Abstract
Objective
The Ossabaw pig is emerging as an attractive model of human cardiometabolic disease due to its size and susceptibility to atherosclerosis, among other characteristics. Here we investigated the relationship between adipose tissue inflammation and metabolic dysfunction in this model.
Methods
Young female Ossabaw pigs were fed a western-style high-fat diet (HFD) (n=4) or control low-fat diet (LFD) (n=4) for a period of 9 months and compared for cardiometabolic outcomes and adipose tissue inflammation.
Results
The HFD-fed “OBESE” pigs were 2.5 times heavier (p<0.001) than LFD-fed “LEAN” pigs and developed severe obesity. HFD-feeding caused pronounced dyslipidemia, hypertension, insulin resistance (systemic and adipose) as well as induction of inflammatory genes, impairments in vasomotor reactivity to insulin and atherosclerosis in the coronary arteries. Remarkably, visceral, subcutaneous and perivascular adipose tissue inflammation (via FACS analysis and RT-PCR) was not increased in OBESE pigs, nor were circulating inflammatory cytokines.
Conclusions
These findings reveal a disconnect between adipose tissue inflammation and cardiometabolic dysfunction induced by western diet feeding in the Ossabaw pig model.
Keywords: macrophages, metabolic syndrome, obesity phenotypes, thrifty phenotype hypothesis, vascular disease
Introduction
As obesity continues to increase, so does the prevalence of cardiometabolic diseases including coronary artery disease, stroke, peripheral vascular disease and type 2 diabetes. These disorders are major causes of overall morbidity and mortality in the U.S. and worldwide. Importantly, as obesity leads to cardiometabolic disease in some but not all cases (1), it is imperative that the mechanisms linking obesity to disease be better understood. Moreover, as obesity is now prevalent in children, it is becoming increasingly important to study the consequences of early life-onset obesity on cardiometabolic disease development (2).
It is currently accepted that visceral obesity and insulin resistance (IR) form the ‘common soil’ from which cardiometabolic diseases develop, and that a central feature to this metabolic milieu is adipose tissue (AT) inflammation (3). Visceral AT inflammation, including inflammatory macrophage (Mφ) polarization, is predictive of metabolic dysfunction in several models with the majority of those conducted in rodents (4, 5); relationships have also been observed between AT inflammation and metabolic dysfunction in humans (6). Although research strides have been made to better understand such mechanisms, the vast majority of work has been done using rodents, whose size and rapid rate of maturation limits their ability to adequately model human obesity. Additionally, unlike humans, rodents do not develop atherosclerotic lesions unless genetically modified. The Ossabaw pig model is attractive because, similar to humans, when exposed to caloric excess and physical inactivity, they develop obesity and its metabolic consequences including IR, dyslipidemia, hypertension, and atherosclerosis (7). The pig more closely resembles the human in terms of its size, growth rate, and development of cardiovascular disease and is emerging as a more appropriate obesity model (8). The Ossabaw pig is characterized by the “thrifty phenotype” whereby this breed has adapted to store large amounts of energy during caloric excess (9). Our group (10, 11) and others (12, 13) have been utilizing the Ossabaw as a model of cardiometabolic disease development. We previously demonstrated that significant metabolic changes, as well as AT (10) and vascular (11) transcriptional alterations, occur early in the development of obesity in this model. Interestingly, the obesity that developed over that early period was not associated with increased expression of inflammatory genes conventionally viewed as being associated with obesity in visceral AT (10) and coronary perivascular AT (PVAT) (11).
Although the Ossabaw is emerging as an important model of cardiometabolic dysfunction, the relationship between visceral AT inflammation and metabolic function in this model remains poorly understood. Here, we sought to extend our previous work in juvenile Ossabaw swine (10, 11) and determine the effects of prolonged high fat diet (HFD) feeding through development and maturation (puberty around 5–6 month in swine) on AT inflammation and cardiometabolic disease. We hypothesized that chronic HFD feeding of female Ossabaw pigs would result in significant cardiometabolic dysfunction in the absence of robust changes in AT inflammation owing to the thrifty phenotype and associated less harmful adipocyte expansion.
Methods
Animals, Diets, Blood Pressure, Body Composition, and Tissue Sampling
All procedures were approved by the ACUC at the University of Missouri. Juvenile (5–6 wk-old) female Ossabaw pigs (n=8) obtained from Michael Sturek, Ph.D. at the Ossabaw Swine Resource, Comparative Medicine Program at Purdue University and Indiana University School of Medicine were housed under temperature-controlled (20–23 °C) conditions with a 12 h/12 h light-dark cycle. Pigs were either limit-fed regular miniature pig chow diet (5L80, Lab Diet; 3.03 kcal/g; 10.5% fat, “LEAN” n=4) or a western HFD (5B4L, Lab Diet; 4.14 kcal/g; 40.8% fat and 17.8% high fructose corn syrup, “OBESE” n=4) for nine months. Since the Ossabaw swine will over-consume when given free food access, limit-feeding the LEAN as well as the OBESE group was necessary.. Blood pressure was assessed by tail cuff method (GE Dash 3000) in conscious pigs after 8 ½ months of intervention and was the average of three measurements with at least a 10 minute separation between measurements. Following the intervention, pigs were weighed, body composition assessed via DXA (Hologic, QDR-1000), sedated by intramuscular injection of Telazol (5 mg/kg) and Xylazine (2.2 mg/kg) following an 18–20 hour fast, and blood collected for serum analyses via jugular vein. The pigs were then euthanized by IV injection of Telazol (10 mg/kg) and Xylazine (2.2 mg/kg) and removal of the heart. Subcutaneous abdominal (SQAT), visceral omental (OMAT), and PVAT surrounding the left anterior descending (LAD) coronary artery were harvested and processed or immediately frozen. The distal portion of the LAD coronary artery was dissected and used for vasomotor function experiments.
Serum and AT-Conditioned Media Analysis
Fasting serum glucose, NEFAs, total cholesterol, LDL, HDL, and TGs were analyzed as previously described (11). Insulin was measured using commercial kits (Porcine Insulin ELISA, Mercodia, #10-1200-01). Serum and AT-conditioned media concentrations of interferon gamma (IFN-γ), interleukin (IL) 1 (IL-1β), IL-1 receptor antagonist (IL-1RA), IL-6, IL-10, and tumor necrosis factor (TNF-α) were determined using a porcine-specific multiplex assay (Millipore Multiplex; Billerica, MA, #PCYTMAG-23K). All assays were run in duplicate.
