higher end of the range. To balance feasibility with the likelihood of seeing a clinically significant effect, we targeted an effect size of 50%. This degree of improvement is greater than the within-person variability of FMD (20–40%) and correlates with the 2% absolute change in vessel diameter felt to be clinically significant by experts in the field.30,38,40 Thirteen subjects provided 80% power to detect a 50% improvement in FMD over six weeks. Seventeen participants were targeted for enrollment to allow for a 20% loss to follow-up rate.
Analytic techniques
A pre-specified, as-treated analysis was performed, excluding subjects who did not remain on the study regimen (including any lapse of study treatment for ≥14 consecutive days) and/or did not have an observed primary endpoint. A supplemental intent-to-treat analysis was planned but not applicable (see Results, below).
Continuous variables are reported as median and IQR, and nominal data as absolute values and percentages. Pairwise comparisons were performed using the Wilcoxon signed rank test. Correlations were assessed using Spearman's rho. All statistical tests are two-sided with a nominal alpha level of 0.05. Since this is a pilot study, analyses were exploratory, without adjustment for multiple testing. Due to the small sample size (n=17), multivariate analysis was not feasible. However, bivariate logistic regression was performed to determine associations between clinical and demographic characteristics and outcome variables.
RESULTS
Participant characteristics
Twenty-three participants were screened, 17 of whom were eligible and enrolled. Baseline demographic and clinical characteristics are detailed in Table 1. Participants were predominantly male (88%), with median age 60 years, SBP 130 mmHg, DBP 72 mmHg, CD4+ T lymphocyte count 625 cells/mm3 and time since HIV diagnosis 19 years. Twenty-nine percent of participants had a pre-existing AIDS diagnosis. Regarding ART use, 71% were on a PI (75% ritonavir-boosted), 29% a non-nucleoside reverse transcriptase inhibitor (NNRTI), 29% integrase inhibitor, 29% abacavir and 65% tenofovir.
Table 1.
Baseline demographic and clinical characteristics.*
| Participants enrolled | N=17 |
|---|---|
|
| |
| Male sex | 88% (15) |
|
| |
| Race | |
| African American | 24% (4) |
| Hispanic | 41% (7) |
| White | 35% (6) |
|
| |
| Age (years) | 60 (54, 63) |
|
| |
| Body mass index (kg/m2) | 27 (27, 30) |
|
| |
| Current tobacco use | 18% (3) |
|
| |
| Blood Pressure (mmHg) | |
| Systolic | 130 (122, 138) |
| Diastolic | 72 (66, 83) |
|
| |
| Framingham risk score (%) | 10 (7, 12) |
|
| |
| CD4+ T lymphocyte count (cells/mm3) | 625 (413, 729) |
|
| |
| PI | 71% (12) |
| Atazanavir/ritonavir | 18% (3) |
| Atazanavir | 18% (3) |
| Darunavir/ritonavir | 18% (3) |
| Fosamprenavir/ritonavir | 6% (1) |
| Lopinavir/ritonavir | 12% (2) |
| NNRTI | 29% (5) |
| Efavirenz | 24% (4) |
| Nevirapine | 6% (1) |
| NRTI | 94% (16) |
| Abacavir | 29% (5) |
| Emtricitabine | 59% (10) |
| Lamivudine | 29% (5) |
| Tenofovir | 65% (11) |
| Zidovudine | 6% (1) |
| Integrase inhibitor (raltegravir) | 29% (5) |
| Entry inhibitor (maraviroc) | 6% (1) |
|
| |
| Glucose (mg/dL) | 96 (92, 105) |
|
| |
| Total cholesterol (mg/dL) | 166 (145,194) |
| Triglycerides (mg/dL) | 107 (79, 145) |
| LDL cholesterol (mg/dL) | 97 (70, 111) |
| HDL cholesterol (mg/dL) | 44 (39, 52) |
|
| |
| Diabetesa | 12% (2) |
|
| |
| Hypertensiona | 65% (11) |
|
| |
| Hyperlipidemiaa | 82% (14) |
Values are expressed as median (interquartile range) or percent (n).
Defined as self-reported diagnosis or on-therapy at baseline.
PI: protease inhibitor, NNRTI: non nucleoside reverse transcriptase inhibitor, NRTI: nucleoside reverse transcriptase inhibitor, LDL: low-density lipoprotein, HDL: high-density lipoprotein
CVD risk factors included 18% current smoking, 12% diabetes mellitus, 65% hypertension and 82% hyperlipidemia. Eleven of the seventeen participants (65%) were taking lipid-lowering therapy: nine participants (53%) were taking statins (six atorvastatin, two pravastatin, one rosuvastatin), three (18%) gemfibrozil and two (12%) niacin. Two participants were taking both statin and niacin therapy, and one participant was taking both a statin and gemfibrozil. Aspirin therapy was taken by eight (47%) participants, five of whom were also statin-treated. No significant differences between PI-treated and non-PI-treated participants were observed in CVD risk factor prevalence, age, baseline BP, lipid profile or HIV-related parameters. Participants treated with abacavir and tenofovir generally had similar characteristics, except abacavir-treated persons more frequently had diabetes mellitus (abacavir 40% (n=2) vs. tenofovir 0% (n=0), p=0.02) and had higher baseline SBP (138 mmHg vs. 125 mmHg, p=0.01) and DBP (87 mmHg vs. 67 mmHg, p=0.004).
Effects of telmisartan
All 17 participants received telmisartan 80 mg daily for six weeks. No treatment discontinuations or telmisartan-related adverse events occurred. One subject was diagnosed with a brachial vein thrombus after his week 0 FMD procedure that remained present at week 6. The thrombus was not believed to be related to study drug, but the week 6 cuff inflation was not performed for patient safety.
