Abstract
Activating mutations in the HER2 tyrosine kinase have been identified in human breast cancers that lack HER2 gene amplification. These patients are not candidates for HER2 targeted drugs under current standards of care, but preclinical data strongly suggest that these patients will benefit from anti-HER2 drugs. In this case report, we describe a young woman with metastatic breast cancer whose tumor was found to carry a HER2 L755S mutation, which is in the kinase domain of HER2. Treatment with the second generation HER2/EGFR tyrosine kinase inhibitor, neratinib, resulted in partial response and dramatic improvement in the patient’s function status. This partial response lasted 11 months and when the patient’s cancer progressed, she was treated with neratinib plus capecitabine and her cancer again responded. This second response parallels the benefit seen with continuing trastuzumab in HER2 amplified breast cancer after disease progression. This case is the first report, to our knowledge, of successful single agent treatment of HER2 mutated breast cancer. Two clinical trials of neratinib for HER2 mutated, metastatic breast cancer are currently enrolling patients. Further, data from The Cancer Genome Atlas project have identified HER2 mutations in a wide range of solid tumors, including bladder, colorectal, and non-small cell lung cancer, suggesting that clinical trials of neratinib or neratinib-based combinations for HER2 mutated solid tumors is warranted.
Breast cancer genome sequencing has identified HER2 activating mutations in cancers that are HER2 negative by immunohistochemistry (IHC) or fluorescence in-situ hybridization (FISH).1,2 These HER2 activating mutations cause oncogenic transformation of breast epithelial cells in tissue culture and increase tumor growth in xenograft models.2,3 The sensitivity of these HER2 activating mutations to HER2 targeted drugs has been measured 2,4 and two clinical trials are screening metastatic breast cancer patients for HER2 mutations and treating the HER2 mutation positive cases with the second generation HER2/EGFR tyrosine kinase inhibitor, neratinib (HKI-272).5,6 In this report, we describe the first case of a HER2 mutated breast cancer that clinically benefited from neratinib monotherapy. When this patient’s cancer progressed, she was placed on the neratinib plus capecitabine combination and her cancer again responded.
Case Presentation
The patient was diagnosed with stage IV, invasive ductal carcinoma in 2003. She was 43 years old and presented with a 2 cm mass in the left breast and bone metastases. Biopsy demonstrated that this tumor was estrogen receptor positive (ER+), progesterone receptor (PR) negative, and HER2 negative by IHC. Her family history was negative for breast and ovarian cancer and the patient tested negative for germline mutations of BRCA1 and BRCA2. She was treated with oophorectomy, letrozole and zoledronic acid with an excellent clinical and radiological response. In 2005, a new breast mass was found in the same breast and biopsy revealed invasive duct carcinoma that was hormone receptor negative and HER2 negative by IHC. A modified radical mastectomy was done, and chemotherapy with doxorubicin and cyclophosphamide was administered. Therapy with letrozole and zoledronic acid was also continued. In 2010, liver metastases were diagnosed and changing letrozole to tamoxifen produced disease stabilization. In May 2011, massive hepatic progression was noted with obstructive jaundice, ascites and pleural effusion. Treatment with capecitabine and intensive supportive measures resulted in slow resolution of the jaundice, normalization of liver function tests and decline of tumor markers. The ascites did not resolve and analysis revealed chylous content with no malignant cells, possibly due to secondary cirrhosis. Treatment with capecitabine was discontinued and fulvestrant 500mg every 4 weeks was initiated.
In October 2012, progressive disease was observed with peritoneal and omental metastases, a left adrenal mass, and enlargement of liver metastases. Hormonal treatment was stopped and oral vinorelbine was started with no response. Liver biopsy showed metastatic breast cancer that was ER, PR, and HER2 negative by IHC and next generation sequencing by a Clinical Laboratories Improvement Amendments (CLIA)–certified commercial laboratory (Foundation Medicine, Cambridge, MA) identified HER2 L755S mutation, amplifications of the MDM2 and MYC genes, and APC I1307K mutation. The HER2 L755S mutation is an activating mutation that is located in the tyrosine kinase inhibitor binding site of the HER2 kinase domain. It produces resistance to lapatinib, but in pre-clinical studies, it is highly sensitive to neratinib, a second generation HER2/EGFR tyrosine kinase inhibitor. 2,4 This mutation is different than the EGFR gatekeeper mutation T790M, which would be T798M in HER2.7 HER2 L755S has been observed in patients who have not received prior lapatinib, and this patient had never been treated with lapatinib.