Histology
SQAT, OMAT, and LAD coronary artery rings were fixed and stained with hemotoxylin and eosin, as previously described (10). Digital images were captured with an Olympus BX60 light microscope and Olympus SC 100 camera (Waltham, MA). Adipocyte size was calculated based on 100 adipocytes/animal from six fields of view using Image J software as described previously (14). Separate slides were stained with porcine-specific anti-scavenger receptor class A (SRA) (Anti MSR-A/CD204, 1:100, Cosmo Bio USA, #KAL-KT022) antibody and examined by an investigator blinded to the treatment groups.
Fluorescence Associated Cell Sorting (FACS)
The stromal vascular cell (SVC) fraction was isolated from whole AT extracted from SQAT, OMAT, and PVAT depots via collagenase digestion as previously described (14) with slight modifications. The following porcine-specific fluorophore-conjugated antibodies were used: CD3ε-PerCP-Cy5.5 (BD Pharmingen, #561478), CD4a- PECy7 (BD Pharmingen, #561473), CD8a-Alexa Fluor 647 (BD Pharmingen, #561475), and CD68- FITC (Santa Cruz Biotechnology, # sc-7083 FITC). Gating strategies included dead cell discrimination and lymphocyte quantification based on forward/side scatter and included unstained cells, single stain, and FMO controls. Cells were immunophenotyped using a CyAN ADP Analyzer (Beckman Coulter, Inc.) and data analyzed using Summit 5.2 (Beckman Coulter).
RNA Extraction and Quantitative Real-time RT-PCR
Quantitative real-time PCR was performed as previously described (11) and reactions were performed in duplicate. Primer sequences are available upon request. 18S was used as house-keeping control gene and cycle thresholds (CT) were not different between groups across for any tissues. mRNA expression values are presented as 2ΔCT whereby ΔCT = 18S CT – gene of interest CT and normalized to LEAN, set at 1.
Cytokine Secretion from AT
A portion of SQAT, OMAT, and PVAT surrounding the LAD coronary artery were incubated in Medium 199 (pH 7.4, 24 hrs) (100 mg AT /500 μl) under standard culture conditions (37°C, 5% CO2) as described (15) to produce AT-conditioned media.
Vasomotor Function Experiments in LAD Coronary Artery Rings
Distal end of the LAD coronary arteries were exposed from the heart and microdissected in the chamber at 4°C. Coronary ring segments were cut into 2-mm rings and mounted in a myograph chamber (Model 610M, Danish Myo Technology, Aarhus, Denmark) containing physiological salt solution gassed with 95% O2-5% CO2 at 37°C, as previously described (11). After a 30-minute equilibration period, an optimal tension (25mN) was applied and then another 30 minutes of equilibration followed. Rings were stimulated with cumulative addition of K+ (30–120 mM) to assess vessel viability. Coronary rings were preconstricted with 10nM U-46619 to induce ~70–80% maximal contraction (i.e. relative to maximal U-46619-induced contraction; data not shown). Concentration-response curves were obtained by cumulative addition of either bradykinin (10−12 to 10−7M), insulin (1 to 1000 μIU/mL) or sodium nitroprusside (10−9 to 10−5M). Relaxation at each concentration was measured and expressed as percent maximum relaxation, where 100% is equivalent to loss of all tension developed in response to U-46619.
Statistical Analysis
Between group differences were determined using Student’s two-tailed t-tests and considered statistically significant if P<0.05. Statistical analysis was performed using SPSS 22.0; all data are presented as mean ± SEM.
Results
HFD Induces Obesity, IR, and Dyslipidemia
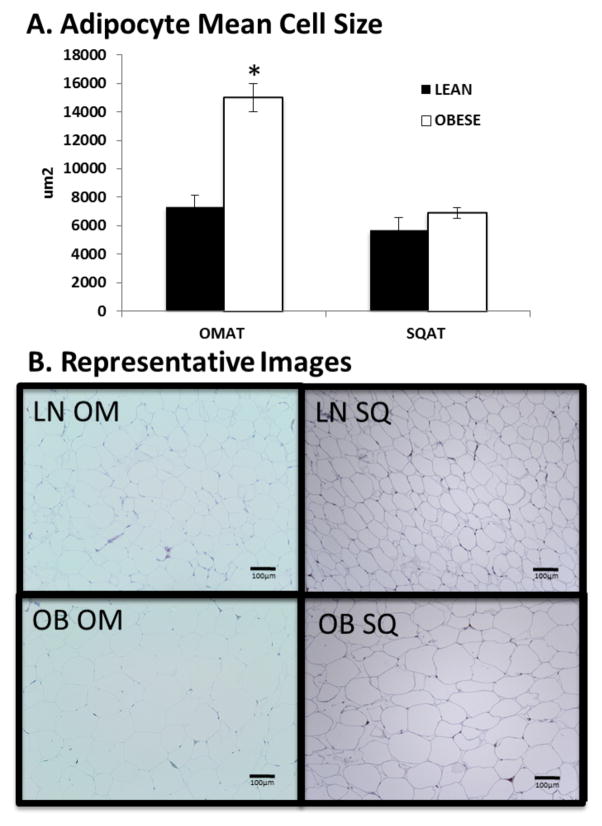
The average body weight for the Ossabaw swine before randomization was 4.9±0.3 kg. Throughout the study, LEAN pigs were limit-fed to an average of 600 g food/day; whereas the OBESE pigs were limit-fed to an average of 1200 g/day. Compared to LEAN, OBESE were ~2.5X heavier and had 45% more body fat (Table 1). OBESE were considerably larger animals, indicated not only by greater adiposity but also by greater length, lean, bone, and heart mass (Table 1). Adipocytes from OMAT were more than twice as large in the OBESE than in the LEAN group (p<0.001, Figure 1). However, adipocytes from SQAT were not different in size between groups (p=0.32, Figure 1). Compared to LEAN, OBESE also had higher fasting total cholesterol, HDL, LDL, NEFAs and TGs (all P<0.001, Table 1). OBESE were also considered diabetic based on elevated fasting plasma glucose and were significantly more insulin resistant based on the homeostatic model assessment (HOMA-IR (16)) and adipocyte IR (Adipo-IR (17)) (Table 1). Confirming what others have previously reported (12), despite only obtaining blood pressure measurements on a single day, OBESE also had significantly elevated systolic and diastolic blood pressure compared to LEAN.
