Abstract
Skin surface temperature has been proposed as an in vivo clinical biomarker for monitoring the detrimental effect of biostimulatory laser applications. In some cases, such as wound healing and cosmetic applications, the target of the irradiation is the skin surface. In other cases, the light has to reach deeper tissues, for instance, during the irradiation of internal body organs. Prerequisite for reproducible biostimulatory effects is that the light intensity surpasses a minimum threshold. Because of the loss of light intensity caused by absorption and scattering, targeting deeper tissues always implies that the intensity at the skin surface will be much higher than that at the target site. Derived from laboratory experiments which showed that virtually the same light which produces biostimulatory effects in cells in vitro and tissues in vivo is instrumental in reducing the viscous friction in nanoconfined systems, we arrive to a new understanding of the effect of biostimulatory levels of light on mitochondria. One immediate result is insight into strategies which promise to maximize the biostimulatory effect and minimize potential phototoxic effects during treatment of deeper tissues. Such optimization strategies are also promising for experimental and therapeutic in vitro applications, in particular in combination with cell-friendly microenvironments.
Keywords: Laser, LED, biostimulation, biomodulation, low level laser therapy (LLLT), mitochondria, adenosine triphosphate (ATP), ATP synthase, cytochrome c oxidase, reactive oxygen species (ROS), gene expression, cognitive improvement, skin rejuvenation, IVF
In their recent article on near-infrared (NIR) laser phototoxicity, published in Scientific Reports, Khan et al. (1) suggest that “monitoring surface temperature could be a real time, in vivo clinical biomarker to monitor the detrimental (phototoxic) effect of NIR laser applications.” Exemplarily, the authors report the irradiation of the shaved dorsal skin of a mouse with laser light (distance between laser probe and mouse 2 cm, wavelength 810 nm, irradiance 1 W/cm2, fluence 21 J/cm2). Concurrently, the skin surface temperature was monitored by an infrared camera model ICI 7640. As documented in the supplementary videoJeny (1), the skin surface temperature increased during irradiation to values above 55 °C—a potentially lethal temperature for mammalian cells (2), and clear evidence of thermal damage, as additionally documented by photographs of the treated area (1). Nonetheless, temperatures ≥55 °C seem to be unusual for the communicated irradiation data. Unfortunately, the limited setup description does not allow us to build a solid conclusion on the actual diameter of the non scattered laser spot on the mouse, as shown in the supplementary videoJeny (1). Irradiances about 1 W/cm2 are not uncommon in clinical applications of NIR laser light. Such irradiances can be easily reached using 50 mW lasers. Interestingly, reports regarding pain caused by treatment with NIR lasers utilized in low level laser therapy (LLLT) are lacking in the literature, even for extended exposure. This point leads to some confusion especially when we consider the reported heat pain threshold (3). Regrettably, the authors fail to include the relevant study of Joensen et al. (4), which focuses on the thermal effects of low intensity laser light, including 810 nm, for various human skin colors and reports for an irradiance of 6.37 W/cm2 a temperature increase up to 42–43 °C on dark skin, reaching the thermal pain threshold. Likewise, the authors fail to address the treatment doses recommended for LLLT (5). It is further interesting that Khan et al. deviate in their understanding of the Arndt-Schulz rule from its interpretation in clinical laser biostimulation (6-8). Furthermore, the authors review the literature for detrimental phototoxic effects of low power lasers, and cite the work of Demidova-Rice et al. (9). Careful inspection of the cited work reveals, however, no explicit data on detrimental phototoxic effects of low level light. Further, Khan et al. investigate the potential of laser biostimulation in damaging DNA: “This also implies that high NIR laser doses can be phototoxic without being genotoxic or mutagenic, indicating they can be safely used for clinical applications.” Moreover, previous work demonstrated that low level laser light has genoprotective properties (10). Importantly, recent work reports on DNA damage in blood cells exposed to low level laser (11). These results clearly indicate that there is an urgent need for a careful definition of “low” (including the parameters wavelength, irradiance, fluence and time of irradiation), before generalizing safety prospects. This discrimination is of particular importance when we extend our consideration to the mechanism of laser biostimulation. From this viewpoint the statement “The primary photochemical event mediating PBM (photobiomodulation) appear to involve generation of ROS following absorption by various cellular chromophores, especially cytochrome C oxidase in the mitochondria.” suggests that the copper enzyme plays a basic role in the mechanism of LLLT. Its role as primary photoacceptor for visible to NIR light was proposed by Karu et al. (12). However, its implication in the mechanism