Abstract
Purpose of review
Conquering allograft rejection remains an elusive goal in spite of recent breakthroughs in the field of immunosuppression. Much of the problem lies in the toxicity and side effects of long-term use of systemic immunosuppressant drugs, which are sometimes ineffective in controlling rejection, but also hinder establishment of transplant tolerance. In this review, we discuss novel technologies that use grafts engineered with immunomodulatory molecules as a means of inducing tolerance.
Recent findings
Several recent studies have demonstrated the feasibility of engineering cells, tissues, or solid organ grafts with immunoregulatory biologics to achieve long termgraft survival without the use of chronic immunosuppression. This approach was shown to primarily change the ratio of T effector versus CD4+CD25+FoxP3+ T regulatory cells within the graft microenvironment in favor of attaining localized tolerance induction and maintenance.
Summary
Localized immunomodulation using biologic-engineered allografts represent a new paradigm for achieving long term graft survival in the absence of chronic use of immunosuppression. The manipulation of the graft, rather than the recipient, not only ensures short and long-term safety by minimizing the adverse effects of immunosuppression, but also allows retention of immune competency critical for the ability of the recipient to fight infections and cancer.
Keywords: SA-FasL, CD95, localized immunomodulation, transplantation tolerance, ProtEx™ technology, FasL
INTRODUCTION
Despite impressive advancements in the development of new immunosuppressants targeting the effector mechanisms of graft rejection, indefinite graft survival remains an unattainable goal due to chronic rejection. Currently, this necessitates the use of standard maintenance immunosuppression for the graft life. However, the chronic use of immunosuppression leads to drug related-toxicities and immune incompetence associated with opportunistic infections and malignancies. Moreover, the continuous use of standard immunosuppression has dual disadvantages: it does not always control acute graft rejection, and contributes to chronic rejection by interfering with the development of tolerogenic mechanisms. The reduction or elimination of long-term immunosuppressants is a difficult barrier to overcome due to persistent generation and high frequencies of alloantigen-specific pathogenic CD4+ and CD8+ T effector (Teff) cells in the absence of active tolerogenic mechanisms, such as protective regulatory T cells, in the recipient.
There is overwhelming evidence in the current literature pointing to localization of pathogenic and tolerogenic immune responses in the target tissues [1]. These findings emphasize the importance of understanding the nature of immune responses within the graft, and thus the exciting possibility of targeted manipulation of intra-graft immune responses for achieving safe, long-term graft survival without chronic use of immunosuppression. We herein review and discuss several novel technologies designed to engineer grafts with biologics for immunomodulation to overcome rejection. The emphasis will primarily be on the review of recent literature focusing on the application of these technologies to cellular and tissue allografts with a limited reference to solid organs.
IMMUNOMODULATION USING GRAFTS ENGINEERED TO DISPLAY BIOLOGICS ON THEIR SURFACE
Localizing and limiting the tolerogenic mechanisms to the graft while leaving the systemic immune responses unrelated to the graft largely intact is best accomplished using the graft itself as a tolerogenic entity. This can be best achieved by modifying the surface of the graft with immunomodulatory biologics involved in induction of tolerance. This approach is conceptually attractive and provides two distinct advantageous over systemic treatment of the graft recipients: i) the modified graft operating as an immunomodulatory entity has better chance to overcome rejection by local graft protection through immunoregulatory, rather than destructive, alloreactive responses, and ii) the invoked immunoregulatory responses may be localized to the graft, thereby retaining better immune competency. Such localized immune strategies have been utilized in nature, as when bacteria encapsulate themselves to avoid immune protection, or when immune-privileged organs maintain increased expression of immunoregulatory factors, such as TGF-β and FasL, to protect against excess inflammation.
In this review, we will describe two distinct approaches targeting the engineering of grafts with biologics for immunomodulation: i) those using various chemistries to modify the surface of pancreatic islets with immunomodulatory molecules, and ii) the ProtEx™ technology developed by Yolcu et al. [2] as a practical and targeted approach for positional display of immunological ligands on the surface of cells, tissues, and solid organs.
IMMUNOMODULATION WITH THROMBOMODULIN
The transplantation of pancreatic islets into type I diabetic patients to achieve euglycemia is an important therapeutic approach to reconstitute endogenous insulin homeostasis. In the clinical setting, islets are prevalently transplanted through the portal vein, initiating an immediate blood-mediated inflammatory reaction (IBMIR) to the graft [3, 4]. IBMIR is responsible for 50–80% loss of infused islets, a major obstacle for early islet engraftment [4–6]. Significant islet loss caused by IBMIR necessitates transplantation of islets from several donors to achieve requisite islet dose needed for euglycemia,. The canonical features of this thrombotic/inflammatory reaction are rapid activation of platelets, coagulation and complement, resulting in acute leukocyte infiltration and islet damage. In fact, coagulation is fostered by the grafted islets through tissue factors expressed in response to stimuli associated with donor death, islet isolation and local injury to endothelial cells of islet microvasculature [4]. Tissue factor mediates the conversion of prothrombin to thrombin, initiating the coagulation cascade, innate, and proinflammatory responses.
