Abstract
Objective
To test the hypothesis that individuals who are active but who decrease physical activity (PA) over time have a higher risk of becoming obese in young adulthood, when compared to individuals who are consistently active throughout childhood and adolescence.
Methods
Iowa Bone Development Study cohort members (242 males and 251 females) participated in accelerometry assessments, dual-energy X-ray absorptiometry scans, and dietary questionnaire surveys at ages 5, 8, 11, 13, 15, 17, and 19 years. Group-based trajectory analyses identified distinct trajectory patterns of moderate- to vigorous-intensity PA (MVPA), percentage of body fat (%BF), and energy intake. A multivariable logistic regression model was fit to estimate the odds of “becoming obese” based on the MVPA trajectories, adjusted for mother’s education, somatic maturation, and energy intake.
Results
Among males, 74.7% had a “normal” body fat pattern, 14.6% had a “becoming obese” pattern, and 10.7% had a “consistently obese” pattern, while among females, the percentages were 58.6%, 28.6% and 12.8%, respectively. Participants who were active (≥45 minutes MVPA) as children but decreased MVPA with age were more likely to become obese, compared to consistently active participants (adjusted OR=2.77; 95% CI=1.16, 6.58).
Conclusions
An active lifestyle throughout childhood and adolescence could prevent obesity development in young adulthood.
Keywords: group-based trajectory, physical activity, adiposity, youth, accelerometers, DXA
Introduction
Population-based surveillance data indicate that children reduce their physical activity (PA) as they age,1 and this reduction is believed to be a risk factor for adult obesity. Despite this belief, longitudinal studies2–4 have produced mixed results, including two studies3,4 that reported no difference in the likelihood of being obese in adulthood between those who consistently active and those who were active but decreased their activity. The mixed results may be partly due to use of less accurate measures, such as self-reported PA and body mass index (BMI), and no consideration of energy intake. In addition, these three studies utilized PA data measured only at two time points (baseline and follow-up), which could not address how PA level changes over time.
Several longitudinal studies have examined the role of childhood PA in adolescent adiposity development using repeatedly-measured objective PA data.5–8 These studies have produced mixed results, with at most a minimal effect size.9 These findings are reflected in reviews by Wilks et al.,9,10 which concluded that PA is not an important predictor of change in adiposity among children and adolescents. However, this conclusion could be premature since the developmental pattern of PA behavior and adiposity is yet to be characterized. To illustrate the analytic limitations of the current literature, we describe two longitudinal study examples by Moore et al.6 and Kwon et al.8 The Moore study,6 which aimed to examine the effects of PA on changes in body fat from the preschool years to early adolescence, showed an inverse association between PA and body fat. Their study quantified longitudinal PA data in two ways: a rank (low, medium, and high tertiles) based on the mean of annually measured PA levels and a rank at each assessment. Although there is much to be admired about this study (e.g., its long follow-up period and annual measures of PA), the analytic approach of defining subgroups using prior analysis and subjective classification rules (i.e., tertile groups by the distribution of PA levels) has statistical dangers, including the dual risks of creating groups that reflect only random variation, and failing to identify important but atypical developmental patterns.11 The Kwon study,8 which aimed to examine whether MVPA or sedentary behaviors are more strongly associated with adiposity during adolescence, showed that MVPA was more strongly linked. The growth model approach used in the Kwon study8 cannot identify distinct developmental patterns of moderate- to vigorous-intensity PA (MVPA) or adiposity. In addition, the results from the growth model could not directly address whether consistently active youth are less likely to become obese, because the growth model assesses the association between MVPA and adiposity variables at each measurement time point (variable-centered approach), rather than the adiposity development of subgroups with unique MVPA patterns (person-centered approach).
Group-based trajectory modeling can complement the two analytic approaches mentioned above. Group-based trajectory models provide an empirical method to identify groups of participants who follow typical and atypical developmental trajectories of PA and adiposity11 and allow for a latent expression of the data and characterize the heterogeneity of the study population. To advance the understanding of the role of PA in obesity development, this study used group-based trajectory analytic methodology to investigate the association between accelerometry-derived PA and DXA-derived percentage of body fat (%BF) developmental patterns over 14 years from age 5 to 19 years. The study hypothesis was that individuals who are active but who become less active as they age have a higher risk of becoming obese in young adulthood when compared to individuals who are consistently active throughout childhood and adolescence.
Methods
Study Sample
Data were obtained from the Iowa Bone Development Study (IBDS), an ongoing cohort study of bone health from childhood through young adulthood. Between 1998 and 2000, the IBDS recruited participants among a subset (n=890) of the Iowa Fluoride Study (IFS) birth cohort that originally included 1,882 families recruited from eight Iowa hospital postpartum wards.12 Assessments were conducted between 1998 and 2014 when participants were approximately 5, 8, 11, 13, 15, 17, and 19 years of age (Waves 1 to 7, respectively). The IBDS uses rolling admission to allow IFS cohort members to participate in any follow-up examinations. A total of 630 cohort members (312 females; 50.5%) completed at least one accelerometry assessment. Based on a family demographic questionnaire completed in 2007. Additional information about the IBDS and its participants can be found in previous publications.13,14 Informed written consent and assent were obtained. Of the 630 IBDS participants, 493 (50.9% females) were included in the current data analysis. Those who were excluded (n=137) had similar levels of family income and maternal education as those who were included.15 The University of Iowa Institutional Review Board (IRB) approved the IBDS.