of LLLT was challenged by Lubart et al. (13). Indeed, predictive models identify as root cause for the entire spectrum of biostimulatory effects the increase in the synthesis of mitochondrial adenosine triphosphate (ATP)—the cell’s major energy currency—by facilitating the performance of the ATP synthase (14-16). In this way alternative biostimulatory mechanisms such as the generation of reactive oxygen species (ROS) (17,18), typically a fast process (19), and the upregulation of gene expression, reportedly a relatively slow process (20,21), seem to accompany the biosynthesis of ATP in an intensity and dose dependent manner, however, without playing a causal role in cell activation. Of particular interest in this context is the function of low and high levels of ROS, and their interplay with the normal function of the ATP synthase. Further advance in the field should emerge from the analysis of biological effects related to the extremely short lifetimes of ROS and their main physicochemical effect consisting in raising the hydrophilic nature of surfaces, a process resulting in an increase in interfacial viscosity (14,16), a state predicted to cause an instant drop in the output capacity of the ATP synthase. For instance, exceeding the energy density threshold defined by the Arndt-Schulz rule by extending the exposure of the cells to biostimulatory light intensities, which normally activate cellular functions, results recurrently in an inhibition of cellular functions. The inhibition can be understood by considering that during longer exposure to the relevant ROS, the surfaces within and proximal to the mitochondrial rotary motor collect more ROS, which by increasing the interfacial hydrophilicity results in an increase in interfacial viscosity. For shorter irradiation times the inhibitory effect of ROS is negligible due to the short lifetimes of ROS. Probably, longer irradiation times (at biostimulatory light intensities) are associated with longer ROS lifetimes due to transient immobilisation of the reactive oxygen molecules in surfaces and interfaces, whereas for shorter irradiation times inhibitory ROS effects vanish because the number of molecules which prevail in an excited state is smaller; accordingly, while diffusing from the site of their generation, less molecules are likely to reach the relevant surfaces and interfaces in a reactive state, in accordance with the characteristic lifetimes of ROS. The amply documented increase in ATP synthesis in vitro in response to biostimulatory levels of red to NIR light as the predominant mechanism in LLLT is in agreement with the observational evidence condensed in the statement “Weaker initial ATP synthesis results in a higher positive laser effect” (7,22) and can be derived from laboratory experiments (14,16) showing that the same light which increased ATP levels in cells is instrumental in reducing the viscosity of interfacial water.
Summarizing, the article of Khan et al. is pushing toward a major advance in LLLT. Its initial aim is of extreme importance for the clinical application of both low intensity lasers and LEDs, and it stimulates research regarding monitoring skin temperatures in LLLT. One way forward consists in the rigorous design of suitable animal experiments and clear definition of standards for the extrapolation of temperatures obtained in animal models to human skin. Special attention must be paid to oxidative stress related damages caused by excess ROS and protein denaturation. In contrast with the aforementioned mechanisms the new model suggests practical strategies for minimizing damages potentially induced during biostimulation. For a better understanding of the pivotal importance of the correct assessment of the skin surface temperature (and its equilibrium value) in response to laser irradiation it may be sufficient to address clinical applications where the aim is to deliver biostimulatory levels of light to deeper tissues. A better understanding of the light/tissue-interaction mechanism is conditional for the prospect of progress in existing methods and the justification of novel LLLT indications both in superficial and deep tissue biostimulation. Superficial applications include, for instance, accelerated wound healing (6) and skin rejuvenation (23). Deep tissue applications suggest themselves from the possibility to refill depleted mitochondrial ATP reservoirs with light, in particular for biological systems which are ATP hungry such as the brain or which consume large amounts of ATP in a short period of time such as sperm. From the typical concentration ratio of intracellular and extracellular ATP of nearly 1 million (24), it is clear that exogenous supplementation with ATP will hardly replenish intracellular ATP deficiencies. By noting that the metabolic agents glucose and oxygen are the primary fuel for brain function Owen and Sunram-Lea reasoned that modes of producing ATP and interventions which improve metabolic function or prop up ATP production may have a vast number of medical implications, in particular for the brain which is the most metabolically active organ in the body and is as such particularly vulnerable to disruption of energy resources (25). Thus, proper administration of laser or LED light might produce effects similar to cognitive enhancing drugs.