This initial period of islet destruction can be ameliorated by natural anticoagulants in the form of heparin sulfate or thrombomodulin. Thrombomodulin activates protein C following pairing with thrombin, reducing blood clotting and inflammation [7–10] through negative regulation of the coagulation pathway, inhibition of innate and adaptive immune responses [11] and expansion of CD4+CD25+FoxP3+ T regulatory (Treg) cells [12–14]. Focusing on the therapeutic efficacy of thrombomodulin in prevention of IBMIR, systemic administration of liposomal thrombomodulin was shown to significantly improve intraportal allogeneic islet engraftment and survival in models of murine chemical diabetes [7]. These positive effects of modulation with thrombomodulin were associated with significant reduction in fibrin deposition, graft-infiltrating neutrophils, and TNF-α and IL-β levels in the liver.
Further procedural improvements are used to directly engineer islet surface with thrombomodulin, such as chemical conjugation performed by Chaikof’s group [15, 16]. These approaches involve attachment of azido-functionalized thrombomodulin molecule onto the islet surface (using Staudinger ligation to a bifunctional poly(ethylene glycol) [15] and streptavidin-biotin bridge [16, 17]). Islets engineered with thrombomodulin displayed increased activated protein C production and reduced thrombogenicity [7, 15, 15, 16]. In another approach, conjugation of both thrombomodulin and urokinase onto the surface of islets using polyethylene glycol-conjugated phospholipids was shown to have no detrimental impact on islet function [18]. Although promising, the use of these approaches to overcome IBMIR and enhance graft survival in vivo remains to be demonstrated.
IMMUNOMODULATION WITH TGF-β
Numerous preclinical models showed that long-term tolerance to allografts is strongly associated with the presence of Treg cells within graft microenvironment [1, 19–21]. Although intuitive, the mechanistic basis of this observation is not fully elucidated and may be caused by presentation of alloantigens by the graft and induce localized amplification of Treg cells. This notion is consistent with studies demonstrating that antigen-specific Treg cells have better efficacy than polyclonal cells in graft protection from rejection [22]. In addition, it has been demonstrated that Treg cells traffic to and reside in the graft, thus preventing rejection by effector cells and contributing to long-term graft survival [1, 19, 20, 23]. Indeed, coating the surface of pancreatic islets with Treg cells prolongs survival in allogeneic recipients [24]. Therefore, localized immunomodulation using biologics involved in generation of Treg cells presents an important approach with a significant potential of efficacy to induce tolerance.
TGF-β1 is critical for the development, expansion, and function of Treg cells. A series of recent studies have shown the capacity of TGF-β1 to mediate tolerance to allo and self-antigens in various transplantation and autoimmune settings. For example, ectopic expression of TGF-β in heart allografts resulted in long-term survival [25], and self-tolerance established using anti-CD3 antibodies was found to depend on TGF-β in NOD mice [26]. These effects may be primarily mediated by the critical role of TGF-β1 in the development, homeostasis, and expansion of Treg cells. Consistently, mice deficient for TGF-β1 display reduced Treg numbers in the periphery due to TGF-β1 regulation of FoxP3 [27]. TGF-β1 also plays an important role in the homeostasis and function of Treg cells in NOD mice [28]. NOD mice have significantly reduced absolute numbers of Treg cells as compared with disease-resistant strains [29, 30]. This reduction results from a decline in the cell surface expression of TGF-β1, which in turn results in reduced expression of FoxP3 and altered function of Treg cells that coincide with the disease onset [28, 29, 31]. TGF-β1 also plays an important role in the conversion of naïve CD4+CD25− T conventional cells into Treg cells by inducing FoxP3 expression [32–34]. Interestingly, a recent study has shown that transient expression of TGF-β1 in inflamed islets of NOD was effective in delaying the disease, not by the enhancement of Treg cells, but by blocking the development and maintenance of pathogenic CD8+ T effector cells [35]. Taken together, these studies demonstrate that TGF-β1 confers immune regulation at multiple levels.
Systemic use of TGF-β1 for immunomodulation has been associated with fibrosis that negatively impacts long-term graft survival [36]. This negative effect of TGF-β1 can potentially be ameliorated by immobilizing this molecule on the target cells/tissues. To test the feasibility and efficacy of engineering the cell surface with TGF-β1 as a means of immunomodulation, Yang et al. generated a TGF-β1 chimeric isoform which has a 1-methyl-2-diphenylphosphino-terephthalate moiety, allowing its binding to PEGylated cells or beads through Staudinger ligation [37]. Antigen-presenting cells coated with the TGF-β1 protein were able to convert conventional CD4+ T cells into induced Treg cells in response to antigens. The engineered cells were also effective in converting polyclonal naïve CD4+ T cells into nonspecific Treg cells in response to costimulation with αCD3/CD28 beads. Induced Treg cells were able to suppress the proliferation of naïve CD4+ T cells in a manner similar to that achieved using naturally occurring Treg cells. However, the efficacy of allografts engineered with TGF-β1 in overcoming rejection remains to be demonstrated.