Blood pressure
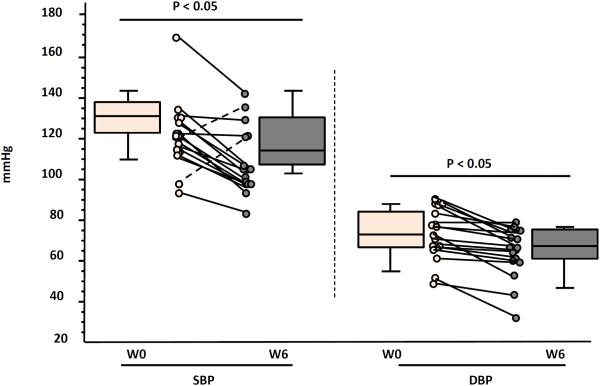
At week 6, SBP and DBP both significantly decreased (p≤0.005), with median declines of 16 mmHg (IQR −6.3, −23.8) and −6.0 mmHg (IQR −2.0, −14.5), respectively (Figure 1).
Figure 1.
Median six-week changes in blood pressure with telmisartan therapy.
FMD
FMD testing was performed for all participants at weeks 0 and 6, with the exception of the participant with the brachial vein thrombus. Median baseline brachial artery diameter and FMD were 4.8 mm (IQR 4.4, 4.9) and 2.7% (IQR 1.4, 3.9), respectively. Baseline brachial artery diameter did not vary by CVD risk factor profile or ART type. Baseline FMD tended to be lower in PI-treated vs. non-PI-treated participants (PI 2.7% vs. non-PI 8.4%, p=0.14), but did not vary by CVD risk factor profile (including current smoking status) or between abacavir vs. tenofovir co-treatment. A sub-analysis of the nine statin treated participants did not show a significant change in maximum relative FMD after six weeks of telmisartan therapy (p=0.26), and change in maximum relative FMD was not significantly different between statin-treated and non-statin-treated participants (p=0.19).
Six-week changes in maximum relative FMD are detailed in Table 2. Overall, no significant changes in brachial artery diameter (0% change from baseline, p=0.72) or FMD (0.7% absolute increase, 26% increase from baseline, p=0.60) were observed. No significant correlations were observed between change in FMD and any clinical or biological parameter. Specifically, no significant correlation was observed between the six-week change in maximum relative FMD and the 6-week change in SBP (p=0.65) or DBP (p=0.63).
Table 2.
Median baseline and six-week changes in brachial artery flow-mediated dilation on telmisartan therapy.*
| Overall | FMD (%) | Relative change | p-value£ |
|---|---|---|---|
| Week 0 | 2.7 (1.4, 3.9) | ||
| Week 6 | 3.5 (1.6, 6.0) | ||
| 6-week change | 0.7 (−1.3, 1.9) | 26% | 0.60 |
| On abacavir † | |||
| Week 0 | 2.7 (1.4, 3.4) | ||
| Week 6 | 5.7 (5.3, 6.3) | ||
| 6-week change | 2.3 (−1.2, 4.0) | 85% | 0.31 |
| On tenofovir | |||
| Week 0 | 2.9 (2.1, 8.4) | ||
| Week 6 | 3.5 (2.3, 3.8) | ||
| 6-week change | 0.8 (−0.3, 1.1) | 28% | 0.57 |
| On PI ⌘ | |||
| Week 0 | 2.7 (1.2, 3.5) | ||
| Week 6 | 3.8 (1.4, 6.3) | ||
| 6-week change | 1.1 (0.4, 4.0) | 41% | 0.08 |
| Not on PI | |||
| Week 0 | 8.4 (2.1, 10.4) | ||
| Week 6 | 3.3 (1.7, 3.5) | ||
| 6-week change | −1.2 (−4.9, −0.3) | −14% | 0.19 |
| Current smoker # | |||
| Week 0 | 2.1 (1.0, 10.4) | ||
| Week 6 | 1.7 (1.4, 3.3) | ||
| 6-week change | −0.3 (−7.1, 0.4) | −14% | 0.75 |
| Non-smoker | |||
| Week 0 | 2.9 (1.4, 3.9) | ||
| Week 6 | 3.8 (2.3, 6.3) | ||
| 6-week change | 1.0 (−1.2, 2.3) | 34% | 0.38 |
Values expressed as median (interquartile range).
Wilcoxon signed rank value for six-week change.
p=0.52 vs. tenofovir
p=0.02 vs. not on a protease inhibitor
p=0.15 vs. non-smoker
FMD: flow-mediated dilation; PI: protease inhibitor
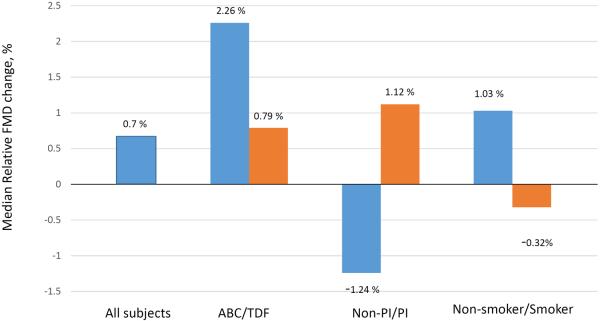
In subset analysis, a 41% six-week increase in FMD was observed among PI-treated participants (p=0.08) that was significantly different than the 14% decline observed among non-PI-treated participants (p=0.19; between-group p=0.02, Figure 2). Additionally, an 85% six-week increase in FMD was observed among abacavir-treated participants (p=0.31). As most abacavir-treated participants (n=4/5) were also PI-treated, we divided the twelve PI-treated participants into abacavir-treated (n=4) and non-abacavir-treated (n=8) groups, and the increase in FMD remained greater among PI-treated participants who were also abacavir-treated (103% vs. 43%, p=0.34). Non-smokers had an increase in FMD compared to current smokers (34% vs. −14%, p= 0.15).
