Abstract
Objective
Several studies have shown decreased insulin clearance rate (ICR) in individuals with obesity, but it remains unclear whether this is predominately due to obesity-associated insulin resistance (IR) or obesity itself. We aimed to clarify the complex interrelationship that exists between obesity, IR and ICR.
Methods
Healthy volunteers (n = 277) had measurement of IR and ICR using the insulin suppression test (IST). IR was quantified by determining the steady-state plasma glucose (SSPG) during the IST. ICR was estimated by dividing the insulin infusion rate by the steady-state plasma insulin concentration. We performed our analysis by stratifying the experimental population into 4 dichotomous categories, varying in obesity and IR. Obesity was defined as a body mass index (BMI) ≥ 30 kg/m2, and IR was defined as SSPG ≥ 150 mg/dl.
Results
Individuals with obesity had higher fasting insulin compared with individuals without obesity, regardless of IR. ICR was similar between individuals with and without obesity but was higher in IR individuals compared with insulin sensitive individuals. In multivariate analysis, both fasting insulin and SSPG were significantly associated with ICR. No significant relationships were observed between BMI and ICR.
Conclusions
Reduced ICR in obesity is secondary to IR, not excess adiposity.
Keywords: Insulin clearance, insulin resistance, obesity, hyperinsulinemia
Introduction
There is general agreement (1–3) that hyperinsulinemia in individuals with obesity is related to increases in insulin secretion and decreases in insulin clearance rate (ICR). Individuals with obesity tend to be insulin resistant, and there is substantial evidence that insulin resistance is also associated with an increase in insulin secretion and a decrease in ICR (1–3). What remains controversial is whether increased insulin secretion and decreased ICR in individuals with obesity is primarily a function of their insulin resistance, as compared to the view that obesity, per se, independent of insulin resistance, can decrease insulin clearance (1–7).
The aim of this study was to take a different approach in an effort to clarify the complex interrelationship that exists between obesity, insulin resistance and ICR. Specifically, the majority of prior studies used linear associations to dissect the degree to which these 3 metabolic variables were related. We began our analysis by stratifying the experimental population into 4 dichotomous categories, varying in adiposity and insulin sensitivity. In addition, the insulin suppression test (IST) was used to obtain a direct measurement of insulin-mediated glucose disposal in all participants (8–11). Since octreotide is infused during the IST to suppress endogenous insulin secretion, this approach should also provide a more precise estimate of ICR.
Methods
Subjects
Experimental data were obtained from 126 males and 151 females in our ongoing registry who responded to newspaper advertisements describing studies related to the effects of insulin resistance on glucose and insulin metabolism. Subjects ranged from 19–69 years of age, were overweight or obese according to body mass index ([BMI]: 25–40 kg/m2), and were of a non-Hispanic white race/ethnicity. Individuals were recruited between 2003 and 2013. Nondiabetic status of the subjects was determined based on the following criteria: no known medical history of diabetes, no use of medications known to affect carbohydrate metabolism, and fasting glucose levels < 126 mg/dL. All individuals also had no history of coronary artery, kidney, or liver disease, and all underwent the insulin suppression test to quantify insulin resistance and ICR. All subjects provided written informed consent approved by the Stanford Intuitional Review Board prior to participating in any experimental procedures.
Experimental Endpoints
Insulin suppression test (IST)
Whole body insulin action was directly measured using a modified version (8) of the IST (9); the values for insulin resistance obtained with this approach are highly correlated (r ~ 0.9) with those obtained using the hyperinsulinemic-euglycemic clamp technique (10, 11). Briefly, after an overnight fast, an intravenous catheter was placed in each arm of the subjects; one catheter was used to administer a 180-min infusion of octreotide (0.27 Hg/m2/min), insulin (32 mU/m2/min), and glucose (267 mg/m2/min), while the other catheter was used for the collection of blood samples. Blood samples were drawn at 10-min intervals during the last 30 minutes to measure the steady-state plasma glucose (SSPG) and steady-state plasma insulin (SSPI) concentrations. Since SSPI concentrations are similar in all subjects, the SSPG concentration provides a direct and specific measure of insulin-mediated glucose disposal; the higher the SSPG concentration, the greater the insulin resistance.
