Abstract
Chlorin e6 is a tricarboxylic acid degradation product of chlorophyll a. Four chlorin e6 bis(amino acid) conjugates were regioselectively synthesized bearing two aspartate conjugates in the 131,173- and 152,173-positions, or at the 131,152 via an ethylene diamine linker. One additional conjugate bearing two different amino acids, lysine at 131 via an ethylene diamine linker and an aspartate at 152 via a β-alanine linker was also synthesized. The cytotoxicity and uptake of four di(amino acid) chlorin e6 conjugates were investigated in human HEp2 cells, and compared with chlorin e6. The most cytotoxic and most taken up conjugates were the zwitterionic 131,152-disubstituted conjugates 28 and 33; these also localized in multiple organelles. In contrast, the tetra-anionic 131,173- and 152,173-di-aspartyl chlorin e6 conjugates 12 and 13 showed low dark cytoxicity and lower phototoxicity compared with chlorin e6.
Graphical Abstract
Regioselective syntheses of chlorin e6 bis(amino acid) conjugates bearing aspartates in the 131,173-, 152,173- and 131,152-positions, and their cell studies are reported.
Introduction
Photodynamic therapy (PDT) is a special form of cancer photochemotherapy involving a photosensitizer and light. Selective uptake of a photosensitizer into tumor cells followed by irradiation with laser light within the “therapeutic window” (λmax 600-800 nm) produces singlet oxygen and other reactive oxygen species (ROS).1-5 Singlet oxygen, in particular, readily reacts with nearby local biomolecules (unsaturated lipids, amino acids and DNA) to cause destruction of apparatus within the cell. Because of limited diffusion of singlet oxygen through tissue, the destructive PDT effects are mainly localized to the photosensitizer-containing cells, thus minimizing the possibility of damage to normal cells near the tumor. Two essentials for the success of PDT treatment are: (i) the tumor-targeting ability of the photosensitizer, and (ii) the wavelength of the light required to activate the photosensitizing drug. Researchers aim for minimal “dark” toxicity, a high quantum yield of triplet state formation upon excitation, high selectivity for tumor cells compared with normal cells, reasonably rapid clearance from normal tissues, and a strong absorption peak between 600 and 800 nm to facilitate maximum light penetration through tumor and normal tissues.
Photofrin is a porphyrin-based photosensitizer mixture that was developed and approved in several countries for the PDT of melanoma, lung, digestive tract, genitourinary tract and for treatment of Barrett’s esophagus.3-6 It suffers from the fact that it is ineffective at wavelengths above 630 nm, and that it clears from tissues so slowly that patients can suffer from residual skin photosensitivity for weeks after treatment. Thus, a number of so-called second generation tetrapyrrole photosensitizers have been invented, these usually possessing strong red-shifted absorption maxima at the red end of the therapeutic window. Examples are tetra(meta-hydroxyphenyl)chlorin, (mTHPC or Temoporfin), 2-(1-hexyloxyethyl)-2-devinyl-pyropheophorbide a (HPPH or Photochlor), mono-L-aspartylchlorin e6 (LS-11, or Talaporfin) and a phthalocyanine called “Pc4”.3-6 Of these, chlorins (dihydroporphyrins) have received special attention for PDT because of their intense absorptions above 640 nm. Indeed, chlorophyll a derivatives are amphiphilic tetrapyrroles that have been extensively investigated and shown to exhibit low dark toxicities while at the same time able to generate cytotoxic ROS upon irradiation with light.7-9 Both HPPH and Talaporfin are in advanced-stage clinical trials for oncologic PDT treatment;10-13 they exhibit superior PDT activity, and are rapidly cleared from tissue such that residual photosensitivity in patients is minimized compared with Photofrin.
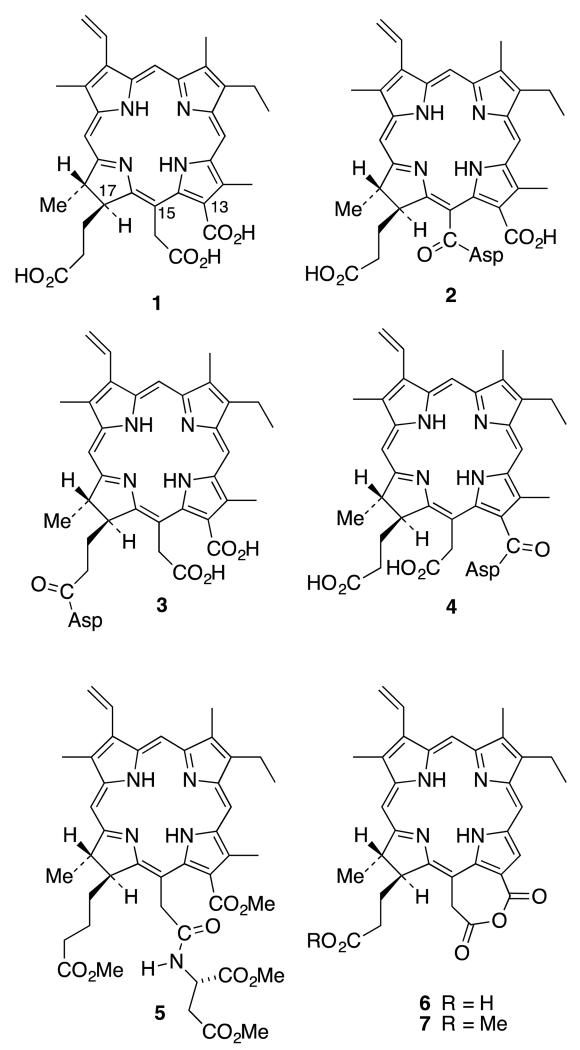
Chlorin e6 (1) is a particularly interesting chlorophyll a derivative because it possesses no less than three chemically different carboxylic acid functions (that could potentially be differentially functionalized with biomolecules): a nuclear aromatic acid in position 131, a nuclear acetic acid in position 152, and a propionic side-chain acid that is truly aliphatic, in position 173. Conjugations of peptides, sugars, lipoproteins, polyethylene glycols, and polyamines with chlorin e6 have been reported.14-22 Amino acid conjugations, in particular, have been shown to improve the PDT effects of tetrapyrrole compounds, and the type and position on the macrocyclic core can have a major impact upon efficacy in PDT. Talaporfin (2) was originally formulated in 1987 as the 173-aspartyl derivative (3) of chlorin e6. Gomi et al.23 used spectroscopic techniques (mostly) to propose that Talaporfin was in fact (2), not (3), but this work was almost universally ignored until our own group confirmed by total synthesis of the three possible isomers (2-4) (and comparison with authentic material), that (2) was indeed the correct structure for Talaporfin.24 A definitive X-ray structure of the tetramethyl ester (5) of Talaporfin finally ended all conjecture.24 It was proposed that the anhydride (6) is an intermediate in the formation of (2) whereby the aspartic acid is not involved in the first step of the reaction. The anhydride (6) is formed by dehydration using only DCC or EDC, and then the aspartic acid nucleophile opens the anhydride ring by attacking the more electrophilic aliphatic carbonyl at position 152.24 This point of view was solidified by isolation of the anhydride, a single crystal X-ray structure of the anhydride methyl ester (7), and the demonstration that ring-opening of the anhydride with eight different nucleophiles resulted only in formation of the 152-conjugate (8).25 Bis-conjugation was shown to be possible (at 173 and 152) in one case,25 or when two equivalents of DCC or EDC were used,26 but at no time was the 173-conjugate the sole product of the conjugation.
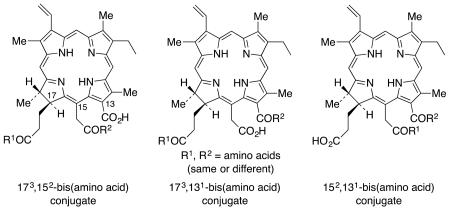
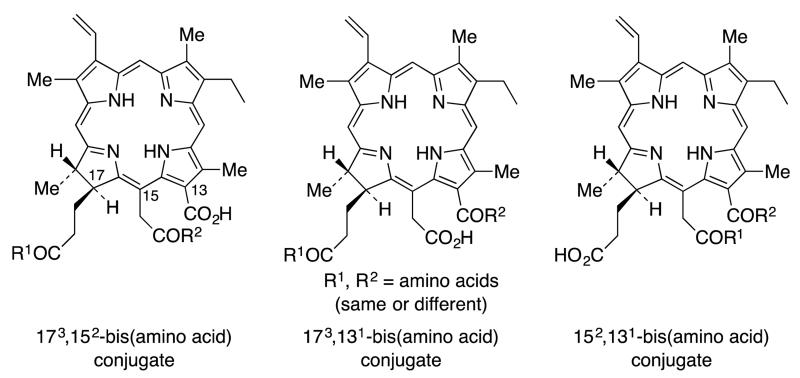
Making use of the differential conjugation methods developed in our earlier work,24 regioselective amino acid conjugates at the 173, 152 and 131 positions of chlorin e6 were synthesized27 to evaluate the effect in vitro of the conjugation site and structure of the amino acid on the PDT efficacy of the conjugates, using human carcinoma HEp2 cells. Syntheses of the 173 and 131 aspartyl regioisomers of mono-L-aspartylchlorin e6 were developed, along with the corresponding cationic lysine derivatives (which would be expected to show strong interactions with anionic biomolecules and membranes, resulting in enhanced PDT effectiveness.28 In addition, molecules with an ethylene diamine or β-alanine spacer group between the macrocycle and the amino acid were also synthesized. Based on in vitro biological evaluations, it was concluded that the site of amino acid conjugation, rather than the nature of the amino acid conjugated, was the major determinant of phototoxicity.27 The most phototoxic compounds were found to be the 131-regioisomers, bearing either an aspartic acid or a lysine residue directly conjugated to position 13 of the chlorin macrocycle, or connected via a β-alanine or ethylene diamine spacer. The most phototoxic compound of this series, and the most promising for PDT applications, was 131-aspartylchlorin e6. Unfortunately, poor solubility of these molecules was one of the major drawbacks in the cellular studies, and some of the sensitizer molecules tended to self-aggregate easily, resulting in lower cell uptake. In the present work, in order to overcome these shortcomings, we report the syntheses and biological evaluations of a new series of chlorin e6 derivatives that possess two amino acids in a single chlorin e6 molecule (Figure 1).
Figure 1.
All possible bis-amino acid regioisomers of chlorin e6
Kimani et al.19 recently reported the synthesis of mono-, di- and tri-PEGylated chorin e6 photosensitizers with tri(ethylene glycol) attached at the three carboxylic acid positions in chlorin e6.19 These were tested for solubility and hydrolytic stability, as well as phototoxicity, cell uptake and localization in ovarian OVCAR-5 cancer cells. Their results showed that increasing the number of PEG groups increased the water solubility and cellular uptake of the compounds. Computational studies also indicated that the PEG groups prevent formation of aggregates by π-π stacking, by wrapping onto the chlorin ring. Most significantly an increased number of PEG groups increased the phototoxicity, and cellular uptake. We therefore anticipated that an increase in the overall charge of the molecule and resulting amphiphilicity, might increase the water solubility29,30 and concomitantly decrease the self-aggregation in biological media.31 Our hypothesis was that a higher number of charged substituents would increase both the phototoxicity and the cellular uptake. To test this hypothesis all three possible regioisomers of diamino acid chlorin e6 derivatives were synthesized (Figure 1). It was found from our previous study, that the position of the amino acid plays a vital role in determining the conformation of the molecule.27 Therefore new synthetic routes were developed to prepare all three regioisomers. Both the 152,173- and 131,173-di(amino acid) derivatives were synthesized starting from chlorin e6 (1) and the 131,152-diamino derivatives were synthesized from pheophytin a. Thus, the aims of the present work were to determine: (i) the degree to which di-(amino acid)-chlorin e6 derivatives were taken up and localized in cancer cells, (ii) the main site(s) of intracellular accumulation, and (iii) whether the position and charge of the amino acid substituents influences the dark and photo-toxicity.
Results and Discussion
Synthesis of 152,173-diaspartylchlorin e6 derivative
A major side product in the synthesis of 152-mono-aspartylchlorin e6 derivative (2) was the 152,173-diaspartylchlorin e6 derivative 8,26 the yield of which can be increased by increasing the number of equivalents of coupling reagent and aspartic acid. According to our proposed mechanism,24 DCC and DMAP activated the acetic side chain in 1 and formed the anhydride intermediate 6. The excess coupling reagent can further activate the propionic acid side chain to form the O-acylisourea anhydride intermediate 9 (Scheme 1).25
Scheme 1.
Proposed intermediates in the formation of 152,173 di-aspartic acid derivative 8.
Thereafter, the free amine group of the amino acid can attack both carbonyl groups in intermediate 9, one in the anhydride ring and the other one in the O-acylisourea group. Mechanistic studies revealed the nucleophile first attacks the more reactive 152 position of the anhydride ring instead of the 131 position, regardless of the size or nucleophilicity of the molecule. Then the second equivalent can react with the O-acylisourea group to form diaspartylchlorin e6 derivative 8.