Table 1.
Body composition and metabolic characteristics of LEAN and OBESE pigs
| LEAN (n=4) | OBESE (n=4) | P Value | |
|---|---|---|---|
| Body weight, kg | 37.3±1.51 | 100.4±2.00 | 0.0001 |
|
| |||
| Length, inches | 44.0±1.0 | 56.1±1.1 | 0.001 |
| Percent body fat, % | 29.2±3.13 | 41.9±1.15 | 0.009 |
| Percent lean mass, % | 72.0±3.6 | 56.6±0.9 | 0.006 |
| Blood pressure (Systolic/Diastolic mmHg) | 110±3/72±4 | 130±4/99±8 | <0.05 |
| Bone mass, kg | 0.80±0.06 | 1.37±0.065 | 0.001 |
| Heart mass, kg | 0.13±0.013 | 0.20±0.008 | 0.001 |
| Total cholesterol, mg/dl | 80.0±5.64 | 189.5±35.2 | 0.022 |
| LDL-c, mg/dl | 31.5±1.5 | 104.0±21.3 | 0.014 |
| HDL-c, mg/dl | 40.5±3.80 | 56.25±3.80 | 0.026 |
| LDL-c:HDL-c | 0.79±0.06 | 1.80±0.3 | 0.007 |
| Total cholesterol:HDL-c | 1.99±0.07 | 3.29±0.4 | 0.015 |
| NEFA, mmol/l | 0.223±0.085 | 2.572±0.353 | 0.001 |
| Triglycerides, mg/dl | 27.5±7.5 | 77.5±14.0 | 0.02 |
| Glucose, mg/dl | 121±10.0 | 308±47.5 | 0.008 |
| Insulin, μg/l | 0.104±0.017 | 0.287±0.009 | 0.017 |
| HOMA-IR | 0.91±0.18 | 6.26±0.96 | 0.001 |
| Adipo-IR | 0.023±0.009 | 0.736±0.096 | <0.001 |
Values are means ± SEM. LDL, low-density lipoprotein; HDL, high-density lipoprotein, NEFA, nonesterified fatty acids; HOMA-IR, homeostatic model assessment; Adipo-IR, adipocyte IR.
Figure 1. OBESE pigs have greater mean adipocyte cell size in omental visceral AT (OMAT), but adipocytes from subcutaneous AT (SQAT) were not different between LEAN and OBESE.
A: Mean adipocyte cell size calculated based on counts for 100 adipocytes/animal; n=4 animals/group. B: Representative images for each depot. Data expressed as mean ± SEM; * P<0.05; LN = LEAN; OB=OBESE; OM = OMAT; SQ = SQAT.
Markers of Inflammation Not Increased in Circulation, AT, or AT-Conditioned Media of OBESE
Of the circulating cytokines measured (IL-10, TNF-α, IFN-γ, IL-1β, IL-1RA, IL-6), only IL-6 was different between OBESE and LEAN with OBESE having ~60% lower circulating values (0.0425±0.006 (LEAN) vs. 0.0165±0.003 (OBESE) pg/mL, P=0.012) (Supplementary Figure 1). When media conditioned with AT from LEAN and OBESE was assessed for cytokines (i.e., as indication of AT cytokine production), no between-group differences were observed in any of the cytokines measured. Similarly, no differences in AT immune cell infiltration were observed between OBESE and LEAN pigs. From the SVC fraction, CD68+SVCs (Mφs) and CD3+, CD3/4+, CD3/8+SVCs (T lymphocytes) were isolated from AT harvested from OMAT, SQAT, and PVAT and quantified via FACS. In concordance with the lack of systemic inflammation in the OBESE, we did not detect increased AT T lymphocytes or Mφs in the OBESE compared to the LEAN in any of the depots. Finally, in accordance with the lack of evidence of AT inflammatory cell infiltration, it did not appear that OBESE OMAT or SQAT displayed increased Mφ content as measured via SRA (Mφ marker) immunostaining (Supp. Fig. 1).
Little Evidence of AT Inflammation in OBESE Via Gene Expression
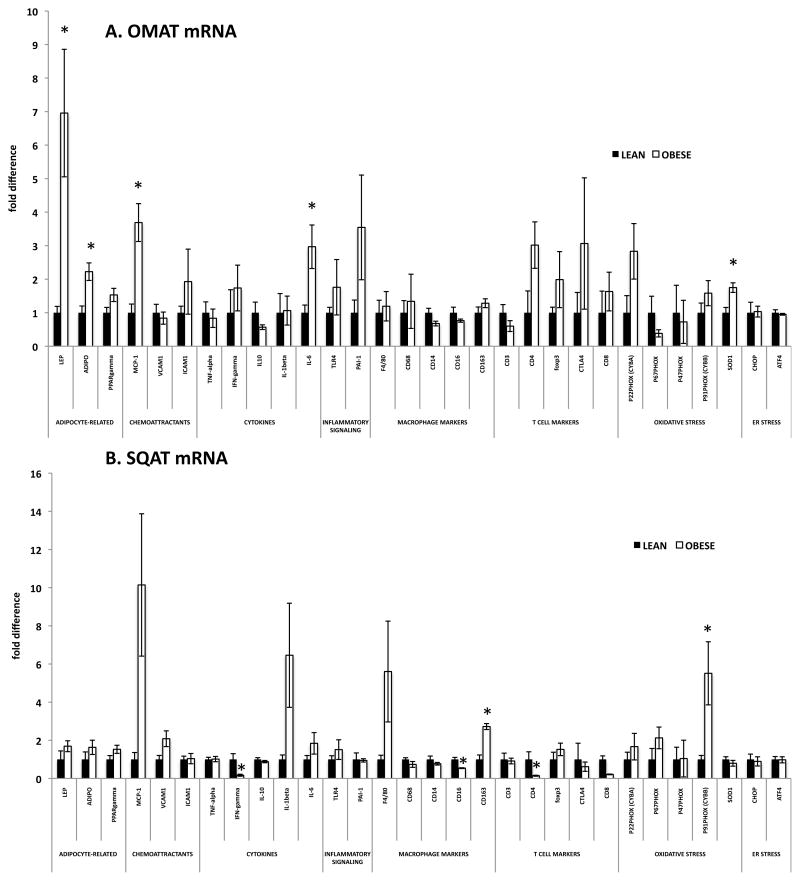
To further examine the inflammatory profile of AT from OBESE and LEAN, a comprehensive gene expression panel was analyzed in OMAT and SQAT (Figure 2). In OMAT, only five genes were significantly up-regulated in OBESE pigs. Adiponectin, an AT-secreted protein known to be insulin sensitizing and anti-inflammatory, was elevated ~2-fold and leptin, another AT-secreted protein important in metabolic homeostasis, was ~7-fold higher. IL-6, a cytokine secreted by immune cells as well as adipocytes that is thought to be “immunomodulatory” was ~3-fold higher in OMAT from OBESE animals. No other inflammatory markers were elevated (TNF-α, IFN-γ, toll-like receptor (TLR4), inflammatory T cell markers) except for monocyte chemoattractant protein (MCP-1), important in drawing in Mφs, which was ~4-fold elevated in OBESE. The T helper cell marker, CD4, trended to be higher among OBESE (P=0.076). Interestingly, the naturally occurring anti-oxidant molecule, superoxide dismutase (SOD1) was also marginally elevated in OBESE OMAT as was PPARλ (P=0.08), a nuclear receptor known to enhance adipocyte insulin sensitivity and reduce inflammation (18) (Figure 2A). No markers of Mφ infiltration were elevated in OBESE compared to LEAN OMAT, while the Mφ markers CD14 and CD16 were marginally suppressed in OBESE OMAT.