Measurements
Physical activity
PA level was assessed in the autumn season (September to November) using Actigraph accelerometers (model 7164 at Waves 1 to 4, GT1M at Wave 5, and GT3X+ at Waves 6 and 7; Pensacola, FL). The different versions of Actigraph accelerometers have been reported to be fairly interchangeable.16 Participants received an accelerometer via the United States Postal Service (USPS) and were asked to wear the monitors during waking hours for four consecutive days (including one weekend day) for Waves 1 and 2 and for five consecutive days (including both weekend days) for all other waves. The accelerometry epoch length was set at one minute for Waves 1 to 4 and five seconds for Wave 5. Raw data were collected for Waves 6 and 7. For data analysis, the five-second epoch and raw data were reintegrated into one-minute epoch data. A non-wear period was estimated using accelerometry data with the following definition: a period of ≥60 consecutive minutes of zero accelerometry counts (with an allowance for two nonzero interruptions) in the accelerometry data array.17 Participants were considered to have valid PA data for a particular wave if the participant wore the accelerometer at least three days during that wave.18 A day was considered valid if the estimated accelerometer wear time was at least 8 hours.18 The accelerometry count cut-off point used was 2,296 counts/minute for moderate or higher intensity, and 4,012 counts/minute for vigorous intensity.19,20 MVPA (minutes/day) was defined as daily accumulated minutes in moderate- or higher-intensity activity. Vigorous-intensity PA (VPA; minutes/day) was defined as daily accumulated minutes in vigorous-intensity activity. Overall activity (light- or higher-intensity activity) counts (counts/day) were defined as daily accumulated counts when ≥100 counts/minute.
Percentage of body fat
Fat mass and body mass were measured using whole body DXA scan data. Participants visited the University of Iowa Clinical Research Unit where one of three trained technicians conducted a DXA scan. DXA models were upgraded over time: Hologic QDR2000 model (Hologic Inc., Bedford, MA) at Waves 1 and 2, Hologic QDR4500 Delphi A model at Waves 3 to 6, and Discovery A model at Wave 7. The scans were analyzed using APEX software version 4.0/13.4. Standard quality control procedures were performed daily using spine and step phantoms. To adjust for differences between the QDR2000 and the QDR4500 Delphi A, translational equations to convert from QDR2000 DXA measures to QDR4500A DXA measures were developed in a subsample of 60 IBDS participants who were scanned on both machines during one study visit.14,21 To adjust for differences between the QDR4500 Delphi A and the Discovery A, a step phantom was scanned on both machines for whole body soft tissue calibration. In addition, 12 human volunteers were scanned on both machines within 60 days. Total body fat mass, visceral fat mass, and body mass were derived from the DXA scan images. %BF was calculated using fat mass and body mass data. Pediatric obesity was defined as ≥ 80th percentile of %BF.22 Because the population reference of %BF percentile is unavailable for children younger than 8 years of age, %BF at age 8 was used to classify adiposity status in young childhood (obese if ≥33.4%BF for boys and ≥37.4%BF for girls at age 8).23 Adult obesity was defined as >25%BF for adult males and >32%BF for adult females.24
Energy intake
Dietary intakes of IBDS cohort members were assessed using three different instruments across the examinations: targeted nutrient questionnaire at Waves 1 and 2, Block Kids’ Food Questionnaire25 at Waves 3 to 6, and National Cancer Institute Diet History Questionnaire II26,27 at Wave 7. The targeted nutrition questionnaire was not designed to estimate energy intake; therefore the dietary data collected at Waves 1 and 2 were not used for the analyses. The Block Kids’ Food Questionnaire contained 75 questions regarding food intake during the previous week. Completed Block Kids’ Food Questionnaires were reviewed by registered dietitians for completeness before analyses by NutritionQuest (Berkeley, CA). In a subsample of 129 IBDS participants, the correlation between energy intake estimated from 3-day diary records and the Block Kids’ Food Questionnaire was statistically significant, but low (r=0.26; p<0.001).25 The Diet History Questionnaire II contained questions regarding the portion size and intake frequency of 144 food items. In the Diet History Questionnaire validity study, the correlation between energy intake estimated from a four-day 24-hour recall and the Diet History Questionnaire was moderate (deattenuated correlation P=0.48).27 Because the absolute value of estimated energy intake (kcal) from different dietary assessment tools used across waves was not comparable, a quartile rank within a wave (coded as 1, 2, 3, or 4) was used as an energy intake indicator.
Somatic maturity
Anthropometry (sitting and standing height measured in centimeters) was used to estimate the age of peak height velocity for each participant aged ≥11 years. A Harpenden stadiometer (Holtain, UK) and the predictive equations established by Mirwald and colleagues were used for this prediction.28
Statistical Analysis
IBDS participants who had ≥3 waves of valid accelerometry data and ≥3 waves of %BF data were included in the data analysis. The criterion of having ≥3 data points allowed for the consideration of quadratic trajectory models. Descriptive analyses for demographic characteristics and exposure and outcome variables were performed by sex and by wave, if appropriate.