PROTEX™ TECHNOLOGY AS AN EFFECTIVE AND PRACTICAL MEANS OF TRANSIENTLY DISPLAYING IMMUNOLOGICAL LIGANDS ON BIOLOGICAL MEMBRANES FOR IMMUNOMODULATION
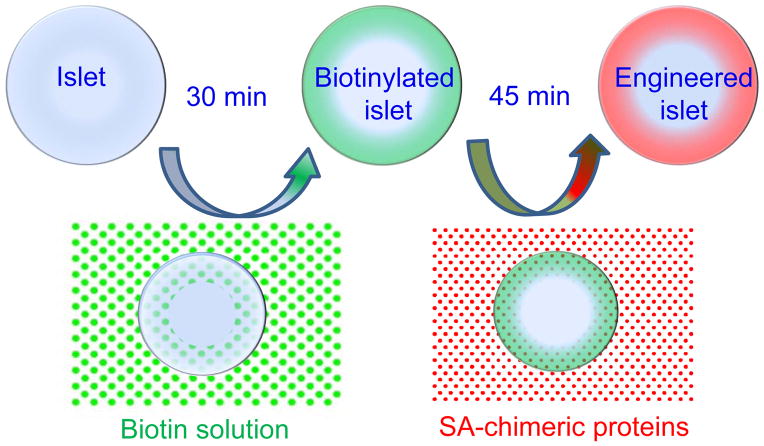
Immune responses are initiated, coordinated, and resolved by complex interactions between cell surface receptors and ligands. Therefore, Yolcu et al. envisioned that transient display of immunological ligands on the cell surface may provide an effective means of immunomodulation and designed a transient protein display technology called ProtEx™ [2]. The concept involves generation of chimeric proteins composed of a functional domain of immunological ligands fused to a modified form of streptavidin. These proteins can then be displayed on the surface of any biological membrane by taking advantage of the high affinity interaction (Kd = 10−15 M) between biotin and streptavidin (Fig. 1). The process of tissue engineering is practical, performed within 2.5 hours, and has an almost absolute efficiency in decorating the targeted tissues. Furthermore, this technology allows for simultaneous display of several chimeric immunological ligands on the cell surface at desired densities with complementary/synergistic functions for better immunomodulatory efficacy [38]. Most importantly, the transient presence of immunological ligands on the cell surface may also obviate the undesired effects arising from gene-therapy based long-term expression of immunological ligands having pleiotropic functions.
Figure 1. ProtEx™ technology.
Biological surfaces of interest are modified with biotin followed by decoration with SA-chimeric proteins under physiological conditions. This concept is schematically shown for pancreatic islets, but is also applicable to cells, tissues, and solid organs.
Chimeric proteins exist as tetramers and oligomers, owing to the structural features of streptavidin, which forms stable tetramers and oligomers under physiological conditions [39]. SA-chimeric immunological proteins were demonstrated to have robust activity whether displayed on the cell membrane or in soluble forms [2]. The improved immunological function of the chimeric ligands as compared with the soluble forms of natural ligands is conceivably due to their ability to oligomerize and crosslink their respective receptors on immune cells for better signal transduction. Immune decisions are made through cell surface receptor/ligand interactions and such interactions are short in duration (minutes to hours). Therefore, the persistence of chimeric immunological ligands on the cell surface for days, if not hours, is sufficient for effective transduction of immunological signals and driving the ensuing immune responses.
SYSTEMIC IMMUNOMODULATION WITH SA-FASL-ENGINEERED CELLS
Activation-induced cell death is critical to immune homeostasis, tolerance to self-antigens, and acquired tolerance to transplantation antigens [40, 41]. Fas/FasL interaction plays an important role in activation induced cell death [42–47]. As such, this molecule has been extensively exploited for tolerance induction to self and alloantigens using gene therapy [47–50]. Ectopic, persistence expression of FasL in cells and tissues of interest using gene therapy for immunomodulation is technically challenging and imposes safety concerns. However, FasL can transiently be displayed as a protein on any biological membrane, such as cells, pancreatic islets and vascular endothelium [21, 51], using the ProtEx™ technology. This approach benefits from the advantage of using SA-FasL-engineered cells, tissues, or solid organs for targeted delivery of apoptotic signals in responding alloreactive cells, thereby avoiding non-selective immune suppression.
