Abstract
Diphencyprone (DPCP) is a hapten that induces delayed-type hypersensitivity (DTH) reactions. MicroRNAs (miRNAs) are short non-coding RNAs that negatively regulate gene expression, and have been implicated in various inflammatory skin diseases, but their role in DTH reactions is not well understood. We generated global miRNA expression profiles (using next-generation sequencing) of DPCP reactions in skin of 7 healthy volunteers at 3, 14, and 120 days after challenge. Compared to placebo-treated sites, DPCP-challenged skin at 3 days (peak inflammation) had 127 miRNAs significantly deregulated. At 14 days (during resolution of inflammation), 43 miRNAs were deregulated and, at 120 days (when inflammation had completely resolved), 6 miRNAs were upregulated. While some miRNAs have been observed in psoriasis or atopic dermatitis, most of the deregulated miRNAs have not yet been studied in the context of skin biology or immunology. Across the three time points studied, many but not all miRNAs were uniquely expressed. As various miRNAs may influence T cell activation, this may indicate that the miRNAs exclusively expressed at different time points function to promote or resolve skin inflammation, and therefore may inform on the paradoxical ability of DPCP to treat both autoimmune conditions (alopecia areata) and conditions of ineffective immunity (melanoma).
Keywords: microRNA, delayed-type hypersensitivity, next-generation sequencing, diphencyprone
INTRODUCTION
DPCP is a hapten that induces DTH reactions in skin and is used therapeutically for warts (1), melanoma metastases (2), and alopecia areata (3). The mechanisms by which DPCP is able to both increase local immune responses for the treatment of warts and melanoma metastases, yet also decrease pathogenic immunity for the promotion of hair growth in alopecia areata, are incompletely understood. Previous work from our group has demonstrated that human skin responses to DPCP evolve from an inflammatory/effector peak at 3 days post-challenge to a more regulated immune response at 14 days. This study included gene expression and immunohistochemical profiling of biopsies from DPCP-challenged healthy volunteer skin at 3 days (peak reaction), 14 days (actively resolving reaction), and 120 days (4–8 months; fully resolved reaction) (4). However, this work did not examine the potential contribution of microRNAs (miRNAs), whose roles in skin biology and immunology are only recently being characterized (5).
miRNAs are short (19–24 nucleotide) endogenous, non-protein coding RNAs that negatively regulate gene expression at the post-transcriptional level. They do this by sequence-specific base pairing, typically within the 3’-untranslated region of the target mRNA, resulting in transcript cleavage or translational repression. By current estimates, more than 60% of human protein-coding genes contain miRNA target sites (6), and are therefore potentially regulated by miRNAs either physiologically or in disease. Since individual miRNAs target multiple, functionally related (as opposed to single) genes, they are of great interest from a therapeutic perspective (7). The potential of modulating miRNA activity in a dermatological disease was recently demonstrated by Guinea-Viniegra et al., who showed that inhibition of miR-21 leads to amelioration of psoriasis pathology (8).
Despite a number of studies on the involvement of miRNAs in inflammatory skin diseases such as psoriasis, previous work on the role of miRNAs in DTH inflammatory reactions is limited. Vennegaard et al. have previously shown by microarray and PCR approaches that miRs-21, -142-3p, -142-5p, and -223 are significantly upregulated in DPCP-challenged human skin as well as mouse skin challenged with a similar hapten, dinitrofluorobenzene (9). However, this study only examined peak reactions and therefore does not inform on potential immunoregulatory mechanims during a resolving DTH reaction. Here, we obtain comprehensive miRNA expression profiles of DPCP challenge reactions at three different time points via deep sequencing to better understand mRNA regulation in DPCP responses over time.
METHODS
Study subjects and skin samples
Skin biopsies were obtained from 7 volunteers under a protocol approved by The Rockefeller University’s Institutional Review Board. Written, informed consent was obtained from all subjects and the study adhered to the Declaration of Helsinki Principles. Each volunteer was sensitized to DPCP (in a topical gel formulation) and then two weeks later, two challenge applications of DPCP as well as two placebo applications (identical formulation but without DPCP) were applied. Six mm full thickness punch biopsies were taken of DPCP-treated and placebo-treated sites both at 3 days and 14 days after challenge, and a further biopsy of a DPCP-treated site was taken 120 days after challenge, as described (4). Normal skin biopsies were taken from two healthy volunteers enrolled in a separate study that also was approved by The Rockefeller University’s Institutional Review Board, with written, informed consent and adherence to the Declaration of Helsinki Principles.