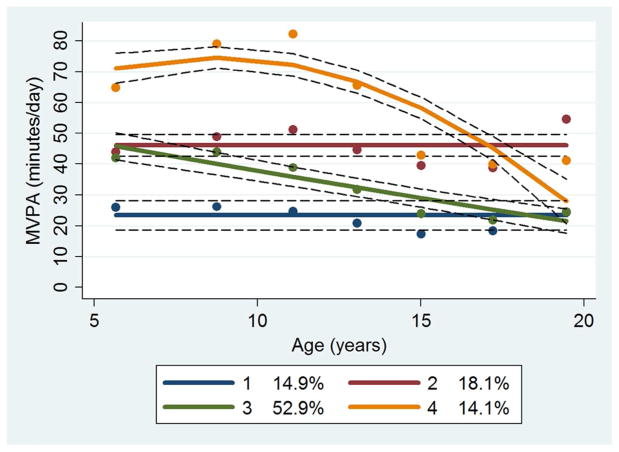
To identify distinct %BF trajectory groups, sex-specific group-based trajectory analysis was conducted in STATA TRAJ.29 Sex-specific analysis was necessary because body fat development differs biologically by sex, and the definition of adult obesity based on %BF levels is sex-specific. In the process of determining the number of %BF trajectory groups, a quadratic model was initially used for all groups. The final number of groups was determined based on the Bayesian Information Criterion, similarity of trajectory shapes, and the proportion of cohort members in each group.30 After determining the final number of groups, the degree of the polynomial for each group (i.e., quadratic, linear, and constant) was reduced until the parameter estimate for the highest degree polynomial effect had an associated p-value<0.01. Model adequacy was evaluated using four diagnostic measures: average posterior probability of assignment for each group of 0.7 or higher; odds of correct classification of 5.0 or higher; the proportion of a sample assigned to a certain group close to the proportion estimated from the model; and 99% confidence intervals (CIs) for the estimated proportion that are reasonably narrow.11 Each participant was assigned to one of the trajectory groups, based on the individual’s maximum posterior probability estimated from the final models. We considered the %BF levels of groups 1, 2, and 3 at age 5 to be normal, and the %BF level of group 4 at age 5 to be obese (Figure 2). We labeled groups 1 and 2 as “normal,” group 3 as “becoming obese,” and group 4 as “consistently obese.” We repeated group-based trajectory analysis for energy intake quartiles (1, 2, 3, and 4, as a continuous variable). Participants were also assigned to one of four MVPA trajectory groups, termed “consistently inactive,” “substantially decreasing from a high level of MVPA (substantially decreasing),” “consistently moderately active (consistently active; 45 minutes of daily MVPA),” and “decreasing from a moderate level of MVPA (decreasing),” based on MVPA trajectory models identified in our previous publication (Figure 1).15
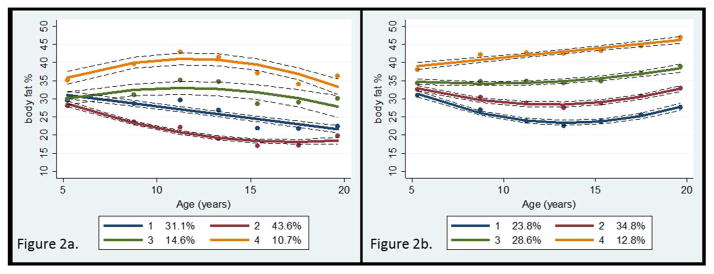
Figure 2.
Percentage of body fat trajectory groups
a. Male
b. Female
Dots indicate actual mean percentage of body fat, solid lines indicate estimated percentage of body fat, and dotted lines indicate 95% confidence intervals of estimated percentage of body fat.
Figure 1.
Moderate- to vigorous-intensity physical activity trajectory groups
MVPA, Moderate- to vigorous-intensity physical activity
Dots indicate actual mean MVPA minutes, solid lines indicate estimated MVPA minutes, and dotted lines indicate 95% confidence intervals of estimated MVPA minutes.
Excluding those who were considered to be obese at baseline (n=60), we conducted a multivariable logistic regression analysis to predict obesity development based on the four MVPA trajectory groups. The “consistently active” group was selected as the reference group. A previous review31 of the association between PA and adiposity in youth emphasized the methodological importance of accounting for potential confounding effects such as socioeconomic status, maturational timing, and dietary patterns. Therefore, we included sex, maternal education level (≥4-year college degree vs. <4-year college degree), age of peak height velocity (years), and energy intake quartile trajectory group in our models. However, accelerometry wear time was not included as a covariate, because additional logistic regression analysis that included the mean wear time variable showed no significant association between wear time and %BF trajectory. Sedentary time was not included as a covariate because two previous studies8,32 showed no independent effect of sedentary time on adiposity after adjusting for MVPA the, and because the additional logistic regression analysis that included the sedentary time trajectory variable showed no significant association between sedentary time trajectory and %BF trajectory. No significant interaction was identified between sex and age of height velocity, between sex and PA trajectory, or between sex and energy intake trajectory.