Yolcu et al. generated an SA-chimeric FasL molecule (SA-FasL) [2] and used the ProtEx™ technology to display this protein on the surface of various cells for immunomodulation in autoimmunity and transplantation settings. Graft recipients systemically treated with SA-FasL-engineered donor splenocytes achieved tolerance to cardiac and pancreatic islet allografts in the absence of chronic immunosuppression [21, 52]. In addition to targeted depletion of effector cells, tolerance was maintained by induction/expansion of Treg cells [21, 52], which are relatively resistant to apoptosis by SA-FasL [17]. This observation resulted in the opportunity of engineering Treg cells with SA-FasL to improve their regulatory function. Treg cells displaying SA-FasL on their surface, referred to as killer Treg cells, had robust regulatory function as compared with unmodified Treg cells [53]. Importantly, SA-FasL-engineered Treg cells when adoptively transferred into prediabetic and new onset diabetic NOD mice homed to the pancreas and regional lymph nodes, proliferated, and induced apoptosis in T effector cells, resulting in delayed and lower incidence of diabetes as compared with controls [53, 53, 54]. Similar efficacy of adoptively transferred SA-FasL-engineered Treg cells was also shown for established inflammatory colitis [55] and acute mouse graft-versus-host disease [56].
LOCALIZED IMMUNOMODULATION WITH SA-FASL-ENGINEERED PANCREATIC ISLETS AND CARDIAC GRAFTS
The ProtEx™ technology to engineer target cells, tissues, and organs with SA-FasL for localized immunomodulation has significant potential for translational efficacy and safety. Quite extensive studies showed that pancreatic islets from various species can be effectively engineered with SA-FasL without a detrimental effect on their viability, function, and in vivo engraftment. Islets engineered with SA-FasL, when transplanted under a transient coverage of rapamycin, had robust efficacy in inducing tolerance in a chemically diabetic allogeneic mouse model [21, 57]. This protocol was also effective in preventing the rejection of xenogeneic rat islets transplanted into mice (Zhao et al., manuscript in preparation). Recipients of long-term islet grafts had almost normal peripheral responses to donor antigens in ex vivo mixed lymphocyte assays and rejected donor skin grafts in an acute fashion. Importantly, the frequency of Treg cells was increased in graft-draining lymph nodes and within the graft. Depletion of Treg cells early or late post-transplantation resulted in islet graft rejection, demonstrating the importance of these cells for the induction as well as maintenance of tolerance. In an adoptive transfer study, Treg cells isolated from graft-draining lymph nodes were effective in preventing the rejection of unmodified donor islets [21]. These observations indicate the localized nature of tolerance, which was further confirmed by demonstrating that long-term graft survivors rejected a second set unmodified donor islet grafts transplanted under the contralateral kidney capsule [21]. Interestingly, local tolerance was so robust that rejection of the second set of islet grafts did not affect the survival of the long-term primary grafts (> 100 days).
ProtEx™ technology is not only applicable to engineering cells and tissues, but also organs. Ex vivo engineering of the heart by perfusion with reactive biotin followed by SA-FasL protein under conditions of extracorporeal organ preservation resulted in effective display of the protein on heart vasculature with a half-life of 9 days in vivo. The engineering process did not significantly affect the function of the heart nor showed toxicity as demonstrated by the indefinitely survival of the engineered heart in syngeneic recipients [51]. Most significant was the ability of the SA-FasL-engineered heart to overcome acute rejection in allogeneic recipients [51]. Taken together, these data demonstrate the utility of ProtEx™ technology as a practical, safe, and effective alternative to DNA-based gene therapy for engineering cells, tissues, and organ for immunomodulation with demonstrated efficacy and potential application to the treatment of various acquired immune disorders and graft rejection.
CONCLUSION
While current clinical practices in transplantation focus on the systemic nonspecific control of the immune system to achieve graft survival, targeted approaches using engineered cells, tissues, and vasculature of solid organs may achieve safer and better outcomes by controlling early inflammation and inducing active immunoregulatory mechanisms. Immunomodulatory molecules, such as SA-FasL and TGF-β, displayed on biological surfaces of the grafts may confer localized immune privilege and long-term survival without the need for chronic immunosuppression. Simultaneous transient display of multiple proteins with synergistic mechanisms of action has significant potential for clinical success. In this context, the ProtEx™ technology presents a novel and practical approach with demonstrated efficacy in inducing robust tolerance at the local and systemic levels in preclinical models. The translation of this concept to the clinic and its efficacy are exciting future propositions. If effective in the clinic, this concept will have a significant impact on clinical practice of transplantation.
KEY POINTS.
Engineering graft with immunomodulatory proteins represents a novel, attractive, and safe means of immunomodulation
Grafts engineered with immunomodulatory proteins allow for localized immunomodulation, without impairing immune competence of the recipient
ProtEx™ technology has the advantage of directional positioning of multiple molecules simultaneously on the surface of the graft for a more effective immunomodulation
Tolerance may be boosted by systemic interventions to induce selected non-responsiveness to donor alloantigens
A major mechanism of tolerance achieved by SA-FasL involves the activity of CD4+CD25+FoxP3+ T regulatory cells
Acknowledgments
We thank Drs. Nadir Askenasy, Longshan Liu, William Bowen, and Changxi Wang for critical reading of the manuscript and Orlando Grimany-Nuno for his technical contribution.