Figure 2.
Median six-week changes in brachial artery flow-mediated dilation with telmisartan therapy.
Metabolic and inflammatory parameters
Six-week changes in metabolic, immunologic and inflammatory parameters are detailed in Table 3. Overall, no significant changes in fasting total cholesterol, LDL cholesterol, HDL cholesterol, triglycerides, glucose, insulin, HOMA-IR score or adiponectin were observed after six weeks of telmisartan therapy, although a trend towards an increase in adiponectin (p=0.06) was seen among PI-treated participants. No significant overall changes in hyaluronic acid, sCD14, sCD163, IL-6, RANKL, OPG, RANKL/OPG ratio or activated T lymphocyte and monocyte populations were observed. However, in the subgroup of PI-treated individuals, a significant six-week decline in RANKL (p=0.008) was observed without a significant change in OPG level or RANK/OPG ratio. In the subgroup of abacavir-treated patients, IL-6 decreased significantly (p=0.04) and hyaluronic acid tended to increase (p=0.08).
Table 3.
Median baseline and six-week changes in metabolic and immuno-inflammatory markers with telmisartan therapy.*
| Week 0 | Week 6 | p-value† | |
|---|---|---|---|
| Total cholesterol (mg/dL) | 166.0 (148.0, 193.0) | 154.0 (138.0, 181.0) | 0.65 |
| LDL cholesterol (mg/dL) | 97.0 (70.0, 111.0) | 90.0 (78.0, 121.0) | 0.34 |
| HDL cholesterol (mg/dL) | 44.4 (38.7, 52.1) | 44.1 (39.7, 49.0) | 0.83 |
| Triglycerides (mg/dL) | 107.0 (80.0, 132.0) | 116.0 (73.0, 141.0) | 0.81 |
| Glucose (mg/dL) | 96.0 (92.0, 104.0) | 97.0 (88.0, 105.0) | 1.0 |
| HOMA-IR score | 2.7 (2.1, 7.0) | 2.6 (1.7, 6.0) | 0.19 |
| Insulin (uU/mL) | 12.1 (9.2, 27.1) | 12.3 (8.2, 19.8) | 0.22 |
| Adiponectin (ng/mL) | 9645.8 (6565.2, 13618.9) | 9560.1 (6195.7, 15841.0) | 0.19 |
| Hyaluronic acid (ng/mL) | 47.0 (33.9, 61.8) | 52.9 (43.1, 80.0) | 0.15 |
| IL-6 (pg/L) | 2.4 (1.5, 3.8) | 2.5 (1.8, 3.7) | 0.96 |
| RANKL (pmol/L) | 155.6 (90.6, 189.8) | 148.4 (62.9, 174.9) | 0.15 |
| OPG (pmol/L) | 6.1 (4.7, 7.3) | 5.4 (4.4, 7.1) | 0.93 |
| RANKL/OPG ratio | 25.4 (16.5, 35.5) | 21.6 (10.6, 37.5) | 0.35 |
| sCD14 (ng/mL) | 1844.5 (1662.4, 2196.4) | 1842.6 (1682.4, 2201.2) | 0.93 |
| sCD163 (ng/mL) | 739.2 (630.9, 1033.4) | 699.3 (613.2, 1005.7) | 0.22 |
| CD8+CD38+HLA-DR+ T lymphocytes (%) | 12.0 (9.7, 15.8) | 10.4 (7.5, 14.7) | 0.17 |
| CD4+CD38+HLA-DR+ T lymphocytes (%) | 11.0 (6.6, 12.5) | 8.8 (6.6, 20.5) | 0.75 |
| CD14+CD16+ monocytes (%) | 4.4 (3.1, 6.8) | 3.7 (2.6, 4.7) | 0.10 |
| CD14+lowCD16+high monocytes (%) | 31.0 (25.6, 54.1) | 37.8 (25.4, 56.2) | 0.89 |
Values are expressed as median (interquartile range).
Wilcoxon signed rank test value for six-week change.
LDL: low-density lipoprotein, HDL: high-density lipoprotein, HOMA-IR: homeostatic model assessment of insulin resistance, IL-6: interleukin-6, RANKL: receptor activator of nuclear factor-kappa-B ligand, OPG: osteoprotegerin, sCD14: soluble CD14, sCD163: soluble CD163
DISCUSSION
To our knowledge, this is the first interventional study to assess the impact of standard-dose telmisartan on brachial artery FMD in older HIV-infected individuals with traditional CVD risk factors. Our participants were primarily at risk for CVD from dyslipidemia (82%) and obesity (41%). The median baseline FMD in our cohort was low (2.7%) and suggestive of endothelial dysfunction. Sawada et al.19 reported a similar FMD value (2.3%) in HIV-uninfected patients with acute coronary syndrome or stable angina and mean age 68.9 years. Participants in our study were almost 10 years younger (mean 59.2 years), highlighting the increased risk of CVD for age previously reported in HIV infection.5
We also observed a trend towards lower baseline FMD in PI-treated (2.7 %) vs. non-PI-treated (8.4%) persons. Stein et al.7 demonstrated a similar FMD impairment among PI-treated persons (2.6% vs. 8.1% non-PI-treated), although PI-treated persons in that study had higher total cholesterol and triglyceride levels. In our small study, the number and type of CVD risk factors, age, blood pressure, lipid profile, body mass index and prevalence of lipodystrophy did not vary by PI treatment status, suggesting PI-treated persons have impaired endothelial function that cannot be explained by traditional CVD or other HIV-specific risk factors. This finding may have a unique physiological basis in treated HIV infection, as PIs may increase CVD risk via activation of the reninangiotensin system (RAS).41
Interestingly, we also observed greater improvements in endothelial function in PI-treated and abacavir-treated participants and non-smokers, suggesting that these groups may receive greater benefit from telmisartan. Activation of the RAS by PIs and the persistent impairment of endothelial function by cigarette smoking are potential explanations for the effects seen in these sub-groups. Additionally, PI therapy may increase oxidative stress,42 increasing oxidized LDL levels and foam cell formation and facilitating atherogenesis. AT1R blockade with telmisartan may interrupt oxidized LDL-induced foam cell formation,43 and PI-associated lipid perturbations (and their downstream pro-inflammatory effects) may be mediated through PPAR-ɣ,44 the sum of which may help explain why PI-treated participants in our study experienced greater FMD improvements with telmisartan therapy.