Results
Participant characteristics are presented in Table 1. Of the 493 participants, 33% completed all seven of the MVPA assessments, 31% completed six, 16% completed five, 13% completed four, and 7% completed three assessments. Similarly, 30% completed all seven of the body fat assessments, 29% completed six, 22% completed five, 12% completed four, and 6% completed three assessments. The mean accelerometer wear time per day at each wave ranged from 12.0 to 12.7 hours. As shown in Table 2, mean %BF was the highest at age 5 for males and at age 19 for females. Four %BF trajectory patterns were identified for both males and females (Figure 2). The %BF trajectory groups 1, 2, and 3 started at a similar level of %BF at age 5, but diverged over time. Importantly, the trajectory model for group 3 reflected a level of %BF at age 19 above the criterion for obesity (estimated mean %BF: 28.6% among males and 37.9% among females), while groups 1 and 2 reflected a normal level of %BF at age 19. For example, estimated %BF at age 19 was 22.1% for males in group 1 and 32.0% for females in group 2. Group 4, to which 10.7% of males (n=27) and 12.8% of females (n=33) were assigned, maintained a high level of %BF throughout childhood and adolescence. Among “consistently active” participants, 9.5% were in the “becoming obese” group, while among participants who followed the “decreasing” activity pattern, 23.8% were in the “becoming obese” group (p<0.01; Table 3). A higher proportion of the “consistently obese” participants (88.3%) were in the “consistently inactive” or “decreasing” activity groups, when compared to “normal” participants (64.1%; p<0.0001).
Table 1.
Characteristics of Iowa Bone Development Study participants by sex
| Males | Females | |||
|---|---|---|---|---|
|
| ||||
| n | % or mean±SD | n | % or mean±SD | |
|
| ||||
| Total | 242 | 251 | ||
| Annual household income in 2007 | ||||
| <$80,000 | 126 | 52.1 | 129 | 51.4 |
| ≥$80,000 | 107 | 44.2 | 110 | 43.8 |
| Missing | 9 | 3.7 | 12 | 4.8 |
| Mother’s education level in 2007 | ||||
| <4-year college degree | 126 | 52.1 | 129 | 51.4 |
| ≥4-year college degree | 116 | 47.9 | 122 | 48.6 |
| Age of peak height velocity, years | 242 | 13.6±0.8 | 251 | 11.8±0.6 |
Table 2.
Physical activity, energy intake, and adiposity levels by sex and wave
| Physical Activity from Accelerometry | Energy Intake from Dietary Questionnaire a | Adiposity from DXA | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| MVPA min/d | VPA min/d | Overall PA × 103 counts/d | Energy intake kcal/d | %BF % | Visceral fat mass grams | ||||
|
| |||||||||
| n | mean±SD | mean±SD | mean±SD | n | mean±SD | n | mean±SD | mean±SD | |
| Males
| |||||||||
| Age 5 | 176 | 48±20 | 10±7 | 474±128 | --b | --b | 183 | 29.6±3.5 | --c |
| Age 8 | 227 | 56±25 | 15±12 | 474±145 | --b | --b | 224 | 28.1±6.0 | --c |
| Age 11 | 231 | 57±25 | 18±13 | 479±154 | 214 | 1,806±487 | 218 | 28.6±7.7 | 225±98 |
| Age 13 | 219 | 46±22 | 14±11 | 391±140 | 214 | 1,890±652 | 222 | 26.3±8.6 | 243±119 |
| Age 15 | 192 | 33±17 | 9±8 | 288±109 | 189 | 1,969±688 | 193 | 22.2±7.2 | 235±99 |
| Age 17 | 180 | 31±18 | 9±10 | 276±107 | 156 | 1,862±663 | 158 | 21.8±6.7 | 265±103 |
| Age 19 | 144 | 37±24 | 13±13 | 298±125 | 116 | 2,586±1167 | 127 | 23.4±6.5 | 303±141 |
|
| |||||||||
| Females
| |||||||||
| Age 5 | 203 | 38±16 | 7±5 | 432±13 | --b | --b | 212 | 33.4±3.6 | --c |
| Age 8 | 242 | 39±18 | 9±6 | 413±124 | --b | --b | 241 | 32.4±5.7 | --c |
| Age 11 | 241 | 35±16 | 9±6 | 370±109 | 226 | 1,614±459 | 234 | 31.0±6.9 | 160±133 |
| Age 13 | 219 | 29±16 | 8±7 | 314±117 | 214 | 1,538±509 | 223 | 30.3±7.0 | 184±147 |
| Age 15 | 186 | 22±14 | 6±7 | 230±89 | 193 | 1,409±436 | 200 | 31.2±6.8 | 192±132 |
| Age 17 | 186 | 22±14 | 5±6 | 235±82 | 178 | 1,370±523 | 187 | 33.0±6.8 | 226±149 |
| Age 19 | 171 | 29±22 | 8±12 | 252±120 | 139 | 1,551±706 | 150 | 34.4±6.9 | 256±191 |
DXA, dual-energy X-ray absorptiometry, MVPA, moderate- to vigorous-intensity physical activity; PA, physical activity; SD, standard deviation; VPA, vigorous-intensity physical activity; %BF, percentage of body fat.
Different dietary instruments were used across the study waves.
Dietary data collected at ages 5 and 8 years were limited and did not allow for the estimation of energy intake.
DXA scans obtained at ages 5 and 8 did not allow for the estimation of visceral fat mass.
Table 3.