RNA extraction and quantification
Total RNA was extracted using the miRNeasy Mini Kit (Qiagen, Valencia, CA) according to the manufacturer’s protocol with on-column DNase digestion. The amount of RNA was assessed by NanoDrop 1000 spectrophotometer (Thermo Fisher Scientific Inc., Wilmington, DE). The quality of extracted RNA was examined using Agilent Bioanalyzer 2100 (Agilent Technologies, Palo Alto, CA).
Barcoded small RNA sequencing
Barcoded small RNA sequencing was performed using a modified version of an established protocol (10). Briefly, 100 ng total RNA from each sample was subjected to 3’ and 5’ adapter ligation and RT-PCR amplification. The resulting cDNA library was sequenced using Illumina sequencing technology (Illumina, Inc., San Diego, CA). The bioinformatic analysis of the small RNA sequence data was performed as described previously (11). Barcodes were used to mark individual samples so that up to 20 could be processed simultaneously (12).
Bioinformatic and statistical analysis
Sequence data were imported into the R working environment (www.r-project.org) and analysed using the edgeR package from Bioconductor (http://bioconductor.org). edgeR supports differential expression analysis and is based on a negative binomial distribution. It adjusts any differential expression analysis for varying sequencing depths as represented by differing library sizes (13). Only human miRNAs complying with a threshold of 100 sequence reads per miRNA in a minimum of 50% of the samples within each group were included in the further analysis. Unsupervised principal component analysis (PCA) and Pearson’s correlation analysis were applied to explore the association between the samples. False discovery rate < 0.05 was considered as statistically significant.
qRT-PCR analysis
Individual qRT-PCR assays were used to validate the expression levels of miR-21, -7, -503 and -383 using the TaqMan MicroRNA Reverse Transcription Kit (Applied Biosystems, Carlsbad, CA) followed by PCR using TaqMan MicroRNA assays (Applied Biosystems). Samples were analyzed using the 7900HT sequence detection system (Applied Biosystems) according to the manufacturer’s directions. The qRT-PCR data were processed using the ΔΔCT method in order to obtain expression fold changes (14), with RNU6B used as the reference gene.
RESULTS
To define miRNA expression profiles of DPCP reactions, we sensitized seven healthy volunteers to topical DPCP. Two weeks later, we applied challenge doses of DPCP and topical placebo gel to corresponding normal skin sites, so that each subject served as his or her own control. Punch biopsies were taken of placebo-treated skin as well as DPCP-treated sites at three different time points post-challenge: 3 days, 14 days, and 120 days, as previously described (4). Following RNA extraction and quality control, comprehensive miRNA expression profiles were generated through barcoded small RNA sequencing.
Our previous work has demonstrated that skin reactions to DPCP peak at 3 days by clinical scoring (of erythema and induration) and immune activation marker expression (including IFNγ, IL-2, IL-2RA, IL-13, IL-9, IL-17A, and IL-22), suggesting presence of all major defined T cell subsets (Th1, Th2, Th9, Th17, and Th22). Yet, T cell and dendritic cell infiltrates persist through 14 days post-challenge, and resolve by 120 days post-challenge, thus suggesting the involvement of active negative regulatory mechanisms to suppress T cell activation in this DTH reaction (4). The potential contributions of miRNAs to this immunoregulation were not explored in this prior study.