The mechanism underlying the greater improvement in FMD in abacavir-treated participants is less clear and beyond the scope of this study. Additionally, there are no published data on potential interactions between abacavir and either the RAS or PPAR–ɣ. However, endothelial damage promotes the mobilization and recruitment of inflammatory cells to the vascular wall, and angiotensin II stimulates AT1R-mediated leukocyte-endothelial cell interactions. Although data are conflicting,45,46 increasing evidence suggests that abacavir may contribute to endothelial dysfunction via increased leukocyte-endothelial cell interactions,47,48 and we cannot rule out the possibility that this is mediated through interactions with the RAS. Additionally, abacavir may increase platelet activation by blunting the effect of nitric oxide on platelets,49 and telmisartan-induced nitric oxide release could possibly overcome this effect of abacavir.
A secondary endpoint of our study was to explore changes in circulating inflammatory biomarkers and T lymphocyte and monocyte profiles with telmisartan treatment, with the goal of gaining mechanistic insights into observed changes in FMD. While studies have reported anti-inflammatory effects of ARBs beyond their blood pressure-lowering effects,50–52 we did not observe any overall, six-week impact of telmisartan on circulating inflammatory biomarkers or peripheral T lymphocyte or monocyte activation. One explanation for this finding may be the short treatment period of this study, as most clinical studies have demonstrated anti-inflammatory benefits after 3–6 months of treatment.53–55
Despite a lack of statistically significant changes in measures of inflammation and immune activation in the overall group, a significant six-week decrease in IL-6 was observed in abacavir-treated participants. IL-6 is a pro-inflammatory cytokine that has been associated with CVD and mortality in HIV infection.56 HIV-infected individuals initiating abacavir-containing ART may experience smaller decreases in systemic inflammation compared to non-abacavir-containing regimens;57 therefore, this group could derive additional benefit from telmisartan therapy. However, these findings and hypotheses warrant further exploration.
PI-treated individuals experienced significant reductions in RANKL levels without changes in OPG or RANKL/OPG ratios. RANKL/OPG axis dysregulation has been postulated to mediate vascular calcification58–60 and plaque destabilization and rupture.61 Additionally, in vitro experiments have demonstrated that angiotensin II induces vascular calcification through RANKL activation, and that RANKL enhances AT1R expression.62 Thus, RAS inhibition with telmisartan could affect vascular remodeling through RANKL/OPG axis modulation. We observed a decrease in RANKL with telmisartan therapy in PI-treated participants only, and published data linking the RANKL/OPG axis with CVD in the setting of HIV infection are conflicting.63,64 As such, further studies are needed.
Finally, we observed a trend towards increased adiponectin levels in PI-treated participants. Adiponectin is regulated by PPAR-γ and its anti-inflammatory effects result from increased nitric oxide production.65 Lower adiponectin levels have been associated with increased coronary artery calcification in HIV-infected men.66 Our data suggest that telmisartan could affect FMD through nitric oxide release and inhibition of vasoconstriction. However, in our population with known CVD risk factors, simultaneous exposure to other drugs with anti-inflammatory properties (aspirin, statins, anti-hypertensive agents) could confound results, and our sample size was too small to fully adjust for concomitant anti-inflammatory medication use. While the anti-inflammatory benefits of ARBs may overlap or be intertwined with those of PPAR-γ agonism,67–70 larger, longitudinal studies are needed to better assess the impact of telmisartan on inflammatory and immune markers.
This pilot study has obvious limitations. FMD was low at baseline, making an effect size of 50% difficult to achieve with a small sample size. The initial sample size calculation was based upon the observed effects of telmisartan on FMD in HIV-uninfected persons with essential hypertension over a similar time frame; however, since participants in our study were normotensive or had controlled hypertension at baseline and we primarily aimed to assess effects of telmisartan on endothelial function beyond blood pressure-lowering effects, a smaller observed effect size not unexpected. The small sample size also prevented effective multivariable modeling and more elaborate sub-group characterization. The short follow-up time prevented assessment of longer-term effect(s) of telmisartan therapy on vascular function, a relevant issue given the increasing life expectancy of HIV-infected adults on suppressive ART. Although brachial artery ultrasound represents a useful method to identify persons with asymptomatic atherosclerosis and increased risk of athero-thrombotic complications, its use in research and routine clinical practice remains limited due to intrinsic variability and the need for skilled operators. Lastly, while sub-group analysis suggested that PI-treated, abacavir-treated and non-smoking persons may receive greater benefit from telmisartan therapy, these analyses were exploratory and must be interpreted with caution. Thus, further studies are needed to confirm the benefit of telmisartan on endothelial function in treated HIV infection, particularly in older persons at risk for CVD.