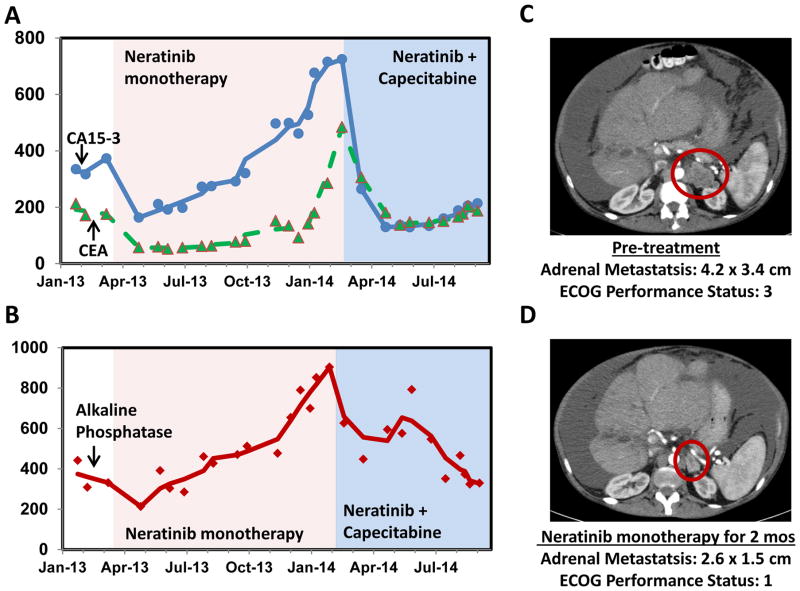
Her ECOG performance status deteriorated to 3 and she was essentially home bound with massive ascites and profound weakness. Neratinib (240 mg orally daily) was obtained through a compassionate access protocol (with approval of local and central IRB), and was started in April 2013. Within two months, her performance status dramatically improved to 1, she was able to resume daily activities including gardening, and she was able to travel abroad on long trips. Her liver enzymes and tumor markers improved (Figure 1) and CT scan done in June 2013 showed 30% reduction of the left adrenal mass corresponding to a partial response by RECIST 1.0. This response persisted for 11 months. In Feb. 2014, clinical worsening was noted with elevated LFT’s and tumor markers, and worsening ascites. Capecitabine (3000 mg daily, days 1–14 of a 21 day cycle) was added. The patient’s tumor again responded with significant improvement of LFT’s and tumor markers. Treatment with both neratinib monotherapy and neratinib plus capecitabine combination was very well tolerated by the patient. She received diarrhea prophylaxis for the first three days (loperamide 4mg loading dose followed by 2mg every 4 hours for three days) and was then tapered off loperamide. With this prophylactic regimen, the patient experienced no diarrhea.
Figure 1.
Laboratory and imaging results with neratinib treatment. A, CA 15-3 (blue) and CEA (green) tumor markers. B, Alkaline phosphatase values (red). The trend line in both A and B represents a 3 point moving average for the period before neratinib (Feb. – Mar. 2013), on neratinib monotherapy (June 2013 – Feb. 2014), and on the neratinib + capecitabine combination (June – Sept. 2014). The time period of neratinib monotherapy and neratinib + capecitabine (Ner. + Cap.) combination therapy is marked by pink and blue shading, respectively. CA27.29 testing is not routinely available in Israel and no CA27.29 values were available for the patient. C, CT imaging on prior to neratinib therapy. D, CT imaging after two months of neratinib monotherapy.
