Abstract
Various neurodegenerative disorders are ascribed to pathogenic molecular processes involving conformational transitions of amyloidogenic proteins into toxic aggregates characterized by their β structures. Accumulating evidence indicates that neuronal cell membranes provide platforms for such conformational transitions of pathogenic proteins as best exemplified by amyloid β (Aβ). Therefore, membrane-bound Aβ species can be promising targets for the development of novel drugs for Alzheimer’s disease. In the present study, solid-state nuclear magnetic resonance spectroscopy has elucidated the membrane-induced conformation of Aβ, in which the disordered N-terminal segment is followed by the stable C-terminal β strand. The data provides an insight into the molecular processes of the conformational transition of Aβ coupled with its assembly into parallel β structures.
Introduction
Various neurodegenerative disorders are ascribed to pathogenic molecular processes involving conformational transitions of amyloidogenic proteins into toxic aggregates characterized by their β structures [1,2]. Amyloid β (Aβ) is a major player in the onset and development of Alzheimer’s disease (AD) [3]. The major isoform of this protein is composed of 40 or 42 amino acid residues, which is proteolytically cleaved from its precursor membrane protein. Aβ has a high propensity to form cross-β-fibrils [4–11], which are supposed to be a major component of senile plaque as a hallmark of AD. Accumulating evidence indicates that neuronal cell membranes provide platforms for conformational transition and subsequent aggregation of pathogenic proteins, including Aβ [12]. In particular, the formation of toxic Aβ aggregates is facilitated in membrane environments containing GM1 ganglioside, a glycosphingolipid abundant in neuronal cell membranes [13–15]. Therefore, membrane-bound Aβ species can be promising targets for the development of novel anti-AD drugs.
To characterize the membrane-bound conformation of Aβ, spectroscopic approaches, including solution nuclear magnetic resonance (NMR), circular dichroism (CD), and Fourier transform infrared spectroscopy (FT-IR), have been attempted by employing membrane mimics typified by aqueous micelles and even organic solvents [16–20]. The data thus obtained have underscored the formation of α-helical structures of Aβ in membrane-like environments. However, it has been suggested that the Aβ conformation is influenced by the size of micelles and the Aβ density thereon [21]. For example, Aβ has been reported to exhibit a thioflavin T-reactive β structure under conditions where Aβ density on micelles is high [21], although detailed structural information remains unavailable. These data emphasize the necessity of the conformational characterization of Aβ bound to large vesicles as more realistic model membranes. However, the membrane-bound Aβ molecules are not accessible with solution NMR techniques because of its slower molecular tumbling. In contrast, solid-state NMR studies have elucidated various structures of Aβ fibrils formed in solution [5,6,8,9,11] and membrane-associated Aβ fibrils [10,22–24], and also membrane perturbations caused by interactions of Aβ with lipid bilayers [25–28]. However, few detailed structural data have been available for the membrane-bound Aβ molecules prior to formation of amyloid fibrils.
Hence, in this study, we applied solid-state NMR spectroscopy to characterize the conformation of Aβ(1–40) bound to multilamella vesicles (MLVs) composed of 1,2-dimyristoyl-sn-glycero-3-phosphocholine (DMPC).
