Abstract
Bipolar disorder is a severe and enduring psychiatric condition which in many cases starts during early adulthood and follows a relapsing and remitting course throughout life. In many patients the disease follows a progressive path with brief periods of inter-episode recovery, sub-threshold symptoms, treatment resistance and increasing functional impairment in the biopsychosocial domains. Knowledge about the neurobiology of bipolar disorder is increasing steadily and evidence from several lines of research implicates immuno-inflammatory mechanisms in the brain and periphery in the etiopathogenesis of this illness and its comorbidities. The main findings are an increase in the levels of proinflammatory cytokines during acute episodes with a decrease in neurotrophic support. Related to these factors are glial cell dysfunction, neuro-endocrine abnormalities and neurotransmitter aberrations which together cause plastic changes in the mood regulating areas of the brain and neuroprogression of the bipolar diathesis. Research in the above mentioned areas is providing an opportunity to discover novel biomarkers for the disease and the field is reaching a point where major breakthroughs can be expected in the not too distant future. It is hoped that with new discoveries fresh avenues will be found to better treat an otherwise recalcitrant disease.
Keywords: Bipolar disorder, Inflammation, Biomarkers, Cytokines, Neurotrophins
INTRODUCTION
Bipolar disorder (BD) is a chronic medical condition with the usual onset in adolescence and early adult life.1 It is marked by recurrent mood exacerbations which can be of opposite polarity, ranging from major depressive episodes to manic episodes. In many patients the disease is characterized by a progressively severe course with frequent episodes, incomplete inter-episode recovery, sub-threshold affective symptoms and refractoriness to treatment measures.2 This is accompanied with impairment in biological, psychological and social functioning of the patients. The bipolar diathesis exerts a pervasive effect and causes adverse consequences for all aspect of life. BD has a very high heritability of approximately 80% and the disease represents a complex interplay of genes and environment.3 Life expectancy of bipolar patients is shortened by 10 to 15 years not just because of an increased suicide rate but by the presence of such medical comorbidities as cardiovascular disease, diabetes and other metabolic conditions.4
Knowledge about the neurobiology of bipolar disorder is expanding rapidly and an increasing body of evidence points to the fact that inflammatory processes play a major role in the pathophysiology of this condition.5 The later include aspects of innate and adaptive immunological responses which cause damage to the central nervous system and the peripheral organs. The inflammatory immune responses result in such manifestations as neuroprogression of the disease as well as accelerated atherosclerosis, dyslipidemia, insulin resistance and premature mortality.6
In the past few years several studies have documented increased levels of circulating proinflammatory cytokines in different phases of bipolar disorder.7 These are polypeptide molecules secreted by macrophages, T lymphocytes and endothelial cells and sub serve such diverse functions as activation of neutrophils, proliferation of B cells, synthesis of acute-phase proteins, and increased vascular permeability. Examples of cytokines include interleukins (ILs), tumor necrosis factors (TNFs), interferons (INFs), transforming growth factors (TGFs) and chemokines. Cytokines can gain access to the CNS where they activate microglia which are one of the three types of glial cells, the others being astroglia and oligodendrocytes.8 The microglia act as resident macrophages in the brain and participate in inflammation in response to injury, infection or insult to the CNS. In the acute phase these cells act to contain neuronal damage but if activated chronically as happens in repeated mood episodes of bipolar disorder the homeostatic balance is overwhelmed and there exists a constant inflammatory environment which causes damage to the neurons.9 As a result, there is failure of neuronal function because of decreased synptogenesis, dendritic loss, oxidative stress, mitochondrial dysfunction and ultimate activation of the apoptotic cascade leading to death of the neurons.10
CYTOKINE MARKERS AND PERIPHERAL INFLAMMATION IN BIPOLAR DISORDER
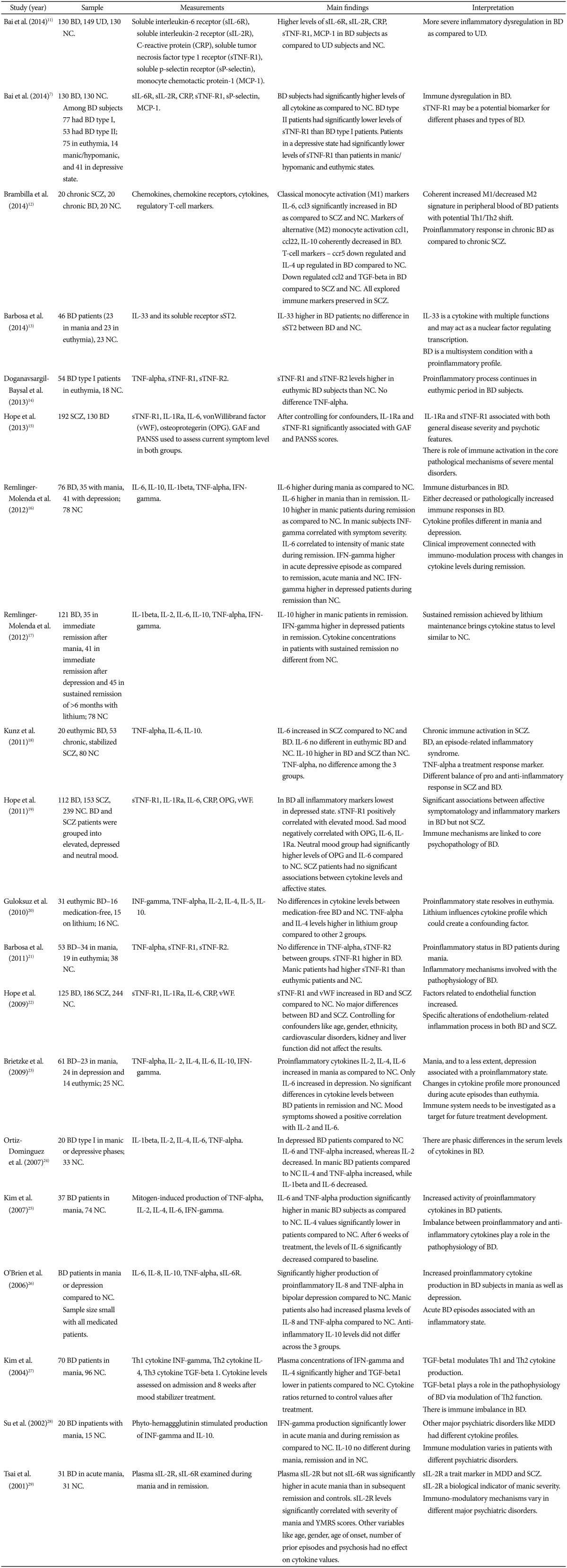
Recent studies have fully demonstrated the association between manic and depressive episodes and a pro-inflammatory state involving both the innate and adaptive immune system. See the table for a detailed overview of immunological abnormalities in bipolar disorder (Table 1). In particular, T-helper cells 1 (Th1) are known to mediate cellular immune reactions and include production of cytokines IL-1, IL-2, IL-6, interferon-gamma, and TNF-alpha. Th2 cells enhance antibody-mediated immune reactions and the production of cytokines IL-4, IL-5, and IL-10 while Th3 cells heighten production of TGF-beta. Elevated levels of TNF-alpha have been reported in manic and depressive episodes in bipolar disorder patients.24 High levels of IL-6 and TNF-alpha have also been reported during mania with IL-6 levels returning to baseline after treatment with mood stabilizers while TNF-alpha continuing to remain high.25 During mania IL-2, IL-6, IL-8 and INF-gamma were the increased pro-inflammatory cytokines, while only IL-6 was found to be increased during depression.23 Moreover bipolar depression was characterized by an altered balance between IL-6 and the anti-inflammatory IL-10.23 Conversely, elevated levels of the anti-inflammatory IL-4 were noticed in bipolar subjects in studies that did not utilize mitogen stimulation.30 IL-4 induces transformation of naïve T-helper cells into Th2 cells and reduces production of Th1 cells and macrophages. As such IL-4 is a key switch regulating the balance between cellular and antibody-based immunity. It might be speculated that IL-4 elevation in bipolar disorder may be of a compensatory nature, to buffer against the increase of proinflammatory cytokines seen in the condition. The findings imply that mania and to a lesser extent bipolar depression, are associated with a pro-inflammatory state.21 Overall, the data suggest that successful treatment with mood stabilizers leading to a euthymic state may reverse inflammation and normalize peripheral levels of inflammatory mediators.20
Table 1. Circulating cytokine abnormalities in bipolar disorder patients.