Figure 2. Little change in AT inflammatory gene expression in OBESE pigs.
A: Omental AT (OMAT) gene expression. B: Subcutaneous AT (SQAT) gene expression. Data expressed as mean ± SEM; * P<0.05
In SQAT, two genes were significantly higher in OBESE: CYBB (GP91-phox), an NADPH oxidase subunit indicative of oxidative stress, and the alternative/anti-inflammatory Mφ marker known to produce anti-inflammatory cytokines, CD163 (Figure 2B). In stark contrast to other animal models of obesity, gene expression of CD4 (indicative of T helper cells) and CD8 (indicative of cytotoxic T cells) were lower in OBESE compared to LEAN as was the pro-inflammatory cytokine, IFNγ, which is indicative of inflammatory T cell activation (19) and the Mφ marker, CD16. Two markers indicative of T regulatory cell (Treg) activation, Foxp3 and CTLA4, were not suppressed in OBESE, however. Tregs have been shown to have anti-inflammatory and insulin-sensitizing properties in AT (20). However, no differences were observed in CD3, CD4, or CD8+ T cells via FACS in SQAT between groups. These findings indicate that the OBESE pigs studied here did not experience increased SQAT T cell and/or Mφ influx.
OBESE Have Impaired Insulin-Stimulated Vasorelaxation and Atherosclerotic Lesion Formation in LAD Coronary Arteries Despite no Increase in PVAT Inflammation
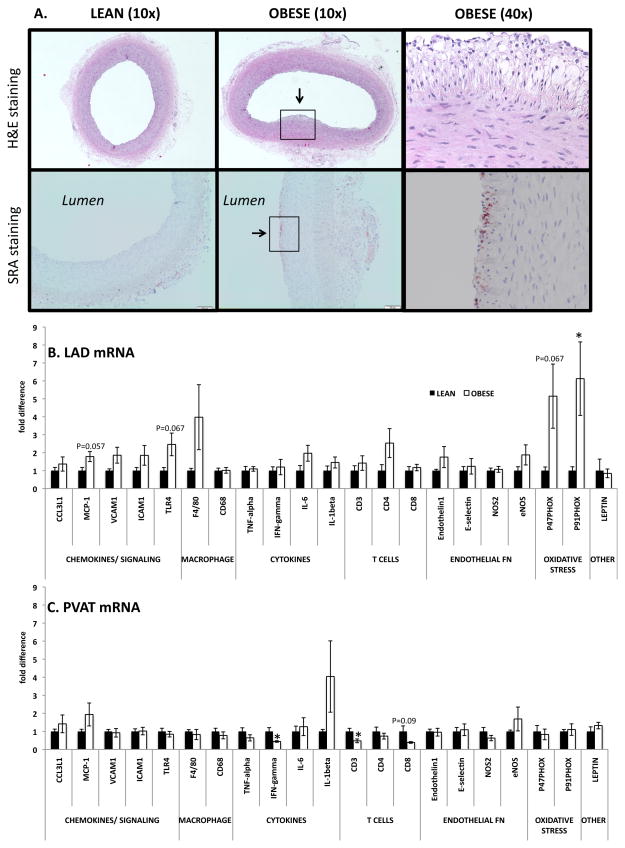
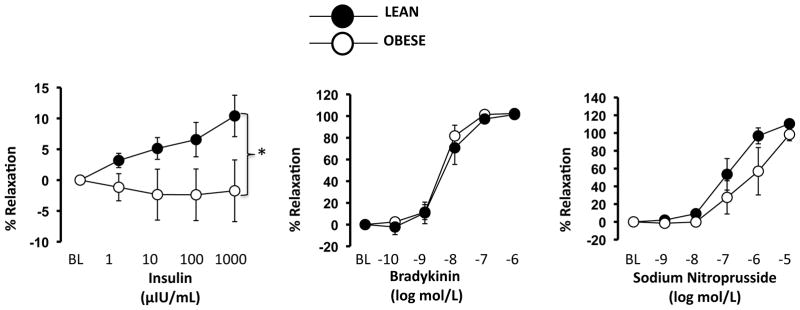
Upon histological examination, the OBESE pigs exhibited early evidence of atherosclerotic lesion formation in the LAD coronary arteries. Specifically, as shown in a representative 40x H&E-stained image, we observed foam cell formation in the subendothelial space and intima-medial thickening of the artery wall (Figure 3A, top panels). In addition, we noted positive SRA staining on the luminal surface of LAD coronary arteries from OBESE pigs, indicative of greater inflammatory Mφs (Figure 3A, bottom panels). These are considered early-stage lesions based on the histological classification of atherosclerosis published by the American Heart Association Committee on Vascular Lesions. Similarly, several inflammatory genes were, or trended toward being, up-regulated in the LAD coronary artery of OBESE vs. LEAN including the chemokines, MCP-1 (P=0.057), VCAM1 (P=0.11), and ICAM (P=0.18), the Mφ marker, F4/80 (P=0.15), and NADPH oxidase subunits, p47Phox (P=0.067) and p91Phox (P<0.05) (Figure 3B). We also measured expression of the same genes in PVAT adjacent to the LAD coronary artery. Similar to the lack of inflammation detected in other depots, the PVAT of the OBESE did not express higher inflammatory gene expression (Figure 3C). No genes were significantly different between OBESE and LEAN with the exception of CD3 (P<0.05), CD8 (trending at P=0.09), and IFN-γ (P<0.05), which all were down-regulated in OBESE. As shown in Figure 4, insulin-stimulated relaxation, but not bradykinin or sodium nitroprusside-induced relaxation, in the LAD coronary artery was blunted in OBESE compared to LEAN.