Results and Discussion
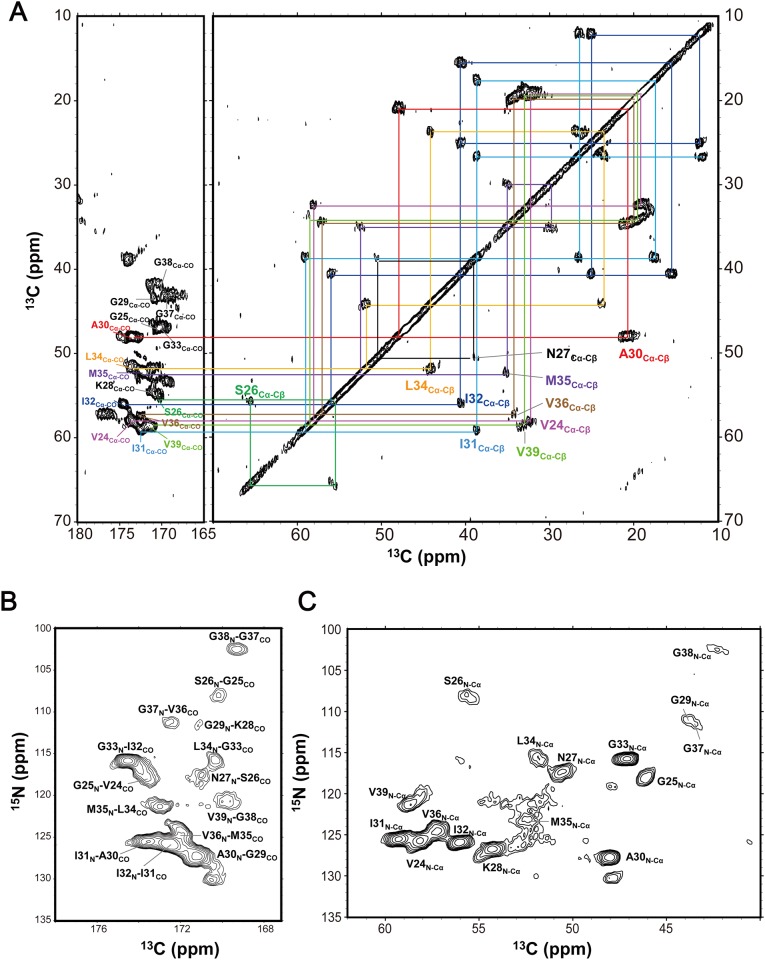
Fig 1A shows the spectrum of uniformly 13C- and 15N-labeled Aβ(1–40) bound to DMPC lipid bilayers in lyophilized and dry state obtained by the 13C-constant time universal cross peak-COSY (CTUC-COSY) [29] homonuclear correlation experiment at 20°C. We observed 22 cross peaks, indicating that the Aβ(1–40) molecule bound to the DMPC membrane has a heterogeneous nature in terms of conformational variability. To identify the well-ordered region(s) of the membrane-induced Aβ(1–40) molecule, we attempted to assign the observed cross peaks based on through-bond connectivities. Sequential signal assignments were achieved by the 13C-CTUC-COSY homonuclear correlation experiment in conjunction with 13C observed NCO and NCA heteronuclear correlation experiments using 13C-15N double cross polarization (DCP) [30] as shown in Fig 1B and 1C. The peak assignments of Aβ(1–40) bound to DMPC lipid bilayers are summarized in Table 1. The results indicate that the observed peaks originated exclusively from the C-terminal segment of Aβ(1–40), i.e., Val24–Val39. The conformational-dependent isotropic chemical shift values of the peaks thus assigned were inspected using TALOS-N [31], revealing that the C-terminal segment exhibited a β structure (S1 Fig). In general, the β strands of Aβ peptides are stabilized through either intra- or intermolecular hydrogen-bonding interactions [4–11,14,32–36]. For example, it has been suggested that SDS can induce Aβ(1–42) oligomerization forming β-strands, Val18-Asp23 and Lys28-Gly33, which are arranged into strand-turn-strand structure [35]. Furthermore, an Aβ(1–40) oligomer induced by epigallocatechin-3-gallate adopts an intramolecular anti-parallel β-sheet formed between Glu22-Asp23 and Gly29-Val39 [36]. However, β-strand conformation has been continuously observed for the Gly25–Val39 segment as mentioned in present study (S1 Fig). Thus, the possibility of an anti-parallel β-sheet structure through intramolecular hydrogen bonds at this C-terminal regions was excluded. Moreover, previous 2H and 31P NMR data have demonstrated that Aβ(1–40) was not inserted in neutral lipid bilayers [26,27]. Based on these data, we concluded that the Aβ(1–40) molecules bound to DMPC lipid bilayers exhibit dichotomous conformation, in which the disordered N-terminal segment is followed by the stable C-terminal β structure.
Fig 1. Solid-state NMR correlation spectra of [U-13C, 15N] Aβ(1–40) bound to DMPC bilayers for signal assignments.
(A) 13C-CTUC-COSY homonuclear correlation spectrum. (B) NCO and (C) NCA heteronuclear correlation spectra based on DCP. All measurements were performed at 20°C for the sample (DMPC/Aβ(1–40) molar ratio = 10/1) in lyophilized and dry state.