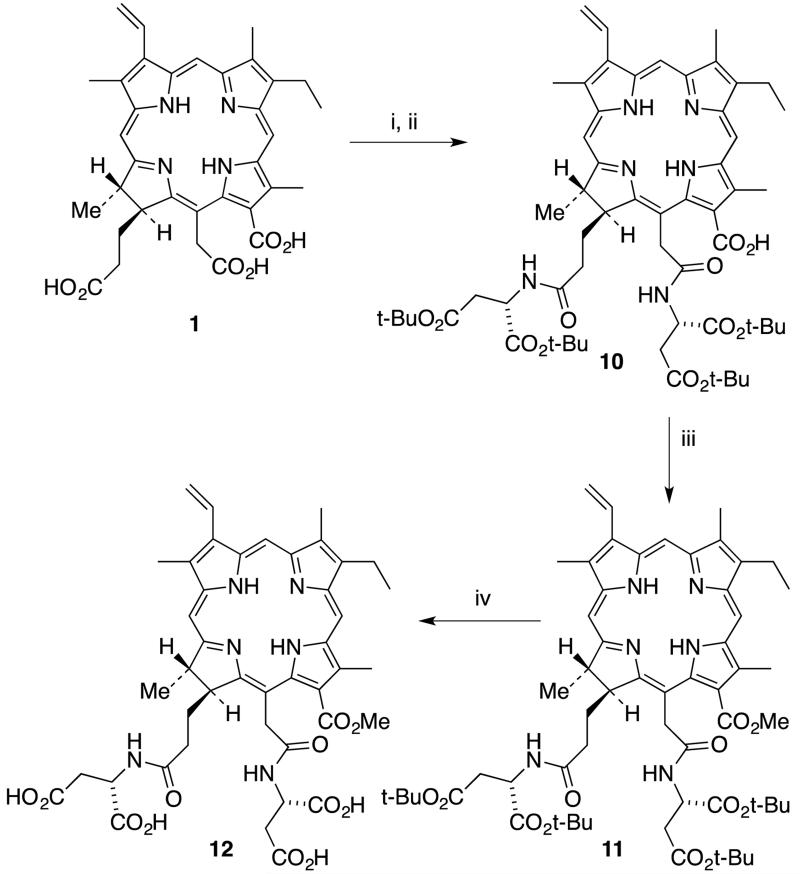
The detailed synthetic route to 152,173-diaspartylchlorin e6 methyl ester (12) is shown in Scheme 2. It was possible to obtain diaspartyl methyl ester 11 in 33% overall yield from chlorin e6 (1). The optimal yield of diaspartyl chlorin e6 12 (26%) was obtained upon activation of chlorin e6 with three equivalents of DCC and DMAP in CH2Cl2 at room temperature for two hours followed by coupling with 2.5 equivalents of di-tert-butyl protected aspartic acid for 24 hours. Less than three equivalents of DCC and aspartic acid tended to form significant amounts of the 152-monoaspartic acid conjugate as a side product. When the number of equivalents of either DCC or aspartic acid was increased, the yield of diaspartyl chlorin e6 10 improved, but it was not possible to prevent the formation of mono-aspartyl conjugate as a side product. Longer reaction times also helped to increase the yield of diaspartyl derivative 10. After confirming the formation of tert-butyl protected diaspartyl chorin e6 10 by TLC and mass spectrometry, freshly prepared diazomethane gas was bubbled through the mixture for five minutes to convert the remaining free acid groups into methyl esters. This reaction was monitored by TLC. It was possible to see two new spots in the TLC plate. A brighter spot with lower RF value belonged to the desired product 152,173-diaspartylchlorin e6 11 and the second spot had a higher RF value similar to that of 152-monoaspartlychlorin e6 methyl ester. Purification was achieved via silica gel column chromatography and the identity of the esterified product 11 was confirmed using 1H NMR and mass spectrometry. 1H NMR spectroscopy showed four singlets between 3.0 and 4.5 ppm each integrating to three protons. Of the four peaks, three belong to the methyl groups directly connected to the macrocycle and the remaining peak belongs to the 131 methyl ester group. The most downfield singlet at δ 4.25 confirmed the formation of the 131 methyl ester because this peak is unique to the methyl group on the formic (13-) side chain of the trimethyl ester of chlorin e6 (1). This observation confirmed that aspartic acid coupled with the acetic (15-) and propionic (17-) side chains of the molecule.
Scheme 2.
Synthesis of 152,173-diaspartylchlorin e6 methyl ester (12).(Reagents: i. 3 equiv. DCC, DMAP, CH2Cl2, 2 h; ii. 3 equiv. Asp di-t-Bu.HCl, DIEA, rt, 24 h; iii. CH2N2, CH2Cl2, 5 min, 30% (3 steps); iv. TFA/CH2Cl2 (1:3), thioanisole, 6 h, rt, 88%.)
In the final step, all four tert-butyl esters in molecule 11 were removed to give 12 in a TFA/CH2Cl2 mixture for six hours at room temperature. TFA and CH2Cl2 were first removed and the product was freeze-dried by dissolving it in a water/acetonitrile mixture. Once the sample was neutralized the color changed from purple to dark green. The identity of product 12 was confirmed by 1H NMR, 13C NMR and mass spectrometry.
Synthesis of 131,173-diaspartylchlorin e6 derivative
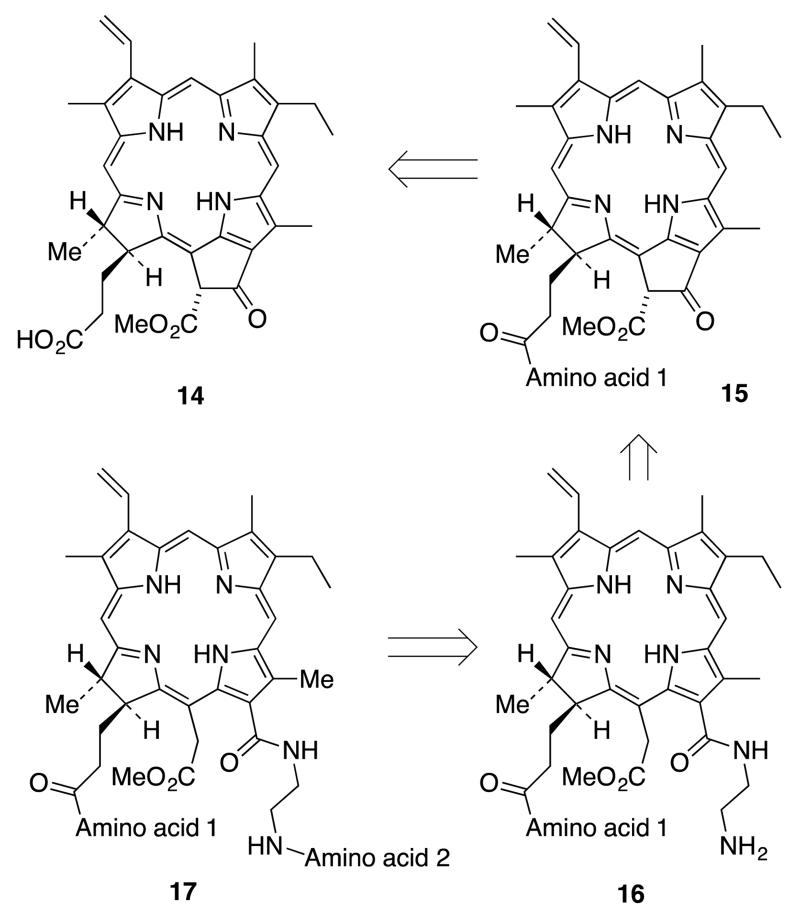
There are two possible routes to synthesize the 131,173-chlorin e6 conjugate 13: 1) coupling of the first amino acid to the propionic acid chain of pheophorbide a (14) and subsequent isocyclic ring opening of pheophorbide 15 with ethylene diamine to give 16, followed by coupling of the carboxylate of a second amino acid to the free amine group of the resulting chlorin derivative, to give 17 (Scheme 3), or 2) selective esterification of the acetic acid side chain (152 ester) of chlorin e6 (1) followed by coupling with two equivalents of an identical amino acid to afford the 131,173-di(amino acid) derivative (Scheme 4).
Scheme 3.
Retrosynthetic route to 131,173-di(amino acid) derivatives, Method 1.
Scheme 4.
Retrosynthetic route to 131,173-di(amino acid) derivatives, Method 2
In the first method, pheophorbide a (14) can be obtained by selective hydrolysis of the phytyl ester group of pheophytin a (obtained from Spirulina maxima or S. pacifica alga32) using a procedure developed by Wasielewski and Svec.33 Then the amino acid can be coupled to the propionic side chain to obtain the pheophorbide derivative 15. The pheophorbide isocyclic ring in 15 can be cleaved, using ethylene diamine,34 to provide 16 bearing a tethered free amine group able to couple with the carboxylic group of a second (potentially different) amino acid.
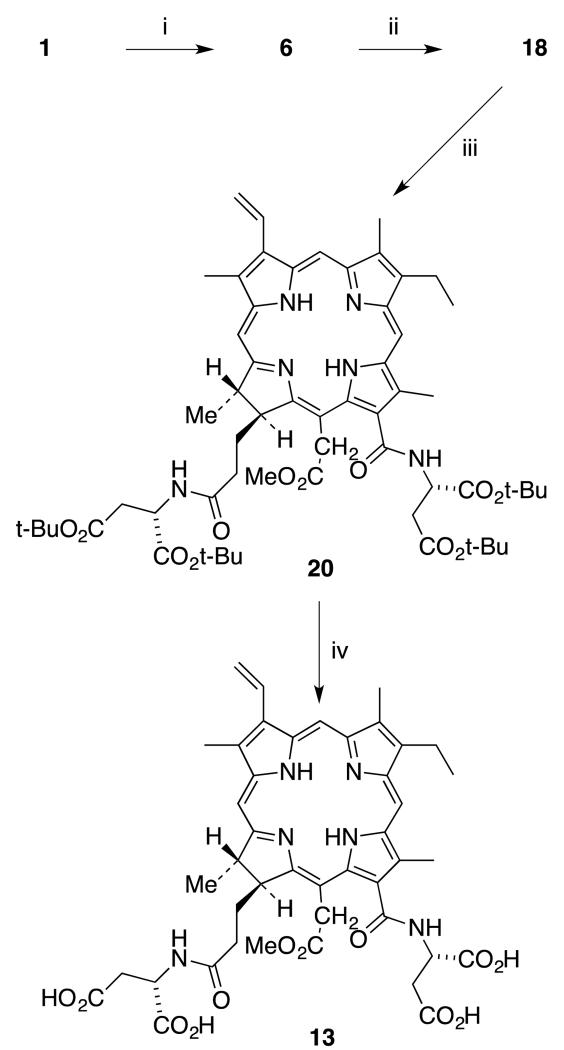
The key step in the second method (Scheme 4) is the selective protection of the acetic acid side chain of chlorin e6 (1) in the presence of the formic and propionic carboxylic side chains. From the previous work, it was found that the acetic acid group of chlorin e6 can be activated selectively by forming a cyclic anhydride. Chlorin e6 was activated using one equivalent of DCC and DMAP to form the anhydride intermediate (6).24 Formation of cyclic anhydride 6 can be confirmed by using UV-Vis spectroscopy. Then freshly prepared 0.5 M sodium methoxide was added until the color of the reaction mixture changed from brown-purple to dark green. Mass spectroscopy confirmed the formation of desired product 18. Purification of di-acid 18 was challenging due to its high polarity. After work-up it was possible to obtain a 1H NMR spectrum of the crude product and this was clear enough to identify the unique peak for the 152 methyl ester, and indicated it was sufficiently pure for the next step. Appearance of a new peak at 3.7 ppm for 3 protons provided evidence that esterification took place uniquely at the acetic acid side chain. Then tert-butyl-protected aspartic acid was coupled with crude 152-chlorin e6 monomethyl ester (18). The optimal yield was obtained with HOBt and TBTU as coupling reagents at room temperature for 48 hours.35 This reaction was monitored by TLC. The product was purified via silica gel column chromatography and the first moving band with 5% methanol/DCM was collected. The structure of product 20 was confirmed by mass and 1H NMR spectroscopy. Purified tert-butyl protected diaspartyl chlorin e6 20 was treated with TFA/CH2Cl2 mixture for six hours at room temperature to deprotect all four carboxylic acid groups. Pure compound 13 was obtained after removal of TFA. The final product, 131,173-diaspartyl chlorin e6 13, was obtained in 28% yield over four steps (Scheme 5).
Scheme 5.
Synthesis of 131,173-diaspartyl chlorin e6 methyl ester (13). (Reagents: i. DCC, DMAP, CH2Cl2, 1 h; ii. 0.5 M NaOMe/MeOH, 0 °C, 99% (2 steps); iii. HOBt, TBTU, DMF, L-Asp di-t-Bu.HCl, DIEA, CH2Cl2, rt, 48 h, 48%; iv. TFA/CH2Cl2, thioanisole, 6 h, rt, 53%.).
Synthesis of 131,152-di(amino acid) chlorin e6 derivatives
Previous studies have revealed that the formic and acetic acid derivatives of chlorin e6 are more potent photosensitizers than the propionic acid conjugates.27 Thus it was assumed that the 131,152-di(amino acid) derivatives may show more potent photosensitizing ability in this di(amino acid) series compared to the two other regioisomers (Figure 1). Therefore two different amino acid conjugates of the 131,152 regioisomer were synthesized.