The distribution of percentage of body fat development patterns by physical activity trajectory groups
| Percentage of Body Fat Trajectory Group | ||||
|---|---|---|---|---|
|
| ||||
| Normal | Becoming Obese | Consistently Obese | Total | |
|
| ||||
| MVPA Trajectory Group | n (%) | n (%) | n (%) | n (%) |
| Consistently active† | 61 (82.4%) | 7 (9.5%) | 6 (8.1%) | 74 (100%) |
| Substantially decreasing†† | 57 (83.8%) | 10 (9.6%) | 1 (1.5%) | 68 (100%) |
| Decreasing††† | 186 (63.3%) | 70 (23.8%) | 38 (12.9%) | 294 (100%) |
| Consistently inactive†††† | 25 (43.9%) | 17 (22.8%) | 15 (26.3%) | 57 (100%) |
|
| ||||
| Total | 329 (66.7%) | 104 (21.1%) | 60 (12.2%) | 493 (100%) |
MVPA, moderate- to vigorous-intensity physical activity.
Group 2 in MVPA group-based trajectory analysis (Figure 1)
Group 4 in MVPA group-based trajectory analysis (Figure 1)
Group 3 in MVPA group-based trajectory analysis (Figure 1)
Group 1 in MVPA group-based trajectory analysis (Figure 1)
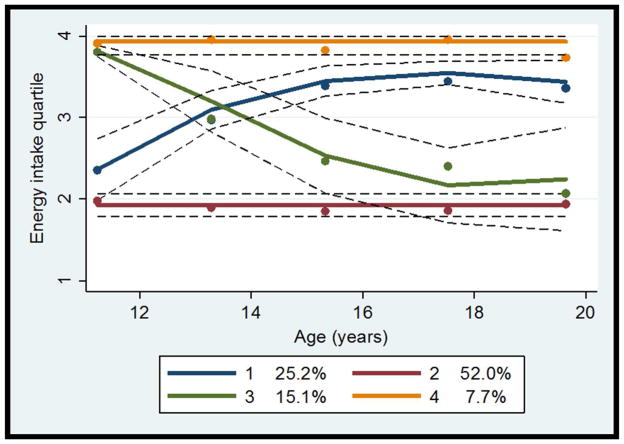
As presented in Figure 3, four energy intake patterns were identified: “increasing” (group 1), “consistently low” (group 2), “decreasing” (group 3), and “consistently high” (group 4). Chi-square tests showed that, when compared to the “consistently low” energy intake group, a higher proportion of the “increasing” energy intake group were in the “becoming obese” (p=0.06) or “consistently obese” group (p<0.01). However, between the “decreasing” and “consistently high” energy intake groups, the percentage of those in the “becoming obese” and “consistently obese” groups were not different (p=0.11 and p=0.98, respectively).
Figure 3.
Energy intake quartile trajectory groups
Dots indicate actual mean energy intake quartile rank, solid lines indicate estimated energy intake quartile rank, and dotted lines indicate 95% confidence intervals of estimated energy intake quartile rank.
The multivariable logistic regression model showed that participants with “decreasing” activity had higher odds of “becoming obese,” relative to “consistently active” participants (adjusted OR=2.77; 95% CI=1.16, 6.58; referent group=%BF trajectory groups 1 and 2 (“normal”); %BF trajectory group 4 (“consistently obese”) were excluded in the analysis; Table 4). Additional analyses were conducted using different adiposity (BMI, fat mass index [FMI], visceral fat mass) and PA indicators (VPA and overall activity). When BMI and FMI were used, those with “decreasing” activity tended to have a higher likelihood of becoming obese, but the ORs were statistically insignificant (BMI analysis: OR=1.92; 95% CI=0.63–5.89; FMI analysis: OR=2.58; 95% CI=0.66–10.04), partly due to a small proportion of the “becoming obese” group. Group-based trajectory analyses for visceral fat mass, VPA, and overall activity did not produce novel heterogeneous patterns of these variables and are not presented in this report.
Table 4.
Multivariable logistic regression model to predict the odds of being in the “becoming obese” trajectory group (n=433)
| Effect | Adjusted OR | 95% CI |
|---|---|---|
| Sex | ||
| Male | 1.00 | Reference |
| Female | 0.42 | 0.17, 1.08 |
| Mother’s education level | ||
| <4-year college degree | 1.00 | Reference |
| ≥4-year college | 1.22 | 0.76, 1.95 |
| Somatic maturity | ||
| Age of peak height velocity (years) | 0.43 | 0.28, 0.66 |
| Energy intake quartile trajectory group | ||
| Consistently low | 1.00 | Reference |
| Increasing | 0.74 | 0.39, 1.40 |
| Decreasing | 1.19 | 0.61, 2.30 |
| Consistently high | 0.64 | 0.22, 1.92 |
| MVPA trajectory group | ||
| Consistently active† | 1.00 | Reference |
| Substantially decreasing†† | 1.99 | 0.68, 5.82 |
| Decreasing††† | 2.77 | 1.16, 6.58 |
| Consistently inactive†††† | 3.79 | 1.31, 10.99 |
Group 2 in MVPA group-based trajectory analysis (Figure 1)
Group 4 in MVPA group-based trajectory analysis (Figure 1)
Group 3 in MVPA group-based trajectory analysis (Figure 1)
Group 1 in MVPA group-based trajectory analysis (Figure 1)
CI, confidence interval; MVPA, moderate- to vigorous-intensity physical activity; OR, odds ratio. Multivariable logistic model: percentage of body fat trajectory group (becoming obese vs. normal [reference group]) = mother’s education level (≥4-year college degree vs. <4-year college degree) + estimated age of peak height velocity (years) + energy intake trajectory group (increasing, decreasing, consistently high, vs. consistently low [reference group]) + MVPA trajectory group (substantially decreasing, decreasing, consistently inactive, vs. consistently active [reference group]).