Conclusions
Our pilot study did not show an overall effect of telmisartan therapy on FMD; however, in older HIV-infected adults with traditional CVD risk factors, persons treated with PIs and abacavir as well as non-smokers received greater benefit. Since CVD is a leading cause of morbidity and mortality in HIV-infected persons, effective CVD prevention and long-term management strategies are needed. Telmisartan's dual AT1R blockade and PPAR-γ agonism makes it an appealing potential therapy for CVD and other metabolic disorders in treated HIV infection, but additional, prospective, randomized, longitudinal studies are needed to better define the potential clinical benefits of telmisartan in this population.
REFERENCES
- 1.Currier JS, Taylor A, Boyd F, et al. Coronary heart disease in HIV-infected individuals. Journal of acquired immune deficiency syndromes. 2003;33(4):506–512. doi: 10.1097/00126334-200308010-00012. [DOI] [PubMed] [Google Scholar]
- 2.Guaraldi G, Orlando G, Zona S, et al. Premature age-related comorbidities among HIV-infected persons compared with the general population. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America. 2011;53(11):1120–1126. doi: 10.1093/cid/cir627. [DOI] [PubMed] [Google Scholar]
- 3.Durand M, Sheehy O, Baril JG, Lelorier J, Tremblay CL. Association between HIV infection, antiretroviral therapy, and risk of acute myocardial infarction: a cohort and nested case-control study using Quebec's public health insurance database. Journal of acquired immune deficiency syndromes. 2011;57(3):245–253. doi: 10.1097/QAI.0b013e31821d33a5. [DOI] [PubMed] [Google Scholar]
- 4.Islam FM, Wu J, Jansson J, Wilson DP. Relative risk of cardiovascular disease among people living with HIV: a systematic review and meta-analysis. HIV medicine. 2012;13(8):453–468. doi: 10.1111/j.1468-1293.2012.00996.x. [DOI] [PubMed] [Google Scholar]
- 5.Currier JS. Update on cardiovascular complications in HIV infection. Topics in HIV medicine : a publication of the International AIDS Society, USA. 2009;17(3):98–103. [PubMed] [Google Scholar]
- 6.Friis-Moller N, Sabin CA, Weber R, et al. Combination antiretroviral therapy and the risk of myocardial infarction. The New England journal of medicine. 2003;349(21):1993–2003. doi: 10.1056/NEJMoa030218. [DOI] [PubMed] [Google Scholar]
- 7.Stein JH, Klein MA, Bellehumeur JL, et al. Use of human immunodeficiency virus-1 protease inhibitors is associated with atherogenic lipoprotein changes and endothelial dysfunction. Circulation. 2001;104(3):257–262. doi: 10.1161/01.cir.104.3.257. [DOI] [PubMed] [Google Scholar]
- 8.Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. The Journal of clinical endocrinology and metabolism. 2007;92(7):2506–2512. doi: 10.1210/jc.2006-2190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Celermajer DS, Sorensen KE, Georgakopoulos D, et al. Cigarette smoking is associated with dose-related and potentially reversible impairment of endothelium-dependent dilation in healthy young adults. Circulation. 1993;88(5 Pt 1):2149–2155. doi: 10.1161/01.cir.88.5.2149. [DOI] [PubMed] [Google Scholar]
- 10.Vogel RA, Corretti MC, Plotnick GD. Changes in flow-mediated brachial artery vasoactivity with lowering of desirable cholesterol levels in healthy middle-aged men. The American journal of cardiology. 1996;77(1):37–40. doi: 10.1016/s0002-9149(97)89131-x. [DOI] [PubMed] [Google Scholar]
- 11.Ludmer PL, Selwyn AP, Shook TL, et al. Paradoxical vasoconstriction induced by acetylcholine in atherosclerotic coronary arteries. The New England journal of medicine. 1986;315(17):1046–1051. doi: 10.1056/NEJM198610233151702. [DOI] [PubMed] [Google Scholar]
- 12.Glagov S, Weisenberg E, Zarins CK, Stankunavicius R, Kolettis GJ. Compensatory enlargement of human atherosclerotic coronary arteries. The New England journal of medicine. 1987;316(22):1371–1375. doi: 10.1056/NEJM198705283162204. [DOI] [PubMed] [Google Scholar]
- 13.Bentzon JF, Otsuka F, Virmani R, Falk E. Mechanisms of plaque formation and rupture. Circulation research. 2014;114(12):1852–1866. doi: 10.1161/CIRCRESAHA.114.302721. [DOI] [PubMed] [Google Scholar]
- 14.Charakida M, Masi S, Luscher TF, Kastelein JJ, Deanfield JE. Assessment of atherosclerosis: the role of flow-mediated dilatation. European heart journal. 2010;31(23):2854–2861. doi: 10.1093/eurheartj/ehq340. [DOI] [PubMed] [Google Scholar]
- 15.Patel S, Celermajer DS. Assessment of vascular disease using arterial flow mediated dilatation. Pharmacological reports : PR. 2006;58(Suppl):3–7. [PubMed] [Google Scholar]
- 16.Celermajer DS, Sorensen KE, Gooch VM, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340(8828):1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 17.Clarkson P, Celermajer DS, Donald AE, et al. Impaired vascular reactivity in insulin-dependent diabetes mellitus is related to disease duration and low density lipoprotein cholesterol levels. Journal of the American College of Cardiology. 1996;28(3):573–579. doi: 10.1016/0735-1097(96)82380-1. [DOI] [PubMed] [Google Scholar]