A closer consideration of the aforementioned effect of ROS on the synthesis of ATP leads us to propose and justify the use of the pulsed irradiation mode in LLLT. Figure 1 is a visual synopsis of an optimal interplay between the production of ATP by the mitochondria and its consumption by the cell, particularly under conditions of oxidative stress. The process of optimization involves the experimental evaluation of two parameters: the period of time needed for light of a certain wavelength and intensity to elevate the mitochondrial ATP levels in a well defined environment, and the period of time in which the cells exhaust the ATP stores. Once the individual parameters are know it is possible to adjust the duration of the light pulse and the dark period between pulses to a balanced ratio accounting for the specific energy demand of the cell. Extending the duration of the light pulse means extra oxidative stress due to ROS generation—extending the dark period beyond a critical limit (ATP deprivation) is expected to reduce cell viability (26). Concomitance of both extremes could result in a synergistic effect irreversibly damaging oxidatively stressed cells. We hope that our comments will contribute to progress in the field of low intensity medical lasers and complement the instructive, well written, and comprehensive article of Khan and his team.
Figure 1.
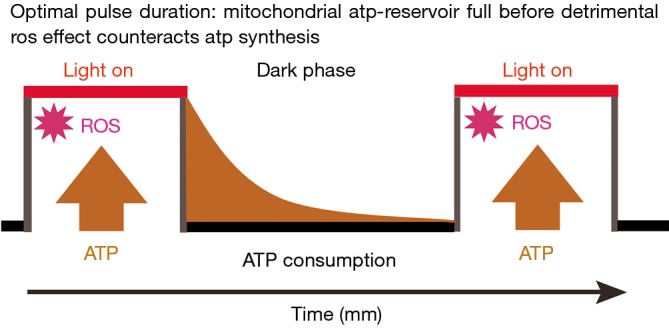
Self-explanatory illustration of the dynamic balance between exogenously controlled ATP synthesis during exposure of a cell to red to NIR light, and endogenous ATP consumption. The effect of the light is to reduce the viscous friction within and around the mitochondrial rotary motor thereby facilitating its normal function. Neither the cytochrome c oxidase dogma (12), nor the ROS theory (17,18) which works without ATP, nor the upregulation of gene expression (20,21), provides the understanding of the advantages of pulsed irradiation in biostimulation which eventually allows for the systematic optimization of the irradiation parameters for maximal ATP production and minimal ROS generation as well as minimal thermal damage. In combination with cell-friendly biomimetic surfaces (involved in minimizing contributions from exogenous ROS) the mechanism derived from the predictive model discussed here promises progress in the biostimulation of cells containing mitochondria. ROS, reactive oxygen species; NIR, near-infrared; ATP, adenosine triphosphate.
Acknowledgements
None.
Footnotes
Provenance: This is a Guest Editorial commissioned by the Section Editor Hui Kong, MD. PhD (Department of Respiratory Medicine, The First Affiliated Hospital of Nanjing Medical University, Nanjing , China).