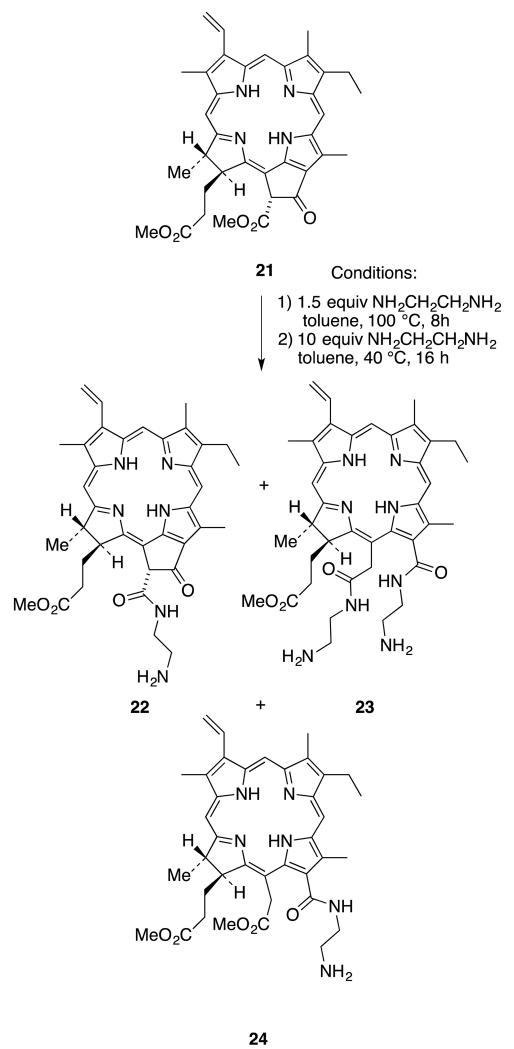
The main challenge was to protect the propionic side chain in the presence of the two other carboxyl groups. There is no known chemical method to selectively methylate the propionic side chain of chlorin e6 without methylating the acetic side chain. Esterification with methanol under acidic conditions will form the 152,173-chlorin e6 dimethyl ester.36 Diazomethane will methylate all three carboxylic groups to form chlorin e6 trimethyl ester.37 But in the recent literature there is a reported chemo-selective aminolysis of the β-keto ester of methyl pheophorbide a (21).38-40 This opens up a new route to synthesize 131,152 chlorin e6 derivative from methyl pheophorbide a (21).
Previously, Shinoda and Osuka reported a facile transesterification of the β-keto ester of methyl pheophorbide a.41 These authors were able to introduce various alcohols and steroid groups in the presence of 2-chloro-1-methylpyridinium iodide (CMPI) and of 4-(N,N-dimethylamino)pyridine (DMAP) to the 132-position of pheophorbide. Haavikko and coworkers also reported a selective aminolysis of the β-keto ester of the methyl pheophorbide a (21).38 They used various primary, secondary and aromatic amines for the aminolysis of the β-keto ester. It is known that aminolysis of the β-keto ester is usually facile compared to inactivated esters.42,43 In the previous literature the formation of β-enaminoesters by reaction between secondary amines and β-ketoesters at low temperatures is described.44 It was noticed that at higher temperatures they tend to form substantial amounts of the corresponding β-ketoamide along with the expected enamino-ester.
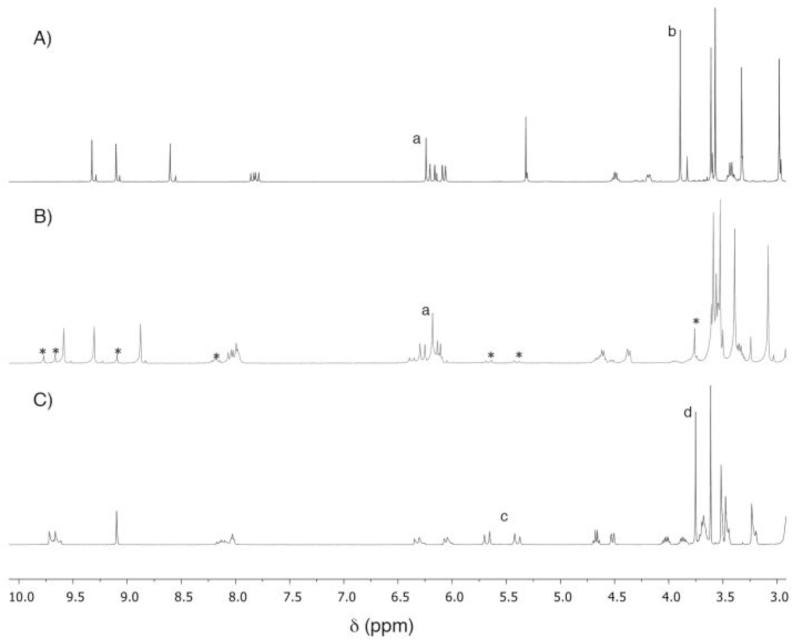
We have observed the same behavior with pheophorbide a.27 In our previous work,25 it was possible to open the isocylic ring of pheophorbide a (by cleaving the β-keto ester) using ethylene diamine at room temperature. It was noticed that at higher temperatures (90 °C in toluene) the reaction with ethylene diamine tended to form two major products (two new very close spots in TLC). Both products were isolated using preparative TLC and characterized by 1H NMR and mass spectroscopy. One of the products still clearly shows the unique peak for the pheophorbide isocylic ring: the proton appears as a sharp singlet at δ 6.26 (Figure 2). The disappearance of the peak for the methyl group of the β-ketoester and new peaks for the ethylene diamine methylene groups confirmed the formation of β-ketoamide 22. The second product was identified as the expected chlorin e6 derivative 23. As reported in the literature, temperature and the concentration of the amine play vital roles in deciding the major product.38 A large excess of amine and mild heating always produced the classical ring-opened product 23, and a stoichiometric amount of amine in refluxing toluene yielded β-ketoamide 22 as the major product.
Figure 2.
1H NMR spectral comparison of: A) methyl pheoporbide a (21); B) β-ketoamide 22 (*contains 10% of ring-opened product 24); and C) 131-ethylene-diaminyl-chlorin e6 dimethyl ester 24. Assignments: a, 132 CH; b, 133 CO2CH3; c, 151 CH2; d, 152 CO2CH3.
The first attempt to introduce the ethylene diamine to both ester and keto groups of methyl pheophorbide 21 to form the 131,152-disubstituted chlorin e6 derivative 23 was unsuccessful. Both β-keto-ester aminolysis and ring-opening reactions were attempted in one pot. First methyl pheophorbide 21 and ethylene diamine were refluxed in toluene until disappearance of the starting material, as monitored by TLC. Then excess amine was added and the mixture was stirred at 40 °C for 12 hours to complete the ring cleavage reaction. Unfortunately, this process resulted in a mixture of compounds, including the desired product 23. Mass spectrometry revealed three major compounds (22-24) in this mixture, as shown in Scheme 6. Purification of these compounds was challenging due to the high polarity of the free amine groups.
Scheme 6.
Attempted synthesis of 131,152-bis-ethylenediamine substituted chlorin e6 derivative 23
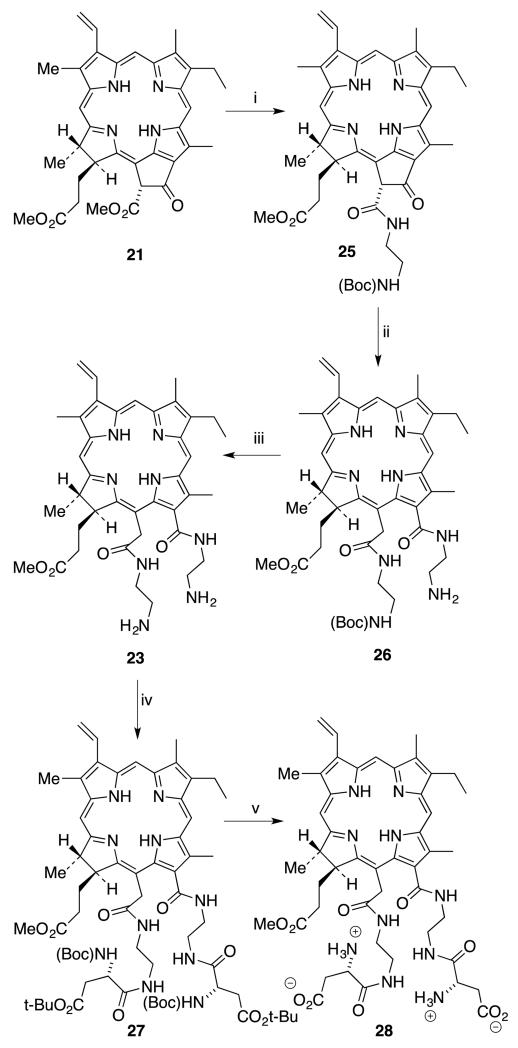
It was decided to use half-protected ethylene diamine to simplify the purification process. As indicated by TLC, aminolysis of the β-keto-ester of methyl pheophorbide a (21) with protected ethylene diamine was completed after 12 hours. But the second step ring-cleavage was unsuccessful with Boc-protected ethylene diamine, possibly due to steric bulkiness of the amine. Thus, product 25 was isolated and purified before going on to the ring cleavage reaction. As it was possible to achieve the isocyclic ring cleavage using free ethylene diamine, unprotected ethylene diamine was used for the second step and the diethylene diamine substituted product 26 was obtained. Product 26 was purified using a short silica gel column and its identity was confirmed using mass and 1H NMR spectroscopy. Boc deprotection was achieved using TFA to obtain the free amines (23) for the subsequent coupling reaction. Coupling with protected aspartic acid, to give 27 followed by deprotection produced the desired product 28, in 15% yield over 5 steps from methyl pheophorbide a (21) (Scheme 7).
Scheme 7.
Synthesis of 131,152-di(ethylenediaminylaspartyl)chlorin e6 28, (Reagents: i. 1.5 equiv (Boc)NHCH2CH2NH2, toluene, 100 °C, 12-16 h, 65%; ii. 10 equiv NH2CH2CH2NH2, toluene, 45 °C, 12 h, 64%; iii. TFA, CH2Cl2 1:3, 6 h, rt, 88%; iv. Boc-Asp(O-tBu)OH, DIEA, CH2Cl2, TBTU, DMF, 40%; iv. TFA/CH2Cl2 1:3, 6 h, rt, 70%.)
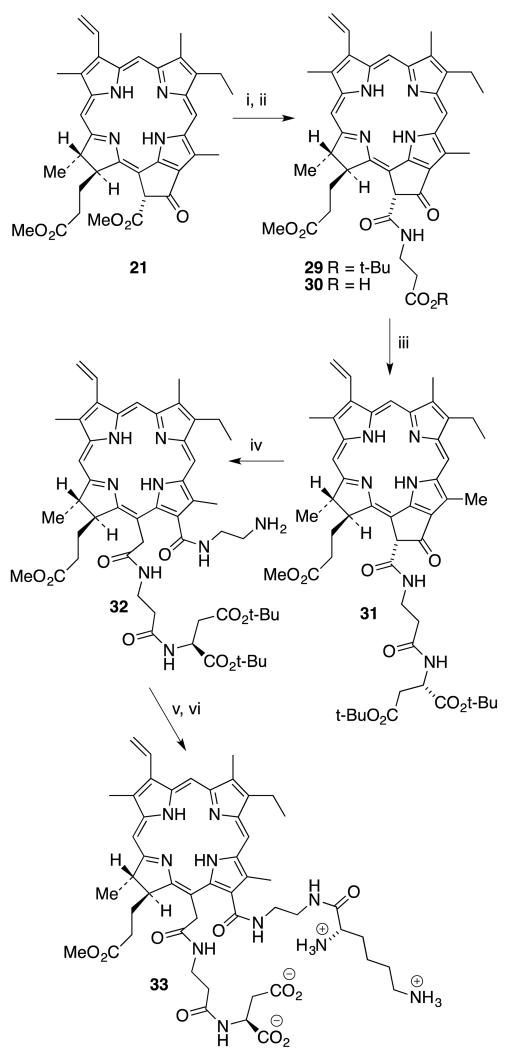
Advantage was taken of a previous route to introduce two types of amino acids to the 131 and 152 positions. Protected β-alanine was used for the aminolysis reaction of 21 as it greatly reduces the unwanted ring-opened product and creates a carboxylic end to couple aspartic acid through its amino group. Subsequent aminolysis with protected β-alanine, to give 29, followed by deprotection of the tert-butyl ester provided free acid 30. Its identity was confirmed by mass spectrometry. Free acid 30 was activated and coupled with protected aspartic acid to obtain pheophorbide derivative 31. This was purified and characterized by 1H NMR and mass spectroscopy. A classical ring-opening reaction was performed with excess ethylene diamine in toluene at 40 °C to provide the chlorin e6 derivative 32. The reaction was monitored using UV-Vis spectroscopy, and the color changed from dark green to bright green during isocyclic ring-opening. 1H NMR spectroscopy shows the new peaks for ethylene diamine and mass spectroscopy confirmed the identity of the product. Then Boc-protected lysine was coupled with the free amine group of chlorin e6 derivative 32 to produce the desired 131-ethylenediaminyl-lysyl-152-β-alanylaspartylchlorin e6 tert-butyl methyl ester. This product was purified using silica gel chromatography. Finally, global deprotection with TFA in CH2Cl2 produced the final product 33 in 9% yield over six steps starting from methyl pheophorbide a (21) (Scheme 8).
Scheme 8.
Synthesis of 131,152-di(amino acid) derivative 33. (Reagents: i. 1.5 equiv NH2CH2CH2CO2t-Bu, toluene, 100 °C, 12-16 h, 54%; ii. TFA, CH2Cl2 1:3, 6 h, rt, 99%; iii. L-Asp di-t-Bu.HCl, DIEA, CH2Cl2, HOBt, TBTU, DMF, 59%; iv. 10 equiv NH2CH2CH2NH2, toluene, 45 °C, 12 h, 58%; v. Boc-Lys(Boc)-OH, DCHA, DIEA, CH2Cl2, HOBt, TBTU, DMF; vi. TFA, CH2Cl2 1:3, 6 h, rt, 88%; iv. Boc-Asp(O-t-Bu)OH, DIEA, CH2Cl2, TBTU, DMF, 40%; iv. TFA/CH2Cl2 1:3, 12 h, rt, 48% (2 steps).