- 18.Hafner F, Kieninger A, Meinitzer A, et al. Endothelial dysfunction and brachial intima-media thickness: long term cardiovascular risk with claudication related to peripheral arterial disease: a prospective analysis. PloS one. 2014;9(4):e93357. doi: 10.1371/journal.pone.0093357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sawada T, Emoto T, Motoji Y, et al. Possible association between non-invasive parameter of flow-mediated dilatation in brachial artery and whole coronary plaque vulnerability in patients with coronary artery disease. International journal of cardiology. 2013;166(3):613–620. doi: 10.1016/j.ijcard.2011.11.101. [DOI] [PubMed] [Google Scholar]
- 20.Anderson TJ, Uehata A, Gerhard MD, et al. Close relation of endothelial function in the human coronary and peripheral circulations. Journal of the American College of Cardiology. 1995;26(5):1235–1241. doi: 10.1016/0735-1097(95)00327-4. [DOI] [PubMed] [Google Scholar]
- 21.Takase B, Uehata A, Akima T, et al. Endothelium-dependent flow-mediated vasodilation in coronary and brachial arteries in suspected coronary artery disease. The American journal of cardiology. 1998;82(12):1535–1539. A1537–1538. doi: 10.1016/s0002-9149(98)00702-4. [DOI] [PubMed] [Google Scholar]
- 22.Charakida M, Donald AE, Green H, et al. Early structural and functional changes of the vasculature in HIV-infected children: impact of disease and antiretroviral therapy. Circulation. 2005;112(1):103–109. doi: 10.1161/CIRCULATIONAHA.104.517144. [DOI] [PubMed] [Google Scholar]
- 23.Torriani FJ, Komarow L, Parker RA, et al. Endothelial function in human immunodeficiency virus-infected antiretroviral-naive subjects before and after starting potent antiretroviral therapy: The ACTG (AIDS Clinical Trials Group) Study 5152s. Journal of the American College of Cardiology. 2008;52(7):569–576. doi: 10.1016/j.jacc.2008.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Blanco JJ, Garcia IS, Cerezo JG, et al. Endothelial function in HIV-infected patients with low or mild cardiovascular risk. The Journal of antimicrobial chemotherapy. 2006;58(1):133–139. doi: 10.1093/jac/dkl190. [DOI] [PubMed] [Google Scholar]
- 25.Hurlimann D, Chenevard R, Ruschitzka F, et al. Effects of statins on endothelial function and lipid profile in HIV infected persons receiving protease inhibitor-containing anti-retroviral combination therapy: a randomised double blind crossover trial. Heart. 2006;92(1):110–112. doi: 10.1136/hrt.2004.056523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Masia M, Martinez E, Padilla S, Gatell JM, Gutierrez F. Endothelial function in HIV-infected patients switching from a boosted protease inhibitor-based regimen to raltegravir: a substudy of the SPIRAL study. The Journal of antimicrobial chemotherapy. 2013;68(2):409–413. doi: 10.1093/jac/dks412. [DOI] [PubMed] [Google Scholar]
- 27.Naseem KM. The role of nitric oxide in cardiovascular diseases. Molecular aspects of medicine. 2005;26(1–2):33–65. doi: 10.1016/j.mam.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 28.Polikandriotis JA, Mazzella LJ, Rupnow HL, Hart CM. Peroxisome proliferator-activated receptor gamma ligands stimulate endothelial nitric oxide production through distinct peroxisome proliferator-activated receptor gamma-dependent mechanisms. Arteriosclerosis, thrombosis, and vascular biology. 2005;25(9):1810–1816. doi: 10.1161/01.ATV.0000177805.65864.d4. [DOI] [PubMed] [Google Scholar]
- 29.Lai KN, Chan LY, Guo H, Tang SC, Leung JC. Additive effect of PPAR-gamma agonist and ARB in treatment of experimental IgA nephropathy. Pediatr Nephrol. 2011;26(2):257–266. doi: 10.1007/s00467-010-1703-y. [DOI] [PubMed] [Google Scholar]
- 30.Benndorf RA, Appel D, Maas R, Schwedhelm E, Wenzel UO, Boger RH. Telmisartan improves endothelial function in patients with essential hypertension. Journal of cardiovascular pharmacology. 2007;50(4):367–371. doi: 10.1097/FJC.0b013e31811dfbe7. [DOI] [PubMed] [Google Scholar]
- 31.Jung AD, Kim W, Park SH, et al. The effect of telmisartan on endothelial function and arterial stiffness in patients with essential hypertension. Korean circulation journal. 2009;39(5):180–184. doi: 10.4070/kcj.2009.39.5.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wago T, Yoshimoto T, Akaza I, et al. Improvement of endothelial function in patients with hypertension and type 2 diabetes after treatment with telmisartan. Hypertension research : official journal of the Japanese Society of Hypertension. 2010;33(8):796–801. doi: 10.1038/hr.2010.107. [DOI] [PubMed] [Google Scholar]
- 33.Perl S, Schmolzer I, Sourij H, et al. Telmisartan improves vascular function independently of metabolic and antihypertensive effects in hypertensive subjects with impaired glucose tolerance. International journal of cardiology. 2010;139(3):289–296. doi: 10.1016/j.ijcard.2008.10.048. [DOI] [PubMed] [Google Scholar]
- 34.Vecchiet J, Ucciferri C, Falasca K, Mancino P, Di Iorio A, De Caterina R. Antihypertensive and metabolic effects of telmisartan in hypertensive HIV-positive patients. Antiviral therapy. 2011;16(5):639–645. doi: 10.3851/IMP1809. [DOI] [PubMed] [Google Scholar]
- 35.Ucciferri C, Falasca K, Mancino P, Di Iorio A, Vecchiet J. Microalbuminuria and hypertension in HIV-infected patients: a preliminary study of telmisartan. European review for medical and pharmacological sciences. 2012;16(4):491–498. [PubMed] [Google Scholar]