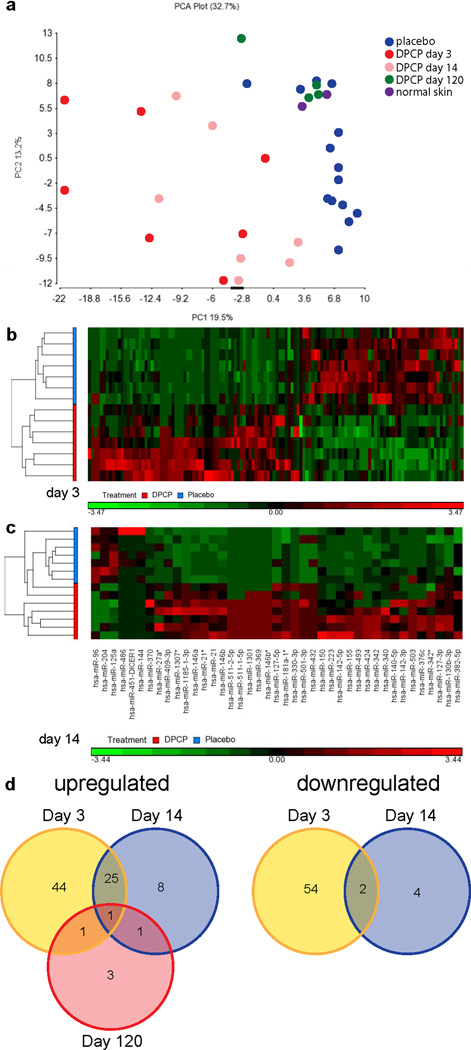
By unsupervised principal component analysis (PCA), there was no miRNA separation due to gender, age, or race (volunteer demographics and clinical scoring of DPCP-induced inflammation provided in Table 1). DPCP challenge biopsies, both at 3 days and 14 days, were clearly separated from placebo-treated skin, with the day 3 samples having a more distinct miRNA expression profile. The DPCP challenge biopsies taken at day 120 clustered with placebo-treated samples. Both of these sample types also clustered with normal skin samples taken from a separate study, therefore confirming that placebo-treated skin and DPCP challenge biopsies taken at 120 days (both of which clinically appear normal) are similar to normal skin (Figure 1a). This was further supported by a sample correlation matrix analysis, which showed a strong correlation across the normal appearing skin samples compared with DPCP days 3 and 14 samples (Figure S1). In fact, there were no significantly deregulated miRNAs between normal skin samples and placebo-treated skin. Compared to placebo-treated skin, we found 127 significantly deregulated miRNAs at day 3 after DPCP challenge and 43 at day 14 after challenge (Figure 1b and c). Table 2 lists the top 10 upregulated miRNAs at days 3 and 14 (full miRNA counts and read frequency lists in Tables S1 and S2, raw data deposited in Gene Expression Omnibus accession number pending). There were 6 significantly deregulated miRNAs at day 120 (all upregulated, fold changes in parentheses): miR-193a-3p (4.01), -136-5p (3.79), -377 (2.67), -140-5p (2.29), -376c (2.19), -17 (2.12).
Table 1.
Demographics and clinical scoring of inflammatory reactions induced by DPCP in all subjects (n=7)
| Subject ID | Gender | Age | Race | day 31 | day 141 | day 3 score | day 14 score |
|---|---|---|---|---|---|---|---|
| 012 | M | 44 | White | 3/2 | 2/1 | 5 | 3 |
| 013 | F | 46 | Black | 3/3 | 2/1 | 6 | 3 |
| 014 | M | 20 | Asian | 3/3 | 2/0 | 6 | 2 |
| 015 | M | 55 | Black | 2/2 | 1/0 | 4 | 1 |
| 016 | F | 29 | Black | 3/1 | 1/0 | 4 | 1 |
| 020 | M | 43 | Black | 3/3 | 1/1 | 6 | 2 |
| 021 | M | 40 | Black | 4/4 | 2/2 | 8 | 4 |
| Average | 5.6 | 2.3 |
p = 2.6×10−5 for DPCP day 3 vs day 14 score comparison (paired two-tailed Student's t-test).
Erythema/induration (0–4 scale for each) - scores are sums of these two measures.
Figure 1.
(a) Principal component analysis (PCA) of global miRNA data for DPCP-treated skin at times indicated, placebo-treated skin, and normal skin. Heat maps of day 3 (b) and day 14 (c) samples. Ordinate shows individual volunteers treated with placebo or DPCP and abscissa displays individual miRNA expression levels. (d) Venn diagrams showing overlaps of significantly upregulated (left) and downregulated (right) miRNAs at the three time points post-challenge: 3 days, 14 days, and 120 days.
Table 2.
