Abstract
Background
To evaluate the clinical value of circulating tumor cells (CTC) count in peripheral venous blood of patients with non-small cell lung carcinoma (NSCLC).
Methods
A total of 50 NSCLC patients who were diagnosed in Wuxi No. 2 People’s Hospital from January 2013 to December 2013 were selected as the NSCLC group, 35 patients with lung benign tumor as the benign group, and 28 healthy subjects as the normal control group. Venous blood samples (3 mL) were collected in all subjects for counting the CTC, and a result of ≥8.7 was judged to be positive. The relationships between the positive rate of CTC and the age, sex, pathological type, and clinical stage of NSCLC were analyzed.
Results
CTC count was significantly higher in NSCLC group than in benign group and normal control group. In NSCLC patients, CTC count was not significantly correlated with sex, age, or the pathological type (P>0.05) but was closely related to clinical stage (P<0.01). Among NSCLC patients, CTC count significantly increased along with tumor progression.
Conclusions
CTC count shows certain correlation with the clinical features of NSCLC and thus can, to certain extent, reflect the status of the disease.
Keywords: Circulating tumor cells (CTCs), non-small cell lung carcinoma (NSCLC), clinical features
Introduction
Lung cancer is the leading malignancy with the highest morbidity and mortality in the Chinese populations (1), among which non-small cell lung cancer (NSCLC) accounts for over 80% of all lung cancer cases. Advanced NSCLC has a poor prognosis, with a 5-year survival of less than 20% (2).
Circulating tumor cells (CTC) refers to the tumor cells exist outside the primary and metastatic tumors. In fact, the invasive tumor cells originated from the solid tumor proliferate constantly; they become CTC with invasion and metastasis potentials after leaving the tumor tissue and entering the circulation (3,4). The occurrence of CTC in blood is a signal for distant metastasis, which may not be found by routine medical imaging, histology, and cytology.
It has been documented that CTC detection is a simple, repeatable, and minimally invasive diagnostic method (5,6). In our current study, we analyzed the relationship between CTC and the clinical features of NSCLC, with an attempt to inform the clinical prediction of disease outcomes and the tailored treatment protocol.
Subjects and methods
Clinical data
A total of 50 NSCLC patients who were diagnosed in our hospital from January 2013 to December 2013 were enrolled in this study. The diagnosis of NSCLC was based on the Guidelines on the Clinical Management of Non-small Cell Lung Cancer (2011 edition). There were 27 males and 23 females aged 47-81 years (median, 62 years). The pathological types included both adenocarcinoma (n=25) and squamous cell carcinoma (n=25). There were 9 cases with stage I, 11 cases with stage II, 7 cases with stage III, and 23 cases with stage IV. The inclusion criteria were as follows: (I) pathologically confirmed NSCLC; and (II) newly diagnosed and naive to radiochemotherapy. The exclusion criteria were as follows: (I) accompanied with end-stage liver disease or kidney disease; and (II) accompanied with other malignant tumors in the past 5 years. Meanwhile, 35 patients [21 men and 14 women aged 34-83 years (median, 61 years)] with benign lung diseases (pneumonia lesions, n=19; pulmonary tuberculosis, n=7; hamartoma, n=5; and pulmonary cysts, n=4) and 28 healthy subjects [13 men and 15 women aged 28-62 years (median, 40 years)] were enrolled as the benign group and normal control group, respectively.
Methods
Reagents and equipment
(I) Reagents: the CytoploRare kit, which includes red blood cell lysis buffer, reaction buffer, anti-CD45 immunomagnetic beads, anti-CD14 immunomagnetic beads, probe liquid (complex binding both folic acid conjugates of tumor-specific ligand and synthesized oligonucleotides), cell activation liquid, cell cleaning solution, cell washing solution, neutralization buffer, PCR reaction liquid, primer working fluid, and deionized water, was purchased from GenoSaber (Shanghai, China). (II) Equipment: the ABI 7000 Real Time PCR system was used.
Principle of detection
The folate receptor is highly expressed on the surface of lung cancer cells but is not or poorly expressed on the surface of normal cells in peripheral blood (7-10). In our current experiment, we firstly used the kit to enrich the CTC in the peripheral blood of NSCLC patients, followed by the labeling of CTC using specific small-molecule probe and then the forming of complex; finally, using the specific primers, we carried out PCR quantitative detection for the oligonucleotides on the probe, so as to calculate the CTC count in peripheral blood.