Measurement of ICR
ICR (units; L/min/m2) was estimated by dividing the insulin infusion rate by the SSPI concentration. Insulin determinations were made with the ultrasensitive insulin assay [Cat#33410] on the Access 2 immunoassay system (Beckman coulter), and had an inter-assay CV of 6.43 and an intra-assay CV of 5.61. The glomerular filtration rate (GFR) was calculated using the abbreviated Modification of Diet in Renal Disease formula: estimated GFR (eGFR) = 186.3 × SCR −1.154 × age−0.203 (or × 0.742 if female), where SCR is serum creatinine expressed in milligrams per deciliter.
Definition of insulin resistance and obesity
Insulin resistance was defined as an SSPG concentration ≥ 150 mg/dL; a cut-point shown in prospective studies to identity apparently healthy individuals who developed clinical syndromes related to insulin resistance (12, 13). Body mass index (BMI) was used to classify individuals as obese (BMI ≥ 30 kg/m2) or non-obese (BMI< 30 kg/m2). With these criteria, participants were placed into 4 experimental groups: non-obese/ insulin-sensitive; obese/ insulin-sensitive; non-obese/ insulin-resistant; and obese/ insulin-resistant.
Statistical analysis
All data are presented as mean ± standard deviations (SD) unless stated otherwise. If necessary, a logarithmic transformation was performed to achieve a normal distribution. The Chi squared (χ2) and independent t-tests were used to compare the proportions and means, respectively, between the groups. Pearson’s correlation coefficients between ICR and experimental variables were calculated. Multiple linear regression models were used to identify factors associated with ICR. Potential predictors of ICR evaluated were age, sex, BMI or waist circumference (WC), eGFR, alanine aminotransferase (ALT), and fasting plasma insulin. eGFR and ALT were added as surrogates of kidney and liver function, respectively, since both organs play vital roles in insulin clearance (14). All data were analyzed using the SPSS statistical package (SPSS Inc.; Chicago, IL, USA). P value < 0.05 was considered to indicate statistical significance and was not adjusted for multiple comparisons.
Results
Anthropometric and metabolic characteristics of the 4 experimental groups are presented in Table 1. By selection SSPG concentrations are increased approximately 2-fold in both of the insulin resistant groups. However, SSPG concentrations do not vary as a function of differences in obesity in either the insulin resistant or insulin sensitive subgroups. Focusing initially on the insulin sensitive groups, the subgroup with obesity had significantly higher values for BMI, WC, and fasting plasma insulin concentration. However, the values for ICR were essentially identical in the groups with and without obesity who were insulin sensitive. In the insulin resistant groups, fasting insulin concentration was also higher in the obese subgroup but ICR was not different between the groups with and without obesity. ALT was significantly higher in the group with obesity.
Table 1.
Anthropometric and biochemical characteristics of the study subjects stratified according to the insulin sensitivity and obesity.