Top 10 upregulated miRNAs in DPCP day 3 and DPCP day 14 samples vs placebo-treated skin
| microRNA | fold change | p-value | FDR | Total RF |
|---|---|---|---|---|
| (a) top upregulated miRNAs at day 3 | ||||
| hsa-miR-223 | 20.87 | 0.00 | 0.00 | 252065 |
| hsa-miR-150 | 15.71 | 0.00 | 0.00 | 519815 |
| hsa-miR-21* | 15.20 | 0.00 | 0.00 | 25877 |
| hsa-miR-142-5p | 14.45 | 0.00 | 0.00 | 224392 |
| hsa-miR-142-3p | 14.26 | 0.00 | 0.00 | 153335 |
| hsa-miR-7-2 | 12.79 | 0.00 | 0.00 | 5941 |
| hsa-miR-7-1 | 12.69 | 0.00 | 0.00 | 6128 |
| hsa-miR-7-3 | 12.69 | 0.00 | 0.00 | 5839 |
| hsa-miR-503 | 9.37 | 0.00 | 0.00 | 6563 |
| hsa-miR-150* | 8.55 | 0.00 | 0.00 | 3935 |
| (b) top upregulated miRNAs at day 14 | ||||
| hsa-miR-21* | 8.08 | 0.00 | 0.00 | 10890 |
| hsa-miR-146b | 5.89 | 0.00 | 0.00 | 195770 |
| hsa-miR-21 | 5.49 | 0.00 | 0.00 | 5895379 |
| hsa-miR-155 | 5.25 | 0.00 | 0.00 | 33337 |
| hsa-miR-142-3p | 4.82 | 0.00 | 0.00 | 99152 |
| hsa-miR-1185-1-3p | 4.68 | 0.00 | 0.00 | 3420 |
| hsa-miR-146b* | 4.04 | 0.00 | 0.00 | 2868 |
| hsa-miR-369 | 3.96 | 0.00 | 0.00 | 2836 |
| hsa-miR-223 | 3.93 | 0.00 | 0.00 | 76363 |
| hsa-miR-142-5p | 3.70 | 0.00 | 0.00 | 134138 |
Abbreviations: FDR, false discovery rate; RF, read frequencies.
Although the lists of top upregulated miRNAs at days 3 and 14 have several miRNAs in common, each of the three time points studied has a unique miRNA profile. For instance, of the 69 upregulated miRNAs at day 3, only 25 are also upregulated at day 14. Figure 1d provides Venn diagrams showing overlaps of significantly deregulated miRNAs at the three time points. One miRNA in particular, miR-140-5p, increased in expression over time, with fold changes increasing from 1.92 to 2.25 to 2.29 from day 3 to 14 to 120. We used qRT-PCR analysis to corroborate selected miRNA expression changes found by deep sequencing. miRNA-21, one of the top upregulated miRNAs at both days 3 and 14, as well as a novel potential therapeutic target in psoriasis (8), was also found to be upregulated at both of these time points by qRT-PCR analysis (fold changes of 1.9 and 5.4, respectively; P<0.005 for both). miR-7 and miR-503, both among the top 10 upregulated miRNAs at day 3, were also validated by qRT-PCR (fold changes of 4.3 and 5.2, respectively; P<0.001 for both), and these miRNAs have not been previously studied in skin inflammation. We also confirmed the top downregulated miRNA at day 3, miR-383 (fold change 0.26; P<0.005), another previously understudied miRNA (Table 3).
Table 3.
qRT-PCR confirmation of selected miRNAs in DPCP day 3 samples vs placebo-treated skin
| microRNA | fold change | p-value1 |
|---|---|---|
| hsa-miR-503 | 5.2 | 0.0009 |
| hsa-miR-7 | 4.3 | 0.0006 |
| hsa-miR-21 | 1.9 | 0.004 |
| hsa-miR-383 | 0.26 | 0.001 |
paired two-tailed Student's t-test
DISCUSSION
Among the top 10 miRNAs we found upregulated at both days 3 and 14, miR-21, miR-142-3p, miR-142-5p, and miR-223 have all previously been found to be significantly upregulated in both human and mouse DTH reactions (9). However, our current study had substantially higher fold changes for the same miRNAs than the study previously published, perhaps in part due to methodological differences (deep sequencing in the current vs microarray in the previous). Deep sequencing not only allows for studies of differential expression, but also facilitates determination of nucleotide variation and discovery of novel miRNAs. These four miRNAs have been shown to be related to T cells and T cell activation, in line with the fact that DTH reactions are mediated by T cells. Furthermore, upregulation of miR-223, miR-142-3p, and miR-142-5p has been reported in atopic dermatitis (15) and in psoriasis (16). miR-21 has been shown to be increased in psoriatic lesional skin, with evidence suggesting a causal role for this miRNA in the disease’s epidermal hyperplasia (8). Despite this, many of the top deregulated miRNAs found in our study have not previously been studied in the context of skin biology or immunology, highlighting the emerging nature of miRNA research. Our qRT-PCR data validated sequencing results both for miRNAs previously examined in the skin, such as miR-21, as well as for miRNAs that, to our knowledge, have never been described in the skin.