Cell Culture Studies
Cytotoxicity
The dark cytotoxicity and phototoxicity (~1.5 J/cm2) of chlorin e6 (1) and its di(amino acid) conjugated derivatives 12, 13, 28 and 33 were evaluated in HEp2 cells using a Cell Titer Blue assay. Significant differences were observed in the dark and phototoxicity of the di(amino acid) conjugates, as shown in Table 1. These differences are attributed to both the sites of conjugation (131,152 or 131,173 or 152,173) and the overall charge and amphiphilic character of the chlorin e6 derivatives. While conjugation of two aspartate groups via their α-amine groups gave tetra-anionic conjugates 12 and 13, the conjugation of two aspartates via the carboxylate groups gave zwitterionic 28. On the other hand compound 33 bearing an aspartate conjugated via the α-amine group and a lysine conjugated via the carboxylate group, is also zwitterionic.
Table 1.
Dark and phototoxicity (~ 1.5 J/cm2) for chlorin e6 and its di-amino acid derivatives in HEp2 cells, using a Cell Titer Blue assay
| Compound | Dark toxicity (IC50, μM) |
Phototoxicity (IC50, μM) |
Ratio |
|---|---|---|---|
| Chlorin e6 (1) | 350 | 14.5 | 24.1 |
| 152,173-di(Asp)Ce6 MME (12) | >400 | 35 | >11 |
| 131,173-di(Asp)Ce6 MME (13) | 305 | 26 | 11.7 |
| 131,152-di(EDAsp)Ce6 MME (28) | 70 | 11 | 6.4 |
| 131-EDLys-152-β-AlaAspCe6 MME (33) |
65 | 9.1 | 7.2 |
In comparison with chlorin e6 (1), the zwitterionic 131,152-disubstituted conjugates 28 and 33 showed higher phototoxicity while the tetra-anionic 12 and 13 showed lower phototoxicity. These results are in agreement with our previous observations that 131- and 152-mono(amino acid) chlorin e6 derivatives tend to show higher phototoxicities than the corresponding 173 derivatives.27 This might in part be due to the different molecular conformations conferred by 131, 152 or 173 substitutions; while the 173 derivatives tend to assume L-shape conformations with the side group positioned perpendicular, or folded over the macrocycle, the 152 and in particular the 131 derivatives tend to assume linear conformations with the side groups extending away from the macrocycle.27 Such linear conformations might favor binding to specific intracellular components, inducing higher phototoxicity. However, the zwitterionic 131,152 conjugates also showed about 5-fold higher dark cytotoxicity than chlorin e6 and the tetra-anionic conjugates, and lower dark/photo cytotoxicity ratio. The observed higher dark and photo cytotoxicities of conjugates 28 and 33 might in part be due to their higher cellular uptake (see below).
Time-dependent Cellular Uptake
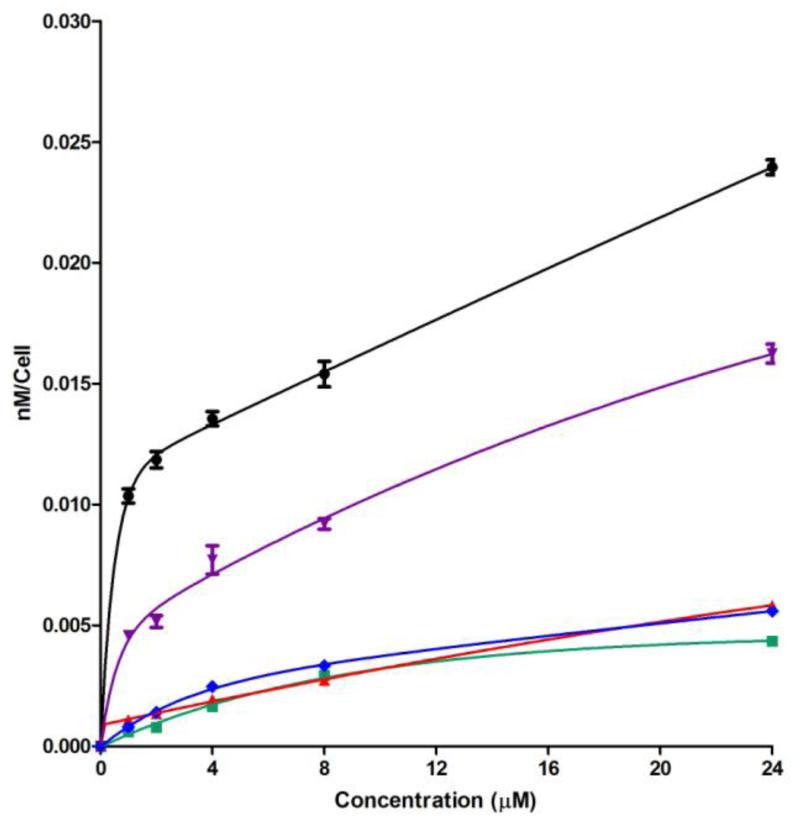
The results obtained for the time-dependent uptake of chlorin e6 and its di(amino acid) derivatives at a concentration of 10 μM in human carcinoma HEp2 cells are shown in Figure 3. Conjugates 12 and 13 showed similar uptake to chlorin e6, probably due to their low interaction and low permeability across the cell membrane as a result of their negative charge. In addition to phosphate groups, the plasma membranes of tumor cells generally contain higher net negative charge compared with normal cells because of over-expression of polysialic acid residues.45 On the other hand, the zwitterionic conjugates 28 and 33, showed much higher cellular uptake at all time points investigated, and after 24 h about 4-fold and 6-fold higher cellular uptake were observed for 28 and 33 than chlorin e6, respectively. Although the 131,152-substituted conjugates 28 and 33 were found to have similar cytotoxicities (see Table 1), conjugate 33 bearing two different amino acid residues, lysine and aspartate, had significant higher uptake than 28 at all times investigated, maybe due to its higher amphiphilicity conferred by substitution with two different amino acids.
Figure 3.
Time-dependent uptake of chlorin e6 (1, green) and its derivatives 152,173-di(Asp)Ce6 MME (12, blue), 131,173-di(Asp)Ce6 MME (13, red), 131,152-di(EDAsp)Ce6 MME (28, purple) and 131-EDLys-152-β-AlaAspCe6 MME (33, black), at 10 μM by HEp2 cells.
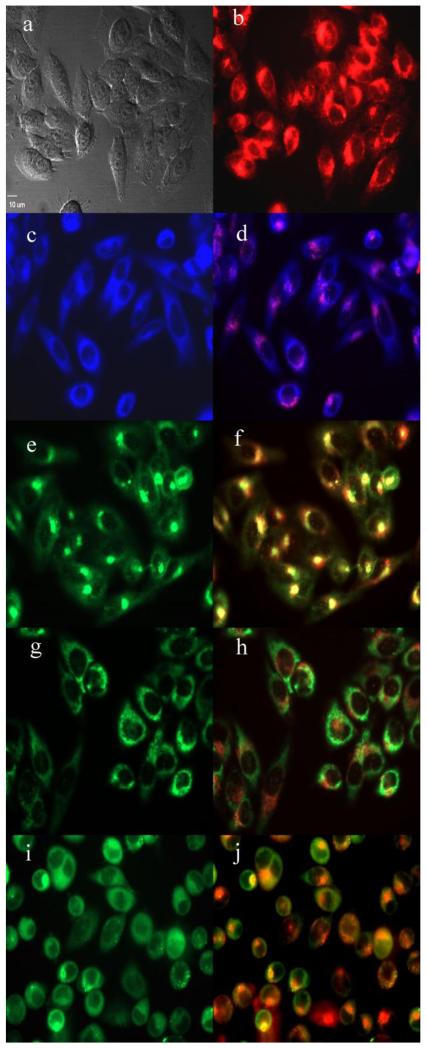
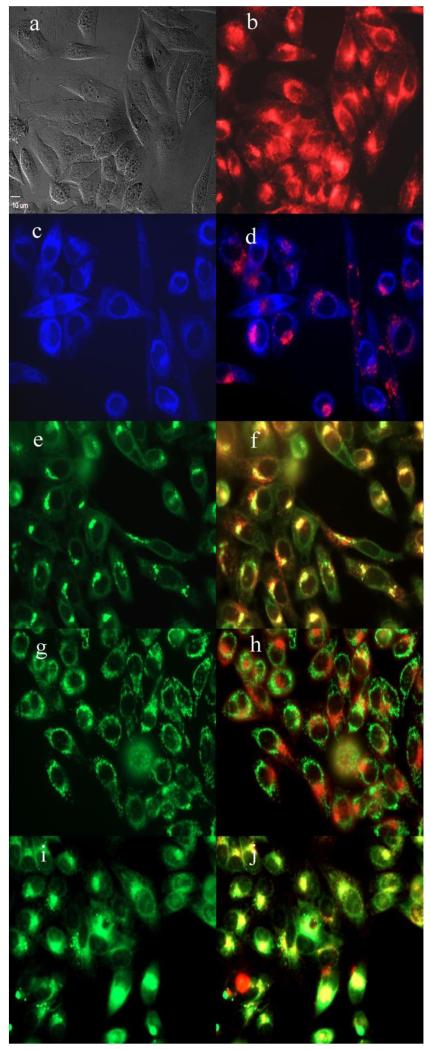
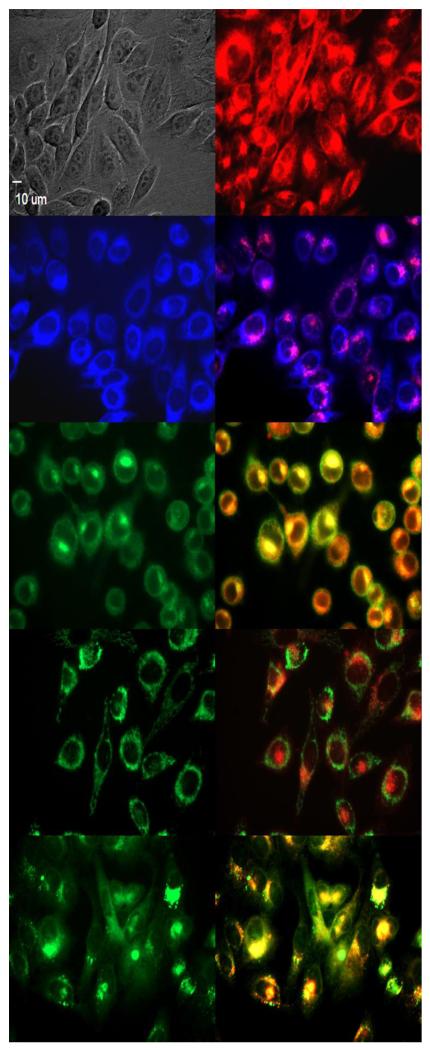
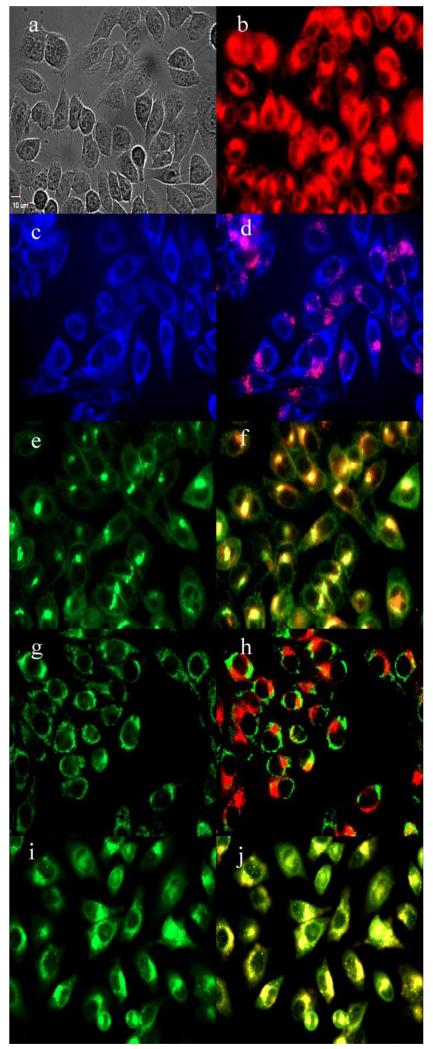
The preferential sites of subcellular localization of the di(amino acid) conjugates of chlorin e6 were evaluated by fluorescence microscopy, and the results are shown in Table 2 and Figures 4-7. The organelle specific probes BODIPY Ceramide (Golgi), LysoSensor Green (Lysosomes), MitoTracker Green (mitochondria), and ER tracker Blue/White fluorescence (ER) were used in the overlay experiments. All conjugates localized in the lysosomes and the Golgi apparatus, and to a smaller extent in the ER. In addition, conjugate 33 bearing lysine and aspartate residues, was also observed in mitochondria, and this might in part be responsible for its higher dark and phototoxicities. These results are in agreement with the known preferential localization of LS-11 (152-aspartyl chlorin e6) in the lysosomes,46 and with our previous observations of 131, 152 and 173 mono(amino acid) conjugates of chlorin e6 localizing in lysosomes, ER and Golgi.27
Table 2.
Major and minor subcellular sites of localization for chlorin e6 and its di-amino acid derivatives in HEp2 cells
| Compound | Lyso | ER | Golgi | Mito |
|---|---|---|---|---|
| Chlorin e6 (1) | + | ++ | − | − |
| 152,173-di(Asp)Ce6 MME (12) | + | + | +++ | − |
| 131,173-di(Asp)Ce6 MME (13) | +++ | + | +++ | − |
| 131,152-di(EDAsp)Ce6 MME (28) | +++ | + | +++ | − |
| 131-EDLys-152-β-AlaAspCe6 MME (33) |
+++ | + | +++ | + |
Figure 4.