Table 1. Summary of 13C, 15N isotropic chemical shifts with assignments of peaks for [U-13C, 15N] Aβ(1–40) incorporated into DMPC bilayers obtained by solid-state NMR.
| Residue | Chemical shifts (ppm) | |||||
|---|---|---|---|---|---|---|
| CO | Cα | Cβ | Cγ | Cδ | N | |
| V24 | 173.6 | 58.1 | 32.4 | 19.7 | 125.7 | |
| G25 | 170.2 | 46.1 | 118.1 | |||
| S26 | 171.0 | 54.8 | 65.6 | 107.9 | ||
| N27 | 172.0 | 50.6 | 39.0 | 117.4 | ||
| K28 | 171.2 | 54.3 | 126.7 | |||
| G29 | 171.2 | 44.0 | 111.6 | |||
| A30 | 173.5 | 48.0 | 20.7 | 127.7 | ||
| I31 | 172.5 | 59.3 | 38.7 | 26.6, 17.5 | 12.0 | 125.5 |
| I32 | 174.5 | 56.0 | 40.6 | 25.1, 15.5 | 12.1 | 125.9 |
| G33 | 170.4 | 47.2 | 115.7 | |||
| L34 | 173.0 | 51.8 | 44.2 | 23.9 | 26.8 | 115.8 |
| M35 | 172.0 | 52.3 | 35.4 | 29.7 | 121.3 | |
| V36 | 172.6 | 57.2 | 34.4 | 21.3 | 124.6 | |
| G37 | 169.3 | 43.7 | 111.3 | |||
| G38 | 170.0 | 42.3 | 102.5 | |||
| V39 | 171.4 | 58.6 | 33.4 | 19.8 | 120.8 | |
| V40 | ||||||
13C and 15N isotropic chemical shifts were referenced to 1H TMS by using secondary reference of adamantane and glycine for 13C and 15N, respectively.
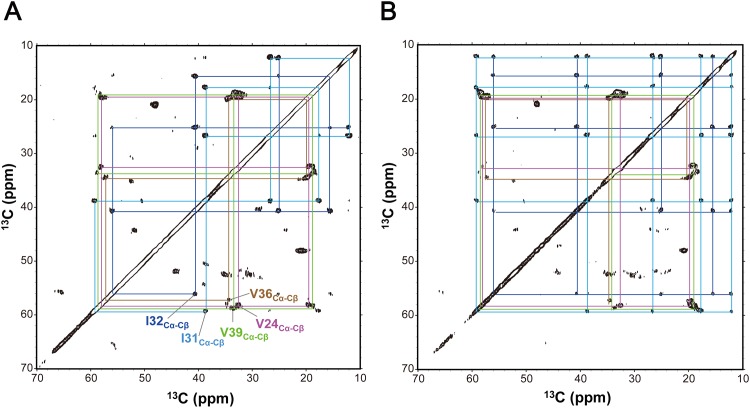
The remaining possibilities of the β-strand structures due to intermolecular hydrogen bonding, i.e., parallel and/or anti-parallel β-sheet arrangements, were inspected using dipolar-assisted rotational resonance [37]/RF-assisted diffusion [38] (DARR/RAD) experiments. Huang et. al. indicated that, in 150-kDa oligomers of Aβ(1–42), its C-terminal β-strand is arranged into an intermolecular antiparallel β-sheet based on detection of inter-residue cross peaks in DARR spectra [32]. To clarify intermolecular proximity among Aβ(1–40) peptides in the DMPC-induced structure, we employed DARR/RAD experiments at various mixing times as shown in Fig 2 and S2 Fig. The 13C-homonuclear through-space DARR/RAD correlation spectra with mixing time up to 400 ms gave only intra-residue cross peaks and inter-residue cross peaks between adjacent residues as a hallmark of the anti-parallel β structure, suggesting that the C-terminal segment of Aβ(1–40) forms a parallel β structure in the membrane environment.
Fig 2. Solid-state NMR 13C homonuclear through-space correlation spectra of [U−13C, 15N] Aβ(1–40) bound to DMPC MLVs acquired by DARR/RAD with mixing times of (A) 10 ms and (B) 100 ms, respectively.
All measurements were performed at 20°C for the sample (DMPC/Aβ(1–40) molar ratio = 10/1) in lyophilized and dry state.