BD: bipolar disorder, UD: unipolar depression, SCZ: schizophrenia, MDD: major depressive disorder, NC: normal controls, GAF: global assessment of functioning, PANSS: Positive and Negative Syndrome Scale, YMRS: Young Mania Rating Scale, TNF: tumor necrosis factors, TGF: transforming growth factors, IL: interleukins
Elevated IL-6 has been one of the most consistent findings in BD.16 IL-6 is a pleitropic cytokine that is produced at sites of inflammation, secreted by T-cells and macrophages. The role of IL-6 is to stimulate the B and T lymphocytes and hepatocytes to produce acute inflammatory proteins such as high-sensitivity C reactive protein (hs-CRP). Furthermore, it has been suggested that cytokines such as IL-1, IL-6 and TNF-alpha act in a concerted manner augmenting the immune response and help in the elimination of pathogens and the resolution of the inflammatory challenge.31 On the other hand, inflammatory cytokines are a known cause of diminished sensitivity to glucocorticoid and insulin receptors.32 Combined with autonomic disturbance seen in bipolar disorder, increased platelet/endothelial aggregation and unhealthy lifestyle, elevated inflammation may contribute to substantially increased risk of cardiovascular disease.33 The cumulative effect of hypercortisolemia as a result of impaired hypothalamic-pituitary-adrenal (HPA) axis regulation combined with compromised glucocorticoid and insulin receptor activity, aggravated by inflammatory cytokines might explain the high rate of metabolic syndrome, dyslipidemia and diabetes in the bipolar population.34 In addition, increased peripheral inflammation has been associated with numerous symptoms of mood disorders such as malaise, fatigue, anhedonia, impairment of concentration, anxiety, irritability, social disconnection, hopelessness, suicidal ideation, bodily aches, and disturbance in sleep and appetite.35
INFLAMMATORY MEDIATORS AND THE CENTRAL NERVOUS SYSTEM
Peripheral inflammatory signals can gain access to the CNS through several pathways including: 1) areas of the brain not covered by the blood-brain barrier (BBB), such as the circumventricular organ, 2) afferent vagal fibers may convey the peripheral cytokines and other inflammatory mediators to their nuclei, including nucleus tractus solitararius, 3) BBB cells have the ability to import cytokines via active transport, 4) peripheral immune cells such as macrophages, T-lymphocytes and monocytes may gain access to the CNS and release the inflammatory mediators, and 5) BBB cells such as endothelial cells and pericytes can be induced to release inflammatory signals. Elevation of cerebrospinal fluid (CSF) inflammatory cytokines such as IL-1 beta has been substantiated in bipolar patients, especially if they have experienced recent manic episodes, compared with healthy volunteers.36
Inflammatory cytokines activate microglia in the brain causing them to intensify the inflammatory response by releasing reactive oxygen species, reactive nitrogen species, cytokines and chemokines.37 This chemical milieu of oxidative stress and inflammatory signals precipitates a change in astroglial function. Glial indoleamine 2, 3-dioxygenase, a tryptophan metabolizing enzyme is up-regulated, resulting in greater production of neurotoxic kynurenine metabolites and quinolinic acid (QA) as opposed to 5HT synthesis.38 In addition, altered astroglia diminish their neurotrophic production including brain derived neurotrophic factor (BDNF) and glial cell line-derived neurotrophic factor (GDNF) and start extruding cytokines and glutamate. Glutamate released from the astroglia accesses extra-synaptic N-methyl D-aspartate (NMDA) receptors, causing suppression of BDNF synthesis and activation of the proapoptotic cascade. QA is a potent NMDA agonist that may further potentiate excitotoxicity.39
At supraphysiological levels proinflammatory cytokines can directly injure neurons. TNF-alpha, for instance interacts with two neuronal receptors, p55 (TNF-RI) and p75 (TNF-RII) and ligand binding to either receptor can activate an apoptotic signaling cascade. The TNF-R then links with the TNF receptor-associated death domain resulting in recruitment and internalization of Fas, activation of caspase-8, and cell death.40
Proinflammatory cytokines increase the expression of serotonin and dopamine transporters, disrupting monoamine signaling. Furthermore, increased oxidative stress may compromise monoamine synthesis by depleting tetrahydrobiopterin, a key co-enzyme in monoamine production.41 Less efficient monoamine signaling is correlated with higher levels of CSF IL-6 and greater severity of depressive symptoms. In a group of suicidal patients that included several bipolar subjects elevated CSF IL-6 was associated with higher 5HT and dopamine turnover, as evidenced by increased level of their metabolites.42 In summary, immune dysregulation in BD is associated with alterations in monoamine and glutamate signaling, impaired neuroplasticity and neurotrophic support, contributing to the symptomatic expression of the disease and its comorbidities.
INFLAMMATION AND CHANGES IN NEUROPLASTICITY
The role of BDNF has received more attention in mood disorder research than other members of the neurotrophin family. It is involved in neuronal maturation, differentiation and survival, synaptic plasticity, and long-term memory consolidation.43 There is compelling preclinical evidence which suggests that BDNF plays an important role in regulating the release of serotonin, glutamate, and gamma-amino butyric acid (GABA).44 Furthermore, BDNF even acts as an immunomodulator in the periphery of the body. There seems to be a bidirectional communication between the immune system and neuroplasticity regulators.45 Recent preclinical research has identified microglia originated BDNF as a key contributor to neuronal tropomyosin-receptor-kinase-B (TrkB) phosphorylation and ensuing changes in synaptic plasticity. Thus, microglia BDNF release appears to have a central role in learning and memory-related synaptic plasticity.46 BDNF is released from the neurons in two forms: as pro-BDNF (pBDNF) and it's chemically abbreviated version, mature BDNF (mBDNF). These two molecules participate in opposing functions: pBDNF binds to p75 receptor initiating apoptosis or shriveling of neurons, whereas mBDNF has primary affinity for the TrkB receptor which mediates neuroplasticity and resilience.47
Several studies have demonstrated decreased levels of BDNF in bipolar depressed and manic subjects and low levels of this factor are correlated with clinical severity of the episodes. Although diminished levels of BDNF have been reported in both treated and untreated patients, one study found normal levels of BDNF in euthymic, treated patients suggesting a potential neurotrophic benefit of pharmacotherapy.48 A reciprocal relationship between BDNF and inflammatory mediators is an important marker of the progression of bipolar diathesis. Chronicity of bipolar illness, repeated episodes, and decreased responsiveness to treatment all have a synergistic impact on a decline of neurotrophic signaling and increase in inflammation. In the latter stages of BD an imbalance between inflammatory cytokines, in particular TNF-alpha, mediators of oxidative stress and BDNF persists even between mood exacerbations and is clinically manifested as incomplete inter-episode recovery, sub-syndromal symptoms, and rapid cycling.49 With progression of the disease there are accompanying structural brain changes with decreased neural regeneration of the hippocampus, neuronal loss in key mood regulating areas and abnormalities in circuits connecting limbic and para-limbic regions.50
NEUROENDOCRINE AND AUTONOMIC DYSREGULATION
Alterations in HPA axis function in bipolar disorder have been well substantiated.51 Exaggerated release of corticotrophin-releasing factor contributes to increased adrenocorticotropic hormone secretion and a subsequent elevation of circulating cortisol. These disturbances are most likely attri-butable to deficits in cortico-limbic regulation with consequent amygdala over activity and a compromised hippocampal regulatory role.52 Moreover, glucocorticoid receptors appear to have diminished sensitivity in mood disorders possibly due to elevation of inflammatory cytokines, thereby disrupting physiological feedback regulation on the HPA axis and immune system.53 Indeed, even euthymic bipolar patients show dysregulation of the HPA axis with reduced diurnal variation of serum cortisol and flattening of the cortisol curve. These abnormalities are further exacerbated in subjects with repeated mood episodes resulting in higher overall cortisol level which is an ominous indicator of compromised overall health.54 Highlighting the relevance of these neuroendocrine anomalies, a recent study has associated elevated evening cortisol levels in bipolar individuals with a history of suicidal behavior.55
In addition to HPA axis irregularities, bipolar disorder may be associated with excessive sympathetic nervous system (SNS) activity. For example, extra-neuronal norepinephrine was reported to be elevated in a group of bipolar patients relative to healthy controls.56 Autonomic dysregulation, more generally reflected by decreased parasympathetic activity and elevated sympathetic activity may be a trait marker for bipolar disorder as indicated by a report of markedly lower heart rate variability in euthymic bipolar patients than in healthy controls.57 The constellation of SNS over activity, parasympathetic withdrawal, glucocorticoid receptor insufficiency, and elevated inflammatory signaling may help account, at least in part for the increased risk of metabolic syndrome, endocrine disorders, and vascular disease seen in bipolar patients.58 Underscoring the relevance of this pattern of neuroendocrine, autonomic and immune changes is the fact that vascular disease has recently been identified as the leading cause of excess mortality in bipolar disorder.59