Discussion
These results support the study hypothesis that individuals who are active but who become less active as they age have a higher risk of becoming obese in young adulthood when compared to individuals who are consistently active throughout childhood and adolescence. The current study also adds new knowledge that maintaining 45 minutes of accelerometry-measured daily MVPA throughout childhood and adolescence represents an active life style for obesity prevention in young adulthood.
Obesity is a significant public health problem that affects one in three American adults.33 Obesity also contributes substantially to an immense social burden.34 Obesity-related illness was estimated to cost 20.6% of U.S. national health expenditures in 2012.34 This study is one of only a few studies to directly demonstrate that, when compared to individuals who decrease their PA over time, individuals who are active throughout childhood and adolescence have a lower risk of obesity in young adulthood. In this study, the group of consistently active participants maintained approximately 45 minutes of daily MVPA based on accelerometry data, which is lower than the current U.S. federal PA guidelines for youth35 which call for at least 60 minutes of MVPA each day. However, those guidelines were primarily established using self-report PA data.36 This study supports the current U.S. federal PA guidelines that call for sustained MVPA throughout the growing years, and provides valuable information that can be used to develop PA guidelines for obesity prevention based on accelerometry-measured movement.36
To our knowledge, this is the first study to report the development of DXA-measured %BF from childhood to young adulthood. Among the males in our study, the typical development of %BF was of a continuous decrease over time, while a persistent or even increased level of %BF until the approximate age of 13 years resulted in obesity in young adulthood. Among females, the typical development of %BF reflected a ‘U-shaped’ pattern with a rebound at approximately age 13 years, while a continuous increase in %BF over time resulted in obesity in young adulthood. These trajectories suggest that the major difference between typical and atypical patterns appears at age 13 or younger in both males and females. Adiposity development at age 13 or younger could be critical to determining obesity development in young adulthood. This finding sheds light on the importance of adiposity development during pre-puberty and puberty, and supports current public health efforts to prevent obesity by focusing on early childhood.
Our study findings do not rule out the potential reverse causal relationship that excess adiposity status leads to unhealthy PA behavior or a positive feedback loop.21,37,38 We found that most participants (88%) who presented with the “consistently obese” pattern disproportionally followed unhealthy activity patterns (“consistently inactive” or “decreasing”), consistent with the reverse causality hypothesis.
This study used a comprehensive longitudinal dataset of objective PA measures and accurate adiposity measures assessed over 14 years in a fairly large sample of youth. There are multiple fat mass-based adiposity indicators, including %BF and FMI. There is no consensus on the most robust indicator. We selected %BF as a main outcome, because established %BF-based obesity definition for adults exists. We observed a similar level of ORs in the association of obesity development with %BF and with FMI, although FMI analysis was statistical insignificant. Future research should follow to confirm our results. Another strength is that this study applied an innovative analytic approach to identify heterogeneous developmental trajectories of MVPA, energy consumption and adiposity. However, several limitations of this study should also be acknowledged. First, because a group-based trajectory model assumes that missing data are random, non-random missing data over time could have biased the study findings. However, the majority participants completed at least six of the seven assessment. Second, the IBDS cohort is comprised of homogenous White, high(er) socioeconomic families. Therefore, the results are not generalizable to other populations. Third, different dietary instruments were used across the study waves. Fourth, the effects of genetic predisposition and other residual and unmeasured confounding on obesity development were not taken into account. Lastly, because trajectory group membership was determined based on estimated probabilities from maximum likelihood analysis, not all group members perfectly followed their assigned group’s trajectory. Therefore, use of the posterior probability-based classification that does not account for the uncertainty in group classification is prone to classification error of the group.39 For example, the fact that an increasing MVPA trajectory pattern was not identified in the group-based trajectory analysis does not mean that there were zero participants who actually increased MVPA over time. However, we used this method because the posterior probability-based classification provides a straightforward basis for producing profiles of trajectory members and an easy and transparent communication of the findings.11
Conclusion
The study results provide evidence that an active lifestyle (maintaining 45 minutes of accelerometry-measured daily MVPA) throughout childhood and adolescence can prevent the development of obesity in young adulthood.
Study Importance Questions.
What is already known about this subject?
Self-reported PA data have shown that, compared to youth who become less active, youth who are active in both adolescence and adulthood have a lower risk of obesity in adulthood.
What does your study add?
Applying an innovative analytic approach, our study provides evidence that maintaining 45 minutes of daily moderate- to vigorous-intensity physical activity [accelerometry-measured] throughout childhood and adolescence prevents the development of obesity in young adulthood.
Acknowledgments
FUNDING: This study was supported by the National Institutes of Health R03 HD078966, R01 DE12101, R01 DE09551, UL1 RR024979 and UL1 TR000442, M01 RR00059, the Roy J. Carver Charitable Trust, and Delta Dental of Iowa Foundation.
Footnotes
DISCLOSURE: None of authors has a conflict of interest to declare.
AUTHOR CONTRIBUTIONS: SK conceived of the study, requested the dataset from the IBDS, performed statistical analyses, and drafted the manuscript. KJ helped to draft and critically reviewed the manuscript. EL prepared the dataset and critically reviewed the manuscript. TB and SL critically reviewed the manuscript. All authors were involved in writing the paper and gave final approval for the submitted and published versions.