Subcellular localization of 152,173-di(aspartate) chlorin e6 12 in HEp2 cells at 10 μM for 6h, (a) phase contrast, (b) overlay of 12 and phase contrast, (c) ER Tracker Blue, (d) overlay of 12 and ER Tracker Blue, (e) BODIPY Ceramide, (f) overlay of 12 and BODIPY Ceramide, (g) MitoTracker Green, (h) overlay of 12 and MitoTracker Green, (i) LysoSensor Green, (j) overlay of 12 and LysoSensor Green. Scale bar: 10 μm.
Figure 7.
Subcellular localization of 152,173-di(aspartate) chlorin e6 33 in HEp2 cells at 10 μM for 6h, (a) phase contrast, (b) overlay of 33 and phase contrast, (c) ER Tracker Blue, (d) overlay of 33 and ER Tracker Blue, (e) BODIPY Ceramide, (f) overlay of 33 and BODIPY Ceramide, (g) MitoTracker Green, (h) overlay of 33 and MitoTracker Green, (i) LysoSensor Green, (j) overlay of 33 and LysoSensor Green. Scale bar: 10 μm.
Experimental
General
All air and moisture sensitive reactions were performed in dried and distilled solvents under an argon atmosphere. All solvents and reagents were purchased from commercial sources, unless otherwise stated. Silica gel 60 (230×400 mesh, Sorbent Technologies) was used for column chromatography. Analytical thin-layer chromatography (TLC) was carried out using polyester backed TLC plates 254 (precoated, 200 μm) from Sorbent Technologies. NMR spectra were recorded on an AV-400 spectrometer (400 MHz for 1H, 100 MHz for 13C). The chemical shifts are reported in δ ppm using the following partially deuterated solvents as internal references: CD2Cl2 5.32 ppm (1H), 54 ppm (13C); d-DMSO 2.49 ppm (1H), 39.5 ppm (13C); d-CH3OH 4.78 ppm (1H), 49.0 ppm (13C); CDCl3 7.26 ppm (1H), 77.16 ppm (13C); (CH3)2CO 2.50 ppm (1H), 29.84 ppm (13C). Electronic absorption spectra were measured on an Agilent 8453 UV/Vis spectrophotometer. Mass spectra were obtained on a Bruker Omniflex MALDI Time-of-Flight Mass Spectrometer. Spirulina pacifica alga was purchased as a spray-dried powder from Cyanotech, Hawaii. Pheophytin a (1) was extracted from Spirulina pacifica alga as previously published and its spectroscopic characterization agreed with the published data.34 All compounds synthesized were purified and isolated in ≥ 95% purity, as evidenced by analytical TLC in at least two solvent systems, and confirmed by the absence of extraneous tetrapyrrole resonances in 1H- and 13C-NMR spectra.
Synthetic procedures and characterization
152,173-Diaspartylchlorin e6 tetra(tert-butyl)-monomethyl ester (11)
Chlorin e6 (1, 100 mg, 0.168 mmol) was dissolved in dry CH2Cl2 (7 mL) and DIEA (0.06 mL, 0.34 mmol) was added. A mixture of DCC (105 mg, 0.51 mmol) and DMAP (163 mg, 0.51 mmol) in CH2Cl2 (8 mL) was added and the mixture was allowed to stir for 2 h. Then aspartic acid di(tert)butyl ester hydrochloride (125 mg, 0.445 mmol) and DIEA (0.075 ml) were mixed in CH2Cl2 (2 ml) and added to the reaction mixture. The solution was allowed to stir overnight at room temperature and after 12 h it was treated with excess ethereal diazomethane. Then the mixture was diluted with CH2Cl2 and washed with 5% aqueous citric acid, followed by a wash with brine and finally water. It was dried over anhydrous Na2SO4 and the solvent was evaporated. The residue was dissolved in 5% methanol/CH2Cl2 and purified via silica gel column chromatography with the same mobile phase to afford 152,173-diaspartylchlorin e6 tera(tert-butyl)-monomethyl ester (11 , C59H80N6O12, 54 mg, 0.051 mmol, 30% yield); UV-Vis (acetone): λmax (ε/mM−1cm−1) 664 nm (52), 608 (3.9), 528 (3.6), 500 (13), 400 (170); 1H NMR (acetone-d6, 400 MHz): δ 9.80 (s, 1H), 9.63 (s, 1H), 9.06 (s, 1H), 8.15 (dd, J = 17.9, 11.6 Hz, 1H), 7.37 (d, J = 7.9 Hz, 1H), 7.23 (d, J = 8.0 Hz, 1H), 6.37 (dd, J = 17.9, 1.5 Hz, 1H), 6.10 (dd, J = 11.6, 1.4 Hz, 1H), 5.37 (d, J = 12.0 Hz, 2H), 4.67 (s, 2H), 4.26 (s, 3H), 3.77 (d, J = 7.6 Hz, 2H), 3.57 (s, 3H), 3.48 (s, 4H), 3.25 (s, 3H), 2.83 – 2.63 (m, 3H), 2.52 – 2.28 (m, 2H), 2.14 (s, 2H), 1.89 – 1.79 (m, 2H), 1.78 (d, J = 7.1 Hz, 3H), 1.69 (t, J = 7.6 Hz, 3H), 1.43 (s, 9H), 1.35 (s, 9H), 1.26 (s, 9H), 1.16 (s, 9H), −1.36 (s, 1H), −1.57 (s, 1H); MS (MALDI-TOF) m/z 1065.591 [M+H]+, calcd. for C59H81N6O12 1065.591.
152,173-Diaspartylchlorin e6 methyl ester (12)
152,173-Diaspartylchlorin e6 tetra(tert-butyl)-monomethyl ester (11, 54 mg, 0.051 mmol) was dissolved in 2 mL of dry CH2Cl2 in an ice bath under argon and TFA (1 mL) was added and the reaction mixture was allowed to stir overnight. The solvent was rotevaporated several times with diethyl ether to remove residual TFA. The residue was washed with CH2Cl2 several times. The final product was re-dissolved in a water/acetonitrile mixture and freeze dried to afford 152,173-diaspartylchlorin e6 methyl ester (12, C43H48N6O12, 38 mg, 0.045 mmol, 88% yield); UV-Vis (MeOH): λmax (ε/mM−1cm−1) 661 nm (71.6), 607 (11), 527 (9.3), 499 (22.4), 399 (172.3); 1H NMR (acetone-d6, 400 MHz): δ 9.88 (s, 1H), 9.66 (s, 1H), 9.21 (s, 1H), 8.11 (dd, J = 17.8, 11.6 Hz, 1H), 6.35 (d, J = 17.8 Hz, 1H), 6.14 (d, J = 11.6 Hz, 1H), 5.35 (t, J = 22.7 Hz, 2H), 4.81 (br. s, 1H), 4.65 (br. s, 1H), 4.63 (br. S, 1H), 4.25 (s, 3H), 3.70 (d, J = 7.9 Hz, 2H), 3.54 (s, 3H), 3.46 (s, 3H), 3.18 (s, 3H), 3.06 – 2.60 (m, 5H), 2.27 (m, 2H), 1.81 (d, J = 7.1 Hz, 3H), 1.55 (t, J = 7.4 Hz, 3H). 2.00 – 1.50 (m, 4H) 1.25 (m, 2H); 13C NMR (acetone-d6, 101 MHz) δ 193.12, 192.77, 191.95, 191.88, 191.84, 191.04, 188.72, 168.55, 164.14, 163.25, 160.42, 158.10, 157.50, 155.68, 155.26, 155.20, 151.92, 150.02, 149.04, 145.83, 142.64, 137.66, 134.79, 124.88, 121.07, 117.59, 116.00, 73.49, 73.03, 69.43, 69.24, 68.92, 59.56, 55.70, 53.44, 52.59, 50.75, 42.86, 39.10, 36.79, 31.75, 31.58, 30.41. MS (MALDI-TOF) m/z 840.451 [M]+, calcd. for C43H49N6O12 840.333
131,173-Diaspartylchlorin e6 tetra(tert-butyl)-monomethyl ester (20)
Chlorin e6 (1, 100 mg, 0.168 mmol) was dissolved in methanol (5 mL). DCC (35 mg, 0.17 mmol) and DMAP (21 mg, 0.17 mmol) were added and the mixture was stirred until the anhydride intermediate was observed in the TLC. After 1 h, freshly prepared sodium methoxide (0.34 mL of a 0.5 M solution) was added into the reaction mixture dropwise until the color changed from brown to light green. The reaction was monitored by UV-Vis spectroscopy. The solution changed from brown to light green as the anhydride ring opened. The mixture was diluted with ethyl acetate and then washed with 5% aqueous citric acid, followed by a wash with brine and finally with water. It was dried over anhydrous Na2SO4 to afford chlorin e6 152-methyl ester (18, C35H39N4O6, 101 mg, 0.165 mmol, 100% yield). Purification was challenging due to the polarity induced by the two free acid groups; thus, the crude product was subjected to the next reaction without purification. A 1H NMR spectrum of the crude product confirmed the methylated acetic side chain. [MS (MALDI-TOF) m/z 633.311 [M+Na]+, calcd. for C35H39N4O6 633.269]. Chlorin e6 monomethyl ester (18, 101 mg, 0.165 mmol) was dissolved in dry DMF (5 mL). A mixture of HOBt (46 mg, 0.34 mmol), TBTU (109 mg, 0.34 mmol) and DIEA (0.06 ml, 0.34 mmol) in DMF (5 ml) was added and the mixture was allowed to stir for 30 min at room temperature. Then aspartic acid di-tert-butyl ester hydrochloride (125 mg, 0.445 mmol) and DIEA (0.08 ml, 0.445 mmol) were mixed in CH2Cl2 (2 mL) and added to the reaction mixture. The solution was allowed to stir for 48-72 h until formation of the desired product was confirmed by TLC. Then the reaction mixture was washed with 5% aqueous citric acid, followed by a wash with brine and then water. The organic phase was dried over anhydrous Na2SO4 and solvent was evaporated. The residue was dissolved in 5% methanol/CH2Cl2 and purified via chromatography on a short silica gel column with the same mobile phase to afford 152,173-diaspartylchlorin e6 tetra(tert-butyl)-monomethyl ester (20, C59H80N6O12, 85 mg, 0.079 mmol, 48% yield); UV-Vis (acetone): λmax (ε/mM−1cm−1) 663 nm (54), 607 (3.8), 528 (3.1), 500 (13), 399 (165); 1H NMR (acetone-d6, 400 MHz): δ 9.79 (d, J = 6.1 Hz, 1H), 9.65 (s, 1H), 9.10 (s, 1H), 8.42 (d, J = 7.9 Hz, 1H), 8.12 (dd, J = 17.8, 11.6 Hz, 1H), 7.29 (d, J = 8.2 Hz, 1H), 6.32 (d, J = 17.8 Hz, 1H), 6.06 (d, J = 11.4 Hz, 1H), 5.73 (d, J = 18.9 Hz, 1H), 5.50 – 5.21 (m, 2H), 4.76 – 4.61 (m, 2H), 4.61 – 4.48 (m, 1H), 3.76 (s, 3H), 3.74 – 3.68 (m, 1H), 3.65 (s, 3H), 3.47 (s, 3H), 3.42 (s, 1H), 3.22 (s, 3H), 3.20 – 3.11 (m, 2H), 2.66 (d, J = 5.2 Hz, 2H), 2.46 – 2.27 (m, 1H), 2.24 – 2.14 (m, 1H), 1.83 – 1.72 (m, 2H), 1.66 (s, 9H), 1.55 (s, 9H), 1.41 (s, 9H), 1.35 (s, 9H), 1.72-1.2 (m, 6H), −1.66 (1H), −1.85 (1H); MS (MALDI-TOF) m/z 1065.681 [M+H]+, calcd. for C59H81N6O12 1065.591.
131,173-Diaspartylchlorin e6 methyl ester (13)
152,173-Diaspartylchlorin e6 tetra(tert)butyl methyl ester (20, 50 mg, 0.047 mmol) was dissolved in 2 mL of dry CH2Cl2 in an ice bath under argon. TFA (1 mL) was added and the reaction mixture was allowed to stir overnight. The mixture was rotavaporated several times with diethyl ether to remove residual TFA. The residue was dissolved in water/acetonitrile mixture and freeze dried to obtain 131,173-diaspartylchlorin e6 methyl ester (13, C43H48N6O12, 21 mg, 0.025 mmol, 53%). UV-Vis (MeOH): λmax (ε/mM−1cm−1) 662 nm (75.3), 607 (23), 529 (10), 500 (8.3), 400 (165.2); 1H NMR (MeOD, 400 MHz) δ 10.07 (s, 1H), 9.86 (s, 1H), 9.45 (s, 1H), 8.06 (dd, J = 17.3, 11.9 Hz, 1H), 6.30 (d, J = 17.8 Hz, 1H), 6.24 (d, J = 11.4 Hz, 1H), 5.62 (d, J = 18.9 Hz, 1H), 5.28 (d, J = 18.3 Hz, 2H), 4.78 – 4.76 (m, 2H), 4.76 - 4.56 (m, 3H), 3.80 (s, 3H), 3.59 (s, 3H), 3.52 (s, 3H), 3.21 (s, 3H), 2.87 – 2.69 (m, 2H), 2.68 – 2.47 (m, 1H), 2.21 (d, J = 19.9 Hz, 2H), 1.94 – 1.72 (m, 4H), 1.59 (d, J = 6.9 Hz, 4H), 1.30 – 1.01 (m, 4H). 13C NMR (MeOD, 101 MHz) δ 175.33, 174.90, 174.72, 174.02, 173.88, 173.83, 173.66, 169.35, 143.94, 142.95, 142.47, 140.57, 140.42, 138.22, 138.01, 136.12, 135.46, 135.25, 133.96, 132.61, 131.29, 129.47, 124.69, 107.02, 100.12, 98.81, 97.77, 54.94, 53.05, 51.25, 50.55, 38.89, 36.86, 34.68, 33.77, 26.68, 26.02, 23.70, 20.00, 16.98, 12.22, 10.91, 9.11. MS (MALDI-TOF) m/z 840.532 [M]+, calcd. for C43H49N6O12 840.333.