Figure 3.
OBESE pigs develop signs of coronary artery inflammation and atherosclerosis. A: Representative images of left anterior descending (LAD) coronary artery cross sections indicating lesion formation (indicated by arrow, top panels) and Mφ staining (SRA staining, bottom panels; positive staining indicated by arrow) in OBESE compared to LEAN; the last image in each row is the magnified region (40x) surrounded by the box in the second image which represents one OBESE animal. B: Gene expression analysis of LAD. C: Gene expression analysis of AT surrounding the LAD (i.e., perivascular AT, PVAT). Data expressed as mean ± SEM; * P<0.05.
Figure 4. OBESE pigs exhibit impaired insulin-stimulated relaxation in left anterior descending (LAD) coronary artery.
Data expressed as mean ± SEM; * P<0.05.
Discussion
We previously demonstrated that juvenile HFD-fed Ossabaw swine develop obesity and IR, with minimal evidence of AT inflammation (10). Here, we extend our previous work, demonstrating that, despite the fact that continued overconsumption of HFD into early adulthood causes extreme obesity, dyslipidemia, systemic IR, vascular IR, hypertension, as well as coronary artery inflammation and atherosclerotic lesions, female Ossabaw swine remained largely “protected” from the development of AT and systemic inflammation traditionally viewed as “characteristic” of obesity-associated metabolic impairments.
The HFD-fed OBESE pigs did not exhibit increased visceral AT (i.e., OMAT) Mφ or T cell infiltration assessed by FACS and verified at the level of mRNA in several inflammatory markers including T cell markers (CD3, CD8) and Mφ markers (CD68, CD14, CD16). The OBESE OMAT expressed higher levels of adiponectin and leptin, which often associate with greater adipocyte size, but the vast majority of inflammatory Mφ, T cell, and cytokine markers were not increased. However, the chemoattractant, MCP-1, thought to precede immune cell infiltration into AT, was significantly increased in OBESE AT. This is consistent with other findings in this model in the absence of significant inflammatory Mφ infiltration (12), and may suggest that MCP-1 is recruiting anti-inflammatory rather than inflammatory Mφs since we observed an increase in the alternative Mφ marker, CD163, in OBESE SQAT. Gene expression of the nuclear receptor, PPARλ trended higher in OBESE OMAT (P=0.08) and SQAT (P=0.12). PPARλ associates with greater insulin sensitivity, less dysregulation of adipocyte lipolysis and an anti-inflammatory Mφ profile (18). Also interesting, SOD1 (an antioxidant) was significantly elevated in OBESE OMAT.
Cytokine release from AT explants harvested from the three depots investigated (i.e., SQAT, OMAT, and PVAT) did not differ between groups, nor were there increases in circulating inflammatory cytokines (TNF-α, IFN-γ, IL-1β). Intriguingly, despite increased IL-6 gene expression in OMAT in OBESE pigs, OMAT secretion was unaltered and circulating levels were significantly reduced. This may suggest a disconnect between transcription and translation. IL-6 has been shown to be increased in AT (21) and circulation of obese humans (22) and correlate both inversely (23) and positively (22) with IR. IL-6 has both AT and skeletal muscle origins (24), and considered by some to be both pro- and anti-inflammatory (25). It is possible that reduced skeletal muscle IL-6 may have contributed to reduced circulating levels in the OBESE pigs. This possibility should be addressed in future studies.
Taken together, Ossabaw swine appear to be protected from HFD-induced increases in AT inflammation. These findings correspond with our previous work in juvenile Ossabaw swine fed a HFD shorter-term (10) and another previous report where HFD-feeding (~ 7 months) failed to increase CD203+ Mφ infiltration (i.e., less CD203+SVCs isolated from AT of HFD-fed vs control pigs) (12). In that study, CD203 was used as a marker of mature Mφs similar to the marker we used to identify cells of the Mφ/monocyte lineage, CD68; both are non-specific Mφ markers. Interestingly, although HFD reduced total AT Mφ infiltration in the Faris study, it caused the Mφ phenotype to change such that a greater percentage expressed CD16, a marker thought to be associated with inflammatory Mφ activity (12).
The SQAT is generally considered a healthier AT depot and is characterized by smaller, more insulin sensitive and less inflammatory adipocytes (26). Still, adipocytes in this depot have been shown to expand with obesity in other models, albeit not to the extent to which adipocytes from the visceral region do (6). Reduced expandability of adipocytes from SQAT during the progression of obesity may potentiate ectopic lipid deposition and increase visceral adiposity, all of which contribute to IR (27). The “adipose tissue expandability” hypothesis is that when adipocytes are limited in their ability to expand, this results in adipocyte stress, inflammation and IR (28). The lack of expandability of SQAT adipocytes in the OBESE may have contributed to their larger OMAT adipocytes as well as the increase in systemic IR and dyslipidemia. Our data suggest that this animal model, compared to others, is protected to some degree in terms of AT inflammation and that less harmful adipocyte ‘expandability’ may be contributing to this protection. Importantly, AT inflammation is not always present in obese adults (29, 30) and it recently has been reported that overweight children showed little evidence for Mφs in AT (31). Thus, the lower susceptibility to AT inflammation in the pig may lend support for the use of pig (as opposed to rodent) models to more accurately parallel the metablic manifestations of obesity in humans.