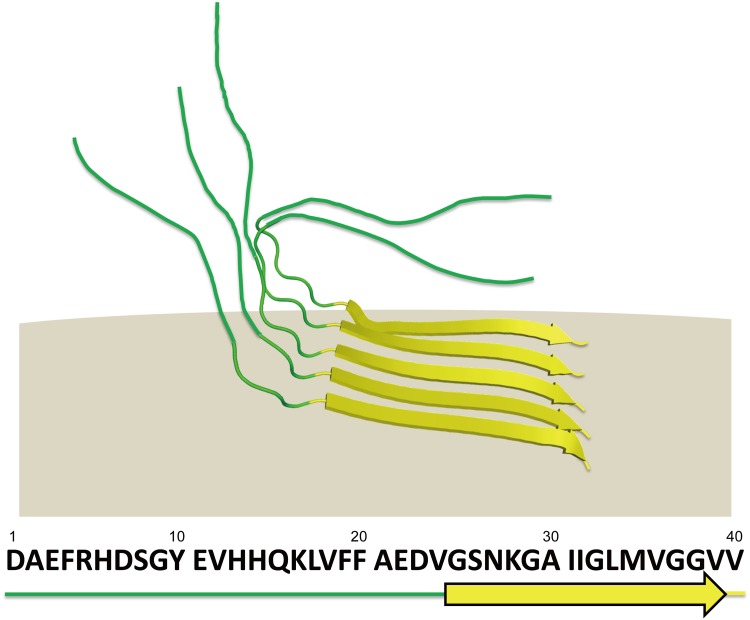
The previously reported NMR studies have demonstrated the conformational versatility of the Aβ(1–40) segments. The free form of Aβ(1–40) is unstructured in an aqueous solution but exhibits an α-helical conformation at Gln15-Asp23 and Ile31-Met35 in organic solvents such as trifluoroethanol [18]. Upon binding to aqueous micelles composed of sodium dodecyl sulfate (SDS) or lyso-GM1, Aβ(1–40) forms discontinuous α-helices at His14-Val24 and Ile31-Val36 under micelle excess conditions [20]. In contrast, the C-terminal hydrophobic dipeptide segment shows two distinct conformational states that are reactive with thioflavin T in GM1 micelles under conditions where the Aβ density on the micelles is high [21]. In the typical mature cross-fibrils, Aβ(1–40) molecules adopt a strand-turn-strand motif at Tyr10-Val24 and Ala30-Val39 and assemble into parallel β sheets [8], although Aβ fibril exhibits polymorph because of the variety in the biological environments [10,39], not only because of the experimental conditions. Niu et. al. has recently reported solid-state NMR study for structure of Aβ(1–40) fibrils formed in the presence of lipid vesicles indicating significant structural differences from the fibrils formed in solution [24]. Furthermore, Qiang et. al. has recently reported solid-state NMR study characterizing the formation of a complex between membrane-associated Aβ(1–40) fibrils and lipids, which contains a typical β-loop-β motif similar to the mature fibril, suggesting that formation of such complex could be associated with lipid uptake and possibly membrane disruption [22]. On the other hand, CD and 2H and 31P NMR data have indicated that Aβ(1–40) could form either an α-helical structure or a β-structure depending on molar ratios of neutral/negatively charged lipids as well as the peptide/lipid molar ratios [26,27]. However, despite these cumulative observations, α-to-β conformational transition processes of Aβ(1–40) remain largely unknown at the atomic level. The present solid-state NMR study identified a partially ordered conformation of membrane-induced Aβ(1–40) molecules, in which only the C-terminal segments are involved in a parallel β structure, while leaving the N-terminal segment disordered (Fig 3). The stabilized C-terminal segment encompasses the C-terminal half of the strand-turn-strand structure in the amyloid fibrils. Based on all these data, we conclude that DMPC vesicles provide large, flat surfaces compared with those of micelles and thereby promote intermolecular interactions of Aβ(1–40) through their C-terminal segments, which form a stable core of the Aβ(1–40) assembly. We suggest that DMPC vesicles capture an intermediate form of Aβ(1–40) during its conformational transition coupled with assembly into the toxic β structure, which could be delineated by our solid-state NMR approach. Our findings will not only provide unique insights into the mechanisms underlying Aβ assembly but also offer structural clues for designing drugs targeting the assembly intermediates of Aβ for the development of anti-AD therapeutics.
Fig 3. Structural model of Aβ(1–40) bound to DMPC bilayers characterized by solid-state NMR analyses together with amino acid sequence of Aβ(1–40).