Ethylenediaminyl(boc) pheophorbide a (25)
Methyl pheophorbide a (21, 100 mg, 0.165 mmol) was dissolved in dry toluene (20 ml) and the mixture was heated to 100 °C under nitrogen. Then, mono-boc protected ethylene diamine (32 mg, 0.20 mmol) was added. The reaction mixture was allowed to stir overnight at 100 °C while monitoring by TLC. Then the solvent was removed and the residue was dissolved in CH2Cl2 and washed with 5% aqueous citric acid, followed by water and brine. It was dried over anhydrous Na2SO4 and the solvent was evaporated. The residue was dissolved in 3% methanol/CH2Cl2 and purified via silica gel column chromatography with the same mobile phase to afford ethylenediaminyl(boc) pheophorbide a (25, C42H50N6O6, 80 mg, 0.109 mmol, 65%); UV-Vis (DCM): λmax (ε/mM−1cm−1) 667 nm (46), 609 (6.5), 535 (8), 505 (10), 413 (99); 1H NMR (acetone-d6, 400 MHz) δ 9.58 (s, 1H), 9.30 (s, 1H), 8.87 (s, 1H), 8.13 – 7.92 (m, 2H), 6.27 (dd, J = 17.9, 1.5 Hz, 1H), 6.18 (s, 1H), 6.12 (dd, J = 11.5, 1.4 Hz, 1H), 6.16 (m, 1H), 4.77 – 4.55 (m, 1H), 4.37 (dt, J = 9.3, 2.5 Hz, 1H), 3.59 (s, 3H), 3.52 (s, 3H), 3.42 – 3.36 (m, 2H), 3.39 (s, 3H), 3.35 (d, J = 6.5 Hz, 3H), 3.08 (s, 3H), 2.74 – 2.57 (m, 2H), 2.46 – 2.33 (m, 1H), 2.31 – 2.19 (m, 1H), 1.84 (d, J = 7.3 Hz, 3H), 1.76 – 1.64 (m, 1H), 1.58 (t, J = 7.6 Hz, 3H), 1.35 (s, 9H), −1.77 – −2.11 (m, 2H); MS (MALDI-TOF) m/z 735.561 [M+H]+, calcd. for C42H51N6O6 735.387
152-Ethylenediaminyl(boc)-131-ethylenediaminylchlorin e6 methyl ester (26)
Ethylenediaminyl(boc) pheophorbide-a (25, 80 mg, 0.109 mmol) was dissolved in toluene (10 mL) and ethylene diamine (30 mg, 0.5 mmol) was added. The reaction mixture was heated at 40 °C overnight. Progress was monitored by TLC and UV-Vis spectrometry. After the reaction was complete by TLC, the solvent was removed and the residue was dissolved in CH2Cl2 and washed with 5% aqueous citric acid to remove excess amine, followed by a wash with brine. The organic phase was dried over anhydrous Na2SO4 and the solvent was evaporated. The residue was dissolved in 5% methanol/CH2Cl2 and eluted from a silica gel column with the same mobile phase. Then the methanol percentage of the mobile phase was gradually increased up to 20% to elute the pure product from the column. The solvent was evaporated and the residue was re-dissolved in 5% acetone/CH2Cl2 and filtered to remove silica from the sample. After evaporation of the solvent pure 152-ethylenediaminyl(boc)-131-ethylenediaminylchlorin e6 methyl ester was obtained (26, C44H58N8O6, 55 mg, 0.069 mmol, 64%); UV-Vis (acetone): λmax (ε/mM−1cm−1) 663 nm (55), 607 (2.6), 528 (1.3), 500 (12), 399 (170); 1H NMR (acetone-d6, 400 MHz) δ 9.74 (s, 1H), 9.73 (s, 1H), 9.13 (s, 1H), 8.36 (br. s, 1H), 8.23 (dd, J = 17.8, 11.5 Hz, 1H), 7.46 (br. s, 1H), 6.64 (br. s, 1H), 6.40 (dd, J = 17.9, 1.5 Hz, 1H), 6.12 (dd, J = 11.7, 1.5 Hz, 1H), 5.55 (d, J = 18.0 Hz, 1H), 5.09 (d, J = 9.3 Hz, 1H), 4.76 – 4.51 (m, 2H), 4.28 (s, 1H), 4.01 – 3.83 (m, 1H), 3.80 – 3.67 (m, 4H), 3.58 (s, 3H), 3.54 (s, 3H), 3.52 (s, 3H), 3.29 (s, 3H), 3.25 – 3.16 (m, 2H), 3.08 (dd, J = 9.1, 4.9 Hz, 1H), 2.78 – 2.64 (m, 1H), 2.43 – 2.21 (m, 2H), 2.00 (d, J = 8.0 Hz, 1H), 1.76 (d, J = 7.1 Hz, 3H), 1.68 (t, J = 7.6 Hz, 3H), 1.20 (s, 9H), 0.97 (m, 3H), −1.66 (s, 1H), −2.02 (s, 1H); MS (MALDI-TOF) m/z 795.651 [M+H]+, calcd. for C44H59N8O6 795.456
131,152-Diethyleneaminylaspartylchlorin e6 di(tert)butyl di(boc) methyl ester (27)
152-Ethylenediaminyl(boc)-131-ethylenediaminylchlorin e6 methyl ester (26, 55 mg, 0.069 mmol) was dissolved in 3 mL of dry CH2Cl2 in an ice bath under argon and 1 m of TFA was added. Then the reaction mixture was allowed to stir overnight. The mixture was rotavaporated several times with diethyl ether to remove residual TFA. The residue was dissolved in water/acetonitrile mixture and freeze dried. Without any further purification the crude product was taken to the next step. (23, C39H50N8O4, 42 mg, 0.06 mmol, 88%), [MS (MALDI-TOF) m/z 695.445 [M+H]+,calcd. for C39H51N8O4 695.403]. (Boc)Asp(tBu)OH (70 mg, 0.24 mmol) was dissolved in dry DMF (5 mL). A mixture of HOBt (32 mg, 0.24 mmol), TBTU (77 mg, 0.24 mmol) and DIEA (0.05 ml, 0. mmol) in DMF (3 mL) was added and the mixture was allowed to stir for 1 h. 131,152-Diethylenediaminylchlorin e6 methyl ester (23, 42 mg, 0.060 mmol) was added to the reaction mixture and stirring was continued for 48 h. The mixture was diluted with CH2Cl2 and then washed with 5% aqueous citric acid, followed by washes with brine and water. The organic layer was dried over anhydrous Na2SO4 and the solvent was evaporated. The residue was dissolved in 6% MeOH/CH2Cl2 and purified via silica gel column chromatography using the same mobile phase to afford 131,152-diethyleneaminyl-diaspartylchlorin e6 di(tert)butyl di(boc) methyl ester (27, C65H92N10O14, 30 mg, 0.024 mmol, 40%); UV-Vis (acetone): λmax (ε/mM−1cm−1) 663 nm (48), 607 (2.1), 528 (1.7), 500 (11), 399 (150); 1H NMR (chloroform-d, 400 MHz) δ 9.68 (s, 1H), 9.61 (s, 1H), 8.79 (s, 1H), 8.07 (dd, J = 17.9, 11.5 Hz, 1H), 7.97 (d, J = 5.7 Hz, 1H), 7.74 (s, 1H), 6.34 (d, J = 17.8 Hz, 1H), 6.13 (d, J = 11.5 Hz, 1H), 5.81 (d, J = 8.3 Hz, 1H), 5.47 (d, J = 18.1 Hz, 1H), 5.28 – 4.88 (m, 2H), 4.67 – 4.30 (m, 3H), 4.10 – 3.68 (m, 10H), 3.65 – 3.12 (m, 5H), 3.54 (s, 3H), 3.48 (s, 3H), 3.31 (s, 3H), 2.93 – 2.69 (m, 2H), 2.67 – 2.53 (m, 1H), 2.23 (m, 3H), 2.04 (s, 1H), 1.90 – 1.55 (m, 7H), 1.31 (s, 18H), 1.14 (s, 18H), 0.94 – 0.80 (m, 1H), −1.62 (s, 1H), −1.80 (s, 1H); MS (MALDI-TOF) m/z 1237.651 [M+H]+, calcd. for C65H93N10O14 1237.687.
131,152-Diethyleneaminyl-diaspartylchlorin e6 methyl ester (28)
131,152-Diethyleneaminyl-diaspartylchlorin e6 di(tert)butyl di(boc) methyl ester (27, 30 mg, 0.024 mmol) was dissolved in 2 mL of dry CH2Cl2 in an ice bath under argon. TFA (1 mL) was added and the reaction mixture was allowed to stir overnight. The reaction mixture was rotavaporated several times with diethyl ether to remove residual TFA. The residue was washed with CH2Cl2 several times. The final product was dissolved in water and freeze dried to obtain 131-152-diethyleneaminyl-diaspartylchlorin e6 methyl ester (28, C47H60N10O10, 16 mg, 0.016 mmol, 70%). UV-Vis (MeOH): λmax (ε/mM−1cm−1) 661 nm (74.7), 606 (8.4), 527 (6.2), 500 (20.5), 400 (190.2); 1H NMR (methanol-d4, 400 MHz) δ 10.18 (s, 1H), 10.03 (s, 1H), 9.47 (s, 1H), 8.20 (dd, J = 17.7, 11.6 Hz, 1H), 6.39 (d, J = 17.8 Hz, 1H), 6.29 (d, J = 11.5 Hz, 1H), 5.59 (d, J = 18.7 Hz, 1H), 5.42 (d, J = 18.7 Hz, 1H), 4.79 (d, J = 7.2 Hz, 1H), 4.54 (d, J = 10.8 Hz, 1H), 4.44 (ddd, J = 24.1, 8.5, 4.2 Hz, 1H), 4.25 – 4.05 (m, 1H), 4.03 – 3.83 (m, 4H), 3.74 (s, 3H), 3.67 (s, 3H), 3.56 (s, 3H), 3.49 (td, J = 10.6, 9.5, 4.6 Hz, 2H), 3.41 – 3.26 (m, 6H), 3.38 (s, 3H), 3.23 – 3.00 (m, 2H), 2.99 – 2.82 (m, 2H), 2.70 (dd, J = 17.9, 9.0 Hz, 1H), 2.59 – 2.47 (m, 1H), 2.46 – 2.31 (m, 1H), 1.91 – 1.51 (m, 3H), 1.83 (d, J = 7.1 Hz, 3H), 1.68 (t, J = 7.3 Hz, 3H), 1.29 (m, 3H); 13C NMR (MeOD, 101 MHz) δ 174.42, 173.97, 173.75, 172.86, 171.55, 171.39, 169.05, 168.72, 168.05, 143.07, 142.13, 141.77, 139.03, 138.76, 137.66, 136.98, 135.23, 134.58, 132.81, 131.33, 130.49, 128.33, 123.54, 105.19, 99.20, 97.43, 97.06, 53.90, 51.08, 49.96, 49.13, 39.50, 39.30, 39.19, 38.70, 34.67, 34.46, 30.47, 29.65, 29.30, 22.83, 22.37, 18.95, 15.79, 11.06, 10.88, 9.85. MS (MALDI-TOF) m/z 947.559 [M+Na]+, calcd. for C47H60N10NaO10 947.439.
β-Alanylpheophorbide a tert-butyl methyl ester (29)
Methyl pheophorbide a (21, 100 mg, 0.165 mmol) was dissolved in dry toluene (10 mL) and the mixture was heated to 100 °C under nitrogen. Then β-alanine(tBu).HCl (45 mg, 0.25 mmol) and DIEA (0.06 ml, 0.33 mmol) were added. The reaction mixture was allowed to stir overnight at 100 °C in an oil bath and was monitored by TLC. Then the solvent was removed and the residue was dissolved in CH2Cl2 and washed with 5% aqueous citric acid followed by water and with brine. The organic layer was dried over anhydrous Na2SO4 and the solvent was evaporated. The residue was dissolved in 5% methanol/CH2Cl2 and purified via silica gel chromatography with the same mobile phase and to afford β-alanylpheoporbide a tert-butyl methyl ester (29, C42H49N5O6, 65 mg, 0.090 mmol, 54%); UV-Vis (DCM): λmax (ε/mM−1cm−1) 667 nm (45), 609 (6.5), 535 (7.6), 505 (10.5), 412 (107); 1H NMR (acetone-d6, 400 MHz) δ 9.33, 9.23* (s, 1H), 8.99, 9.93* (s, 1H), 8.79, 8.77* (s, 1H), 7.97*, 7.92 (t, J = 6.0 Hz, 1H), 7.80 (m, 1H), 6.16, 6.13* (s, 1H), 6.07 (d, J = 9.7 Hz, 1H), 5.99 (dd, J = 11.6, 1.5 Hz, 1H), 4.65*, 4.57 (qd, J = 7.4, 2.0 Hz, 1H), 4.38 (tt, J = 9.7, 2.2 Hz, 1H), 3.68 (qd, J = 6.6, 2.3 Hz, 1H), 3.61 (q, J=6.4, 1H) 3.59*, 3.53 (s, 3H), 3.49 (s, 3H), 3.41 (s, 2H), 3.28*, 3.27 (s, 3H), 2.86*, 2.84 (s, 3H), 2.79 (m, 2H), 2.71 – 2.52 (m, 2H), 2.49 – 2.12 (m, 2H), 1.85 (d, J = 7.3 Hz, 3H), 1.64 (t, J = 7.3 Hz, 3H), 1.48*, 1.49 (s, 9H), −2.00 (s, 1H), −2.16 (s 1H).(* Minor 132 epimer); MS (MALDI-TOF) m/z 720.467 [M+H]+, calcd. for C42H50N5O6 720.368.