Assays
Primer and probe sequences
Reverse transcription primer: 5'-CTCAACTGGTGTCGTGGAGTCGGCAATTCAGTTGAGGGTTCTAA-3'; forward primer: 5'-TATGATTATGAGGCATGA-3'; reverse primer: 5'-GGTGTCGTGGAGTCG-3'; and quantitative fluorescence probe: 5'-FAM-CAGTTGAGGGTTC-MGB-3'.
Separation and enrichment of CTCs
Lysis of red blood cells: Fasting venous blood (3 mL) was collected using EDTA-containing tubes. After 12 mL of red blood cell lysis buffer was added and mixed well, the mixture was inoculated at 4 °C for 15 min before centrifuging for 10 min. After the supernatant was discarded, 10 mL of reaction buffer was added. The sediment was resuspended by gentle pipetting 10 times.
Removal of white blood cells: 150 µL of anti-CD45 immunomagnetic beads and 50 µL of anti-CD14 immunomagnetic beads were added, and then the mixture was inoculated at 4 °C for 20 min.
Labeling the cells with probe: 10 µL of probe liquid was added in the sample after equilibrating at room temperature. The mixture was inoculated at room temperature for 40 min and then centrifuged for 10 min to terminate the reaction.
Washing unbound probe: the mixture was added with 1 mL of cell washing buffer and thoroughly washed three times; then, it was centrifuged.
Elution of probe complexes: after the supernatant was discarded, 120 µL of elution buffer was added to elute the ligand-oligonucleotide complexes bound with folate receptor on cell surface. The mixture was centrifuged after the sediment was resuspended. The supernatant was transferred to a new Ep tube and added with 24 µL of neutralization buffer; PCR was performed after thorough mixing.
Targeted PCR and fluorescence detection
In the reaction tube, 2.5 µL of sample liquid and 22.5 µL of reaction liquid were added, and amplification was performed using the ABI 7000 Real Time PCR system. Meanwhile, six PCR calibration products and three PCR quality control products were set. The reaction conditions were as follows: 95 °C for 2 min, 40 °C for 30 s, 60 °C for 60 s, and 8 °C for 5 min, followed by 40 cycles of 95 °C for 10 s, 35 °C for 30 s, and 72 °C for 10 s. All the operations were in strict accordance with the in accordance with the manufacturer’s instructions (11,12).
Calculation of the detection results
Previous studies have demonstrated that the average amount of folate receptors on tumor cells is 7.5×10-18 mol (12). To facilitate the measurement of CTC during sample detection, the number of lung cancer cells that express the above amount of folate receptors is defined as one CTC unit (CU).
With the labeled value of the PCR calibrated products (concentration: 10-14-10-11.5 M, which equals to 1.39×10 - 4.40×103 CU/mL) as the abscissa and the CT values detected by PCR as the ordinate, we carried out linear regression analysis on the measurement values of the calibrated products and thus obtained the calibrator curve. The detected values of the samples were calculated using the calibrator curve.
Judgment criteria
The results were judged as negative if the CTC amount was <8.7 CU and as positive if ≥8.7 CU.
Statistical analysis
Statistical analysis was performed using the Sigmaplot 12.0 and SPSS 17.0 software. Continuous variables are expressed as mean ± standard deviations. The differences in means and distribution sites between two groups were analyzed using independent t-test or Mann-Whitney U test. Univariate analysis of variance or Kruskal-Wallis H test was applied to compare the differences among multiple groups. A correlation between two continuous variables was analyzed using Spearman method. A P value of <0.05 was considered statistically significant.
Results
Comparison of peripheral CTC values among three groups
The peripheral CTC levels showed skew distribution in the NSCLC group, benign group, and normal control group. The peripheral CTC count was 41.01 CU in the NSCLC group, which was significantly higher than that in the benign group (1.03 CU) and in the normal control group (0.34 CU) (F=37.12, P<0.01) (Figure 1).
Figure 1.
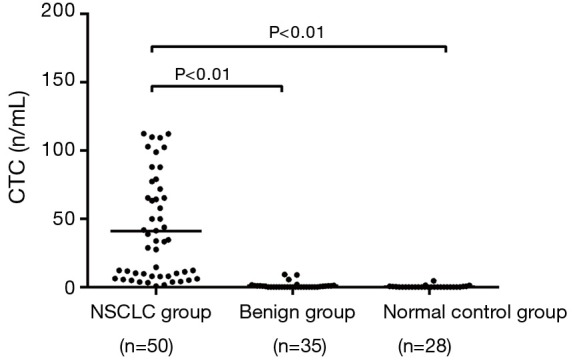
Comparison of CTC count in NSCLC group, lung benign tumor group and control group. The peripheral CTC levels showed skew distribution in the NSCLC group, benign group, and normal control group.