| Insulin Sensitive | Insulin Resistant | |||||
|---|---|---|---|---|---|---|
| Non-obese | Obese | P-value† | Non-obese | Obese | P-value† | |
| N | 97 | 42 | 45 | 93 | ||
| SSPG (mg/dL) | 95.6±25.5 | 109.7±26.6 | 0.093 | 206.5±40.1 | 214.3±39.4 | 0.279 |
| Age (yrs) | 52.3±7.9 | 53.3±8.4 | 0.536 | 53.0±10.0 | 52.6±9.1 | 0.903 |
| Sex (men) | 42 (43.3%) | 20 (47.6%) | 0.588 | 17 (37.8%) | 47 (50.5%) | 0.178 |
| BMI (kg/m2) | 27.5 ±1.4 | 32.6±1.8 | <0.001 | 27.8±1.5 | 33.8±2.6 | <0.001 |
| Waist Circumference (cm) | 95.3±6.6 | 109.0±8.3 | <0.001 | 96.9±6.5 | 109.6±8.5 | <0.001 |
| ALT (IU/L) | 29.4±11.8 | 30.7±11.9 | 0.499 | 30.3±14.5 | 38.0±17.7 | 0.019 |
| eGFR (mL/min/1.73m2) | 95.3±20.0 | 95.8±21.7 | 0.971 | 103.1±21.9 | 93.3±24.0 | 0.072 |
| Fasting Glucose (mg/dL) | 95.7 ±10.1 | 97.6±10.0 | 0.203 | 102.0±10.1 | 101.6±9.6 | 0.817 |
| Fasting Insulin (µU/mL) | 8.27±7.58 | 9.82±5.83 | 0.036 | 14.0±8.37 | 17.99±9.46 | 0.016 |
| Insulin Clearance (L/min/m2) | 0.498±0.131 | 0.509±0.136 | 0.865 | 0.445±0.104 | 0.407±0.156 | 0.145 |
Data are means ± standard deviations
P-values are for comparisons between the non-obese and obese groups
SSPG, steady-state plasma glucose; BMI, body mass index; ALT, alanine aminotransferase; eGFR, estimated glomerular filtration rate
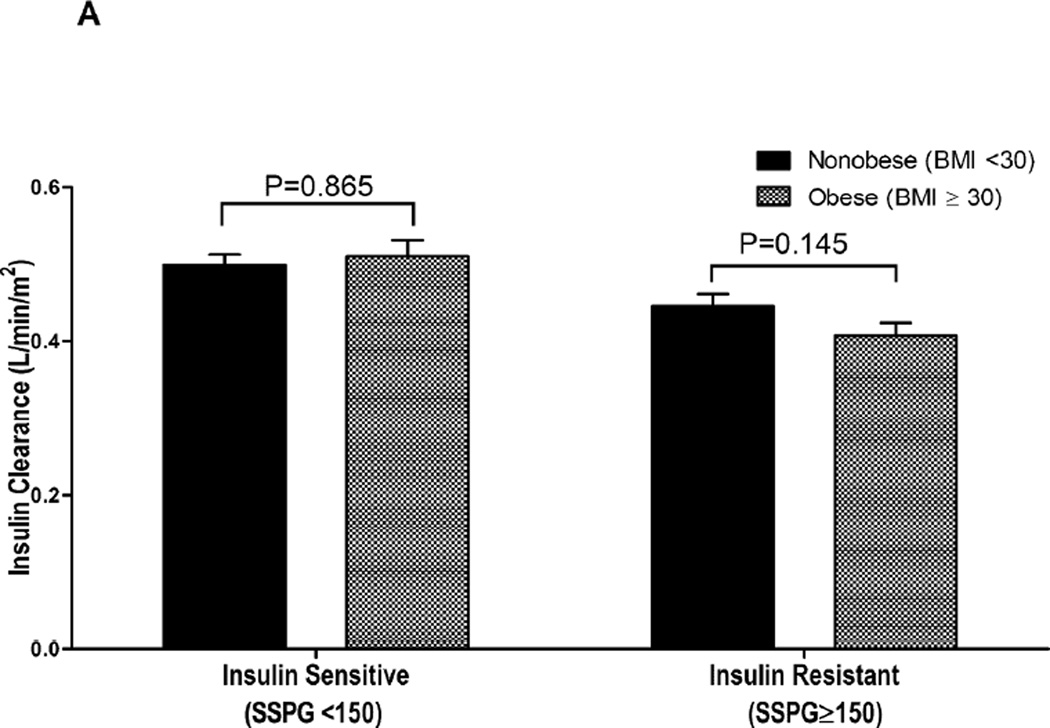
Differences in the impact of obesity (BMI) vs. insulin resistance (SSPG concentration) on ICR and fasting plasma insulin concentration are illustrated in Fig. 1. The results in Fig.1A compare the impact of obesity (BMI) and insulin resistance (SSPG concentration) on ICR. These data demonstrate that ICR values are lower in those who are insulin resistant, whether they are obese on nonobese, but there is no effect of obesity on ICR in either insulin sensitive or insulin resistant individuals. In contrast, Figure 1B demonstrates that fasting insulin concentrations are significantly higher in individuals with obesity, irrespective of their being insulin sensitive or insulin resistant.
Figure 1.