Given the lack of overt AT inflammation, which is the major source of obesity-associated systemic inflammation (32), it was not surprising that OBESE did not have greater systemic inflammation. However, the OBESE pigs developed other features of cardiometabolic dysfunction including hypertension, hyperglycemia, hyperinsulinemia, hypertriglyceridemia, and impaired insulin-stimulated vasodilation in coronary arteries. The OBESE also developed atherosclerotic lesions and increased coronary artery inflammation and oxidative stress. Indeed, the Ossabaw has been described as one of the best porcine models of metabolic syndrome-induced atherosclerosis (33). The role played by PVAT in the pathophysiology of cardiovascular disease is becoming increasingly appreciated with evidence suggesting that inflammatory factors secreted by PVAT promote inflammation and impair vascular function (34, 35). Remarkably, although significant up-regulation of genes associated with inflammation were detected in the LAD, such genes were not elevated, and many reduced, in PVAT. These findings are consistent with recent data showing that most of the PVAT secreted proteins that were altered with obesity in Ossabaw were not related to classic markers of inflammation or oxidative stress (35). Similarly, our previous microarray analysis in coronary PVAT from juvenile lean and obese Ossabaw revealed only 7 genes were significantly altered with obesity (11), none of which were linked to inflammation or oxidative stress pathways. Together, these findings point to a disconnect between AT inflammation and cardiometabolic dysfunction in the Ossabaw model. An important question is whether this disconnect is specific to the Ossabaw or is consistent across swine breeds. Compared to other models, the Ossabaw is arguably the best model of metabolic syndrome-associated cardiometabolic dysfunction for a variety of reasons including practicality of their smaller size and development of human cardiometabolic manifestations. However, the limited data available in other breeds suggests that pigs in general may not be as susceptible to metabolic inflammation. Female HFD-fed White pigs develop some evidence of systemic inflammation, but no increase in IL-6 or AT Mφs (36); Gottingen pigs (37) and a swine model of familial hypercholesterolaemia (38) also appear protected against systemic inflammation. While insufficient data are available to make conclusions regarding the breed-specificity of the lack of AT inflammation documented in our study, the available evidence suggest that the pig model is less susceptible to obesity-induced AT inflammation compared to other models. Another important consideration is that there are known sex differences in obesity-induced AT inflammation such that ovary-intact females are less susceptible to AT inflammation compared to age-matched males; whether this protection would be seen in male Ossabaw pigs is unknown. Unfortunately, the other cited studies (12, 37) investigating metabolic inflammation in pigs were also exclusively in females.
Insulin-stimulated relaxation in the LAD coronary artery was blunted in OBESE versus LEAN pigs in the absence of changes in bradykinin-induced relaxation, a response largely endothelium-dependent. In line with this observation, compelling evidence from studies using obese rodents demonstrate that impairments in insulin-stimulated dilation occur prior to impairments in other endothelium-dependent dilators in both skeletal muscle and coronary arteries (39, 40). Reciprocally, our group found that an improvement in insulin-induced dilation with physical activity-induced weight loss occurs in the absence of changes in acetylcholine-mediated dilation in rats (15). Thus, it appears that obesity-related changes in vascular insulin sensitivity do not always correlate with changes in classic measures of endothelium-dependent dilation.
Remarkably, after nine months of HFD feeding, the Ossabaw pigs studied here were largely “protected” from AT and systemic inflammation despite developing severe obesity with visceral adipoctye size expansion, IR, atherosclerosis, and dyslipidemia. These findings suggest that visceral AT inflammation is not a “hallmark feature” of the development of cardiometabolic disease in the female Ossabaw pig model. Given that AT inflammation has been shown to predict adverse metabolic outcomes in humans and rodents (6), determining what factor(s) is “protecting” the Ossabaw from developing inflammation could lead to therapeutic or preventative strategies applicable to human cardiometabolic disease. We speculate that the Ossabaw pig, and perhaps other swine breeds, have evolved to survive despite gross AT expansion owing to their “thrifty” genotype and postulate that this genetically-selected less harmful AT expansion may buffer the Ossabaw pig from a further exacerbation in cardiometabolic pathologies.
Supplementary Material
The Ossabaw swine model is emerging as an appropriate model for human cardiometabolic disease, but the role of adipose tissue inflammation on the development of cardiometabolic disease in this model has not been extensively investigated. As such, this study’s objective was to investigate the relationship between adipose tissue inflammation and cardiometabolic disease in Ossabaw swine.
Findings demonstrate that feeding Ossabaw swine a western high fat diet, from an early age into adulthood, results in severe obesity and cardiometabolic impairments without increased adipose tissue inflammation.
Future study of the adipose tissue metabolic phenotype of the Ossabaw pig model may lend important insight into the relationship(s) between adipose tissue immunometabolism and human diseases.
Acknowledgments
We thank Miles Tanner, Kayla Kanosky, and Denise Holiman for their technical assistance. In addition, we thank Dr. Doug Bowles for lending us the cuff-based blood pressure system. We acknowledge the support of NIH RR013223 and HL062552 to Dr. Michael Sturek and the Comparative Medicine Center of IUSM and Purdue University for the female Ossabaw swine used in this study. Funding was provided by NIH K01HL125503 (J.P.), University of Missouri Mizzou Advantage (R.S.R.), VA-CDA2-1299 (salary support to R.S.R.), and a grant from the Allen Foundation, INC (R.S.R.). This work was partially supported with resources and the use of facilities at the Harry S Truman Memorial Veterans Hospital in Columbia, MO.
Footnotes
No disclosures.