β-Alanylaspartylpheoporbide a di-tertbutyl methyl ester (31)
β-Alanylpheophorbide a tert-butyl methyl ester (29, 65 mg, 0.090 mmol) was dissolved in 2 mL of dry CH2Cl2 in an ice bath under argon. TFA (1 mL) was added and the reaction mixture was allowed to stir for 6 h. The reaction mixture was diluted with CH2Cl2 and washed with water and then with saturated sodium bicarbonate. This formed a precipitate while washing with sodium bicarbonate then citric acid solution was added until the precipitate dissolved in the organic phase. Then the solution was washed with brine and dried over anhydrous Na2SO4 to give β-alanylpheoporbide a methyl ester (30, C38H41N5O6, 60 mg, 0.09 mmol, 100%). [MS (MALDI-TOF) m/z 686.387 [M+H]+, calcd. for C38H41N5NaO6 686.295]. β-Alanylpheoporbide a methyl ester (30, 60 mg, 0.090 mmol) was dissolved in dry DMF (5 mL). A mixture of HOBt (18 mg, 0.135 mmol), TBTU (43 mg, 0.135) and DIEA (0.03 ml, 0.18 mmol) in DMF (3 mL) was added and the mixture was allowed to stir for 30 min. Then a mixture of aspartic acid di(tert)butyl ester hydrochloride (101 mg, 0.36 mmol) and DIEA (0.06 ml, 0.36 mmol) in CH2Cl2 (3 mL) was added to the reaction mixture. The mixture was allowed to stir for 24 h. The mixture was diluted with CH2Cl2 and then washed with 5% aqueous citric acid, followed by with water and brine. The organic layer was dried over anhydrous Na2SO4 and the solvent was evaporated. The residue was dissolved in 5% methanol/CH2Cl2 and purified via silica column chromatography with the same mobile phase to afford β-alanyl aspartyl pheophorbide a di-tertbutyl methyl ester (31, C50H62N6O9, 48 mg, 0.053 mmol, 59%); UV-Vis (DCM): λmax (ε/mM−1cm−1) 667 nm (41.5), 609 (5.5), 535 (7), 505 (10), 413 (103); 1H NMR (acetone-d6, 400 MHz) δ 9.06, 8.98* (s, 1H), 8.70, 8.59* (s, 1H), 8.69 (s, 1H), 8.2* 8.06 (t, J = 5.9 Hz, 1H), 7.70 – 7.45 (m, 2H), 6.17, 6.08* (s, 1H), 5.93 (dt, J = 17.7, 2.3 Hz, 1H), 5.85 (dd, J = 11.5, 1.4 Hz, 1H), 4.82*, 4.76 (dt, J = 8.3, 5.8 Hz, 1H), 4.64*, 4.55 (tt, J = 9.0, 4.5 Hz, 1H), 4.42*, 4.35 (dt, J = 9.7, 2.5 Hz, 1H), 3.77 (q, J = 6.5 Hz, 2H), 3.63*, 3.59 (s, 3H), 3.42, 3.31* (s, 3H), 3.14 (s, 3H), 3.05 (h, J = 6.8, 6.3 Hz, 3H), 2.78 (dd, J = 5.9, 4.7 Hz, 2H), 2.74 – 2.66 (m, 4H), 2.63 (s, 3H), 2.49 – 2.37 (m, 1H), 2.20 (td, J = 9.0, 3.3 Hz, 1H), 1.85, 1.65* (d, J = 7.3 Hz, 3H), 1.45 (s, 9H), 1.42 (s, 9H), 1.35 (t, J = 7.5 Hz, 2H), −2.17*, −2.33 (s, 2H). ).(* Minor 132 epimer); MS (MALDI-TOF) m/z 890.461 [M]+, calcd. for C50H63N6O9 890.458.
131-Ethylenediaminyl-152-β-alanylaspartylchlorin e6 di-tert-butyl methyl ester (32)
β-Alanylaspartylpeoporbide a di-tert-butyl methyl ester (31, 48 mg, 0.053 mmol) was dissolved in toluene (10 ml) and ethylenediamine (15 mg, 0.26 mmol) was added. The reaction mixture was heated at 40 °C for 24-36 h. It was monitored by TLC and UV-Vis spectroscopy. After reaction was complete as monitored by TLC, the solvent was removed and the residue was dissolved in CH2Cl2 and washed with 5% aqueous citric acid to remove excess amine, followed by a wash with brine. The organic phase was dried over anhydrous Na2SO4 and the solvent was evaporated. The residue was dissolved in 5% methanol/CH2Cl2 and chromatographed on a silica gel column with the same mobile phase. Then the methanol percentage of the mobile phase was gradually increased up to 20% to elute the pure product from the column. The solvent was evaporated and re-dissolved in 5% acetone/CH2Cl2 and filtered to remove silica from the sample. After evaporation of solvent 131-ethylenediaminyl-152-β-alanyl aspartylchlorin e6 di-tert-butyl methyl ester was obtained (32, C52H70N8O9, 30 mg, 0.031 mmol, 58 %). UV-Vis (acetone): λmax (ε/mM−1cm−1) 664 nm (51.5), 607 (2.5), 528 (2), 500 (11), 400 (160); It was not possible to obtain the 1H NMR spectrum and so the crude product was subjected directly to next step. MS (MALDI-TOF) m/z 951.767 [M+H]+, calcd. for C52H71N8O9 951.534.
131-Ethylenediaminyllysinyl-152-β-alanylaspartylchlorin e6 methyl ester (33) via 132-ethylenediaminyllysinyl-152-β-alanylaspartylchlorin e6 di-tert-butyl di-(boc) methyl ester
Boc-Lys(Boc)OH.DCHA (61 mg, 0.116 mmol) was dissolved in dry DMF (5 mL). A mixture of HOBt (16 mg, 0.116 mmol), TBTU (37 mg, 0.116 mmol) and DIEA (0.024 ml, 0.14 mmol) in DMF (3 mL) was added and the mixture was stirred for 30 min. 131-Ethylenediaminyl-152-β-alanylaspartylchlorin e6 di-tertbutyl methyl ester (32, 30 mg, 0.031 mmol) was added to the reaction mixture and it was stirred for 72 h. After the reaction was deemed complete by TLC, the mixture was diluted with CH2Cl2 and then washed with 10% sodium bicarbonate, 5% aqueous citric acid, then followed by washing with brine. The organic phase was dried over anhydrous Na2SO4 and the solvent was evaporated. The residue was dissolved in 10% MeOH/CH2Cl2 and purified via silica gel column chromatography using the same mobile phase to afford 131-ethylenediaminyllysinyl-152-β-alanylaspartylchlorin e6 di-tert-butyl di-tert-butyl carbamates methyl ester (C68H98N10O14, 24 mg, 0.018 mmol, 61%); UV-Vis (acetone): λmax (ε/mM−1cm−1) 663 nm (43.5), 607 (3), 528 (2.5), 500 (10), 400 (136); 1H NMR (acetone-d6, 400 MHz) δ 9.67 (s, 1H), 9.65 (s, 1H), 9.10 (s, 1H), 8.71 (s, 1H), 8.26 (d, J = 5.5 Hz, 1H), 8.17 (dd, J = 17.8, 11.5 Hz, 1H), 7.05 (s, 2H), 6.34 (d, J = 17.8 Hz, 1H), 6.23 (d, J = 8.0 Hz, 1H), 6.07 (d, J = 11.5 Hz, 1H), 5.95 (s, 1H), 5.56 (d, J = 18.9 Hz, 1H), 5.22 (d, J = 19.2 Hz, 1H), 4.68 (q, J = 7.1 Hz, 1H), 4.54 (d, J = 9.6 Hz, 1H), 4.29 (q, J = 3.6 Hz, 1H), 3.98 – 3.62 (m, 9H), 3.57 (s, 3H), 3.48 (s, 6H), 3.25 (s, 3H), 3.02 (d, J = 6.0 Hz, 2H), 2.92 (s, 3H), 2.56 – 2.40 (m, 2H), 2.40 – 2.26 (m, 2H), 2.26 – 2.17 (m, 1H), 1.91 (dt, J = 23.0, 8.6 Hz, 2H), 1.74 (d, J = 7.1 Hz, 3H), 1.66 (t, J = 7.2 Hz, 3H), 1.57 – 1.44 (m, 4H), 1.37 (s, 18H), 1.34 (s, 18H), −1.65 (s, 1H), −1.94 (s, 1H); MS (MALDI-TOF) m/z 1279.775 [M+H]+, calcd. for C68H99N10O14 1279.734.
The abovementioned 131-ethylenediaminyllysinyl-152-β-alanylaspartylchlorin e6 di-tertbutyl di-(boc) methyl ester (24 mg, 0.018 mmol) was dissolved in 2 mL of dry CH2Cl2 in an ice bath under argon. TFA (1 mL) was added and the reaction mixture was allowed to stir overnight. The mixture was rotavaporated several times with diethyl ether to remove TFA and the residue was washed with CH2Cl2 several times. The final product was dissolved in water and then freeze dried to obtain 131-ethylenediaminyllysinyl-152-β-alanylaspartylchlorin e6 methyl ester (33, C50H66N10O10, 14 mg, 0.014 mmol; 79%). UV-Vis (MeOH): λmax (ε/mM−1cm−1) 658 nm (18.9), 635 (67.8), 594 (14), 511 (10), 411 (150.8); 1H NMR (400 MHz, MeOD) δ 9.96 (s, 1H), 9.70 (s, 1H), 9.42 (s, 1H), 7.98 (s, 2H), 6.39 – 6.01 (m, 3H), 5.57 (d, J = 18.5 Hz, 1H), 5.44 (d, J = 18.5 Hz, 1H), 4.80 (d, J = 6.3 Hz, 2H), 4.60 (d, J = 8.7 Hz, 1H), 4.36 (s, 1H), 4.17 (s, 1H), 4.01 (s, 1H), 3.95 – 3.79 (m, 3H), 3, 3.70 (s, 6H), 3.60 (s, 3H), 3.47 (s, 3H), 3.08 (m, 3H), 3.03 (m, 3H), 2.86 (d, J = 7.1 Hz, 1H), 2.78 (s, 1H), 2.60-2.32 (m, 5H), 2.21 – 1.98 (m, 4H), 1.83 (m, 3H), 1.67 (t, J = 6.7 Hz, 3H), 1.56 (br. s, 4H) 1.31 (m, 2H). 13C NMR (MeOD, 101 MHz) δ 174.30, 173.45, 172.41, 171.99, 169.49, 169.40, 169.24, 143.12, 141.31, 138.38, 138.21, 136.42, 134.93, 134.49, 132.42, 130.83, 130.21, 128.13, 123.05, 121.13, 118.21, 115.30, 112.39, 109.05, 104.86, 99.38, 96.88, 53.87, 53.19, 51.07, 49.15, 48.73, 39.86, 39.15, 38.98, 37.50, 36.18, 35.26, 34.82, 30.76, 30.52, 29.83, 26.79, 22.43, 21.66, 18.72, 15.78, 11.06, 10.83, 9.62. MS (MALDI-TOF) m/z 967.602 [M+H]+, calcd. for C50H67N10O10 967.504
Cell Studies
All reagents used in these studies were purchased from Invitrogen. The human HEp2 cells were obtained from ATCC and maintained in a 50:50 mixture of DMEM:AMEM supplemented with 5% FBS and 1% penicillin/streptomycin antibiotic. The cells were sub-cultured twice weekly to maintain sub-confluent stocks.
Time-Dependent Uptake
The HEp2 cells were plated at 7500 cells per well in a Costar 96-well plate and allowed to grow for 48 h. Compound stock solutions were prepared at 32 mM in DMSO and Cremophor (10% of Cremophor in DMSO). Further dilution into the cells of the 96-well plate gave a final concentration of 400 μM with maximum DMSO concentration of 1.95% and Cremophor concentration of 0.05%. Uptake was allowed to continue for 0, 1, 2, 4, 8, 12 and 24 h. The uptake was terminated by removing the loading medium and washing the wells once with PBS. The compound concentration was measured using intrinsic fluorescence as measured with a BMG FLUOstar plate reader equipped with a 355 nm excitation and a 650 nm emission filter. The cells were measured using a CyQuant Cell proliferation assay as per manufacturer’s instructions.
Cytotoxicity
The HEp2 cells were plated as described above for the uptake experiment. The compounds were diluted into media to give 400 μM solution concentrations. Two-fold serial dilutions were then prepared and the cells were incubated overnight. Cell toxicity was measured using Promega’s Cell Titer Blue viability assay47 as per manufacturer’s instructions, with untreated cells considered 100% viable and cells treated with 0.2% saponin as 0% viable.
The cells were prepared as described above with compound concentration range from 0-100 μM. After loading overnight, the medium was replaced with medium containing 50 mM HEPES pH 7.2. The cells were exposed to a 1000 W halogen lamp filtered through a 610 nm liquid filter to provide approximately 1.5 J/cm2 light dose. The cells were kept cool by filtering the IR radiation through 10 mm of water and placing the culture in an Echotherm chilling plate (Torrey Pines Scientific, Inc.). After 20 min exposure to light, the plate was incubated overnight. Cell viability was then measured as described above.