Since our study captured miRNA profiles at three different time points representing distinct phases of a DTH reaction (peak, actively resolving, fully resolved), and because these profiles proved to be unique and not simply subsets of one another, they may inform on positive vs negative immune regulation in this human model of a DTH reaction over time. For instance, miR-150, one of the top 10 upregulated miRNAs at day 3 (15.71-fold), was only slightly upregulated at day 14 (2.96-fold). This preferential expression of miR-150 during the peak reaction complements the fact that this miRNA inhibits DTH reactions in mice (17), and therefore may need to be upregulated early on in a DTH reaction to promote resolution of inflammation. Of the 8 miRNAs uniquely upregulated at day 14, little is known about their roles in immunology, but all have been implicated in decreasing cell proliferation by cancer studies (18)(19)(20)(21)(22)(23)(24)(25). As the day 14 reactions are characterized by active resolution and reduced expression of IL-2 (4), it is possible that these miRNAs are related to inhibition of cell proliferation in this context as well. In addition, the unique miRNA expression profiles at different time points may shed light on the paradoxical ability of DPCP to treat conditions of both autoimmunity (alopecia areata) and ineffective immunity (melanoma and warts). These unique miRNA profiles are paralleled by the unique mRNA profiles we found at days 3 and 14 in our previous study. Interestingly, the day 120 DPCP challenge biopsies, which resemble placebo-treated skin by clinical, histological, and gene expression criteria (4), do still have some miRNAs significantly upregulated compared to placebo-treated skin. This may reflect persistent changes long after clinical resolution of induced inflammation in this DTH reaction.
Certain miRNAs could account for previously identified mRNA expression changes, via sequence complementarity between the miRNA and mRNA. Nevertheless, because miRNAs can exert their effects by repressing translation (26), one may not necessarily expect to see lower target mRNA levels for a given upregulated miRNA. Also, the miRNA changes could be due to immune cell infiltration bringing in the miRNAs, and not actual deregulation within a given cell type. It has been previously demonstrated that interactions between miRNAs and gene expression are confounded by leukocyte infiltration (27), which our prior work has shown to be abundant in our samples (4). One way to address this is by the technique of laser capture microdissection, which allows for study of specific regions or cell populations under microscopic visualization, and which has recently has been applied to psoriasis in conjunction with deep sequencing (28). This prior study demonstrated that the top 2 upregulated miRNAs at both days 3 and 14 (miR-223, miR-150, miR-21, and miR-146b) are all significantly upregulated in reticular dermis compared to epidermis, therefore suggesting an immune as opposed to keratinocyte source for these miRNAs. Although laser capture microdissection limits the amount of cell types being examined in a sample, there is still the concern that observed miRNA changes are simply due to infiltrating leukocytes.
Since our study included inflamed biopsies at different time points, we were more directly able to investigate how miRNAs are modulated in leukocytes over time. We have previously shown that DPCP-challenged skin, at both days 3 and 14, contains many infiltrating leukocytes (including CD11c+ dendritic cells and CD3+ T cells), but that markers of all major defined T cell subsets including Th1 cells decrease from day 3 to 14 (4). miRNA-21, which was significantly upregulated at both days 3 and 14, but more so at day 14 (by both sequencing and qRT-PCR), is known to regulate IL-12p35 expression (29) in dendritic cells (30), and this molecule is key to Th1 cell polarization. Therefore, in our samples, the increase in miR-21 at day 3 compared to placebo-treated skin could simply be due to the influx of many dendritic cells expressing this miRNA. On the other hand, we speculate that the increase in miR-21 expression from day 3 to 14 is due to actual upregulation in the infiltrating cells, and could explain the decrease in Th1 polarization that we previously demonstrated, via decreasing IL-12p35 expression (Figure S2). This effect may also be relevant to the therapeutic potential of this miRNA in psoriasis (8), a disease where Th1 cells play an important pathogenic role. Although previous work, including laser capture microdissection, has implicated an immune source for the primary miRNAs we found to be deregulated in this DTH reaction, future studies with in situ hybridization will be required to determine the exact cellular sources of the different miRNAs. Overall, here we provide a human antigen-specific model system to study miRNA regulation of a prototypic cell-mediated immune reaction over time, thus providing a useful starting point for the determination of roles of different miRNAs in positive vs negative immune regulation.