NEUROGLIAL ABNORMALITIES
Convergent evidence from several areas of research including histopathologic, biochemical and neuroimaging have provided clues to the abnormalities of all three of the glial cell families in bipolar disorder linking the pathogenesis of the condition to structural and functional anomalies in astroglia, oligodendrocytes and microglia.60 Postmortem studies of bipolar patients have noted a reduction in both glial cell numbers and density.61 Glial cell abnormalities have been reported in the subgenual anterior cingulate gyrus, dorsolateral prefrontal cortex, orbitofrontal cortex and the amygdala of unmedicated bipolar patients. Interestingly, one study found evidence that treatment with lithium or valproate may mitigate some of the glial loss.62 Furthermore, a significant 29% reduction in oligodendroglia numerical density in the dorsolateral prefrontal white matter was detected in bipolar patients compared with controls.63 Evidence of diminished myelin staining in the dorsolateral prefrontal cortex and reductions of S100B immune-positive oligodendrocytes in the hippocampus of bipolar subjects further extend these findings.64 Indeed, research evidence indicates that oligodendroglial deficits may be the key CNS cellular abnormality in bipolar disorder.65
A postmortem study of suicidal bipolar, major depressive disorder and schizophrenic patients provides intriguing insights into a possible role of microglia in the pathophysiology of these conditions. Unlike mood-disorder patients who committed suicide, subjects who had the same diagnosis but died of other causes showed no evidence of brain microgliosis. However, suicidal mood disorder patients, including the bipolar group had a substantial elevation of microglia density in the dorsolateral prefrontal cortex, anterior cingulate gyrus and mediodorsal thalamus when compared with controls and mood-disorder patients who did not die of suicide. Given the established role of microglia in CNS inflammation, these findings raise the intriguing possibility that suicidality might literally be the consequence of disease flare-up.66 Supporting the role for these microglia changes in disease pathology is the fact that a remarkable overlap exists between the sites of cellular pathology and the brain regions with altered structure and functioning in neuroimaging studies of bipolar illness.67
In contrast to the evidence for a glial role in bipolar pathogenesis, the data supporting a role for a primary neuronal pathology in the condition are less convincing. With a few notable exceptions, neuronal changes in bipolar disorder are mostly morphological in character, possibly attributable to apoptosis and thinning of interneuronal neuropil, and are much less extensive than glial pathology. In summary, the available evidence does not provide much support for viewing bipolar disorder as a typical neurodegenerative disease. Unlike conventional neurodegenerative disorders, which are associated with marked neuronal loss and prominent gliosis, glial loss seems to be the dominant cellular pathology in bipolar illness. According to the available evidence, bipolar disorder is much more a disease characterized by glial pathology rather than being a neurodegenerative condition.68 Although the clinical correlates of the cellular pathology in BD await better description, there is little doubt that documented histopathological changes in key cortico-limbic areas and the white-matter tracts play an important role in the clinical manifestations of bipolar illness.
ASTROGLIA FUNCTION AND GLUTAMATE ABNORMALITIES
Astrocytes which are frequently found ensheathing synapses are an essential part of this structure along with the presynaptic and postsynaptic neurons. This close proximity has given rise to the term "tripartite synapse". An ongoing molecular dialog between glial cells and neurons has an important role in the regulation of glutamate signaling. Glutamate released from neurons is taken up by astroglia and converted to glutamine before being returned to neurons as the "raw material" for further neurotransmitter synthesis.69 In addition glutamine is a precursor for glutathione which serves as the main antioxidant in the brain. A magnetic resonance spectroscopic (MRS) study has indicated an elevation in glutamine/glutamate ratio in the anterior cingulate gyrus and parieto-occipital cortex of manic subjects compared with matched controls, pointing to excessive glutamatergic activity and/or aberrant glial/neuronal interactions in the context of bipolar illness.69 Several MRS studies and a postmortem study have reported an increase in glutamatergic transmission in the frontal cortex and hippocampus of bipolar subjects relative to control groups.70
Glx is the term used to denote glutamate and related compounds like glutamine, GABA and glutathione in the brain. MRS provides an opportunity for in vivo evaluation of glutamate-related metabolites and depending on field strength and signal-to-noise ratio, glutamate and glutamine can be quantified either separately or as a composite of Glx. Recent comprehensive meta-analyses have identified relatively consistent elevation of Glx in anterior cingulate gyrus, medial prefrontal cortex, dorsolateral prefrontal cortex, parieto-occipital cortex, insula and hippocampus. These findings persisted across the bipolar mood states and even in euthymic bipolar patients, relative to control group.71 Effect sizes related to Glx signal were more robust in mania and depression than in euthymic patients. It can be assumed that at least some of the glutamatergic aberration in bipolar disorder reflects functional and numerical glial abnormalities given their cardinal role in the regulation of glutamate metabolism and signaling.72 Distribution of aberrant Glx signals in bipolar disorder also substantially overlaps with glial alterations reported in postmortem cytological studies. Anatomical structures characterized by anomalous MRS signals in bipolar disorder are some of the key components of the cortico-limbic regulatory pathways involved in regulation of mood, cognitive processing, autonomic and endocrine responses. It would be plausible to speculate that altered glutamatergic signaling in these principal cortico-limbic circuits may be reflected in diverse bipolar clinical symptomatology.
Glutamatergic findings in bipolar disorder are similar whether the patients are medicated or not. Of note, one of the studies demonstrated an inverse relationship between diurnal salivary cortisol levels and hippocampal glutamate concentration in bipolar patients. This finding reaffirms a critical link between neuroendocrine disturbance and glutamate transmission in bipolar disorder, implicating this key area involved in memory, emotional regulation and stress response.73 Overall, multiple, consistent and convergent evidence from genetic (not discussed here), postmortem, biochemical and imaging studies points to a principal role of glutamatergic dysregulation in the etiopathogenesis of bipolar disorder. Moreover, evidence links aberrant glial-neuron interactions and immuno-endocrine dysregulation with alterations in glutamatergic transmission.74
ANTI-INFLAMMATORY TREATMENTS-THE INFLAMMATORY PATHWAY
Immune-inflammatory signaling involves an array of interacting cascading molecules. In brief, the long-chain omega-6 fatty acid arachidonic acid, derived from dietary linoliec acid via a series of transformation reactions by the enzymes desaturase and elongase, becomes acetylated and incorporated into membrane phospholipids. Phospholipid-bound arachidonic acid is mobilized via a calcium-dependant cytosolic isoform of phopholipase A2, and free arachidonic acid is a substrate for cyclooxygenase (COX)-mediated biosynthesis of prostaglandins (i.e. PGH2), thromboxanes and prostacyclins, as well as lipooxygenase-mediated biosynthesis of leukotrienes. COX generated PGH2 is converted to PGE2 via PGE synthase and PGE2 stimulates the biosynthesis of downstream pro-inflammatory cytokines including IL-6 at the level of transcription.75 Pro-inflammatory cytokines including IL-6, IL-1 beta and TNF-alpha in turn stimulate hepatic biosynthesis of acute-phase proteins including CRP. In contrast to arachidonic acid, the long-chain omega-3 fatty acids, including eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) are predominantly anti-inflammatory and EPA competes with arachidonic acid for metabolism by COX enzymes. In addition, COX and lipooxygenase metabolites of DHA and EPA (i.e. D- and E-series resolvins) have potent inflammation-resolving properties.76
ANTI-INFLAMMATORY POTENTIAL OF LITHIUM AND VALPROATE
There is considerable preliminary evidence suggesting that traditional mood stabilizers modulate neuroinflammation. In two recent studies lithium was shown to have neuroprotective activity in preclinical models. In rat glial cells, pretreatment with lithium showed a significant anti-inflammatory potential, decreasing lipopolysacchride-induced secretion of TNF-alpha, IL-1 beta, prostaglandin E2 and nitric oxide.77 Similarly, in an intracerebral hemorrhage model lithium reduced cell death, COX 2 expression, and reactive microglia in perihematomal regions in rats.78 Interestingly, valproate has also shown anti-inflammatory properties in preclinical models, modulating both systemic and CNS responses.79 Nevertheless, not enough clinical evidence exists to support that these medications would exert neuroprotective effects in general, and specifically through immune and inflammatory pathways in particular.