Discussion
This patient’s cancer had a HER2 L755S mutation and she had a partial response from neratinib monotherapy. This response markedly improved the patient’s performance status and quality of life. After 11 months, her cancer progressed and treatment with neratinib plus capecitabine produced another disease response, which is analogous to the benefit seen with continuing trastuzumab after progression in HER2 positive (HER2 gene amplified) breast cancer.8 While a prior case report of HER2 mutated, inflammatory breast cancer describes treatment with chemotherapy and HER2 targeted drugs (lapatinib and trastuzumab)9, this case is the first published report of a patient with HER2 mutated breast cancer responding to treatment with a single agent, HER2 targeted drug. Furthermore, lapatinib would not have been an appropriate drug for our patient since the HER2 L755S mutation alters the kinase inhibitor binding site and causes lapatinib resistance.2,4
Two clinical trials of neratinib monotherapy for HER2 mutated, metastatic breast cancer are currently enrolling patients5,6 and trials of neratinib-based combination regimens for HER2 mutated breast cancer should be considered in the future. Phase I trials of neratinib with the chemotherapy drugs, paclitaxel, capecitabine, or vinorelbine, have demonstrated that these combinations are safe10–12 and neratinib has also been combined with other targeted therapy drugs, such as trastuzumab and temsirolimus.13,14 A phase I trial of a three drug, neratinib-containing regimen (neratinib, trastuzumab, and paclitaxel) for HER2 positive breast cancer showed good patient tolerability.15 The main toxicity was diarrhea. With diarrhea prophylaxis, no cases of grade 3/4 diarrhea were observed and grade 2 and 1 diarrhea rates were 17% and 50%, respectively.15
The Cancer Genome Atlas project has identified HER2 mutations in a wide range of solid tumors, including breast, colorectal, bladder, and non-small cell lung cancers.1,3,16,17 Further, the specific mutations seen in HER2 are highly recurrent, with the most common mutations occurring either in the kinase domain or at residues 309–310 of the extracellular domain.2,3 These mutations can be potently inhibited with neratinib2 and therefore, investigation of neratinib or neratinib-based combinations for the treatment of multiple solid tumors with HER2 mutations is warranted. Continuation of neratinib in a different drug combination after progression should be considered, as this patient had a second response with addition of capecitabine to neratinib.
Acknowledgments
This work was supported by the NIH (grant R01CA161001 to R. Bose and R01CA095614 to M.J. Ellis) and the Siteman Cancer Center-Foundation for Barnes-Jewish Hospital Cancer Frontier Fund.
Footnotes
Disclosures of Potential Conflicts of Interest
C.X. Ma is the principal investigator of a phase II clinical trial funded in part by Puma Biotechnology, the manufacturer of neratinib, and R. Bose and M.J. Ellis are sub-investigators on this trial. N. Efrat Ben-Baruch is an investigator on four clinical trials sponsored by Puma Biotechnology. R. Bose has received honoria from the speaker’s bureaus of Genentech, Novartis, and RGA International. M.J. Ellis has ownership interest (including patents) in Bioclassifer LLC and has performed ad hoc consulting for Puma Biotechnology, Genentech, Novartis, Astra-Zeneca, and Pfizer. S.M. Kavuri has no conflicts of interest.
References
- 1.The Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumors. Nature. 2012;490(7418):61–70. doi: 10.1038/nature11412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bose R, Kavuri SM, Searleman AC, et al. Activating HER2 mutations in HER2 gene amplification negative breast cancer. Cancer Discov. 2013;3(2):224–237. doi: 10.1158/2159-8290.CD-12-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greulich H, Kaplan B, Mertins P, et al. Functional analysis of receptor tyrosine kinase mutations in lung cancer identifies oncogenic extracellular domain mutations of ERBB2. Proc Natl Acad Sci U S A. 2012;109(36):14476–14481. doi: 10.1073/pnas.1203201109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kancha RK, von Bubnoff N, Bartosch N, Peschel C, Engh RA, Duyster J. Differential sensitivity of ERBB2 kinase domain mutations towards lapatinib. PLoS ONE. 2011;6(10):e26760. doi: 10.1371/journal.pone.0026760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ClinicalTrials.gov. [Access date Sept. 28, 2014];Neratinib in Metastatic HER2 Non-amplified But HER2 Mutant Breast Cancer. http://www.clinicaltrials.gov/ct2/show/NCT01670877.