Differences in insulin clearance rate (A) and fasting plasma insulin concentration (B) among non-obese/insulin-sensitive (BMI < 30 kg/m2 and SSPG < 150 mg/dL; n = 97), obese/insulin-sensitive (n=42), non-obese/insulin-resistant (n=45) and obese/insulin-resistant (BMI ≥ 30 kg/m2 and SSPG≥ 150 mg/dL; n=93) groups defined by insulin sensitivity and obesity. *log-transformed fasting insulin
Table 2 presents the univariate and multivariate relationships between ICR and possible modulators of its activity. At the univariate level, every factor other than age was significantly correlated with ICR. However, when adjusted for other relevant covariates, only the relationships between ICR and concentrations of both fasting plasma insulin and SSPG remained statistically significant. It should be emphasized that there was no significant relationship between BMI and ICR, and the same findings were seen when WC was substituted for BMI. We also conducted a simplified regression analysis with only SSPG and BMI as independent variables and ICR as the dependent variable. SSPG was significant (standardized beta coefficient [β] = −0.437, P<0.001) while BMI remained insignificant (β = −0.037, P=0.543).
Table 2.
Univariate and multivariate linear regression analyses of insulin clearance with anthropometric and biochemical parameters
| Univariate analysis | Multivariate analysis | |||
|---|---|---|---|---|
| β | P-value | β | P-value | |
| Age | 0.016 | 0.793 | 0.019 | 0.744 |
| Sex (women vs. men) | −0.177 | 0.003 | −0.133 | 0.059 |
| BMI | −0.233 | <0.001 | −0.012 | 0.836 |
| ALT | −0.142 | 0.020 | 0.033 | 0.563 |
| eGFR | 0.132 | 0.031 | 0.073 | 0.270 |
| Fasting glucose | −0.142 | 0.019 | 0.082 | 0.171 |
| Fasting insulin | −0.490 | <0.001 | −0.301 | <0.001 |
| SSPG | −0.454 | <0.001 | −0.312 | <0.001 |
Beta, standardized beta coefficient; BMI, body mass index; ALT, alanine aminotransferase; eGFR, estimated glomerular filtration rate; SSPG, steady-state plasma glucose
Discussion
At the simplest level, the results presented provide straight-forward answers to some of the issues raised in the Introduction. Thus, the results demonstrated that increases in SSPG concentration, a direct measure of insulin resistance at the whole body level, and increases in plasma insulin concentration were independently related to decreases in ICR. As such, our findings are consistent with previous descriptions of this relationship (2, 15). A second major question addressed in this study was whether obesity was also independently related to increases in plasma insulin concentration and decreases in ICR. In this instance the findings are more complicated. Plasma insulin concentrations were significantly higher in individuals with obesity, whether they were insulin sensitive or insulin resistant (Fig. 1B), However, ICR did not vary as a function of obesity in either the insulin resistant or insulin sensitive subgroups (Fig.1 A), and results of the multiple linear regression analysis (Table 2) displays the lack of an independent relationship between adiposity and ICR.
The relationship among obesity, plasma insulin concentration, and ICR demonstrated in our study raises the following question: in the absence of an effect on ICR, why were plasma insulin concentrations elevated in individuals with obesity? As indicated earlier, there is evidence that obesity is associated with both an increase in insulin secretion and a decrease in ICR (1–7). We did not measure insulin secretion, but since our findings suggest that obesity, per se, did not decrease ICR, the increase in plasma insulin concentration must have been due to an obesity-associated increase in insulin secretion. Although a logical conclusion, hyperinsulinemia in obesity is conventionally assumed to result from increased insulin secretion in an attempt to compensate for obesity-associated insulin resistance (1–3), and we found plasma insulin concentrations to be higher in persons with obesity, whether they were insulin resistant or insulin sensitive. A possible solution to this conundrum can be found in the findings (1) of the European Group for the Study of Insulin Resistance (EGIR). In addition to describing an independent relationship between insulin resistance and insulin secretion, these authors found that degree of adiposity also had an independent relationship with increases in insulin secretion. Therefore, elevated plasma insulin concentration in obesity, irrespective of insulin resistance status and ICR, may relate to enhanced insulin secretion rate.