RE-PURPOSED DRUGS WITH ANTI-INFLAMMATORY PROPERTIES
The adjunctive use of drugs with anti-inflammatory properties, such as omega-3 fatty acids, acetylsalicylic acid, celecoxib and minocycline is another area that has recently started to be explored. Omega-3 is nutritionally important fatty acids that include alpha-linolenic acid, DHA and EPA. A recent meta-analysis showed that EPA is a more effective component in the treatment of major depressive disorder than DHA.80 As alluded to earlier, these molecules are supposed to compete for the biotransformation of eicosanoids such as prostaglandins and leukotrienes. In fact, competition for the biosynthesis of inflammatory mediators could be partially responsible for their anti-inflammatory effects. Although current evidence does not support the adjunctive use of omega-3 in the treatment of bipolar mania, some studies have demonstrated their efficacy in bipolar depression.81
The antibiotic and anti-inflammatory effects of minocycline inhibit apoptosis by attenuating microglial release of proinflammatory cytokines IL-1 beta, TNF-alpha and IL-6, while at the same time promoting the release of anti-inflammatory cytokine IL-10. However, the efficacy of minocycline has not been formally tested in mood disorders. Recently, a clinical trial with minocycline and aspirin was proposed and conducted but final results have not yet been published.82
Acetylsalicylic acid irreversibly inhibits COX-1 and modifies the enzymatic activity of COX-2. COX-1 and COX-2 differentially modulate leukocyte recruitment during neuroinflammation. The clinical use of low dose aspirin has been primarily driven by its role as an antithrombotic and thrombolytic agent. Given the high rates of death from cardiovascular events in bipolar disorder, this action might be potentially advantageous in the management of bipolar disorder. Nevertheless, recent literature also supports the use of low dose aspirin in the management of mood disorders itself, more specifically to ameliorate depressive symptoms.83 The COX-2 inhibitor celecoxib was tested in the treatment of depressive and mixed episodes in bipolar disorder in a short term randomized, controlled trial. That study showed some benefit of celecoxib in the treatment of depressive symptoms, but it remains unclear whether those benefits outweigh the risks at this point.84
DEVELOPMENT OF BIOMARKERS-FOCUS ON CYTOKINES AND BDNF
Biomarker development is a multi-stage process which requires 1) proof of concept, 2) prospective validation in independent populations, 3) documentation of incremental information when added to standard risk markers, 4) assessment of effects on patient management and outcomes, and 5) cost-effectiveness.85 Biomarkers have a variety of potential applications including diagnosis, monitoring of disease burden, prognosis, and prediction of treatment response. Sensitivity, specificity, positive and negative predictive value are statistical properties that are crucial to examine in order to determine the value of incorporating biomarkers into clinical practice. In psychiatry, such approaches appear premature as in-vestigative efforts are currently being directed at acquiring evidence on the most relevant candidates. Nevertheless, the need for biomarkers is widely acknowledged by clinicians, researchers and patients alike. This need is arguably greatest for bipolar disorder, characterized by numerous presentations in terms of mood polarity and exceedingly high rates of comorbidity. The extant literature provides some clues in this respect, as an increasing number of studies point to abnormalities of components of the inflammatory pathway and neurotrophic signaling in different phases of the bipolar illness. This section of the paper attempts to summarize these findings with a focus on peripheral cytokines and brain derived neurotrophic factor measurements in the study populations.
PERIPHERAL CYTOKINES IN BIPOLAR DISORDER
In 2013, three meta-analyses incorporating somewhat different methodologies yielded generally convergent findings. In one report, Munkholm and colleagues examined 13 studies (using non-stimulated in-vivo studies only) published through January 2012 (n=556 BD, n=767 controls) and found that levels of TNF-alpha, soluble TNF receptor type 1 (sTNF-R1) and soluble interleukin-2 receptor (sIL-2R) were significantly elevated among manic BD patients vs. controls. In addition, sTNF-R1 and TNF-alpha were elevated among manic BD patients vs euthymic BD patients. Some studies demonstrated statistical trends towards increased levels of TNF-alpha and IL-6 among depressed BD patients vs. controls, and increased IL-6 in bipolar depression vs bipolar mania, however these findings were not significant in the meta-analysis. Only sTNF-R1 was significantly higher among euthymic BD patients vs controls.86
In their subsequent meta-analysis, including articles up to January 2013, Munkholm and colleagues examined 18 studies (non-stimulated, in vivo; n=761 BD, n=919 controls). They examined all BD subjects together, including those who were manic, depressed, or euthymic and found that sIL-2R, TNF-alpha, sTNF-R1, sIL-6R and IL-4 (an anti-inflammatory cytokine) were higher among BD patients vs controls.30
Finally, Modabbernia and colleagues examined 30 studies published through December 2012 (n=1351 BD, n=1248 controls). The larger number of studies is owing to the inclusion of studies using mitogen-stimulation, in addition to those examining only circulating levels of cytokines. They found that IL-4, IL-6, IL-10, sIL-2R, sIL-6R, IL-1RA (interleukin-1 receptor antagonist), TNF-alpha and sTNF-R1 were significantly elevated in manic BD patients vs controls. Non-significant trends were observed for IL-1beta and IL-6. Heterogeneity of findings was mitigated in subgroup analyses based on mitogen stimulation methodology. There were trends towards higher TNF-alpha and IL-10 levels among depressed BD patients vs controls. Among BD patients, IL-6 tended to be higher during mania than depression.87
SERUM BDNF AS A BIOMARKER FOR BIPOLAR DISORDER
BDNF is important for neurogenesis, synaptic plasticity and dendritic growth. Much of the circulating BDNF originates from CNS neurons and glia. Preclinical models demonstrate high positive correlation between serum BDNF levels and brain BDNF expression.88 Increasing BDNF expression in the hippocampus and prefrontal cortex produces antidepressant effects. The BDNF val66met polymorphism, associated with reduced function, may be implicated among children and adolescents with bipolar disorder and among adults with early onset BD.89 Multiple studies have found reduced circulating BDNF protein levels during acute phases of bipolar disorder.90 Studies have found negative associations between serum BDNF levels and manic and depressive symptoms, although there are also contradictory findings from small studies.91 Most studies include patients taking psychotropic medications, however convergent findings are reported among untreated participants.92 Greater reductions in BDNF are noted in bipolar disorder as compared to major depressive disorder.93 A relatively recent systematic review and meta-regression analysis (n=548 BD, n=565 controls) reported reduced BDNF with large effect sizes (ES) for mania (ES -0.81) and bipolar depression (ES -0.97) versus controls. In contrast, differences in BDNF levels among euthymic BD patients versus controls were not significant and of modest magnitude (ES -0.20). There was substantial variability in findings in the euthymic phase, and both increasing age and longer illness duration were associated with reduced peripheral BDNF levels and contributed to this variability.94
One study, in which first episode bipolar patients in manic phase were examined, showed an increase in BDNF levels with treatment over time and episode remission.95 Another study in which both manic and depressed patients received the same treatment i.e. extended release quetiapine showed an increase in BDNF with treatment in depressed subjects but a decrease in BDNF in manic/mixed episode patients.96 In a study in which serum BDNF levels were investigated in manic patients before and after treatment, an increase in BDNF with lithium administration was seen.97 In general, it can be concluded from the existing literature that BDNF levels are reduced in acute episodes and increase after treatment with mood stabilizing medications.
COMPARISON OF BIOMARKERS BETWEEN BIPOLAR DISORDER AND MAJOR DEPRESSIVE DISORDER
Increasing amounts of data suggest that inflammatory responses have an important role in the pathophysiology of depression. Most of the evidence that links inflammation and MDD comes from three observations:
1) MDD (even in the absence of medical illness) is associated with raised inflammatory markers.
2) Inflammatory medical illnesses-both CNS and peripheral-are associated with greater rates of major depression.
3) Patients treated with cytokines for various illnesses are at increased risk of developing major depressive illness.
Mean array value for inflammatory mediators/markers is higher in MDD than normal, non-depressed subjects. Approximately one-third of people with MDD have higher levels of inflammatory markers compared with normal, non-depressed population. These increases are more modest than in autoimmune or infectious disease-for example, 2-3 times higher than in healthy controls. However, small physiologic differences can have profound consequences over time, especially if they change in a consistent direction. Similar findings have been found in cardiovascular disease, stroke and diabetes.