In comparison with the previously investigated mono-amino acid derivatives of chlorin e6,27 the di-amino acid conjugates appear to be less phototoxic than the 131- and the 152-aspartate derivatives (IC50 = 0.6 and 4.0 uM at 1 J/cm2), probably due to the higher hydrophilicity of the di(amino acid) conjugates.
Microscopy
The Hep2 cells were plated in a 6-well plate and allowed to grow overnight. The cells were exposed to 10 μM of each compound, and then kept at 37 °C for 6 h in 5% CO2 before adding the organelle tracer. The working concentrations of organelle tracers were as follows: LysoSensor Green 50 nM, MitoTracker Green 250 nM, ER Tracker Blue/white 100 nM, and BODIPY FL C5 Ceramide 50 nM. The organelle tracers were diluted in growing medium and the cells were incubated concurrently with the compound and the tracer for 30 min. The loading medium was removed and cells were washed with PBS 3 times before imaging. The images were acquired using a Leica DMRXA2 microscope with a water immersion objective and DAPI, GFP, and TRITC filter cubes (Chroma Technologies).
Conclusions
Previous work27 has shown 131-monoconjugates of chlorin e6 to be more potent PDT sensitizers (with regard to dark- and photo-toxicity) than the corresponding 152-monoconjugates. In the present work a series of chlorin e6 di(amino acid) conjugates were regioselectively synthesized bearing two aspartates in the 131,173- and 152,173-positions, or at the 131,152 via an ethylene diamine linker. One conjugate bearing two different amino acids, lysine at 131 via an ethylene diamine linker and an aspartate at 152 via a β-alanine linker was also synthesized. The conjugation of aspartate residues via the α-amine groups gave anionic compounds while conjugation via the carboxylate gave a zwitterionic compound. The cytotoxicity and uptake of four di(amino acid) chlorin e6 conjugates, two anionic and two zwitterionic, were investigated in human HEp2 cells, and compared with chlorin e6. The most cytotoxic, IC50 (dark) = 65-70 μM and IC50 (light) = 9-11 μM, were the zwitterionic 131,152-disubstituted conjugates 28 and 33; these were also the most taken up by cells and localized in multiple organelles. On the other hand, the tetra-anionic 131,173- and 152,173-di-aspartyl chlorin e6 12 and 13 showed low dark cytoxicity and lower phototoxicity compared with chlorin e6. These di(amino acid) derivatives of chlorin e6 appear to be less phototoxic than previously investigated 131- and 152-mono aspartate chlorin e6 derivatives,27 maybe due to their increased hydrophilicity.
Supplementary Material
Figure 5.
Subcellular localization of 152,173-di(aspartate) chlorin e6 13 in HEp2 cells at 10 μM for 6h, (a) phase contrast, (b) overlay of 13 and phase contrast, (c) ER Tracker Blue, (d) overlay of 13 and ER Tracker Blue, (e) BODIPY Ceramide, (f) overlay of 13 and BODIPY Ceramide, (g) MitoTracker Green, (h) overlay of 13 and MitoTracker Green, (i) LysoSensor Green, (j) overlay of 13 and LysoSensor Green. Scale bar: 10 μm.
Figure 6.
Subcellular localization of 152,173-di(aspartate) chlorin e6 28 in HEp2 cells at 10 μM for 6h, (a) phase contrast, (b) overlay of 28 and phase contrast, (c) ER Tracker Blue, (d) overlay of 28 and ER Tracker Blue, (e) BODIPY Ceramide, (f) overlay of 28 and BODIPY Ceramide, (g) MitoTracker Green, (h) overlay of 28 and MitoTracker Green, (i) LysoSensor Green, (j) overlay of 28 and LysoSensor Green. Scale bar: 10 μm.
Acknowledgements
This research was supported by the following grants from the US National Institutes of Health, R01 CA132861 (KMS) and R01 CA179902 (MGHV).
Footnotes
Electronic Supplementary Information (ESI) available: Copies of 1H NMR spectra, 13C NMR spectra, dark toxicity curves, and subcellular localizations for chlorin e6 (21 pages). See DOI: 10.1039/x0xx00000x
Notes and references
- 1.Dougherty TJ, Gomer CJ, Henderson BW, Jori G, Kessel D, Korbelik M, Moan J, Peng Q. J. Natl. Cancer Inst. 1998;90:889. doi: 10.1093/jnci/90.12.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hahn SM, Glatstein E. Rev. Contemp. Pharm. 1999;10:69. [Google Scholar]
- 3.Pandey RK. J. Porphyrins Phthalocyanines. 2000;4:368. [Google Scholar]
- 4.Brown SB, Brown EA, Walker I. Lancet Oncol. 2004;5:497. doi: 10.1016/S1470-2045(04)01529-3. [DOI] [PubMed] [Google Scholar]
- 5.Josefsen LB, Boyle RW. Theranostics. 2012;2:916. doi: 10.7150/thno.4571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huang Z. Tech. Cancer Res. Treatment. 2005;4:283. doi: 10.1177/153303460500400308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kostenicha GA, Zhuravkina IN, Zhavrid EA. J. Photochem. Photobiol. B: Biol. 1994;22:211. doi: 10.1016/1011-1344(93)06974-8. [DOI] [PubMed] [Google Scholar]
- 8.Allison RR, Downie GH, Cuenca R, Hu X-H, Sibata CH, Childs CJ. Photodiagnosis and Photodynamic Therapy. 2004;1:27. doi: 10.1016/S1572-1000(04)00007-9. [DOI] [PubMed] [Google Scholar]
- 9.Kessel D. Photochem. Photobiol. 2008;49:447. doi: 10.1111/j.1751-1097.1989.tb09193.x. [DOI] [PubMed] [Google Scholar]
- 10.Sunar U, Rohrbach D, Rigual N, Tracy E, Keymel K, Cooper MT, Baumann H, Henderson BW. Optics Express. 2010;18:14969. doi: 10.1364/OE.18.014969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.(a) Usuda J, Ichinose S, Ishizumi T, Hayashi H, Ohtani K, Maehara S, Ono S, Kajiwara N, Uchida O, Tsutsui H. J. Thoracic Oncology. 2010;5:62. doi: 10.1097/JTO.0b013e3181c42287. [DOI] [PubMed] [Google Scholar]; (b) Usuda J, Ichinose S, Ishizumi T, Hayashi H, Ohtani K, Maehara S, Ono S, Honda H, Kajiwara N, Uchida O. Clin. Cancer Res. 2010;16:2198. doi: 10.1158/1078-0432.CCR-09-2520. [DOI] [PubMed] [Google Scholar]
- 12.Wang S, Bromley E, Xu L, Chen JC, Keltner L. Expert Opinion on Pharmacotherapy. 2010;11:133. doi: 10.1517/14656560903463893. [DOI] [PubMed] [Google Scholar]
- 13.Bromley E, Briggs B, Keltner L, Wang S-S. Photodermatology, Photoimmunology Photomedicine. 2011;27:85. doi: 10.1111/j.1600-0781.2011.00573.x. [DOI] [PubMed] [Google Scholar]
- 14.Akhlynina TV, Jans DA, Rosenkranz AA, Statsyuk NV, Balashova IY, Toth G, Pavo I, Rubin AB, Sobolev AS. J. Biol. Chem. 1997;272:20328. doi: 10.1074/jbc.272.33.20328. [DOI] [PubMed] [Google Scholar]
- 15.Soukos NS, Hamblin MR, Hasan T. Photochem. Photobiol. 1997;65:723. doi: 10.1111/j.1751-1097.1997.tb01916.x. [DOI] [PubMed] [Google Scholar]
- 16.Schmidt-Erfurth U, Diddens H, Birngruber R, Hasan T. Brit. J. Cancer. 1997;75:54. doi: 10.1038/bjc.1997.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bisland SK, Singh D, Garieépy J. Bioconjugate Chem. 1999;10:982. doi: 10.1021/bc990020u. [DOI] [PubMed] [Google Scholar]
- 18.Uzdensky AB, Dergacheva OY, Zhavoronkova AA, Reshetnikov AV, Ponomarev GV. Life Sciences. 2004;74:2185. doi: 10.1016/j.lfs.2003.09.053. [DOI] [PubMed] [Google Scholar]
- 19.Kimani S, Ghosh G, Ghogare A, Rudshteyn B, Bartusik D, Hasan T, Greer A. J. Org. Chem. 2012;77:10638. doi: 10.1021/jo301889s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zheng X, Morgan J, Pandey RS, Chen Y, Tracy E, Baumann H, Missert JR, Batt C, Jackson J, Bellnier DA, Henderson BW, Pandey RK. J. Med. Chem. 2009;52:4306. doi: 10.1021/jm9001617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Josefsen LB, Boyle RW. Br. J. Pharmacol. 2008;154:4. doi: 10.1038/bjp.2008.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Giuntini F, Alonso CMA, Boyle RW. Photochem. Photobiol. Sci. 2011;10:759. doi: 10.1039/c0pp00366b. [DOI] [PubMed] [Google Scholar]
- 23.Gomi S, Bnishizuka T, Ushiroda O, Uchida N, Takahashi H, Sumi S. Heterocycles. 1998;48:2231. [Google Scholar]
- 24.Hargus JA, Fronczek FR, Vicente MGH, Smith KM. Photochem. Photobiol. 2007;83:1006. doi: 10.1111/j.1751-1097.2007.00092.x. [DOI] [PubMed] [Google Scholar]
- 25.Chen H, Jinadasa RGW, Jiao L, Fronczek FR, Nguyen AL, Smith KM. Eur. J. Org. Chem. 2015:3661. doi: 10.1002/ejoc.201500478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Roberts WG, Shiau F-Y, Nelson JS, Smith KM, Berns MW. J. Natl. Cancer Inst. 1988;80:330. doi: 10.1093/jnci/80.5.330. [DOI] [PubMed] [Google Scholar]
- 27.Jinadasa RGW, Hu X, Vicente MGH, Smith KM. J. Med. Chem. 2011;54:7464. doi: 10.1021/jm2005139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Park H, Na K. Biomaterials. 2013;34:6992. doi: 10.1016/j.biomaterials.2013.05.070. [DOI] [PubMed] [Google Scholar]
- 29.Pegaz B, Debefve E, Borle F, Ballini JP, Wagnieres G, Spaniol S, Albrecht V, Scheglmann D, Nifantiev NE, van den Bergh H, Konan YN. Photochem. Photobiol. 2005;81:1505. doi: 10.1562/2005-02-23-RA-448. [DOI] [PubMed] [Google Scholar]
- 30.Hamblin MR, Miller JL, Rizvi I, Ortel B, Maytin EV, Hasan T. Cancer Res. 2001;61:7155. [PubMed] [Google Scholar]
- 31.Kuimova MK, Collins HA, Balaz M, Dahlstedt E, Levitt JA, Sergent N, Suhling K, Drobizhev M, Makarov N, Rebane A, Anderson HJ, Phillips D. Org. Biomol. Chem. 2009;7:889. doi: 10.1039/b814791d. [DOI] [PubMed] [Google Scholar]
- 32.Smith KM, Goff DA, Simpson DJ. J. Am. Chem. Soc. 1985;107:4946. [Google Scholar]
- 33.Wasielewski MR, Svec WA. J . Org. Chem. 1980;45:1969. [Google Scholar]
- 34.Ol’shevskaya VA, Savchenko AN, Zaitsev AV, Kononova EG, Petrovskii PV, Ramonova AA, Tatarskiy VV, Jr, Uvarov OV, Moisenovich MM, Kalinin VN, Shtil AA. J. Organomet. Chem. 2009;694:1632. [Google Scholar]
- 35.Meseguer B, Alonso-Díaz D, Griebenow N, Herget T, Waldmann H. Chem. Eur. J. 2000;6:3943. doi: 10.1002/1521-3765(20001103)6:21<3943::aid-chem3943>3.3.co;2-k. H. [DOI] [PubMed] [Google Scholar]
- 36.Smith KM, Lewis WM. Tetrahedron. 1981;37(Supplement 1):399. [Google Scholar]
- 37.Hudlicky M. J. Org. Chem. 1980;45:5377. [Google Scholar]
- 38.Haavikko R, Kavakka JS, Helaja J. Tetrahedron Lett. 2010;51:714. [Google Scholar]
- 39.Belykh DV, Kopylov EA, Gruzdev IV, Kuchin AV. Russ. J. Org. Chem. 2010;46:577. [Google Scholar]
- 40.Dugin NO, Zavialova MG, Novikov RA, Timofeev VP, Misharin AY, Ponomarev GV. Macroheterocycles. 2012;5:146. [Google Scholar]
- 41.Shinoda S, Osuka A. Tetrahedron Lett. 1996;37:4945. [Google Scholar]
- 42.Cossy J, Thellend A. Synthesis. 1989:753. A. [Google Scholar]
- 43.Garcia MJ, Rebolledo F, Gotor V. Tetrahedron Lett. 1993;34:6141. [Google Scholar]
- 44.Labelle M, Gravel D. Chem. Commun. 1985:105. [Google Scholar]
- 45.Janas T, Nowotarski K, Janas T. Biochim. Biophys. Acta. 2011;1808:2322. doi: 10.1016/j.bbamem.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 46.Kessel D. J. Porphyrins Phthalocyanines. 2004;8:1009. doi: 10.1142/s1088424608000273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang JH, Chung TD, Oldenburg KR. J. Biomol. Screen. 1999;4:67. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.