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Khan I, Tang E, Arany P. Molecular pathway of near-infrared laser phototoxicity involves ATF-4 orchestrated ER stress. Sci Rep 2015;5:10581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Roti Roti JL. Cellular responses to hyperthermia (40-46 degrees C): cell killing and molecular events. Int J Hyperthermia 2008;24:3-15. [DOI] [PubMed] [Google Scholar]
- 3.Defrin R, Ohry A, Blumen N, et al. Sensory determinants of thermal pain. Brain 2002;125:501-10. [DOI] [PubMed] [Google Scholar]
- 4.Joensen J, Demmink JH, Johnson MI, et al. The thermal effects of therapeutic lasers with 810 and 904 nm wavelengths on human skin. Photomed Laser Surg 2011;29:145-53. [DOI] [PubMed] [Google Scholar]
- 5.Recommended treatment doses for Low Level Laser Therapy. Available online: http://waltza.co.za/wp-content/uploads/2012/08/Dose_table_780-860nm_for_Low_Level_Laser_Therapy_WALT-2010.pdf
- 6.Mester E, Mester AF, Mester A. The biomedical effects of laser application. Lasers Surg Med 1985;5:31-9. [DOI] [PubMed] [Google Scholar]
- 7.Mester A. Laser Biostimulation. Photomed Laser Surg 2013;31:237-9. [DOI] [PubMed] [Google Scholar]
- 8.Sommer AP, Pinheiro AL, Mester AR, et al. Biostimulatory windows in low-intensity laser activation: lasers, scanners, and NASA's light-emitting diode array system. J Clin Laser Med Surg 2001;19:29-33. [DOI] [PubMed] [Google Scholar]
- 9.Demidova-Rice TN, Salomatina EV, Yaroslavsky AN, et al. Low-level light stimulates excisional wound healing in mice. Lasers Surg Med 2007;39:706-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dube A, Bock C, Bauer E, et al. He-Ne laser irradiation protects B-lymphoblasts from UVA-induced DNA damage. Radiat Environ Biophys 2001;40:77-82. [DOI] [PubMed] [Google Scholar]
- 11.Sergio LP, Silva AP, Amorim PF, et al. DNA damage in blood cells exposed to low-level lasers. Lasers Surg Med 2015;47:361-8. [DOI] [PubMed] [Google Scholar]
- 12.Karu TI, Afanas'eva NI. Cytochrome c oxidase as the primary photoacceptor upon laser exposure of cultured cells to visible and near IR-range light. Dokl Akad Nauk 1995;342:693-5. [PubMed] [Google Scholar]
- 13.Lubart R, Lavi R, Friedmann H, et al. Photochemistry and photobiology of light absorption by living cells. Photomed Laser Surg 2006;24:179-85. [DOI] [PubMed] [Google Scholar]
- 14.Sommer AP, Haddad MKh, Fecht HJ. Tuning the wheel of life with light. In: Proceedings of the International Conference on Laser Applications in Life Sciences; Ulm, Germany; 2014:145. [Google Scholar]
- 15.Sommer AP. On the mechanism of photobiostimulation. In: Proceedings of the International Conference on Laser Applications in Life Sciences; Ulm, Germany; 2104:149. [Google Scholar]
- 16.Sommer AP, Haddad MKh, Fecht HJ. Light Effect on Water Viscosity: Implication for ATP Biosynthesis. Sci Rep 2015;5:12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lubart R, Friedmann H, Lavie R. Photobiostimulation as a function of different wavelengths. Laser Therapy 2000;12:38-41. [Google Scholar]
- 18.Lavi R, Ankri R, Sinyakov M, et al. The plasma membrane is involved in the visible light-tissue interaction. Photomed Laser Surg 2012;30:14-9. [DOI] [PubMed] [Google Scholar]
- 19.Pal G, Dutta A, Mitra K, et al. Effect of low intensity laser interaction with human skin fibroblast cells using fiber-optic nano-probes. J Photochem Photobiol B 2007;86:252-61. [DOI] [PubMed] [Google Scholar]
- 20.Houreld NN, Ayuk SM, Abrahamse H. Expression of genes in normal fibroblast cells (WS1) in response to irradiation at 660nm. J Photochem Photobiol B 2014;130:146-52. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y, Song S, Fong CC, et al. cDNA microarray analysis of gene expression profiles in human fibroblast cells irradiated with red light. J Invest Dermatol 2003;120:849-57. [DOI] [PubMed] [Google Scholar]
- 22.Sommer AP. A mechanism for ultrasound/light-induced biostimulation. Ann Transl Med 2015;3:291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sommer AP, Zhu D. Facial Rejuvenation in the Triangle of ROS. Cryst Growth Des 2009;9:4250-4. [Google Scholar]
- 24.Naviaux RK. Metabolic features of the cell danger response. Mitochondrion 2014;16:7-17. [DOI] [PubMed] [Google Scholar]
- 25.Owen L, Sunram-Lea SI. Metabolic agents that enhance ATP can improve cognitive functioning: a review of the evidence for glucose, oxygen, pyruvate, creatine, and L-carnitine. Nutrients 2011;3:735-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tian J, Zeng X, Xie X, et al. Intracellular Adenosine Triphosphate Deprivation through Lanthanide-Doped Nanoparticles. J Am Chem Soc 2015;137:6550-8. [DOI] [PubMed] [Google Scholar]