Alterations in serum and CSF concentrations of a number of inflammatory markers, including cytokines, chemokines and acute phase reactant proteins, have been found in patients with MDD. The most replicated findings pertain to raised CRP and proinflammatory cytokines-TNF-alpha and IL-6-confirmed by at least two recent meta-analyses.98,99 A meta-analysis of 22 antidepressant treatment studies found that IL-1beta and IL-6 levels (but not TNF-alpha) decreased in response to therapy with SSRIs, along with a reduction in depressive symptoms.100 These findings propose the possibility that inflammatory cytokines contribute to depressive symptoms, and that antidepressants block the effect of inflammatory cytokines in the brain.
The multiple mechanisms through which inflammation may act in precipitating the depression phenotype include:
1) Insensitivity to glucocorticoid inhibitory feedback
2) Reduced parasympathetic signaling
3) Reduced production of BDNF
4) Increased anterior cingulated cortex activity
5) Reduced hippocampal volume
This resonates with the concept of allostatic load. Allostasis has been described as the process of adaptation to acute stress, involving activation of both the sympathetic adrenomedullary and HPA axes in order to restore homeostasis when faced with a challenge. Allostatic load refers to the 'wear and tear' that the body experiences as a result of the activation of the above systems. It is not surprising that the progression of inflammation serves as a key mediator of the process of allostasis. A number of studies have shown a significant association between allostatic load (measured using a composite score of inflammatory and metabolic markers) and medical health (eg, cardiovascular disease) and with mental ill health (such as major depression).
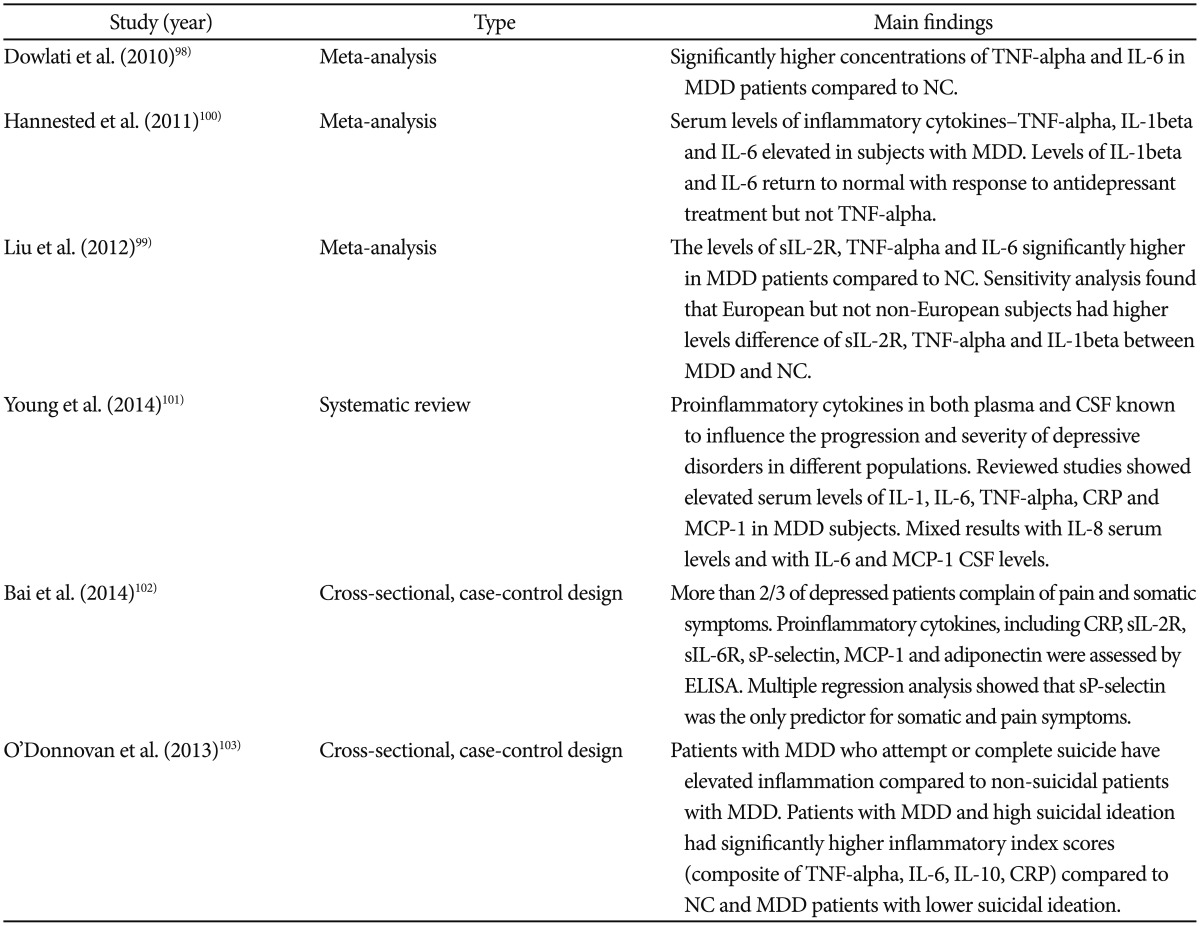
A summary of abnormalities of inflammatory biomarkers in major depressive disorder is given in Table 2.
Table 2. Abnormalities of inflammatory mediators in major depressive disorder.
CRP: C-reactive protein, CSF: cerebrospinal fluid, ELISA: enzyme linked immune-sorbent assay, IL: interleukin, MCP-1: monocyte chemoattractantprotein-1, MDD: major depressive disorder, NC: normal control, TNF: tumor necrosis factor
p11 PROTEIN AND ITS RELATIONSHIP TO MOOD DISORDERS
The etiopathogenesis and treatment of major mood disorders have focused on monoaminergic (5HT, norepinephrine and dopamine) and amino acid (GABA, glutamate) receptors at plasma membrane. The time lag in the neuropsychiatric effects implicates intracellular neuroplasticity as primary in the mechanism of action of antidepressants and mood stabilizers. This review now discusses the function of a small acidic chaperone protein, p11 also known as S100A10 which interacts with a number of serotonin receptors, in particular 5HT1B, 5HT1D and 5HT4 and has been implicated in mood regulation, nociception, ionic calcium uptake and cell polarization.
p11 is expressed in several brain regions implicated in the pathophysiology of mood disorders, including the nucleus accumbens, cerebral cortex and hippocampus.104 Of clinical and translational relevance, p11 mRNA and protein are down-regulated in the anterior cingulate cortex and the ventral striatum from depressed individuals. Studies in human suicide victims have also found reductions of p11 mRNA in hippocampus and amygdala, indicating an important role of p11 in multiple brain regions associated with depression pathophysiology.105 Conversely, antidepressants of several distinct categories-SSRIs, tricyclic antidepressants, monoamine oxidase inhibitors-as well as electroconvulsive therapy, increases p11 expression in frontal cortex and hippocampus of mice and rats. p11 knockout mice exhibit reduced neurogenic and behavioral responsiveness to SSRI administration. p11, by virtue of its ability to promote accumulation of 5HT receptors at the cell surface, serves as an amplification mechanism for 5HT receptor-mediated actions. p11 is expressed in peripheral blood cells and ongoing work should establish whether p11 peripheral expression can serve as a biomarker of depression and/or responsiveness to antidepressant treatment.
CONCLUSIONS
Bipolar disorder is different from other psychiatric conditions in the sense that whereas, most mental illnesses vacillate within a single register between symptom exacerbation and various degrees of recovery, flare ups in BD come in two distinct forms-mania and depression-and that often these episodes can take any of a nearly infinite number of combinations of mood disturbances. In the pathophysiology of bipolar disorder, consistent findings have emerged at a cellular level, providing evidence that BD is reliably associated with dysregulation of glial-neuronal interactions and with abnormalities more apparent in glial elements than in neurons. Among these glial elements are microglia-the brain's primary immune elements, which appear to be overactive in the context of bipolarity. Multiple studies now indicate that inflammation is also increased in the periphery of the body in both the depressive and manic phases of the illness, with at least some return to normality in the euthymic state. These findings are in line with changes in the HPA axis, such as reduced sensitivity to glucocorticoids which are known to drive inflammatory activation. The fact that bipolarity reaches so deeply into the core processes of life itself may enhance the understanding of why the disorder is so reliably associated with immune abnormalities and with a marked escalation in risk for the development of multiple medical conditions that account for much of the increased mortality associated with the disorder.