The second question relates to our inability to replicate the finding of other investigators that obesity, per se, was independently related to a decrease in ICR (4–7). Since these studies differed in overall protocol, we can only speculate upon the potential reasons for disparate findings. For example, Erdmann et al. (4) reported a weight-dependent decrease in ICR in 291 individuals, stratified into 5 BMI groups. In addition, insulin resistance as estimated by HOMA-IR was also greater with increasing BMI. Since the decrease in ICR was not analyzed in the context of the increase in insulin resistance, these results do not necessarily support the notion that obesity, per se, decreases ICR. Marini and colleagues (7) attempted to avoid the potentially confounding issue of insulin resistance by comparing ICR in 3 groups: non-obese/insulin sensitive; obese/insulin sensitive; and obese/insulin resistant. ICR was significantly decreased in only the obese/insulin resistant group. These findings demonstrate that persons with obesity can differ in terms of both insulin resistance and ICR. However, the absence of a non-obese/insulin resistant group does not seem to permit a definitive conclusion as to whether obesity or insulin resistance was responsible for the association with a decrease in ICR.
Dealing with the differences between our findings and those of the IRAS Family Study (5) and the Insulin Resistance Atherosclerosis Study (6) is more complicated. To begin with the methods of assessing insulin resistance (insulin suppression test vs. the frequently sampled intravenous glucose tolerance test, FSIVGTT) and insulin concentration (fasting plasma insulin vs acute insulin response to intravenous glucose) were quite different. Perhaps of greater importance were differences in how ICR was calculated. Our calculations involved dividing the exogenous insulin infusion rate by the steady-state plasma insulin concentration during a constant infusion of glucose and insulin, while endogenous insulin was suppressed. In contrast, the IRAS investigators calculated ICR as the ratio of the dose of an acute injection of insulin over the incremental area under the curve of insulin from 20 min to infinity (5, 6). ICR has been shown to be overestimated when insulin is given as a bolus compared with a constant infusion (16). In addition, impact of endogenous insulin secretion rate on ICR is ignored in most calculations, which may explain the greater variability in ICR with the FSIVGTT (5, 6) as well as the clamp (7). It is obvious that we can only speculate as to how these differences might affect the measurement of ICR. Perhaps more to the point, although the IRAS investigators concluded that both obesity and insulin resistance were independent predictors of ICR in African Americans and Hispanics (5.6), only insulin resistance was an independent predictor in non-Hispanic whites (6). Thus, at least in the case of non-Hispanic whites, our findings and those of the IRAS investigators are in agreement; insulin resistance is an independent predictor of ICR, but obesity is not.
Although the results of our study seem straight-forward, interpretation of our findings is limited to some extent by the nature of the experimental protocol. Most importantly, since we used a cross-sectional design, caution must be exercised in differentiating causal relationships from associations. In addition, participants were non-Hispanic whites, and the present findings cannot be extrapolated to other racial groups. We also were not able to explore additional factors that could modulate ICR such as body composition (17), liver fat content (18, 19), physical fitness (20), and glucose tolerance (21) beyond fasting glucose. Genetic mutations in the insulin receptor gene have also been associated with insulin resistance and decreased ICR, independent of obesity (22). While we could not account for all variables that modulate ICR, our experimental protocol had certain strengths; including a relatively large number of participants, divided into 4 dichotomous groups, use of specific methods to quantify insulin sensitivity and insulin clearance, and the exclusion of individuals with conditions that could affect glucose metabolism.
In conclusion, our results add further evidence that differences in ICR play an important role in regulation of plasma insulin concentration (1–7). In addition, they provide additional support for the notion that decreases in ICR are independently associated with magnitude of insulin resistance, not excess adiposity, in non-Hispanic whites (1,6). However, at least 3 major questions remain concerning the factors that modulate plasma insulin concentration: 1) is the independent relationship between excess adiposity and decreased ICR in African Americans and Hispanics (5, 6) a function of race or methodology; 2) what is responsible for the finding that insulin resistance cannot account for the increased insulin secretion and hyperinsulinemia in non-Hispanic white individuals with obesity (1); and 3) what is the pathophysiological explanation for the association between insulin resistance and ICR. Obviously, answers to these questions will go a long way in our understanding of the association between obesity and hyperinsulinemia—the issue addressed in this study.