Materials and Methods
Sample preparation
Expression and purification of uniformly 13C- and 15N-labeled Aβ(1–40) were performed as previously described [21]. DMPC was purchased from Avanti Polar Lipids, Inc. MLVs were prepared by dissolving 7.0 mg of DMPC in 100 μl of methanol/chloroform (1:1) solution and 3.5 mg of Aβ(1–40) peptide in 500 μl of chloroform (DMPC: Aβ(1–40) = 10:1). The solvents in the DMPC/Aβ(1–40) sample were removed from the sample by nitrogen gas, followed by complete drying under vacuum. The dried DMPC/Aβ(1–40) sample was suspended in a total of 400 μl of ultra-pure water and homogenized by six cycles of successive freezing, thawing, and vortexing for 5 min each. Then, the DMPC/Aβ(1–40) sample was immediately lyophilized to capture the structural state of Aβ(1–40) induced upon the interaction with DMPC bilayers.
Solid-state NMR experiments
All solid-state NMR experiments were performed using a Bruker Avance 600 spectrometer at 1H resonant frequency of 600 MHz, equipped with a 2.5 mm outer diameter 1H-13C-15N triple resonance magic angle spinning (MAS) probe at a spinning frequency of 13.5 kHz. The sample was packed into a 4 mm space at the center of a sample tube using original Diflon spacers of 1 mm thickness to maintain RF homogeneity. Temperatures were controlled to 20°C using a VT controller. Typical RF fields of 1H and 13C pulses were 100 and 88 kHz, respectively. The spinning frequency of the sample was actively controlled by Bruker MAS controller to 13.5 kHz ± 5 Hz. 1H heteronuclear dipolar decoupling was achieved by SPINAL64 [40] at a RF field of 100 kHz. 1H-X RAMP-Cross polarizations [41] were used to enhance the initial magnetization of rare nuclei with 1H RF fields of 100 and 80 kHz for X = 13C, 15N, respectively with repetition time of 2 s.
The 13C CTUC-COSY experiment was acquired at a constant evolution time of 6.96 ms with t1 of 348 and t2 of 2500 points. The number of scans was 1024 for each t1 point. Z-filtering of 1 ms was inserted before detection.
The NCO and NCA-DCP experiments were acquired with t1 of 256 and t2 of 2048 points, respectively. The number of scans was 1024 for each t1 point. Contact times of 1.5 and 5.0 ms were used for 1H-15N and 15N-13C CPs, respectively. 15N-13C CP was achieved by a 13C spin locking field of 20 kHz at -1 SSB HH matching condition with 5% of RF ramp of 15N RF spin locking field. LG decoupling at RF field of 100 kHz was applied during the second CP period.
The 13C DARR/RAD experiments were performed at n = 1 R3 condition for 1H RF field during the mixing period with t1 of 500 and t2 of 2500 points, respectively. The number of scans was 256 for each t1 point. The experiments were performed at mixing times of 4, 10, 100, 200, and 400 ms.
Quadrature detection in the indirect dimensions was achieved by States procedure for all two-dimensional (2D) experiments. Other parameters for 2D experiments are described in individual figure legends. The t1 time domain of 2D FID for 13C CTUC-COSY experiment was extended by forward linear prediction. The t1 time domains of 2D FIDs were apodized by a sin window function. 2D FIDs were zero filled to 4096 for both t1 and t2 time domains prior to Fourier transformation. All data processing was performed using TOPSPIN2.1 (Bruker Biospin, Japan). Spectral analyses were performed using Sparky. Secondary structural elements were identified by TALOS-N software [31] according to 13C and 15N chemical shifts.
Supporting Information
The secondary structure analysis by the TALOS software indicated a β-strand region (yellow shadow and yellow arrows) at the residues of Gly25-Val39. The chemical shift references were corrected before analyses of TALOS-N.
(PDF)
All measurements were performed at 20°C for the sample (DMPC/Aβ(1–40) molar ratio = 10/1) in lyophilized and dry state.