As far as the development of reliable biomarkers for the disease is concerned, recent meta-analyses confirm that proinflammatory markers are increased and BDNF is decreased among adults with BD during acute episodes. Although constrained by a small number of studies with few subjects, there is also preliminary evidence that these markers change over time and in response to treatment. The extant literature stro-ngly suggests that there is ample justification to continue research regarding these markers as potential clinical indicators in BD. For cytokines and BDNF to realize their full potential will require the consistent application of several methodological approaches. These include larger sample sizes, repeated measures and within-subject comparisons, incorporation of important covariates (age, sex, BMI, stressors) and sub-group analyses (BD subtype, family history and comorbidities). Preliminary data suggest that age and stage of illness may contribute to heterogeneity in findings. It is important for the field to consider the possibility that there are developmental and/or stage-related differences in the relationship of these markers to symptoms, course and treatment of BD. Validation of cytokines and BDNF would justify further investigation into whether explicitly attempting to modify levels of these markers, using anti-inflammatory and/or neurotrophin-based interventions, can yield benefits in terms of mood stabilization.
References
- 1.Fagiolini A, Forgione R, Maccari M, Cuomo A, Morana B, Dell'Osso MC, et al. Prevalence, chronicity, burden and borders of bipolar disorder. J Affect Disord. 2013;148:161–169. doi: 10.1016/j.jad.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Vieta E, Reinares M, Rosa AR. Staging bipolar disorder. Neurotox Res. 2011;19:279–285. doi: 10.1007/s12640-010-9197-8. [DOI] [PubMed] [Google Scholar]
- 3.Uher R. Gene-environment interactions in severe mental illness. Front Psychiatry. 2014;5:48. doi: 10.3389/fpsyt.2014.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Laursen TM. Life expectancy among persons with schizophrenia or bipolar affective disorder. Schizophr Res. 2011;131:101–104. doi: 10.1016/j.schres.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 5.Hamdani N, Doukhan R, Kurtlucan O, Tamouza R, Leboyer M. Immunity, inflammation, and bipolar disorder: diagnostic and therapeutic implications. Curr Psychiatry Rep. 2013;15:387. doi: 10.1007/s11920-013-0387-y. [DOI] [PubMed] [Google Scholar]
- 6.Gama CS, Kunz M, Magalhaes PV, Kapczinski F. Staging and neuroprogression in bipolar disorder: a systematic review of the literature. Rev Bras Psiquiatr. 2013;35:70–74. doi: 10.1016/j.rbp.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Bai YM, Su TP, Tsai SJ, Wen-Fei C, Li CT, Pei-Chi T, et al. Comparison of inflammatory cytokines levels among type I/type II and manic/hypomanic/euthymic/depressive states of bipolar disorder. J Affect Disord. 2014;166:187–192. doi: 10.1016/j.jad.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 8.Stertz L, Magalhaes PV, Kapczinski F. Is bipolar disorder an inflammatory condition? The relevance of microglia activation. Curr Opin Psychiatry. 2013;26:19–26. doi: 10.1097/YCO.0b013e32835aa4b4. [DOI] [PubMed] [Google Scholar]
- 9.Watkins CC, Sawa A, Pomper MG. Glia and immune cell signaling in bipolar disorder: insights from neuropharmacology and molecular imaging to clinical application. Transl Psychiatry. 2014;4:e350. doi: 10.1038/tp.2013.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brietzke E, Stabellini R, Grassi-Oliveira R, Lafer B. Cytokines in bipolar disorder: recent findings, deleterious effects but promise for future therapeutics. CNS Spectr. 2011;16:157–168. doi: 10.1017/S1092852912000338. [DOI] [PubMed] [Google Scholar]
- 11.Bai YM, Su TP, Li CT, Tsai SJ, Chen MH, Tu PC, et al. Comparison of pro-inflammatory cytokines among patients with bipolar disorder and unipolar depression and normal controls. Bipolar Disord. 2015;17:269–277. doi: 10.1111/bdi.12259. [DOI] [PubMed] [Google Scholar]
- 12.Brambilla P, Bellani M, Isola A, Bergami A, Marinelli V, Dusi N, et al. Increased M1/decreased M2 signature and signs of Th1/Th2 shift in chronic patients with bipolar disorder, but not in those of schizophrenia. Transl Psychiatry. 2014;4:e406. doi: 10.1038/tp.2014.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barbosa IG, Morato IB, de Miranda AS, Bauer ME, Soares JC, Teixeira AL. A preliminary report of increased plasma levels of IL-33 in bipolar disorder: further evidence of pro-inflammatory status. J Affect Disord. 2014;157:41–44. doi: 10.1016/j.jad.2013.12.042. [DOI] [PubMed] [Google Scholar]
- 14.Doganavsargil-Baysal O, Cinemre B, Aksoy UM, Akbas A, Metin O, Fettahoglu C, et al. Levels of TNF-alpha, soluble TNF receptors (sTNF-R1, sTNF-R2) and cognition in bipolar disorder. Hum Psychopharmacol. 2013;28:160–167. doi: 10.1002/hup.2301. [DOI] [PubMed] [Google Scholar]
- 15.Hope S, Ueland T, Steen NE, Dieset I, Lorentzen S, Berg AO, et al. Interleukin 1 receptor antagonist and soluble tumor necrosis factor receptor 1 are associated with general severity and psychotic symptoms in schizophrenia and bipolar disorder. Schizophr Res. 2013;145:36–42. doi: 10.1016/j.schres.2012.12.023. [DOI] [PubMed] [Google Scholar]
- 16.Remlinger-Molenda A, Wojciak P, Michalak M, Rybakowski J. Activity of selected cytokines in bipolar patients during manic and depressive episodes. Psychiatr Pol. 2012;46:599–611. [PubMed] [Google Scholar]
- 17.Remlinger-Molenda A, Wojciak P, Michalak M, Karczewski J, Rybakowski JK. Selected cytokine profile during remission in bipolar patients. Neuropsychobiology. 2012;66:193–198. doi: 10.1159/000339949. [DOI] [PubMed] [Google Scholar]
- 18.Kunz M, Cereser KM, Goi PD, Fries GR, Teixeira AL, Fernandes BS, et al. Serum levels of IL-6, IL-10 and TNF-alpha in patients with bipolar disorder and schizophrenia: differences in pro- and anti-inflammatory balance. Rev Bras Psiquiatr. 2011;33:268–274. doi: 10.1590/s1516-44462011000300010. [DOI] [PubMed] [Google Scholar]
- 19.Hope S, Dieset I, Agartz I, Steen NE, Ueland T, Melle I, et al. Affective symptoms are associated with markers of inflammation and immune activation in bipolar disorder but not in schizophrenia. J Psychiatr Res. 2011;45:1608–1616. doi: 10.1016/j.jpsychires.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 20.Guloksuz S, Cetin EA, Cetin T, Deniz G, Oral ET, Nutt DJ. Cytokine levels in euthymic bipolar patients. J Affect Disord. 2010;126:458–462. doi: 10.1016/j.jad.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 21.Barbosa IG, Huguet RB, Mendonca VA, Sousa LP, Neves FS, Bauer ME, et al. Increased plasma levels of soluble TNF receptor I in patients with bipolar disorder. Eur Arch Psychiatry Clin Neurosci. 2011;261:139–143. doi: 10.1007/s00406-010-0116-z. [DOI] [PubMed] [Google Scholar]
- 22.Hope S, Melle I, Aukrust P, Steen NE, Birkenaes AB, Lorentzen S, et al. Similar immune profile in bipolar disorder and schizophrenia: selective increase in soluble tumor necrosis factor receptor I and von Willibrand factor. Bipolar Disord. 2009;11:726–734. doi: 10.1111/j.1399-5618.2009.00757.x. [DOI] [PubMed] [Google Scholar]
- 23.Brietzke E, Stertz L, Fernandes BS, Kauer-Sant'anna M, Mascarenhas M, Escosteguy Vargas A, et al. Comparison of cytokine levels in depressed, manic and euthymic patients with bipolar disorder. J Affect Disord. 2009;116:214–217. doi: 10.1016/j.jad.2008.12.001. [DOI] [PubMed] [Google Scholar]