What is already known about this subject?
-
●
Individuals with obesity have increased insulin concentrations compared to their lean counterparts.
-
●
Decreased insulin clearance rate (ICR) may explain the hyperinsulinemia in obesity.
-
●
It remains unclear whether decreased ICR is predominately due to obesity-associated insulin resistance or obesity itself.
What does your study add?
-
●
Individuals with obesity have higher fasting insulin concentrations regardless of insulin resistance.
-
●
Obesity, per se, did not decrease ICR.
-
●
Reduced ICR in person with obesity is secondary to insulin resistance, not excess adiposity.
ACKNOWLEDGEMENTS
The authors would like to thank study volunteers and the staff and nurses in the Stanford Clinical and Translational Research Unit for their invaluable assistance with our metabolic studies.
FUNDING: This project was supported in part by an NIH/NCRR CTSA award number UL1 RR025744.
Footnotes
Disclosure: The authors declared no conflict of interest.
References
- 1.Ferrannini E, Natali A, Bell P, Cavallo-Perin P, Lalic N, Mingrone G. Insulin resistance and hypersecretion in obesity. European Group for the Study of Insulin Resistance (EGIR) J Clin Invest. 1997;100:1166–1173. doi: 10.1172/JCI119628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jones CN, Abbasi F, Carantoni M, Polonsky KS, Reaven GM. Roles of insulin resistance and obesity in regulation of plasma insulin concentrations. Am J Physiol Endocrinol Metab. 2000;278:E501–E508. doi: 10.1152/ajpendo.2000.278.3.E501. [DOI] [PubMed] [Google Scholar]
- 3.Goodarzi MO, Cui J, Chen YD, Hsueh WA, Guo X, Rotter JI. Fasting insulin reflects heterogeneous physiological processes: role of insulin clearance. Am J Physiol Endocrinol Metab. 2011;301:E402–E408. doi: 10.1152/ajpendo.00013.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Erdmann J, Mayr M, Oppel U, Sypchenko O, Wagenpfeil S, Schusdziarra V. Weight-dependent differential contribution of insulin secretion and clearance to hyperinsulinemia of obesity. Regul Pept. 2009;152:1–7. doi: 10.1016/j.regpep.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 5.Lee CC, Haffner SM, Wagenknecht LE, Lorenzo C, Norris JM, Bergman RN, et al. Insulin clearance and the incidence of type 2 diabetes in Hispanics and African Americans: the IRAS Family Study. Diabetes care. 2013;36:901–907. doi: 10.2337/dc12-1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lorenzo C, Hanley AJ, Wagenknecht LE, Rewers MJ, Stefanovski D, Goodarzi MO, et al. Relationship of insulin sensitivity, insulin secretion, and adiposity with insulin clearance in a multiethnic population: the insulin Resistance Atherosclerosis study. Diabetes care. 2013;36:101–103. doi: 10.2337/dc12-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marini MA, Frontoni S, Succurro E, Arturi F, Fiorentino TV, Sciacqua A, et al. Differences in insulin clearance between metabolically healthy and unhealthy obese subjects. Acta diabetologica. 2014;51:257–261. doi: 10.1007/s00592-013-0511-9. [DOI] [PubMed] [Google Scholar]
- 8.Pei D, Jones CN, Bhargava R, Chen YD, Reaven GM. Evaluation of octreotide to assess insulin-mediated glucose disposal by the insulin suppression test. Diabetologia. 1994;37:843–845. doi: 10.1007/BF00404344. [DOI] [PubMed] [Google Scholar]