Footnotes
Conflict of interest: The ProtEx™ technology described in this manuscript is licensed from UofL by FasCure Therapeutics, LLC, Louisville, KY, in which Haval Shirwan and Esma S. Yolcu have significant equity interest. The other authors disclosed no potential conflict of interest.
Financial support and sponsorship: Supported in parts by grants NIH R21 AI113348 and 5T32 HL076138-07 Training Fellowship (H. Zhao), Juvenile Diabetes Research Foundation (17-2012-527), American Diabetes Association (1-12-BS-191), the Commonwealth of Kentucky Research Challenge Trust Fund, and Keck Foundation.
References
- 1.Zhang N, Schroppel B, Lal G, Jakubzick C, Mao X, Chen D, Yin N, Jessberger R, Ochando JC, Ding Y, Bromberg JS. Regulatory T cells sequentially migrate from inflamed tissues to draining lymph nodes to suppress the alloimmune response. Immunity. 2009;30:458–69. doi: 10.1016/j.immuni.2008.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yolcu ES, Askenasy N, Singh NP, Cherradi SE, Shirwan H. Cell membrane modification for rapid display of proteins as a novel means of immunomodulation: FasL-decorated cells prevent islet graft rejection. Immunity. 2002;17:795–808. doi: 10.1016/s1074-7613(02)00482-x. [DOI] [PubMed] [Google Scholar]
- 3.Bennet W, Sundberg B, Groth CG, Brendel MD, Brandhorst D, Brandhorst H, Bretzel RG, Elgue G, Larsson R, Nilsson B, Korsgren O. Incompatibility between human blood and isolated islets of Langerhans: a finding with implications for clinical intraportal islet transplantation? Diabetes. 1999;48:1907–14. doi: 10.2337/diabetes.48.10.1907. [DOI] [PubMed] [Google Scholar]
- 4.Moberg L, Johansson H, Lukinius A, Berne C, Foss A, Kallen R, Ostraat O, Salmela K, Tibell A, Tufveson G, Elgue G, Nilsson EK, Korsgren O, Nilsson B. Production of tissue factor by pancreatic islet cells as a trigger of detrimental thrombotic reactions in clinical islet transplantation. Lancet. 2002;360:2039–45. doi: 10.1016/s0140-6736(02)12020-4. [DOI] [PubMed] [Google Scholar]
- 5.Korsgren O, Nilsson B, Berne C, Felldin M, Foss A, Kallen R, Lundgren T, Salmela K, Tibell A, Tufveson G. Current status of clinical islet transplantation. Transplantation. 2005;79:1289–93. doi: 10.1097/01.tp.0000157273.60147.7c. [DOI] [PubMed] [Google Scholar]
- 6.Bennet W, Sundberg B, Lundgren T, Tibell A, Groth CG, Richards A, White DJ, Elgue G, Larsson R, Nilsson B, Korsgren O. Damage to porcine islets of Langerhans after exposure to human blood in vitro, or after intraportal transplantation to cynomologus monkeys: protective effects of sCR1 and heparin. Transplantation. 2000;69:711–9. doi: 10.1097/00007890-200003150-00007. [DOI] [PubMed] [Google Scholar]
- 7.Cui W, Wilson JT, Wen J, Angsana J, Qu Z, Haller CA, Chaikof EL. Thrombomodulin improves early outcomes after intraportal islet transplantation. Am J Transplant. 2009;9:1308–16. doi: 10.1111/j.1600-6143.2009.02652.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ito T, Kawahara K, Okamoto K, Yamada S, Yasuda M, Imaizumi H, Nawa Y, Meng X, Shrestha B, Hashiguchi T, Maruyama I. Proteolytic cleavage of high mobility group box 1 protein by thrombin-thrombomodulin complexes. Arterioscler Thromb Vasc Biol. 2008;28:1825–30. doi: 10.1161/ATVBAHA.107.150631. [DOI] [PubMed] [Google Scholar]
- 9.Suzuki K, Hayashi T, Nishioka J, Kosaka Y, Zushi M, Honda G, Yamamoto S. A domain composed of epidermal growth factor-like structures of human thrombomodulin is essential for thrombin binding and for protein C activation. J Biol Chem. 1989;264:4872–6. [PubMed] [Google Scholar]
- 10.Wenzel J, Assmann JC, Schwaninger M. Thrombomodulin--a new target for treating stroke at the crossroad of coagulation and inflammation. Curr Med Chem. 2014;21:2025–34. doi: 10.2174/0929867321666131228204839. [DOI] [PubMed] [Google Scholar]
- 11.Morser J. Thrombomodulin links coagulation to inflammation and immunity. Curr Drug Targets. 2012;13:421–31. doi: 10.2174/138945012799424606. [DOI] [PubMed] [Google Scholar]
- 12.Ikezoe T, Yang J, Nishioka C, Yokoyama A. Thrombomodulin alleviates murine GVHD in association with an increase in the proportion of regulatory T cells in the spleen. Bone Marrow Transplant. 2015;50:113–20. doi: 10.1038/bmt.2014.208. [DOI] [PubMed] [Google Scholar]