- 24.Ortiz-Dominguez A, Hernandez ME, Berlanga C, Gutierrez-Mora D, Moreno J, Heinze G, et al. Immune variations in bipolar disorder: phasic differences. Bipolar Disord. 2007;9:596–602. doi: 10.1111/j.1399-5618.2007.00493.x. [DOI] [PubMed] [Google Scholar]
- 25.Kim YK, Jung HG, Myint AM, Kim H, Park SH. Imbalance between pro-inflammatory and anti-inflammatory cytokines in bipolar disorder. J Affect Disord. 2007;104:91–95. doi: 10.1016/j.jad.2007.02.018. [DOI] [PubMed] [Google Scholar]
- 26.O'Brien SM, Scully P, Scott LV, Dinan TG. Cytokine profiles in bipolar affective disorder: focus on acutely ill patients. J Affect Disord. 2006;90:263–267. doi: 10.1016/j.jad.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 27.Kim YK, Myint AM, Lee BH, Han CS, Lee SW, Leonard BE, et al. T-helper types 1, 2, and 3 cytokine interactions in symptomatic manic patients. Psychiatry Res. 2004;129:267–272. doi: 10.1016/j.psychres.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 28.Su KP, Leu SJ, Yang YY, Shen WW, Chou YM, Tsai SY. Reduced production of interferon-gamma but not interleukin-10 in bipolar mania and subsequent remission. J Affect Disord. 2002;71:205–209. doi: 10.1016/s0165-0327(01)00369-x. [DOI] [PubMed] [Google Scholar]
- 29.Tsai SY, Yang YY, Kuo CJ, Chen CC, Leu SJ. Effects of symptomatic severity on elevation of plasma soluble interleukin-2 receptor in bipolar mania. J Affect Disord. 2001;64:185–193. doi: 10.1016/s0165-0327(00)00252-4. [DOI] [PubMed] [Google Scholar]
- 30.Munkholm K, BraunerJV, Kessing LV, Vinberg M. Cytokines in bipolar disorder vs. healthy control subjects: a systematic review and meta-analysis. J Psychiatr Res. 2013;47:1119–1133. doi: 10.1016/j.jpsychires.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 31.Prasad R, Kapoor R, Srivastava R, Mishra OP, Singh TB. Cerebrospinal fluid TNF-alpha, IL-6, and IL-8 in children with bacterial meningitis. Pediatr Neurol. 2014;50:60–65. doi: 10.1016/j.pediatrneurol.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 32.Kontoangelos K, Papageorgiou CC, Raptis AE, Tsiotra P, Boutati E, Lambadiari V, et al. Cytokines, diabetes mellitus and psychopathology: a challenging combination. Neuro Endocrinol Lett. 2014;35:159–169. [PubMed] [Google Scholar]
- 33.Crump C, Sundquist K, Winkleby MA, Sundquist J. Comorbidities and mortality in bipolar disorder: a Swedish national cohort study. JAMA Psychiatry. 2013;70:931–939. doi: 10.1001/jamapsychiatry.2013.1394. [DOI] [PubMed] [Google Scholar]
- 34.Goldstein BI, Kemp DE, Soczynska JK, McIntyre RS. Inflammation and the phenomenology, comorbidity, and treatment of bipolar disorder: a systematic review of the literature. J Clin Psychiatry. 2009;70:1078–1090. doi: 10.4088/JCP.08r04505. [DOI] [PubMed] [Google Scholar]
- 35.Janelidze S, Mattei D, Westrin A, Traskman-Bendz L, Brundin L. Cytokine levels in blood may distinguish suicide attempters from depressed patients. Brain Behav Immun. 2011;25:335–339. doi: 10.1016/j.bbi.2010.10.010. [DOI] [PubMed] [Google Scholar]
- 36.Soderlund J, Olsson SK, Sameulsson M, Walther-Jallow L, Johansson C, Erhardt S, et al. Elevation of cerebrospinal fluid interleukin-1ß in bipolar disorder. J Psychiatry Neurosci. 2011;36:114–118. doi: 10.1503/jpn.100080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Maletic V, Raison C. Integrated neurobiology of bipolar disorder. Front Psychiatry. 2014;5:98. doi: 10.3389/fpsyt.2014.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Myint AM. Kynurenines: from the perspective of major psychiatric disorders. FEBS J. 2012;279:1375–1385. doi: 10.1111/j.1742-4658.2012.08551.x. [DOI] [PubMed] [Google Scholar]
- 39.Jun C, Choi Y, Lim SM, Bae S, Hong YS, Kim JE, et al. Disturbance of the glutamatergic system in mood disorders. Exp Neurobiol. 2014;23:28–35. doi: 10.5607/en.2014.23.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park KM, Bowers WJ. Tumor necrosis factor-alpha mediated signaling in neuronal homeostasis and dysfunction. Cell Signal. 2010;22:977–983. doi: 10.1016/j.cellsig.2010.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Felger JC, Miller AH. Cytokine effects on the basal ganglia and dopamine function: the subcortical source of inflammatory malaise. Front Neuroendocrinol. 2012;33:315–327. doi: 10.1016/j.yfrne.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindqvist D, Janelidze S, Hagell P, Erhardt S, Sameulsson M, Minthon L, et al. Interleukin-6 is elevated in the cerebrospinal fluid of suicide attempters and related to symptom severity. Biol Psychiatry. 2009;66:287–292. doi: 10.1016/j.biopsych.2009.01.030. [DOI] [PubMed] [Google Scholar]
- 43.Grande I, Fries GR, Kunz M, Kapczinski F. The role of BDNF as a mediator of neuroplasticity in bipolar disorder. Psychiatry Investig. 2010;7:243–250. doi: 10.4306/pi.2010.7.4.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deltheil T, Guiard BP, Cerdan J, David DJ, Tanaka KF, Reperant C, et al. Behavioral and serotonergic consequences of decreasing or increasing hippocampus brain-derived neurotrophic factor protein levels in mice. Neuropharmacology. 2008;55:1006–1014. doi: 10.1016/j.neuropharm.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 45.Patas K, Penninx BW, Bus BA, Vogelzangs N, Molendijk ML, Elzinga BM, et al. Association between serum brain-derived neurotrophic factor and plasma interleukin-6 in major depressive disorder with melancholic features. Brain Behav Immun. 2014;36:71–79. doi: 10.1016/j.bbi.2013.10.007. [DOI] [PubMed] [Google Scholar]
- 46.Parkhurst CN, Yang G, Ninan I, Savas JN, Yates JR, 3rd, Lafaille JJ, et al. Microglia promote learning-dependant synapse formation through brain-derived neurotrophic factor. Cell. 2013;155:1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yang J, Harte-Hargrove LC, Siao CJ, Marinic T, Clarke R, Ma Q, et al. proBDNF negatively regulates neuronal remodeling, synaptic transmission, and synaptic plasticity in hippocampus. Cell Rep. 2014;7:796–806. doi: 10.1016/j.celrep.2014.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cunha AB, Frey BN, Andreazza AC, Goi JD, Rosa AR, Goncalves CA, et al. Serum brain-derived neurotrophic factor is decreased in bipolar disorder during depressive and manic episodes. Neurosci Lett. 2006;398:215–219. doi: 10.1016/j.neulet.2005.12.085. [DOI] [PubMed] [Google Scholar]
- 49.Berk M, Kapczinski F, Andreazza AC, Dean OM, Giorlando F, Maes M, et al. Pathways underlying neuroprogression in bipolar disorder: focus on inflammation, oxidative stress and neurotrophic factors. Neurosci Biobehav Rev. 2011;35:804–817. doi: 10.1016/j.neubiorev.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 50.Soares JC. Contributions from brain imaging to the elucidation of pathophysiology of bipolar disorder. Int J Neuropsychopharmacol. 2003;6:171–180. doi: 10.1017/S1461145703003390. [DOI] [PubMed] [Google Scholar]
- 51.Maripuu M, Wikgren M, Karling P, Adolfsson R, Norrback KF. Relative hypo- and hypercortisolism are both associated with depression and lower quality of life in bipolar disorder: a cross-sectional study. PLoS One. 2014;9:e98682. doi: 10.1371/journal.pone.0098682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Drevets WC, Price JL, Furey ML. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct. 2008;213:93–118. doi: 10.1007/s00429-008-0189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pace TW, Hu F, Miller AH. Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav Immun. 2007;21:9–19. doi: 10.1016/j.bbi.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Havermans R, Nicolson NA, Berkhof J, deVries MW. Patterns of salivary cortisol secretion and responses to daily events in patients with remitted bipolar disorder. Psychoneuroendocrinology. 2011;36:258–265. doi: 10.1016/j.psyneuen.2010.07.016. [DOI] [PubMed] [Google Scholar]