(PDF)
Acknowledgments
We thank Ms. Yukiko Isono for her help in preparing recombinant proteins. This work was supported in part by JSPS/MEXT KAKENHI Grant-in-Aid for Yong Scientists (B) (15K21680 to M.Y.-U.), Grant-in-Aid for Scientific research (C) (25410158 to K.N.), Grant-in-Aid for Scientific Research on Innovative Areas (25102001 and 25102008 to K.K.), Research Funding for Longevity Sciences (25–19) from the National Center for Geriatrics and Gerontology, the Nanotechnology Platform Program of MEXT, and the Okazaki ORION project.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported in part by JSPS/MEXT KAKENHI Grant-in-Aid for Young Scientists (B) (15K21680 to MY-U) (https://www.jsps.go.jp/english/e-grants/grants01.html), Grant-in-Aid for Scientific Research (C) (25410158 to KN) (https://www.jsps.go.jp/english/e-grants/grants01.html), Grant-in-Aid for Scientific Research on Innovative Areas (25102001 and 25102008 to KK) (https://www.jsps.go.jp/english/e-grants/grants01.html), Research Funding for Longevity Sciences (25-19) from the National Center for Geriatrics and Gerontology, the Nanotechnology Platform Program of MEXT (http://nanonet.mext.go.jp/english/), and the Okazaki ORION Project (http://www.oib.orion.ac.jp/English/index.html). All these grants were used in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Chiti F, Dobson CM. Protein misfolding, functional amyloid, and human disease. Annu Rev Biochem 2006; 75: 333–366. [DOI] [PubMed] [Google Scholar]
- 2.Irvine GB, El-Agnaf OM, Shankar GM, Walsh DM. Protein aggregation in the brain: the molecular basis for Alzheimer's and Parkinson's diseases. Mol Med 2008; 14: 451–464. 10.2119/2007-00100.Irvine [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hardy JA, Higgins GA. Alzheimer's disease: the amyloid cascade hypothesis. Science 1992; 256: 184–185. [DOI] [PubMed] [Google Scholar]
- 4.Lendel C, Bjerring M, Dubnovitsky A, Kelly RT, Filippov A, Antzutkin O N, et al. A hexameric peptide barrel as building block of amyloid-β protofibrils. Angew Chem Int Ed Engl 2014; 53: 12756–12760. 10.1002/anie.201406357 [DOI] [PubMed] [Google Scholar]
- 5.Luhrs T, Ritter C, Adrian M, Riek-Loher D, Bohrmann B, Dobeli H, et al. 3D structure of Alzheimer's amyloid-β(1–42) fibrils. Proc Natl Acad Sci U S A 2005; 102: 17342–17347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao Y, Ma B, McElheny D, Parthasarathy S, Long F, Hoshi M, et al. Aβ(1–42) fibril structure illuminates self-recognition and replication of amyloid in Alzheimer's disease. Nat Struct Mol Biol 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scheidt HA, Morgado I, Rothemund S, Huster D. Dynamics of amyloid β fibrils revealed by solid-state NMR. J Biol Chem 2012; 287: 2017–2021. 10.1074/jbc.M111.308619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petkova AT, Ishii Y, Balbach JJ, Antzutkin ON, Leapman RD, Delaglio F, et al. A structural model for Alzheimer's β-amyloid fibrils based on experimental constraints from solid state NMR. Proc Natl Acad Sci U S A 2002; 99: 16742–16747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petkova AT, Yau WM, Tycko R. Experimental constraints on quaternary structure in Alzheimer's β-amyloid fibrils. Biochemistry 2006; 45: 498–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lu JX, Qiang W, Yau WM, Schwieters CD, Meredith SC, Tycko R. Molecular structure of β-amyloid fibrils in Alzheimer's disease brain tissue. Cell 2013; 154: 1257–1268. 10.1016/j.cell.2013.08.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paravastu AK, Leapman RD, Yau WM, Tycko R. Molecular structural basis for polymorphism in Alzheimer's β-amyloid fibrils. Proc Natl Acad Sci U S A 2008; 105: 18349–18354. 