- 13.Xue M, Jackson CJ. Activated protein C and its potential applications in prevention of islet beta-cell damage and diabetes. Vitam Horm. 2014;95:323–63. doi: 10.1016/B978-0-12-800174-5.00013-2. [DOI] [PubMed] [Google Scholar]
- 14.Xue M, Dervish S, Harrison LC, Fulcher G, Jackson CJ. Activated protein C inhibits pancreatic islet inflammation, stimulates T regulatory cells, and prevents diabetes in non-obese diabetic (NOD) mice. J Biol Chem. 2012;287:16356–64. doi: 10.1074/jbc.M111.325951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stabler CL, Sun XL, Cui W, Wilson JT, Haller CA, Chaikof EL. Surface re-engineering of pancreatic islets with recombinant azido-thrombomodulin. Bioconjug Chem. 2007;18:1713–5. doi: 10.1021/bc7002814. [DOI] [PubMed] [Google Scholar]
- 16.Wilson JT, Haller CA, Qu Z, Cui W, Urlam MK, Chaikof EL. Biomolecular surface engineering of pancreatic islets with thrombomodulin. Acta Biomater. 2010;6:1895–903. doi: 10.1016/j.actbio.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaminitz A, Askenasy EM, Yaniv I, Stein J, Askenasy N. Apoptosis of purified CD4+ T cell subsets is dominated by cytokine deprivation and absence of other cells in new onset diabetic NOD mice. PLoS One. 2010;5:e15684. doi: 10.1371/journal.pone.0015684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen H, Teramura Y, Iwata H. Co-immobilization of urokinase and thrombomodulin on islet surfaces by poly(ethylene glycol)-conjugated phospholipid. J Control Release. 2011;150:229–34. doi: 10.1016/j.jconrel.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 19.Cobbold SP, Waldmann H. Regulatory cells and transplantation tolerance. Cold Spring Harb Perspect Med. 2013;3 doi: 10.1101/cshperspect.a015545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kendal AR, Chen Y, Regateiro FS, Ma J, Adams E, Cobbold SP, Hori S, Waldmann H. Sustained suppression by Foxp3+ regulatory T cells is vital for infectious transplantation tolerance. J Exp Med. 2011;208:2043–53. doi: 10.1084/jem.20110767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yolcu ES, Zhao H, Bandura-Morgan L, Lacelle C, Woodward KB, Askenasy N, Shirwan H. Pancreatic islets engineered with SA-FasL protein establish robust localized tolerance by inducing T regulatory cells in mice. J Immunol. 2011;187:5901–9. doi: 10.4049/jimmunol.1003266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sagoo P, Ali N, Garg G, Nestle FO, Lechler RI, Lombardi G. Human regulatory T cells with alloantigen specificity are more potent inhibitors of alloimmune skin graft damage than polyclonal regulatory T cells. Sci Transl Med. 2011;3:83ra42. doi: 10.1126/scitranslmed.3002076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee I, Wang L, Wells AD, Dorf ME, Ozkaynak E, Hancock WW. Recruitment of Foxp3+ T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J Exp Med. 2005;201:1037–44. doi: 10.1084/jem.20041709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golab K, Kizilel S, Bal T, Hara M, Zielinski M, Grose R, Savari O, Wang XJ, Wang LJ, Tibudan M, Krzystyniak A, Marek-Trzonkowska N, Millis JM, Trzonkowski P, Witkowski P. Improved coating of pancreatic islets with regulatory T cells to create local immunosuppression by using the biotin-polyethylene glycol-succinimidyl valeric acid ester molecule. Transplant Proc. 2014;46:1967–71. doi: 10.1016/j.transproceed.2014.05.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Josien R, Douillard P, Guillot C, Muschen M, Anegon I, Chetritt J, Menoret S, Vignes C, Soulillou JP, Cuturi MC. A critical role for transforming growth factor-beta in donor transfusion-induced allograft tolerance. J Clin Invest. 1998;102:1920–6. doi: 10.1172/JCI4221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Belghith M, Bluestone JA, Barriot S, Megret J, Bach JF, Chatenoud L. TGF-beta-dependent mechanisms mediate restoration of self-tolerance induced by antibodies to CD3 in overt autoimmune diabetes. Nat Med. 2003;9:1202–8. doi: 10.1038/nm924. [DOI] [PubMed] [Google Scholar]