References
- 1.Wolff-Hughes DL, Bassett DR, Fitzhugh EC. Population-referenced percentiles for waist-worn accelerometer-derived total activity counts in U.S. youth: 2003–2006 NHANES. PLoS One. 2014;9(12):e115915. doi: 10.1371/journal.pone.0115915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang X, Telama R, Viikari J, Raitakari OT. Risk of obesity in relation to physical activity tracking from youth to adulthood. Med Sci Sports Exerc. 2006;38(5):919–925. doi: 10.1249/01.mss.0000218121.19703.f7. [DOI] [PubMed] [Google Scholar]
- 3.Tammelin T, Laitinen J, Näyhä S. Change in the level of physical activity from adolescence into adulthood and obesity at the age of 31 years. Int J Obes Relat Metab Disord. 2004;28(6):775–782. doi: 10.1038/sj.ijo.0802622. [DOI] [PubMed] [Google Scholar]
- 4.Kvaavik E, Tell GS, Klepp KI. Predictors and tracking of body mass index from adolescence into adulthood: follow-up of 18 to 20 years in the Oslo Youth Study. Arch Pediatr Adolesc Med. 2003;157(12):1212–1218. doi: 10.1001/archpedi.157.12.1212. [DOI] [PubMed] [Google Scholar]
- 5.Bandini LG, Must A, Phillips SM, Naumova EN, Dietz WH. Relation of body mass index and body fatness to energy expenditure: longitudinal changes from preadolescence through adolescence. Am J Clin Nutr. 2004;80(5):1262–1269. doi: 10.1093/ajcn/80.5.1262. [DOI] [PubMed] [Google Scholar]
- 6.Moore LL, Gao D, Bradlee ML, et al. Does early physical activity predict body fat change throughout childhood? Pre Med. 2003;37(1):10–17. doi: 10.1016/s0091-7435(03)00048-3. [DOI] [PubMed] [Google Scholar]
- 7.Johnson MS, Figueroa-Colon R, Herd SL, et al. Aerobic fitness, not energy expenditure, influences subsequent increase in adiposity in black and white children. Pediatrics. 2000;106(4):E50. doi: 10.1542/peds.106.4.e50. [DOI] [PubMed] [Google Scholar]
- 8.Kwon S, Burns TL, Levy SM, Janz KF. Which Contributes More to Childhood Adiposity-High Levels of Sedentarism or Low Levels of Moderate-through-Vigorous Physical Activity? The Iowa Bone Development Study. J Pediatr. 2013;162(6):1169–74. doi: 10.1016/j.jpeds.2012.11.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilks DC, Besson H, Lindroos AK, Ekelund U. Objectively measured physical activity and obesity prevention in children, adolescents and adults: a systematic review of prospective studies. Obes Rev. 2011;12(5):e119–129. doi: 10.1111/j.1467-789X.2010.00775.x. [DOI] [PubMed] [Google Scholar]
- 10.Wilks DC, Sharp SJ, Ekelund U, et al. Objectively measured physical activity and fat mass in children: a bias-adjusted meta-analysis of prospective studies. PLoS One. 2011;6(2):e17205. doi: 10.1371/journal.pone.0017205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagin D. Group-based modeling of development. Cambridge:MA: Harvard University Press; 2005. [Google Scholar]
- 12.Levy SM, Warren JJ, Davis CS, Kirchner HL, Kanellis MJ, Wefel JS. Patterns of fluoride intake from birth to 36 months. J Public Health Dent. 2001;61(2):70–77. doi: 10.1111/j.1752-7325.2001.tb03369.x. [DOI] [PubMed] [Google Scholar]
- 13.Janz KF, Burns TL, Levy SM Iowa Bone Development S. Tracking of activity and sedentary behaviors in childhood: the Iowa Bone Development Study. Am J Prev Med. 2005;29(3):171–178. doi: 10.1016/j.amepre.2005.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Janz KF, Kwon S, Letuchy EM, et al. Sustained effect of early physical activity on body fat mass in older children. Am J Prev Med. 2009;37(1):35–40. doi: 10.1016/j.amepre.2009.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwon SJK, Letuchy EM, Trudy BL, Steven LM. Developmental trajectories of physical activity, sports, and television viewing during childhood to young adulthood. BMC Public Health. 2015 Jul 15;15:667. doi: 10.1186/s12889-015-2043-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cain KL, Conway TL, Adams MA, Husak LE, Sallis JF. Comparison of older and newer generations of ActiGraph accelerometers with the normal filter and the low frequency extension. Int J Behav Nutr Phys Act. 2013;10:51. doi: 10.1186/1479-5868-10-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sherar LB, Griew P, Esliger DW, et al. International children’s accelerometry database (ICAD): design and methods. BMC Public Health. 2011;11:485. doi: 10.1186/1471-2458-11-485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mattocks C, Ness A, Leary S, et al. Use of accelerometers in a large field-based study of children: protocols, design issues, and effects on precision. J Phys Act Health. 2008;5 (Suppl 1):S98–111. doi: 10.1123/jpah.5.s1.s98. [DOI] [PubMed] [Google Scholar]
- 19.Evenson KR, Catellier DJ, Gill K, Ondrak KS, McMurray RG. Calibration of two objective measures of physical activity for children. J Sports Sci. 2008;26(14):1–9. doi: 10.1080/02640410802334196. [DOI] [PubMed] [Google Scholar]