Relationship between peripheral CTC count and clinical features in newly diagnosed NSCLC patients
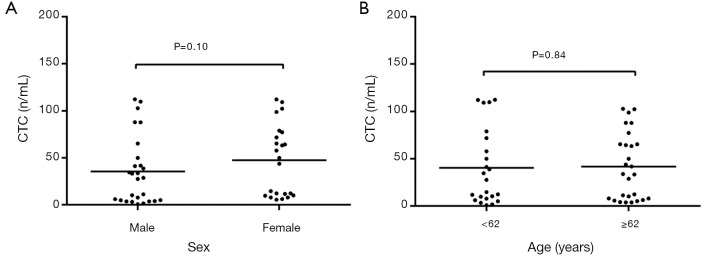
The peripheral CTC count showed no significant correlation with age (t=−1.152, P>0.05) and age (t=−0.125, P>0.05) in the newly diagnosed NSCLC patients (Figure 2A,B).
Figure 2.
Distribution of CTC count in NSCLC patients according to sex and age. (A) Distribution of CTC count in NSCLC patients according to sex; (B) distribution of CTC count in NSCLC patients according to age.
Relationship between peripheral CTC count and pathological types/TNM stages in newly diagnosed NSCLC patients
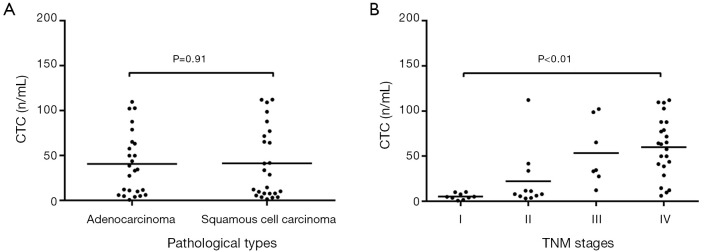
Among NSCLC patients with different pathological types, the peripheral CTC amount was 40.72 CU in adenocarcinoma subgroup and 41.31 CU in squamous cell carcinoma subgroup (t=−0.055, P>0.05) (Figure 3A).
Figure 3.
A distribution of CTC count in NSCLC patients according to pathological type and clinical stage. (A) A distribution of CTC count in NSCLC patients according to pathological type; (B) a distribution of CTC count in NSCLC patients according to clinical stage.
In addition, the CTC amount was 5.38 CU in stage I patients, 22.31 CU in stage II patients, 53.39 CU in stage III patients, and 60.14 CU in stage IV patients. The CTC level gradually increased along with the increase of clinical stage (F=9.01, P<0.01) (Figure 3B).
Discussion
The conventional methods for detecting lung cancer include chest X-ray, chest computed tomography (CT), fiberoptic bronchoscopy, and sputum cytology; however, their applications are limited due to low sensitivity, poor tolerance, and uselessness for early dynamic monitoring (13). As a simple, repeatable, and minimally invasive procedure, peripheral blood CTC detection can serve as an ideal source for sampling.
In our current study, we applied a combined method using both immunomagnetic separation and fluorescence quantitative PCR to detect peripheral CTC amount in NSCLC patients. Using this highly sensitive and specific method, we can identify a very small amount of tumor cells from a large number of peripheral blood cells. We applied this method in 50 NSCLC patients, 35 patients with benign lung diseases, and 28 healthy subjects and found that the peripheral CTC amount was significantly higher in NSCLC group than in the benign group and normal control group, suggesting that blood CTC level might have certain value in the diagnosis of lung cancer. Meanwhile, a small number of CTCs were also detected in stage I lung cancer, indicating lung cancer micrometastasis can occur in the early stages. Further analysis showed that the CTC amount was not significantly correlated with age, age, and pathologic type in NSCLC patients but was significantly correlated with the clinical stage. The blood CTC level was significantly higher in patients with advanced lung cancer than in those with early-stage disease, which was consistent with the findings of Chen et al. (14) and Liu et al. (15). In contrast, Sienel et al. (16) detected CTC amount using an anti-CK antibody-based immunocytochemical method and found that the positive rate of CTC was not significantly correlated with the standard clinical stages. This may be explained by the difference in detection methods. We will further evaluate it in studies with larger sample sizes. Although whether CTC can be used as a standard indicator for staging remains controversial, it is useful to reflect the degree of tumor progression and urge the physicians to take more pro-active treatment strategies.