References
- 1.Shaharyar S, Roberson LL, Jamal O, Younus A, Blaha MJ, Ali SS, et al. Obesity and metabolic phenotypes (metabolically healthy and unhealthy variants) are significantly associated with prevalence of elevated C-reactive protein and hepatic steatosis in a large healthy brazilian population. Journal of obesity. 2015;2015:178526. doi: 10.1155/2015/178526. Epub 2015/04/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adebayo O, Willis GC. The changing face of diabetes in America. Emergency medicine clinics of North America. 2014;32(2):319–27. doi: 10.1016/j.emc.2013.12.004. Epub 2014/04/29. [DOI] [PubMed] [Google Scholar]
- 3.Murdolo G, Smith U. The dysregulated adipose tissue: a connecting link between insulin resistance, type 2 diabetes mellitus and atherosclerosis. Nutrition, metabolism, and cardiovascular diseases : NMCD. 2006;16 (Suppl 1):S35–8. doi: 10.1016/j.numecd.2005.10.016. Epub 2006/03/15. [DOI] [PubMed] [Google Scholar]
- 4.Mraz M, Haluzik M. The role of adipose tissue immune cells in obesity and low-grade inflammation. The Journal of endocrinology. 2014;222(3):R113–R27. doi: 10.1530/JOE-14-0283. Epub 2014/07/10. [DOI] [PubMed] [Google Scholar]
- 5.Vieira-Potter VJ. Inflammation and macrophage modulation in adipose tissues. Cellular microbiology. 2014 doi: 10.1111/cmi.12336. Epub 2014/07/31. [DOI] [PubMed] [Google Scholar]
- 6.Kloting N, Fasshauer M, Dietrich A, Kovacs P, Schon MR, Kern M, et al. Insulin-sensitive obesity. American journal of physiology Endocrinology and metabolism. 2010;299(3):E506–15. doi: 10.1152/ajpendo.00586.2009. Epub 2010/06/24. [DOI] [PubMed] [Google Scholar]
- 7.Dyson MC, Alloosh M, Vuchetich JP, Mokelke EA, Sturek M. Components of metabolic syndrome and coronary artery disease in female Ossabaw swine fed excess atherogenic diet. Comparative medicine. 2006;56(1):35–45. Epub 2006/03/09. [PubMed] [Google Scholar]
- 8.Koopmans SJ, Schuurman T. Considerations on pig models for appetite, metabolic syndrome and obese type 2 diabetes: From food intake to metabolic disease. European journal of pharmacology. 2015 doi: 10.1016/j.ejphar.2015.03.044. Epub 2015/03/31. [DOI] [PubMed] [Google Scholar]
- 9.Neel JV. Diabetes mellitus: a “thrifty” genotype rendered detrimental by “progress”? American journal of human genetics. 1962;14:353–62. Epub 1962/12/01. [PMC free article] [PubMed] [Google Scholar]
- 10.Toedebusch RG, Roberts MD, Wells KD, Company JM, Kanosky KM, Padilla J, et al. Unique transcriptomic signature of omental adipose tissue in Ossabaw swine: a model of childhood obesity. Physiological genomics. 2014;46(10):362–75. doi: 10.1152/physiolgenomics.00172.2013. Epub 2014/03/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Padilla J, Jenkins NT, Lee S, Zhang H, Cui J, Zuidema MY, et al. Vascular transcriptional alterations produced by juvenile obesity in Ossabaw swine. Physiol Genomics. 2013;45(11):434–46. doi: 10.1152/physiolgenomics.00038.2013. Epub 2013/04/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Faris RJ, Boddicker RL, Walker-Daniels J, Li J, Jones DE, Spurlock ME. Inflammation in response to n3 fatty acids in a porcine obesity model. Comparative medicine. 2012;62(6):495–503. Epub 2012/01/01. [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X, Li ZL, Woollard JR, Eirin A, Ebrahimi B, Crane JA, et al. Obesity-metabolic derangement preserves hemodynamics but promotes intrarenal adiposity and macrophage infiltration in swine renovascular disease. American journal of physiology Renal physiology. 2013;305(3):F265–76. doi: 10.1152/ajprenal.00043.2013. Epub 2013/05/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vieira Potter VJ, Strissel KJ, Xie C, Chang E, Bennett G, Defuria J, et al. Adipose tissue inflammation and reduced insulin sensitivity in ovariectomized mice occurs in the absence of increased adiposity. Endocrinology. 2012;153(9):4266–77. doi: 10.1210/en.2011-2006. Epub 2012/07/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crissey JM, Jenkins NT, Lansford KA, Thorne PK, Bayless DS, Vieira-Potter VJ, et al. Adipose tissue and vascular phenotypic modulation by voluntary physical activity and dietary restriction in obese insulin-resistant OLETF rats. Am J Physiol Regul Integr Comp Physiol. 2014;306(8):R596–606. doi: 10.1152/ajpregu.00493.2013. Epub 2014/02/14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–9. doi: 10.1007/BF00280883. Epub 1985/07/01. [DOI] [PubMed] [Google Scholar]
- 17.Malin SK, Kashyap SR, Hammel J, Miyazaki Y, DeFronzo RA, Kirwan JP. Adjusting glucose-stimulated insulin secretion for adipose insulin resistance: an index of beta-cell function in obese adults. Diabetes care. 2014;37(11):2940–6. doi: 10.2337/dc13-3011. Epub 2014/08/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Odegaard JI, Ricardo-Gonzalez RR, Goforth MH, Morel CR, Subramanian V, Mukundan L, et al. Macrophage-specific PPARgamma controls alternative activation and improves insulin resistance. Nature. 2007;447(7148):1116–20. doi: 10.1038/nature05894. Epub 2007/05/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blazquez R, Sanchez-Margallo FM, de la Rosa O, Dalemans W, Alvarez V, Tarazona R, et al. Immunomodulatory Potential of Human Adipose Mesenchymal Stem Cells Derived Exosomes on in vitro Stimulated T Cells. Frontiers in immunology. 2014;5:556. doi: 10.3389/fimmu.2014.00556. Epub 2014/11/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nature medicine. 2009;15(8):930–9. doi: 10.1038/nm.2002. Epub 2009/07/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bastard JP, Jardel C, Bruckert E, Blondy P, Capeau J, Laville M, et al. Elevated levels of interleukin 6 are reduced in serum and subcutaneous adipose tissue of obese women after weight loss. The Journal of clinical endocrinology and metabolism. 2000;85(9):3338–42. doi: 10.1210/jcem.85.9.6839. Epub 2000/09/22. [DOI] [PubMed] [Google Scholar]