Supplementary Material
ACKNOWLEDGMENTS
We thank William R Levis for helpful comments and discussions on this manuscript. This research was supported by National Institutes of Health (NIH) grant UL1 RR024143 from the National Center for Research Resources and the Milstein Medical Program. NG was supported by a Medical Scientist Training Program grant from the National Institute of General Medical Sciences of the NIH under award number T32GM07739 to the Weill Cornell/Rockefeller/Sloan-Kettering Tri-Institutional MD-PhD Program. The content of this study is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ABBREVIATIONS USED
- DPCP
diphencyprone
- DTH
delayed-type hypersensitivity
- miRNA
microRNA
- PCA
principal component analysis
Footnotes
NG and NR performed the research. NG and JGK designed the research study. KMA, NR, and TT contributed essential reagents or tools. MBL and JRZ analysed the data. NG and JGK wrote the paper.
CONFLICT OF INTEREST: TT is a cofounder and scientific advisor to Alnylam Pharmaceutical and scientific advisor to Regulus Therapeutics.
REFERENCES
- 1.Upitis JA, Krol A. The use of diphenylcyclopropenone in the treatment of recalcitrant warts. J Cutan Med Surg. 2002;6:214–217. doi: 10.1007/s10227-001-0050-9. [DOI] [PubMed] [Google Scholar]
- 2.Damian DL, Saw RPM, Thompson JF. Topical immunotherapy with diphencyprone for in transit and cutaneously metastatic melanoma. J Surg Oncol. 2014;109:308–313. doi: 10.1002/jso.23506. [DOI] [PubMed] [Google Scholar]
- 3.Freyschmidt-Paul P, Happle R, McElwee KJ, et al. Alopecia areata: treatment of today and tomorrow. J Investig Dermatol Symp Proc. 2003;8:12–17. doi: 10.1046/j.1523-1747.2003.12165.x. [DOI] [PubMed] [Google Scholar]
- 4.Gulati N, Suárez-Fariñas M, Fuentes-Duculan J, et al. Molecular characterization of human skin response to diphencyprone at peak and resolution phases: therapeutic insights. J Invest Dermatol. 2014;134:2531–2540. doi: 10.1038/jid.2014.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baltimore D, Boldin MP, O’Connell RM, et al. MicroRNAs: new regulators of immune cell development and function. Nat Immunol. 2008;9:839–845. doi: 10.1038/ni.f.209. [DOI] [PubMed] [Google Scholar]
- 6.Friedman RC, Farh KK-H, Burge CB, et al. Most mammalian mRNAs are conserved targets of microRNAs. Genome Res. 2009;19:92–105. doi: 10.1101/gr.082701.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sayed D, Abdellatif M. MicroRNAs in development and disease. Physiol Rev. 2011;91:827–887. doi: 10.1152/physrev.00006.2010. [DOI] [PubMed] [Google Scholar]
- 8.Guinea-Viniegra J, Jiménez M, Schonthaler HB, et al. Targeting miR-21 to treat psoriasis. Sci Transl Med. 2014;6:225re1. doi: 10.1126/scitranslmed.3008089. [DOI] [PubMed] [Google Scholar]
- 9.Vennegaard MT, Bonefeld CM, Hagedorn PH, et al. Allergic contact dermatitis induces upregulation of identical microRNAs in humans and mice. Contact Dermatitis. 2012;67:298–305. doi: 10.1111/j.1600-0536.2012.02083.x. [DOI] [PubMed] [Google Scholar]
- 10.Hafner M, Landgraf P, Ludwig J, et al. Identification of microRNAs and other small regulatory RNAs using cDNA library sequencing. Methods. 2008;44:3–12. doi: 10.1016/j.ymeth.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farazi TA, Brown M, Morozov P, et al. Bioinformatic analysis of barcoded cDNA libraries for small RNA profiling by next-generation sequencing. Methods. 2012;58:171–187. doi: 10.1016/j.ymeth.2012.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hafner M, Renwick N, Farazi TA, et al. Barcoded cDNA library preparation for small RNA profiling by next-generation sequencing. Methods. 2012;58:164–170. doi: 10.1016/j.ymeth.2012.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinforma. 2010;26:139–140. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- 15.Sonkoly E, Janson P, Majuri M-L, et al. MiR-155 is overexpressed in patients with atopic dermatitis and modulates T-cell proliferative responses by targeting cytotoxic T lymphocyte-associated antigen 4. J Allergy Clin Immunol. 2010;126:581.e1–20–589.e1–20. doi: 10.1016/j.jaci.2010.05.045. [DOI] [PubMed] [Google Scholar]