- 36.Centers for Disease C, Prevention State-specific secondhand smoke exposure and current cigarette smoking among adults - United States, 2008. MMWR Morb Mortal Wkly Rep. 2009;58(44):1232–1235. [PubMed] [Google Scholar]
- 37.Donald AE, Halcox JP, Charakida M, et al. Methodological approaches to optimize reproducibility and power in clinical studies of flow-mediated dilation. Journal of the American College of Cardiology. 2008;51(20):1959–1964. doi: 10.1016/j.jacc.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 38.Corretti MC, Anderson TJ, Benjamin EJ, et al. Guidelines for the ultrasound assessment of endothelial-dependent flow-mediated vasodilation of the brachial artery: a report of the International Brachial Artery Reactivity Task Force. Journal of the American College of Cardiology. 2002;39(2):257–265. doi: 10.1016/s0735-1097(01)01746-6. [DOI] [PubMed] [Google Scholar]
- 39.Stein JH, Brown TT, Ribaudo HJ, et al. Ultrasonographic measures of cardiovascular disease risk in antiretroviral treatment-naive individuals with HIV infection. Aids. 2013;27(6):929–937. doi: 10.1097/QAD.0b013e32835ce27e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sorenson KE CD, Spiegelhalter DJ, Georgakopoulos D, Robinson J, Thomas O, Deanfield JE. Non-invasive measurement of human endothelium dependent arterial responses: accuracy and reproducibility. British Heart Journal. 1995;74:247–253. doi: 10.1136/hrt.74.3.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boccara F, Auclair M, Cohen A, et al. HIV protease inhibitors activate the adipocyte renin angiotensin system. Antiviral therapy. 2010;15(3):363–375. doi: 10.3851/IMP1533. [DOI] [PubMed] [Google Scholar]
- 42.Masia M, Padilla S, Bernal E, et al. Influence of antiretroviral therapy on oxidative stress and cardiovascular risk: a prospective cross-sectional study in HIV-infected patients. Clin Ther. 2007;29(7):1448–1455. doi: 10.1016/j.clinthera.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 43.Rafatian N, Milne RW, Leenen FH, Whitman SC. Role of renin-angiotensin system in activation of macrophages by modified lipoproteins. Am J Physiol Heart Circ Physiol. 2013;305(9):H1309–1320. doi: 10.1152/ajpheart.00826.2012. [DOI] [PubMed] [Google Scholar]
- 44.Mencarelli A, Francisci D, Renga B, et al. Ritonavir-induced lipoatrophy and dyslipidaemia is reversed by the anti-inflammatory drug leflunomide in a PPAR-gamma-dependent manner. Antiviral therapy. 2012;17(4):669–678. doi: 10.3851/IMP2039. [DOI] [PubMed] [Google Scholar]
- 45.Wohl DA, Arnoczy G, Fichtenbaum CJ, et al. Comparison of cardiovascular disease risk markers in HIV-infected patients receiving abacavir and tenofovir: the nucleoside inflammation, coagulation and endothelial function (NICE) study. Antiviral therapy. 2014;19(2):141–147. doi: 10.3851/IMP2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hatano H, Scherzer R, Wu Y, et al. A randomized controlled trial assessing the effects of raltegravir intensification on endothelial function in treated HIV infection. Journal of acquired immune deficiency syndromes. 2012;61(3):317–325. doi: 10.1097/QAI.0b013e31826e7d0f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Pablo C, Orden S, Peris JE, Barrachina MD, Esplugues JV, Alvarez A. Profile of leukocyte-endothelial cell interactions induced in venules and arterioles by nucleoside reverse-transcriptase inhibitors in vivo. The Journal of infectious diseases. 2013;208(9):1448–1453. doi: 10.1093/infdis/jit340. [DOI] [PubMed] [Google Scholar]
- 48.De Pablo C, Orden S, Calatayud S, Marti-Cabrera M, Esplugues JV, Alvarez A. Differential effects of tenofovir/emtricitabine and abacavir/lamivudine on human leukocyte recruitment. Antiviral therapy. 2012;17(8):1615–1619. doi: 10.3851/IMP2357. [DOI] [PubMed] [Google Scholar]
- 49.Falcinelli E, Francisci D, Belfiori B, et al. In vivo platelet activation and platelet hyperreactivity in abacavir-treated HIV-infected patients. Thrombosis and haemostasis. 2013;110(2):349–357. doi: 10.1160/TH12-07-0504. [DOI] [PubMed] [Google Scholar]
- 50.De Ciuceis C, Flati V, Rossini C, et al. Effect of antihypertensive treatments on insulin signalling in lympho-monocytes of essential hypertensive patients: a pilot study. Blood pressure. 2014;23(6):330–338. doi: 10.3109/08037051.2014.901021. [DOI] [PubMed] [Google Scholar]
- 51.Yamaguchi K, Wakatsuki T, Soeki T, et al. Effects of telmisartan on inflammatory cytokines and coronary plaque component as assessed on integrated backscatter intravascular ultrasound in hypertensive patients. Circulation journal : official journal of the Japanese Circulation Society. 2014;78(1):240–247. doi: 10.1253/circj.cj-13-0741. [DOI] [PubMed] [Google Scholar]
- 52.Klinghammer L, Urschel K, Cicha I, et al. Impact of telmisartan on the inflammatory state in patients with coronary atherosclerosis--influence on IP-10, TNF-alpha and MCP-1. Cytokine. 2013;62(2):290–296. doi: 10.1016/j.cyto.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 53.Marketou ME, Kontaraki JE, Tsakountakis NA, et al. Differential effect of telmisartan and amlodipine on monocyte chemoattractant protein-1 and peroxisome proliferator-activated receptor-gamma gene expression in peripheral monocytes in patients with essential hypertension. The American journal of cardiology. 2011;107(1):59–63. doi: 10.1016/j.amjcard.2010.08.048. [DOI] [PubMed] [Google Scholar]