- 22.van Beek L, Lips MA, Visser A, Pijl H, Ioan-Facsinay A, Toes R, et al. Increased systemic and adipose tissue inflammation differentiates obese women with T2DM from obese women with normal glucose tolerance. Metabolism: clinical and experimental. 2014;63(4):492–501. doi: 10.1016/j.metabol.2013.12.002. Epub 2014/01/29. [DOI] [PubMed] [Google Scholar]
- 23.Mauer J, Chaurasia B, Goldau J, Vogt MC, Ruud J, Nguyen KD, et al. Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin. Nature immunology. 2014;15(5):423–30. doi: 10.1038/ni.2865. Epub 2014/04/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munoz-Canoves P, Scheele C, Pedersen BK, Serrano AL. Interleukin-6 myokine signaling in skeletal muscle: a double-edged sword? The FEBS journal. 2013;280(17):4131–48. doi: 10.1111/febs.12338. Epub 2013/05/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Starkie R, Ostrowski SR, Jauffred S, Febbraio M, Pedersen BK. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2003;17(8):884–6. doi: 10.1096/fj.02-0670fje. Epub 2003/03/11. [DOI] [PubMed] [Google Scholar]
- 26.Bolinder J, Kager L, Ostman J, Arner P. Differences at the receptor and postreceptor levels between human omental and subcutaneous adipose tissue in the action of insulin on lipolysis. Diabetes. 1983;32(2):117–23. doi: 10.2337/diab.32.2.117. Epub 1983/02/01. [DOI] [PubMed] [Google Scholar]
- 27.Alligier M, Gabert L, Meugnier E, Lambert-Porcheron S, Chanseaume E, Pilleul F, et al. Visceral fat accumulation during lipid overfeeding is related to subcutaneous adipose tissue characteristics in healthy men. The Journal of clinical endocrinology and metabolism. 2013;98(2):802–10. doi: 10.1210/jc.2012-3289. Epub 2013/01/04. [DOI] [PubMed] [Google Scholar]
- 28.Virtue S, Vidal-Puig A. Adipose tissue expandability, lipotoxicity and the Metabolic Syndrome--an allostatic perspective. Biochimica et biophysica acta. 2010;1801(3):338–49. doi: 10.1016/j.bbalip.2009.12.006. Epub 2010/01/09. [DOI] [PubMed] [Google Scholar]
- 29.Apovian CM, Bigornia S, Mott M, Meyers MR, Ulloor J, Gagua M, et al. Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arteriosclerosis, thrombosis, and vascular biology. 2008;28(9):1654–9. doi: 10.1161/ATVBAHA.108.170316. Epub 2008/06/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Le KA, Mahurkar S, Alderete TL, Hasson RE, Adam TC, Kim JS, et al. Subcutaneous adipose tissue macrophage infiltration is associated with hepatic and visceral fat deposition, hyperinsulinemia, and stimulation of NF-kappaB stress pathway. Diabetes. 2011;60(11):2802–9. doi: 10.2337/db10-1263. Epub 2011/10/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tam CS, Tordjman J, Divoux A, Baur LA, Clement K. Adipose tissue remodeling in children: the link between collagen deposition and age-related adipocyte growth. The Journal of clinical endocrinology and metabolism. 2012;97(4):1320–7. doi: 10.1210/jc.2011-2806. Epub 2012/01/20. [DOI] [PubMed] [Google Scholar]
- 32.Festa A, D’Agostino R, Jr, Williams K, Karter AJ, Mayer-Davis EJ, Tracy RP, et al. The relation of body fat mass and distribution to markers of chronic inflammation. International journal of obesity and related metabolic disorders : journal of the International Association for the Study of Obesity. 2001;25(10):1407–15. doi: 10.1038/sj.ijo.0801792. Epub 2001/10/24. [DOI] [PubMed] [Google Scholar]
- 33.Hamamdzic D, Wilensky RL. Porcine models of accelerated coronary atherosclerosis: role of diabetes mellitus and hypercholesterolemia. Journal of diabetes research. 2013;2013:761415. doi: 10.1155/2013/761415. Epub 2013/07/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Villacorta L, Chang L. The role of perivascular adipose tissue in vasoconstriction, arterial stiffness, and aneurysm. Hormone molecular biology and clinical investigation. 2015 doi: 10.1515/hmbci-2014-0048. Epub 2015/02/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Owen MK, Witzmann FA, McKenney ML, Lai X, Berwick ZC, Moberly SP, et al. Perivascular adipose tissue potentiates contraction of coronary vascular smooth muscle: influence of obesity. Circulation. 2013;128(1):9–18. doi: 10.1161/CIRCULATIONAHA.112.001238. Epub 2013/05/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Busnelli M, Manzini S, Froio A, Vargiolu A, Cerrito MG, Smolenski RT, et al. Diet induced mild hypercholesterolemia in pigs: local and systemic inflammation, effects on vascular injury - rescue by high-dose statin treatment. PloS one. 2013;8(11):e80588. doi: 10.1371/journal.pone.0080588. Epub 2013/11/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rodgaard T, Skovgaard K, Moesgaard SG, Cirera S, Christoffersen BO, Heegaard PM. Extensive changes in innate immune gene expression in obese Gottingen minipigs do not lead to changes in concentrations of circulating cytokines and acute phase proteins. Animal genetics. 2014;45(1):67–73. doi: 10.1111/age.12090. Epub 2013/10/11. [DOI] [PubMed] [Google Scholar]
- 38.Simmons GH, Padilla J, Jenkins NT, Laughlin MH. Exercise training and vascular cell phenotype in a swine model of familial hypercholesterolaemia: conduit arteries and veins. Experimental physiology. 2014;99(2):454–65. doi: 10.1113/expphysiol.2013.075838. Epub 2013/11/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Katakam PV, Tulbert CD, Snipes JA, Erdos B, Miller AW, Busija DW. Impaired insulin-induced vasodilation in small coronary arteries of Zucker obese rats is mediated by reactive oxygen species. American journal of physiology Heart and circulatory physiology. 2005;288(2):H854–60. doi: 10.1152/ajpheart.00715.2004. Epub 2005/01/15. [DOI] [PubMed] [Google Scholar]
- 40.Eringa EC, Stehouwer CD, Roos MH, Westerhof N, Sipkema P. Selective resistance to vasoactive effects of insulin in muscle resistance arteries of obese Zucker (fa/fa) rats. American journal of physiology Endocrinology and metabolism. 2007;293(5):E1134–9. doi: 10.1152/ajpendo.00516.2006. Epub 2007/07/12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.