10.1073/pnas.0806270105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fantini J, Yahi N. Molecular insights into amyloid regulation by membrane cholesterol and sphingolipids: common mechanisms in neurodegenerative diseases. Expert Rev Mol Med 2010; 12: e27 10.1017/S1462399410001602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ariga T, McDonald MP, Yu RK. Role of ganglioside metabolism in the pathogenesis of Alzheimer's disease—a review. J Lipid Res 2008; 49: 1157–1175. 10.1194/jlr.R800007-JLR200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yanagisawa K, Odaka A, Suzuki N, Ihara Y. GM1 ganglioside-bound amyloid β-protein (Aβ): a possible form of preamyloid in Alzheimer's disease. Nat Med 1995; 1: 1062–1066. [DOI] [PubMed] [Google Scholar]
- 15.Matsuzaki K, Kato K, Yanagisawa K. Aβ polymerization through interaction with membrane gangliosides. Biochim Biophys Acta 2010; 1801: 868–877. 10.1016/j.bbalip.2010.01.008 [DOI] [PubMed] [Google Scholar]
- 16.Jarvet J, Danielsson J, Damberg P, Oleszczuk M, Gräslund A. Positioning of the Alzheimer Aβ(1–40) peptide in SDS micelles using NMR and paramagnetic probes. J Biomol NMR 2007; 39: 63–72. [DOI] [PubMed] [Google Scholar]
- 17.Wahlström A, Hugonin L, Perálvarez-Marin A, Jarvet J, Gräslund A. Secondary structure conversions of Alzheimer's Aβ(1–40) peptide induced by membrane-mimicking detergents. FEBS J 2008; 275: 5117–5128. 10.1111/j.1742-4658.2008.06643.x [DOI] [PubMed] [Google Scholar]
- 18.Sticht H, Bayer P, Willbold D, Dames S, Hilbich C, Beyreuther K, et al. Structure of amyloid A4-(1–40)-peptide of Alzheimer's disease. Eur J Biochem 1995; 233: 293–298. [DOI] [PubMed] [Google Scholar]
- 19.Coles M, Bicknell W, Watson AA, Fairlie DP, Craik DJ. Solution structure of amyloid β-peptide(1–40) in a water-micelle environment. Is the membrane-spanning domain where we think it is? Biochemistry 1998; 37: 11064–11077. [DOI] [PubMed] [Google Scholar]
- 20.Utsumi M, Yamaguchi Y, Sasakawa H, Yamamoto N, Yanagisawa K, Kato K. Up-and-down topological mode of amyloid β-peptide lying on hydrophilic/hydrophobic interface of ganglioside clusters. Glycoconj J 2009; 26: 999–1006. 10.1007/s10719-008-9216-7 [DOI] [PubMed] [Google Scholar]
- 21.Yagi-Utsumi M, Matsuo K, Yanagisawa K, Gekko K, Kato K. Spectroscopic Characterization of Intermolecular Interaction of Amyloid β Promoted on GM1 Micelles. Int J Alzheimers Dis 2010; 2011: 925073 10.4061/2011/925073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Qiang W, Akinlolu RD, Nam M, Shu N. Structural evolution and membrane interaction of the 40-residue β amyloid peptides: differences in the initial proximity between peptides and the membrane bilayer studied by solid-state nuclear magnetic resonance spectroscopy. Biochemistry 2014; 53: 7503–7514. 10.1021/bi501003n [DOI] [PubMed] [Google Scholar]
- 23.Akinlolu RD, Nam M, Qiang W. Competition between Fibrillation and Induction of Vesicle Fusion for the Membrane-Associated 40-Residue β-Amyloid Peptides. Biochemistry 2015; 54: 3416–3419. 10.1021/acs.biochem.5b00321 [DOI] [PubMed] [Google Scholar]
- 24.Niu Z, Zhao W, Zhang Z, Xiao F, Tang X, Yang J. The molecular structure of Alzheimer β-amyloid fibrils formed in the presence of phospholipid vesicles. Angew Chem Int Ed Engl 2014; 53: 9294–9297. 10.1002/anie.201311106 [DOI] [PubMed] [Google Scholar]
- 25.Nakazawa Y, Suzuki Y, Williamson MP, Saito H, Asakura T. The interaction of amyloid Aβ(1–40) with lipid bilayers and ganglioside as studied by 31P solid-state NMR. Chem Phys Lipids 2009; 158: 54–60. 10.1016/j.chemphyslip.2008.12.001 [DOI] [PubMed] [Google Scholar]
- 26.Terzi E, Holzemann G, Seelig J. Interaction of Alzheimer β-amyloid peptide(1–40) with lipid membranes. Biochemistry 1997; 36: 14845–14852. [DOI] [PubMed] [Google Scholar]