- 16.Zibert JR, Løvendorf MB, Litman T, et al. MicroRNAs and potential target interactions in psoriasis. J Dermatol Sci. 2010;58:177–185. doi: 10.1016/j.jdermsci.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 17.Bryniarski K, Ptak W, Jayakumar A, et al. Antigen-specific, antibody-coated, exosome-like nanovesicles deliver suppressor T-cell microRNA-150 to effector T cells to inhibit contact sensitivity. J Allergy Clin Immunol. 2013;132:170–181. doi: 10.1016/j.jaci.2013.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jiang H, Jin C, Liu J, et al. Next generation sequencing analysis of miRNAs: MiR-127-3p inhibits glioblastoma proliferation and activates TGF-β signaling by targeting SKI. Omics. 2014;18:196–206. doi: 10.1089/omi.2013.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uppal A, Wightman SC, Mallon S, et al. 14q32-encoded microRNAs mediate an oligometastatic phenotype. Oncotarget. 2015;6:3540–3552. doi: 10.18632/oncotarget.2920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu X, Shi H, Liu B, et al. miR-330-3p controls cell proliferation by targeting early growth response 2 in non-small-cell lung cancer. Acta Biochim Biophys Sin. 2015;47:431–440. doi: 10.1093/abbs/gmv032. [DOI] [PubMed] [Google Scholar]
- 21.Chen X-P, Chen Y-G, Lan J-Y, et al. MicroRNA-370 suppresses proliferation and promotes endometrioid ovarian cancer chemosensitivity to cDDP by negatively regulating ENG. Cancer Lett. 2014;353:201–210. doi: 10.1016/j.canlet.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 22.Li S, Meng H, Zhou F, et al. MicroRNA-132 is frequently down-regulated in ductal carcinoma in situ (DCIS) of breast and acts as a tumor suppressor by inhibiting cell proliferation. Pathol Res Pract. 2013;209:179–183. doi: 10.1016/j.prp.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 23.Li C, Nie H, Wang M, et al. MicroRNA-409-3p regulates cell proliferation and apoptosis by targeting PHF10 in gastric cancer. Cancer Lett. 2012;320:189–197. doi: 10.1016/j.canlet.2012.02.030. [DOI] [PubMed] [Google Scholar]
- 24.Jiang N, Chen W-J, Zhang J-W, et al. Downregulation of miR-432 activates Wnt/β-catenin signaling and promotes human hepatocellular carcinoma proliferation. Oncotarget. 2015;6:7866–7879. doi: 10.18632/oncotarget.3492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gu Y, Cheng Y, Song Y, et al. MicroRNA-493 suppresses tumor growth, invasion and metastasis of lung cancer by regulating E2F1. PloS One. 2014;9:e102602. doi: 10.1371/journal.pone.0102602. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 26.Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010;79:351–379. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- 27.Zhu W, Streicher K, Shen N, et al. Genomic signatures characterize leukocyte infiltration in myositis muscles. BMC Med Genomics. 2012;5:53. doi: 10.1186/1755-8794-5-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Løvendorf MB, Mitsui H, Zibert JR, et al. Laser capture microdissection followed by next-generation sequencing identifies disease-related microRNAs in psoriatic skin that reflect systemic microRNA changes in psoriasis. Exp Dermatol. 2014;24:187–193. doi: 10.1111/exd.12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lu TX, Munitz A, Rothenberg ME. MicroRNA-21 is up-regulated in allergic airway inflammation and regulates IL-12p35 expression. J Immunol. 2009;182:4994–5002. doi: 10.4049/jimmunol.0803560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lu TX, Hartner J, Lim E-J, et al. MicroRNA-21 limits in vivo immune response-mediated activation of the IL-12/IFN-gamma pathway, Th1 polarization, and the severity of delayed-type hypersensitivity. J Immunol. 2011;187:3362–3373. doi: 10.4049/jimmunol.1101235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.