- 6.ClinicalTrials.gov. [Access date May 28, 2014];An Open-label, Phase 2 Study of Neratinib in Patients With Solid Tumors With Somatic Human Epidermal Growth Factor Receptor (EGFR, HER2, HER3) Mutations or EGFR Gene Amplification. http://www.clinicaltrials.gov/ct2/show/NCT01953926.
- 7.Rexer BN, Ghosh R, Narasanna A, et al. Human breast cancer cells harboring a gatekeeper T798M mutation in HER2 overexpress EGFR ligands and are sensitive to dual inhibition of EGFR and HER2. Clin Cancer Res. 2013;19(19):5390–5401. doi: 10.1158/1078-0432.CCR-13-1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.von Minckwitz G, du Bois A, Schmidt M, et al. Trastuzumab beyond progression in human epidermal growth factor receptor 2-positive advanced breast cancer: a german breast group 26/breast international group 03–05 study. J Clin Oncol. 2009;27(12):1999–2006. doi: 10.1200/JCO.2008.19.6618. [DOI] [PubMed] [Google Scholar]
- 9.Ali SM, Alpaugh RK, Downing SR, et al. Response of an ERBB2-Mutated Inflammatory Breast Carcinoma to Human Epidermal Growth Factor Receptor 2-Targeted Therapy. J Clin Oncol. 2014 doi: 10.1200/JCO.2013.49.0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chow LW, Xu B, Gupta S, et al. Combination neratinib (HKI-272) and paclitaxel therapy in patients with HER2-positive metastatic breast cancer. Br J Cancer. 2013;108(10):1985–1993. doi: 10.1038/bjc.2013.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Awada A, Dirix L, Manso Sanchez L, et al. Safety and efficacy of neratinib (HKI-272) plus vinorelbine in the treatment of patients with ErbB2-positive metastatic breast cancer pretreated with anti-HER2 therapy. Ann Oncol. 2013;24(1):109–116. doi: 10.1093/annonc/mds284. [DOI] [PubMed] [Google Scholar]
- 12.Saura C, Garcia-Saenz JA, Xu B, et al. Safety and Efficacy of Neratinib in Combination With Capecitabine in Patients With ErbB2-positive Breast Cancer. Cancer Res. 2011;71(24 Suppl):Abstract P1-12-09. doi: 10.1200/JCO.2014.56.3809. [DOI] [PubMed] [Google Scholar]
- 13.Gandhi L, Bahleda R, Tolaney SM, et al. Phase I study of neratinib in combination with temsirolimus in patients with human epidermal growth factor receptor 2-dependent and other solid tumors. J Clin Oncol. 2014;32(2):68–75. doi: 10.1200/JCO.2012.47.2787. [DOI] [PubMed] [Google Scholar]
- 14.Gajria D, King T, Pannu H, et al. Combined inhibition of mTORC1 with Temsirolimus and HER2 with Neratinib: A Phase I/II study in patients with metastatic HER2-amplified or triple-negative breast cancer. Cancer Res. 2011;71(24 Suppl):Abstract PD 09-08. [Google Scholar]
- 15.Jankowitz RC, Abraham J, Tan AR, et al. Safety and efficacy of neratinib in combination with weekly paclitaxel and trastuzumab in women with metastatic HER2positive breast cancer: an NSABP Foundation Research Program phase I study. Cancer Chemother Pharmacol. 2013;72(6):1205–1212. doi: 10.1007/s00280-013-2262-2. [DOI] [PubMed] [Google Scholar]
- 16.The Cancer Genome Atlas Consortium. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487(7407):330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.The Cancer Genome Atlas Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507(7492):315–322. doi: 10.1038/nature12965. [DOI] [PMC free article] [PubMed] [Google Scholar]