- 27.Peng Y, Laouar Y, Li MO, Green EA, Flavell RA. TGF-beta regulates in vivo expansion of Foxp3-expressing CD4+CD25+ regulatory T cells responsible for protection against diabetes. Proc Natl Acad Sci U S A. 2004;101:4572–7. doi: 10.1073/pnas.0400810101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pop SM, Wong CP, Culton DA, Clarke SH, Tisch R. Single cell analysis shows decreasing FoxP3 and TGFbeta1 coexpressing CD4+CD25+ regulatory T cells during autoimmune diabetes. J Exp Med. 2005;201:1333–46. doi: 10.1084/jem.20042398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gregg RK, Jain R, Schoenleber SJ, Divekar R, Bell JJ, Lee HH, Yu P, Zaghouani H. A sudden decline in active membrane-bound TGF-beta impairs both T regulatory cell function and protection against autoimmune diabetes. J Immunol. 2004;173:7308–16. doi: 10.4049/jimmunol.173.12.7308. [DOI] [PubMed] [Google Scholar]
- 30.Wu AJ, Hua H, Munson SH, McDevitt HO. Tumor necrosis factor-alpha regulation of CD4+CD25+ T cell levels in NOD mice. Proc Natl Acad Sci U S A. 2002;99:12287–92. doi: 10.1073/pnas.172382999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.You S, Belghith M, Cobbold S, Alyanakian MA, Gouarin C, Barriot S, Garcia C, Waldmann H, Bach JF, Chatenoud L. Autoimmune diabetes onset results from qualitative rather than quantitative age-dependent changes in pathogenic T-cells. Diabetes. 2005;54:1415–22. doi: 10.2337/diabetes.54.5.1415. [DOI] [PubMed] [Google Scholar]
- 32.Zheng SG, Wang JH, Gray JD, Soucier H, Horwitz DA. Natural and induced CD4+CD25+ cells educate CD4+CD25− cells to develop suppressive activity: the role of IL-2, TGF-beta, and IL-10. J Immunol. 2004;172:5213–21. doi: 10.4049/jimmunol.172.9.5213. [DOI] [PubMed] [Google Scholar]
- 33.Fantini MC, Becker C, Monteleone G, Pallone F, Galle PR, Neurath MF. TGF-beta induces a regulatory phenotype in CD4+CD25− T cells through Foxp3 induction and down-regulation of Smad7. J Immunol. 2004;172:5149–53. doi: 10.4049/jimmunol.172.9.5149. [DOI] [PubMed] [Google Scholar]
- 34.Chen W, Jin W, Hardegen N, Lei KJ, Li L, Marinos N, McGrady G, Wahl SM. Conversion of peripheral CD4+CD25- naive T cells to CD4+CD25+ regulatory T cells by TGF-beta induction of transcription factor Foxp3. J Exp Med. 2003;198:1875–86. doi: 10.1084/jem.20030152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wallberg M, Wong FS, Green EA. An islet-specific pulse of TGF-beta abrogates CTL function and promotes beta cell survival independent of Foxp3+ T cells. J Immunol. 2011;186:2543–51. doi: 10.4049/jimmunol.1002098. [DOI] [PubMed] [Google Scholar]
- 36.Grewal IS, Grewal KD, Wong FS, Wang H, Picarella DE, Janeway CA, Jr, Flavell RA. Expression of transgene encoded TGF-beta in islets prevents autoimmune diabetes in NOD mice by a local mechanism. J Autoimmun. 2002;19:9–22. doi: 10.1006/jaut.2002.0599. [DOI] [PubMed] [Google Scholar]
- 37.Yang EY, Kronenfeld JP, Gattas-Asfura KM, Bayer AL, Stabler CL. Engineering an “infectious” Treg biomimetic through chemoselective tethering of TGF-beta1 to PEG brush surfaces. Biomaterials. 2015;67:20–31. doi: 10.1016/j.biomaterials.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sharma RK, Yolcu ES, Elpek KG, Shirwan H. Tumor cells engineered to codisplay on their surface 4-1BBL and LIGHT costimulatory proteins as a novel vaccine approach for cancer immunotherapy. Cancer Gene Ther. 2010;17:730–41. doi: 10.1038/cgt.2010.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pahler A, Hendrickson WA, Kolks MA, Argarana CE, Cantor CR. Characterization and crystallization of core streptavidin. J Biol Chem. 1987;262:13933–7. [PubMed] [Google Scholar]
- 40.Dhein J, Walczak H, Bäumler C, Debatin K-M, Krammer PH. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95) Nature. 1995;373:438–41. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 41.Ju S-T, Panka DJ, Cul H, Ettinger R, El-Khatib M, Sherr DH, Stanger BZ, Marshak-Rothstein A. Fas(CD95)/FasL interactions required for programmed cell death after T-cell activation. Nature. 1996;373:444–8. doi: 10.1038/373444a0. [DOI] [PubMed] [Google Scholar]