- 27.Bokvist M, Lindstrom F, Watts A, Gröbner G. Two types of Alzheimer's β-amyloid (1–40) peptide membrane interactions: aggregation preventing transmembrane anchoring versus accelerated surface fibril formation. J Mol Biol 2004; 335: 1039–1049. [DOI] [PubMed] [Google Scholar]
- 28.Lau TL, Gehman JD, Wade JD, Masters CL, Barnham KJ, Separovic F. Cholesterol and Clioquinol modulation of Aβ(1–42) interaction with phospholipid bilayers and metals. Biochim Biophys Acta 2007; 1768: 3135–3144. [DOI] [PubMed] [Google Scholar]
- 29.Chen L, Olsen RA, Elliott DW, Boettcher JM, Zhou DH, et al. Constant-time through-bond 13C correlation spectroscopy for assigning protein resonances with solid-state NMR spectroscopy. J Am Chem Soc 2006; 128: 9992–9993. [DOI] [PubMed] [Google Scholar]
- 30.Schaefer J, McKay RA, Stejskal EO. Double-cross-polarization NMR of solids. J Magn Reson 1969; 34: 443–447. [Google Scholar]
- 31.Shen Y, Bax A. Protein backbone and sidechain torsion angles predicted from NMR chemical shifts using artificial neural networks. J Biomol NMR 2013; 56: 227–241. 10.1007/s10858-013-9741-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huang D, Zimmerman MI, Martin PK, Nix AJ, Rosenberry TL, Paravastu A K. Antiparallel β-Sheet Structure within the C-Terminal Region of 42-Residue Alzheimer's Amyloid-β Peptides When They Form 150-kDa Oligomers. J Mol Biol 2015; 427: 2319–2328. 10.1016/j.jmb.2015.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cerf E, Sarroukh R, Tamamizu-Kato S, Breydo L, Derclaye S, Dufrêne Y F, et al. Antiparallel β-sheet: a signature structure of the oligomeric amyloid β-peptide. Biochem J 2009; 421: 415–423. 10.1042/BJ20090379 [DOI] [PubMed] [Google Scholar]
- 34.Chimon S, Shaibat MA, Jones CR, Calero DC, Aizezi B, Ishii Y. Evidence of fibril-like β-sheet structures in a neurotoxic amyloid intermediate of Alzheimer's β-amyloid. Nat Struct Mol Biol 2007; 14: 1157–1164. [DOI] [PubMed] [Google Scholar]
- 35.Yu L, Edalji R, Harlan JE, Holzman TF, Lopez AP, Labkovsky B, et al. Structural characterization of a soluble amyloid β-peptide oligomer. Biochemistry 2009; 48: 1870–1877. 10.1021/bi802046n [DOI] [PubMed] [Google Scholar]
- 36.Lopez del Amo JM, Fink U, Dasari M, Grelle G, Wanker EE, Reif B. Structural properties of EGCG-induced, nontoxic Alzheimer's disease Aβ oligomers. J Mol Biol 2012; 421: 517–524. 10.1016/j.jmb.2012.01.013 [DOI] [PubMed] [Google Scholar]
- 37.Takegoshi K, Nakamura S, Terao T. 13C-1H dipolar-assisted rotational resonance in magic angle spinning NMR. Chem Phys Lett 2001; 344: 631–637. [Google Scholar]
- 38.Morcombe CR, Gaponenko V, Byrd RA, Zilm KW. Diluting abundant spins by isotope edited radio frequency field assisted diffusion. J Am Chem Soc 2004; 126: 7196–7197. [DOI] [PubMed] [Google Scholar]
- 39.Scherpelz KP, Lu JX, Tycko R, Meredith SC. Preparation of Amyloid Fibrils Seeded from Brain and Meninges. Methods Mol Biol 2016; 1345: 299–312. 10.1007/978-1-4939-2978-8_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fung BM, Khitrin AK, Ermolaev K. An improved broadband decoupling sequence for liquid crystals and solids. J Magn Reson 2000; 142: 97–101. [DOI] [PubMed] [Google Scholar]
- 41.Metz G, Wu X, Smith SO. Ramped-amplitude cross polarization in magic-angle-spinning NMR. J Magn Reson Series A 1994; 110: 219–227. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The secondary structure analysis by the TALOS software indicated a β-strand region (yellow shadow and yellow arrows) at the residues of Gly25-Val39. The chemical shift references were corrected before analyses of TALOS-N.
(PDF)
All measurements were performed at 20°C for the sample (DMPC/Aβ(1–40) molar ratio = 10/1) in lyophilized and dry state.
(PDF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.