- 54.Chujo D, Yagi K, Asano A, et al. Telmisartan treatment decreases visceral fat accumulation and improves serum levels of adiponectin and vascular inflammation markers in Japanese hypertensive patients. Hypertension research : official journal of the Japanese Society of Hypertension. 2007;30(12):1205–1210. doi: 10.1291/hypres.30.1205. [DOI] [PubMed] [Google Scholar]
- 55.Lake JE, Tseng CH, Currier JS. A pilot study of telmisartan for visceral adiposity in HIV infection: the metabolic abnormalities, telmisartan, and HIV infection (MATH) trial. PloS one. 2013;8(3):e58135. doi: 10.1371/journal.pone.0058135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kuller LH, Tracy R, Belloso W, et al. Inflammatory and coagulation biomarkers and mortality in patients with HIV infection. PLoS medicine. 2008;5(10):e203. doi: 10.1371/journal.pmed.0050203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hileman CO, Wohl DA, Tisch DJ, Debanne SM, McComsey GA. Short communication: initiation of an abacavir-containing regimen in HIV-infected adults is associated with a smaller decrease in inflammation and endothelial activation markers compared to non-abacavir-containing regimens. AIDS research and human retroviruses. 2012;28(12):1561–1564. doi: 10.1089/aid.2012.0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Anand DV, Lahiri A, Lim E, Hopkins D, Corder R. The relationship between plasma osteoprotegerin levels and coronary artery calcification in uncomplicated type 2 diabetic subjects. Journal of the American College of Cardiology. 2006;47(9):1850–1857. doi: 10.1016/j.jacc.2005.12.054. [DOI] [PubMed] [Google Scholar]
- 59.Abedin M, Omland T, Ueland T, et al. Relation of osteoprotegerin to coronary calcium and aortic plaque (from the Dallas Heart Study) The American journal of cardiology. 2007;99(4):513–518. doi: 10.1016/j.amjcard.2006.08.064. [DOI] [PubMed] [Google Scholar]
- 60.Mohammadpour AH, Shamsara J, Nazemi S, Ghadirzadeh S, Shahsavand S, Ramezani M. Evaluation of RANKL/OPG Serum Concentration Ratio as a New Biomarker for Coronary Artery Calcification: A Pilot Study. Thrombosis. 2012;2012:306263. doi: 10.1155/2012/306263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kiechl S, Schett G, Schwaiger J, et al. Soluble receptor activator of nuclear factor-kappa B ligand and risk for cardiovascular disease. Circulation. 2007;116(4):385–391. doi: 10.1161/CIRCULATIONAHA.106.686774. [DOI] [PubMed] [Google Scholar]
- 62.Osako MK, Nakagami H, Shimamura M, et al. Cross-talk of receptor activator of nuclear factor-kappaB ligand signaling with renin-angiotensin system in vascular calcification. Arteriosclerosis, thrombosis, and vascular biology. 2013;33(6):1287–1296. doi: 10.1161/ATVBAHA.112.301099. [DOI] [PubMed] [Google Scholar]
- 63.Hwang JJ, Wei J, Abbara S, Grinspoon SK, Lo J. Receptor activator of nuclear factor-kappaB ligand (RANKL) and its relationship to coronary atherosclerosis in HIV patients. Journal of acquired immune deficiency syndromes. 2012;61(3):359–363. doi: 10.1097/QAI.0b013e31826a6c16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kelesidis T, Kendall MA, Yang OO, Hodis H, Currier JS. Perturbations of circulating levels of RANKL-osteoprotegerin axis in relation to lipids and progression of atherosclerosis in HIV-infected and -uninfected adults: ACTG NWCS 332/A5078 Study. AIDS research and human retroviruses. 2013;29(6):938–948. doi: 10.1089/aid.2012.0305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Urbich C, Heeschen C, Aicher A, Dernbach E, Zeiher AM, Dimmeler S. Relevance of monocytic features for neovascularization capacity of circulating endothelial progenitor cells. Circulation. 2003;108(20):2511–2516. doi: 10.1161/01.CIR.0000096483.29777.50. [DOI] [PubMed] [Google Scholar]
- 66.Ketlogetswe KS, Post WS, Li X, et al. Lower adiponectin is associated with subclinical cardiovascular disease among HIV-infected men. Aids. 2014;28(6):901–909. doi: 10.1097/QAD.0000000000000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Lindholm LH, Ibsen H, Dahlof B, et al. Cardiovascular morbidity and mortality in patients with diabetes in the Losartan Intervention For Endpoint reduction in hypertension study (LIFE): a randomised trial against atenolol. Lancet. 2002;359(9311):1004–1010. doi: 10.1016/S0140-6736(02)08090-X. [DOI] [PubMed] [Google Scholar]
- 68.Pfeffer MA, Swedberg K, Granger CB, et al. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet. 2003;362(9386):759–766. doi: 10.1016/s0140-6736(03)14282-1. [DOI] [PubMed] [Google Scholar]
- 69.Lithell H, Hansson L, Skoog I, et al. The Study on Cognition and Prognosis in the Elderly (SCOPE): principal results of a randomized double-blind intervention trial. Journal of hypertension. 2003;21(5):875–886. doi: 10.1097/00004872-200305000-00011. [DOI] [PubMed] [Google Scholar]
- 70.Berger JP, Akiyama TE, Meinke PT. PPARs: therapeutic targets for metabolic disease. Trends in pharmacological sciences. 2005;26(5):244–251. doi: 10.1016/j.tips.2005.03.003. [DOI] [PubMed] [Google Scholar]