Our study had some limitations. First, currently there were no internationally recognized criteria for judging the positivity of CTC, and our study was only based on the kit instructions provided by the manufacturer, which could cause false positive or false negative results. Second, the sample size was relatively small in our study.
In summary, since the mechanism of lung cancer metastasis via blood flow remains unclear, the value of CTC in lung cancer diagnosis requires further investigations. It is believed that, along with the improvements in detection techniques and optimization of methodologies, CTC detection and relevant research will facilitate the diagnosis of lung cancer and the early identification of its metastasis, thus becoming a new auxiliary tool in the clinical management of lung cancer.
Acknowledgements
Funding: This work was supported by Science and Technology Projects of Wuxi City (YGZXQ1310).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin 2013;63:11-30. [DOI] [PubMed] [Google Scholar]
- 2.Molina JR, Adjei AA, Jett JR. Advances in chemotherapy of non-small cell lung cancer. Chest 2006;130:1211-9. [DOI] [PubMed] [Google Scholar]
- 3.Joosse SA, Pantel K. Biologic challenges in the detection of circulating tumor cells. Cancer Res 2013;73:8-11. [DOI] [PubMed] [Google Scholar]
- 4.Khan MS, Kirkwood A, Tsigani T, et al. Circulating tumor cells as prognostic markers in neuroendocrine tumors. J Clin Oncol 2013;31:365-72. [DOI] [PubMed] [Google Scholar]
- 5.Bevilacqua S, Gallo M, Franco R, et al. A "live" biopsy in a small-cell lung cancer patient by detection of circulating tumor cells. Lung Cancer 2009;65:123-5. [DOI] [PubMed] [Google Scholar]
- 6.Xenidis N, Ignatiadis M, Apostolaki S, et al. Cytokeratin-19 mRNA-positive circulating tumor cells after adjuvant chemotherapy in patients with early breast cancer. J Clin Oncol 2009;27:2177-84. [DOI] [PubMed] [Google Scholar]
- 7.Parker N, Turk MJ, Westrick E, et al. Folate receptor expression in carcinomas and normal tissues determined by a quantitative radioligand binding assay. Anal Biochem 2005;338:284-93. [DOI] [PubMed] [Google Scholar]
- 8.O'Shannessy DJ, Yu G, Smale R, et al. Folate receptor alpha expression in lung cancer: diagnostic and prognostic significance. Oncotarget 2012;3:414-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nunez MI, Behrens C, Woods DM, et al. High expression of folate receptor alpha in lung cancer correlates with adenocarcinoma histology and EGFR [corrected] mutation. J Thorac Oncol 2012;7:833-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christoph DC, Asuncion BR, Hassan B, et al. Significance of folate receptor alpha and thymidylate synthase protein expression in patients with non-small-cell lung cancer treated with pemetrexed. J Thorac Oncol 2013;8:19-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yu Y, Chen Z, Dong J, et al. Folate receptor-positive circulating tumor cells as a novel diagnostic biomarker in non-small cell lung cancer. Transl Oncol 2013;6:697-702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lou J, Ben S, Yang G, et al. Quantification of rare circulating tumor cells in non-small cell lung cancer by ligand-targeted PCR. PLoS One 2013;8:e80458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu GJ. Progress in the early diagnosis of lung Cancer. Shijiazhuang: Hebei Medical University, 2008:5. [Google Scholar]
- 14.Chen Y, Li H, Qin Q, et al. Relationship between pathological staging and circulating tumor cells in non small cell lung cancer. Clin J Med Offic 2014;42:192-4. [Google Scholar]
- 15.Liu CH, Yang Z, Li Na, et al. The relationship between circulating tumor cells and elinieopathologic characteristics of primary lung cancer patients. Chong Qing Medicine 2014;43:2565-7. [Google Scholar]
- 16.Sienel W, Seen-Hibler R, Mutschler W, et al. Tumour cells in the tumour draining vein of patients with non-small cell lung cancer: detection rate and clinical significance. Eur J Cardiothorac Surg 2003;23:451-6. [DOI] [PubMed] [Google Scholar]