- 20.Trost SG, Loprinzi PD, Moore R, Pfeiffer KA. Comparison of accelerometer cut-points for predicting activity intensity in youth. Med Sci Sports Exer. 2011;43(7):1360–1368. doi: 10.1249/MSS.0b013e318206476e. [DOI] [PubMed] [Google Scholar]
- 21.Kwon S, Janz KF, Burns TL, Levy SM. Effects of adiposity on physical activity in childhood: Iowa Bone Development Study. Med Sci Sports Exer. 2011;43(3):443–448. doi: 10.1249/MSS.0b013e3181ef3b0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Flegal KM, Ogden CL, Yanovski JA, et al. High adiposity and high body mass index-forage in US children and adolescents overall and by race-ethnic group. Am J Clin Nutr. 2010;91(4):1020–1026. doi: 10.3945/ajcn.2009.28589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogden C, Li Y, Freedman D, Flegal K. Smoothed percentage body fat percentiles for U.S. children and adolescents, 1999–2004. National Health Statistics Reports. 2011. 2011 Dec 10;2011 http://www.cdc.gov/nchs/data/nhsr/nhsr043.pdf. [PubMed] [Google Scholar]
- 24.American Council Exercise. ACE lifestyle & weight management consultant manual, the ultimate resource for fitness professionals. San Diego, CA: American Council on Exercise; 2009. [Google Scholar]
- 25.Marshall TA, Eichenberger Gilmore JM, Broffitt B, Stumbo PJ, Levy SM. Relative validity of the Iowa Fluoride Study targeted nutrient semi-quantitative questionnaire and the block kids’ food questionnaire for estimating beverage, calcium, and vitamin D intakes by children. J Am Diet Assoc. 2008;108(3):465–472. doi: 10.1016/j.jada.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Thompson FE, Subar AF, Brown CC, et al. Cognitive research enhances accuracy of food frequency questionnaire reports: results of an experimental validation study. J Am Diet Assoc. 2002;102(2):212–225. doi: 10.1016/s0002-8223(02)90050-7. [DOI] [PubMed] [Google Scholar]
- 27.Subar AF, Thompson FE, Kipnis V, et al. Comparative validation of the Block, Willett, and National Cancer Institute food frequency questionnaires : the Eating at America’s Table Study. Am J Epidemiol. 2001;154(12):1089–1099. doi: 10.1093/aje/154.12.1089. [DOI] [PubMed] [Google Scholar]
- 28.Mirwald RL, Baxter-Jones AD, Bailey DA, Beunen GP. An assessment of maturity from anthropometric measurements. Med Sci Sports Exer. 2002;34(4):689–694. doi: 10.1097/00005768-200204000-00020. [DOI] [PubMed] [Google Scholar]
- 29.Jones B, Nagin D. A Stata plugin for estimating group-based trajectory models. 2012 [Google Scholar]
- 30.Nylund KL, Asparouhov T, Muthen BO. Deciding on the number of classess in latent class analysis and growth mixture modeling: a Monte Carlo Simulation Study. Struct Equ Modeling. 2007;14(4):535–569. [Google Scholar]
- 31.Must A, Tybor DJ. Physical activity and sedentary behavior: a review of longitudinal studies of weight and adiposity in youth. Int J Obes (Lond) 2005;29 (Suppl 2):S84–96. doi: 10.1038/sj.ijo.0803064. [DOI] [PubMed] [Google Scholar]
- 32.Ekelund U, Luan J, Sherar LB, et al. Moderate to vigorous physical activity and sedentary time and cardiometabolic risk factors in children and adolescents. JAMA. 2012;307(7):704–712. doi: 10.1001/jama.2012.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311(8):806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cawley J, Meyerhoefer C. The medical care costs of obesity: an instrumental variables approach. J Health Econ. 2012;31(1):219–230. doi: 10.1016/j.jhealeco.2011.10.003. [DOI] [PubMed] [Google Scholar]
- 35.U.S. Department of Health and Human Services. Physical Activity Guidelines Advisory Committee Report. Washington, DC: U.S. Department of Health and Human Services; 2008. [Google Scholar]
- 36.Troiano RP, McClain JJ, Brychta RJ, Chen KY. Evolution of accelerometer methods for physical activity research. Br J Sports Med. 2014;48(13):1019–1023. doi: 10.1136/bjsports-2014-093546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hjorth MF, Chaput JP, Ritz C, et al. Fatness predicts decreased physical activity and increased sedentary time, but not vice versa: support from a longitudinal study in 8- to 11-year-old children. Int J Obes (Lond) 2014;38(7):959–965. doi: 10.1038/ijo.2013.229. [DOI] [PubMed] [Google Scholar]
- 38.Richmond RC, Davey Smith G, Ness AR, den Hoed M, McMahon G, Timpson NJ. Assessing causality in the association between child adiposity and physical activity levels: a Mendelian randomization analysis. PLoS Med. 2014;11(3):e1001618. doi: 10.1371/journal.pmed.1001618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roeder K, Lynch K, Nagin D. Modeling uncertainty in latent class membership: A case study in criminology. JASA. 1999:766–776. [Google Scholar]