- 42.Hughes PD, Belz GT, Fortner KA, Budd RC, Strasser A, Bouillet P. Apoptosis regulators Fas and Bim cooperate in shutdown of chronic immune responses and prevention of autoimmunity. Immunity. 2008;28:197–205. doi: 10.1016/j.immuni.2007.12.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hutcheson J, Scatizzi JC, Siddiqui AM, Haines GK, III, Wu T, Li QZ, Davis LS, Mohan C, Perlman H. Combined deficiency of proapoptotic regulators Bim and Fas results in the early onset of systemic autoimmunity. Immunity. 2008;28:206–17. doi: 10.1016/j.immuni.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 44.Weant AE, Michalek RD, Khan IU, Holbrook BC, Willingham MC, Grayson JM. Apoptosis regulators Bim and Fas function concurrently to control autoimmunity and CD8+ T cell contraction. Immunity. 2008;28:218–30. doi: 10.1016/j.immuni.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 45.Parijs LV, Ibraghimov A, Abbas AK. The roles of costimulation and Fas in T cell apoptosis and peripheral tolerance. Immunity. 1996;4:321–6. doi: 10.1016/s1074-7613(00)80440-9. [DOI] [PubMed] [Google Scholar]
- 46.Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–7. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 47.Askenasy N, Yolcu ES, Yaniv I, Shirwan H. Induction of tolerance using Fas ligand: a double-edged immunomodulator. Blood. 2004;105:1396–404. doi: 10.1182/blood-2004-06-2364. [DOI] [PubMed] [Google Scholar]
- 48.Matsue H, Matsue K, Kusuhara M, Kumamoto T, Okumura K, Yagita H, Takashima A. Immunosuppressive properties of CD95L-transduced “killer” hybrids created by fusing donor- and recipient-derived dendritic cells. Blood. 2001;98:3465–72. doi: 10.1182/blood.v98.12.3465. [DOI] [PubMed] [Google Scholar]
- 49.Min WP, Gorczynski R, Huang XY, Kushida M, Kim P, Obataki M, Lei J, Suri RM, Cattral MS. Dendritic cells genetically engineered to express Fas ligand induce donor-specific hyporesponsiveness and prolong allograft survival. J Immunol. 2000;164:161–7. doi: 10.4049/jimmunol.164.1.161. [DOI] [PubMed] [Google Scholar]
- 50.Swenson KM, Ke B, Wang T, Markowitz JS, Maggard MA, Spear GS, Imagawa DK, Goss JA, Busuttil RW, Seu P. Fas ligand gene transfer to renal allografts in rats: effects on allograft survival. Transplantation. 1998;65:155–60. doi: 10.1097/00007890-199801270-00002. [DOI] [PubMed] [Google Scholar]
- 51.Askenasy N, Yolcu ES, Wang Z, Shirwan H. Display of Fas Ligand protein on cardiac vasculature as a novel means of regulating allograft rejection. Circulation. 2003;107:41–7. doi: 10.1161/01.cir.0000064893.96179.7e. [DOI] [PubMed] [Google Scholar]
- 52.Yolcu ES, Gu X, Lacelle C, Zhao H, Bandura-Morgan L, Askenasy N, Shirwan H. Induction of tolerance to cardiac allografts using donor splenocytes engineered to display on their surface an exogenous fas ligand protein. J Immunol. 2008;181:931–9. doi: 10.4049/jimmunol.181.2.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kaminitz A, Yolcu ES, Stein J, Yaniv I, Shirwan H, Askenasy N. Killer Treg restore immune homeostasis and suppress autoimmune diabetes in prediabetic NOD mice. J Autoimmun. 2011;37:39–47. doi: 10.1016/j.jaut.2011.03.003. [DOI] [PubMed] [Google Scholar]
- 54**.Kaminitz A, Yolcu ES, Mizrahi K, Shirwan H, Askenasy N. Killer Treg cells ameliorate inflammatory insulitis in non-obese diabetic mice through local and systemic immunomodulation. Int Immunol. 2013;25:485–94. doi: 10.1093/intimm/dxt016. The authors of this paper demonstrate that CD4+CD25+FoxP3+ T regulatory cells engineered with SA-FasL are more potent in reversing insulitis in NOD mice then unmanipulated T regulatory cells, perhaps because of their enhanced ability to kill effector T cells. [DOI] [PubMed] [Google Scholar]
- 55.Kaminitz A, Askenasy N, Yolcu ES. Immunomodulation with regulatory T cells and Fas-ligand ameliorate established inflammatory colitis. Gut. 2013;62:1228–30. doi: 10.1136/gutjnl-2012-304432. [DOI] [PubMed] [Google Scholar]
- 56.Yolcu ES, Kaminitz A, Mizrahi K, Ash S, Yaniv I, Stein J, Shirwan H, Askenasy N. Immunomodulation with donor regulatory T cells armed with Fas-ligand alleviates graft-versus-host disease. Exp Hematol. 2013;41:903–11. doi: 10.1016/j.exphem.2013.04.016. [DOI] [PubMed] [Google Scholar]
- 57.Yolcu ES, Zhao H, Shirwan H. Immunomodulation with SA-FasL protein as an effective means of preventing islet allograft rejection in chemically diabetic NOD mice. Transplant Proc. 2013;45:1889–91. doi: 10.1016/j.transproceed.2013.01.041. [DOI] [PMC free article] [PubMed] [Google Scholar]