- 9.Shen SW, Reaven GM, Farquhar JW. Comparison of impedance to insulinmediated glucose uptake in normal subjects and in subjects with latent diabetes. J Clin Invest. 1970;49:2151–2160. doi: 10.1172/JCI106433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenfield MS, Doberne L, Kraemer F, Tobey T, Reaven G. Assessment of insulin resistance with the insulin suppression test and the euglycemic clamp. Diabetes. 1981;30:387–392. doi: 10.2337/diab.30.5.387. [DOI] [PubMed] [Google Scholar]
- 11.Knowles JW, Assimes TL, Tsao PS, Natali A, Mari A, Quertermous T, et al. Measurement of insulin-mediated glucose uptake: direct comparison of the modified insulin suppression test and the euglycemic, hyperinsulinemic clamp. Metabolism. 2013;62:548–553. doi: 10.1016/j.metabol.2012.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yip J, Facchini FS, Reaven GM. Resistance to insulin-mediated glucose disposal as a predictor of cardiovascular disease. J Clin Endocrinol Metab. 1998;83:2773–2776. doi: 10.1210/jcem.83.8.5005. [DOI] [PubMed] [Google Scholar]
- 13.Facchini FS, Hua N, Abbasi F, Reaven GM. Insulin resistance as a predictor of age-related diseases. J Clin Endocrinol Metab. 2001;86:3574–3578. doi: 10.1210/jcem.86.8.7763. [DOI] [PubMed] [Google Scholar]
- 14.Ferrannini E, Wahren J, Faber OK, Felig P, Binder C, DeFronzo RA. Splanchnic and renal metabolism of insulin in human subjects: a dose-response study. Am J Physiol. 1983;244:E517–E527. doi: 10.1152/ajpendo.1983.244.6.E517. [DOI] [PubMed] [Google Scholar]
- 15.Jones CN, Pei D, Staris P, Polonsky KS, Chen YD, Reaven GM. Alterations in the glucose-stimulated insulin secretory dose-response curve and in insulin clearance in nondiabetic insulin-resistant individuals. J Clin Endocrinol Metab. 1997;82:1834–1838. doi: 10.1210/jcem.82.6.3979. [DOI] [PubMed] [Google Scholar]
- 16.Polonsky KS, Pugh W, Jaspan JB, Cohen DM, Karrison T, Tager HS, et al. Cpeptide and insulin secretion. Relationship between peripheral concentrations of C-peptide and insulin and their secretion rates in the dog. J Clin Invest. 1984;74:1821–1829. doi: 10.1172/JCI111601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yki-Jarvinen H, Koivisto VA, Karonen SL. Influence of body composition on insulin clearance. Clinical physiology. 1985;5:45–52. doi: 10.1111/j.1475-097x.1985.tb00745.x. [DOI] [PubMed] [Google Scholar]
- 18.Kotronen A, Juurinen L, Tiikkainen M, Vehkavaara S, Yki-Jarvinen H. Increased liver fat, impaired insulin clearance, and hepatic and adipose tissue insulin resistance in type 2 diabetes. Gastroenterology. 2008;135:122–130. doi: 10.1053/j.gastro.2008.03.021. [DOI] [PubMed] [Google Scholar]
- 19.Kotronen A, Vehkavaara S, Seppala-Lindroos A, Bergholm R, Yki-Jarvinen H. Effect of liver fat on insulin clearance. Am J Physiol Endocrinol Metab. 2007;293:E1709–E1715. doi: 10.1152/ajpendo.00444.2007. [DOI] [PubMed] [Google Scholar]
- 20.Ahren B, Thorsson O. Increased insulin sensitivity is associated with reduced insulin and glucagon secretion and increased insulin clearance in man. J Clin Endocrinol Metab. 2003;88:1264–1270. doi: 10.1210/jc.2002-021547. [DOI] [PubMed] [Google Scholar]
- 21.Bonora E, Zavaroni I, Coscelli C, Butturini U. Decreased hepatic insulin extraction in subjects with mild glucose intolerance. Metabolism. 1983;32:438–446. doi: 10.1016/0026-0495(83)90004-5. [DOI] [PubMed] [Google Scholar]
- 22.Hojlund K, Wojtaszewski JF, Birk J, Hansen BF, Vestergaard H, Beck-Nielsen H. Partial rescue of in vivo insulin signalling in skeletal muscle by impaired insulin clearance in heterozygous carriers of a mutation in the insulin receptor gene. Diabetologia. 2006;49:1827–1837. doi: 10.1007/s00125-006-0312-6. [DOI] [PubMed] [Google Scholar]