- 55.Kamali M, Saunders EF, Prossin AR, Brucksch CB, Harrington GJ, Langenecker SA, et al. Associations between suicide attempts and elevated bedtime salivary cortisol levels in bipolar disorder. J Affect Disord. 2012;136:350–358. doi: 10.1016/j.jad.2011.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grossman F, Potter WZ. Catecholamines in depression: a cumulative study of urinary norepinephrine and its major metabolites in unipolar and bipolar depressed patients versus healthy volunteers at the NIMH. Psychiatry Res. 1999;87:21–27. doi: 10.1016/s0165-1781(99)00055-4. [DOI] [PubMed] [Google Scholar]
- 57.Latalova K, Prasko J, Diveky T, Grambal A, Kamaradova D, Velartova H, et al. Autonomic nervous system in euthymic patients with bipolar affective disorder. Neuro Endocrinol Lett. 2010;31:829–836. [PubMed] [Google Scholar]
- 58.Taylor V, MacQueen G. Associations between bipolar disorder and metabolic syndrome: a review. J Clin Psychiatry. 2006;67:1034–1041. doi: 10.4088/jcp.v67n0704. [DOI] [PubMed] [Google Scholar]
- 59.Laursen TM, Wahlbeck K, Hallgren J, Westman J, Osby U, Alinaghizadeh H, et al. Life expectancy and death by diseases of the circulatory system in patients with bipolar disorder or schizophrenia in the Nordic countries. PLoS One. 2013;8:e67133. doi: 10.1371/journal.pone.0067133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schroeter ML, Abdul-Khaliq H, Sacher J, Steiner J, Blasig IE, Mueller K. Mood disorders are glial disorders: evidence from in vivo studies. Cardiovasc Psychiatry Neurol. 2010;2010:780645. doi: 10.1155/2010/780645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rajkowska G, Halaris A, Selemon LD. Reductions in neuronal and glial density characterize the dorsolateral prefrontal cortex in bipolar disorder. Biol Psychiatry. 2001;49:741–752. doi: 10.1016/s0006-3223(01)01080-0. [DOI] [PubMed] [Google Scholar]
- 62.Bowley MP, Drevets WC, Ongur D, Price JL. Low glial numbers in the amygdala in major depressive disorder. Biol Psychiatry. 2002;52:404–412. doi: 10.1016/s0006-3223(02)01404-x. [DOI] [PubMed] [Google Scholar]
- 63.Uranova NA, Vostrikov VM, Orlovskaya DD, Rachmanova VI. Oligodendroglial density in the prefrontal cortex in schizophrenia and mood disorders: a study from the Stanley Neuropathology Consortium. Schizophr Res. 2004;67:269–275. doi: 10.1016/S0920-9964(03)00181-6. [DOI] [PubMed] [Google Scholar]
- 64.Gos T, Schroeter ML, Lessel W, Bernstein HG, Dobrowolny H, Schiltz K, et al. S100B-immunopositive astrocytes and oligodendrocytes in the hippocampus are differentially afflicted in unipolar and bipolar depression: a postmortem study. J Psychiatr Res. 2013;47:1694–1699. doi: 10.1016/j.jpsychires.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 65.Vostrikov VM, Uranova NA, Orlovskaya DD. Deficit of perineuronal oligodendrocytes in the prefrontal cortex in schizophrenia and mood disorders. Schizophr Res. 2007;94:273–280. doi: 10.1016/j.schres.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 66.Steiner J, Bielau H, Brisch R, Danos P, Ullrich O, Mawrin C, et al. Immunological aspects in the neurobiology of suicide: elevated microglial density in schizophrenia and depression is associated with suicide. J Psychiatr Res. 2008;42:151–157. doi: 10.1016/j.jpsychires.2006.10.013. [DOI] [PubMed] [Google Scholar]
- 67.Harrison PJ. The neuropathology of primary mood disorder. Brain. 2002;125:1428–1449. doi: 10.1093/brain/awf149. [DOI] [PubMed] [Google Scholar]
- 68.Rajkowaska G. Cell pathology in mood disorders. Semin Clin Neuropsychiatry. 2002;7:281–292. doi: 10.1053/scnp.2002.35228. [DOI] [PubMed] [Google Scholar]
- 69.Ongur D, Jensen JE, Prescot AP, Stork C, Lundy M, Cohen MB, et al. Abnormal glutamatergic neurotransmission and neuronal-glial interactions in acute mania. Biol Psychiatry. 2008;64:718–726. doi: 10.1016/j.biopsych.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hashimoto K, Sawa A, Iyo M. Increased levels of glutamate in brains from patients with mood disorders. Biol Psychiatry. 2007;62:1310–1316. doi: 10.1016/j.biopsych.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 71.Yuksel C, Ongur D. Magnetic resonance spectroscopic studies of glutamate-related abnormalities in mood disorders. Biol Psychiatry. 2010;68:785–794. doi: 10.1016/j.biopsych.2010.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chitty KM, Lagopoulos J, Lee RS, Hickie IB, Hermens DF. A systematic review and meta-analysis of proton magnetic resonance spectroscopy and mismatch negativity in bipolar disorder. Eur Neuropsychopharmacol. 2013;23:1348–1363. doi: 10.1016/j.euroneuro.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 73.Colla M, Schubert F, Bubner M, Heidenreich JO, Bajbouj M, Seifert F, et al. Glutamate as a spectroscopic marker of hippocampal structural plasticity is elevated in long-term euthymic bipolar patients on chronic lithium therapy and correlates inversely with diurnal cortisol. Mol Psychiatry. 2009;14:696–704. doi: 10.1038/mp.2008.26. [DOI] [PubMed] [Google Scholar]
- 74.Scumpia PO, Kelly-Scumpia K, Stevens BR. Alpha-lipoic acid effects on glial functions accompanying double-stranded RNA antiviral and inflammatory signaling. Neurochem Int. 2014;64:55–63. doi: 10.1016/j.neuint.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 75.Wang P, Zhu F, Konstantopoulos K. Prostaglandin E2 induces interleukin-6 expression in human chondrocytes via cAMP/protein kinase A- and phosphatidylinositol 3-kinase-dependant NF-kappaB activation. Am J Physiol Cell Physiol. 2010;298:C1445–C1456. doi: 10.1152/ajpcell.00508.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee HN, Surh YJ. Therapeutic potential of resolvins in the prevention and treatment of inflammatory disorders. Biochem Pharmacol. 2012;84:1340–1350. doi: 10.1016/j.bcp.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 77.Nahman S, Belmaker RH, Azab AN. Effects of lithium on lipopolysacchride-induced inflammation in rat primary glial cells. Innate Immun. 2012;18:447–458. doi: 10.1177/1753425911421512. [DOI] [PubMed] [Google Scholar]
- 78.Kang K, Kim YJ, Kim YH, Roh JN, Nam JM, Kim PY, et al. Lithium pretreatment reduces brain injury after intracerebral hemorrhage in rats. Neurol Res. 2012;34:447–454. doi: 10.1179/1743132812Y.0000000015. [DOI] [PubMed] [Google Scholar]
- 79.Zhang Z, Zhang ZY, Wu Y, Schluesener HJ. Valproic acid ameliorates inflammation in experimental autoimmune encephalomyelitis rats. Neuroscience. 2012;221:140–150. doi: 10.1016/j.neuroscience.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 80.Sublette ME, Ellis SP, Geant AL, Mann JJ. Meta-analysis of the effects of eicosapentaenoic acid (EPA) in clinical trials in depression. J Clin Psychiatry. 2011;72:1577–1584. doi: 10.4088/JCP.10m06634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sarris J, Mischoulon D, Schweitzer I. Omega-3 for bipolar disorder: meta-analyses of use in mania and bipolar depression. J Clin Psychiatry. 2012;73:81–86. doi: 10.4088/JCP.10r06710. [DOI] [PubMed] [Google Scholar]
- 82.Savitz J, Preskorn S, Teague TK, Drevets D, Yates W, Drevets W. Minocycline and aspirin in the treatment of bipolar depression: a protocol for a proof-of-concept, randomized, double-blind, placebo-controlled, 2x2 clinical trial. BMJ Open. 2012;2:e000643. doi: 10.1136/bmjopen-2011-000643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Berk M, Dean O, Drexhage H, McNeil JJ, Moylan S, O'Neil A, et al. Aspirin: a review of its neurobiological properties and therapeutic potential for mental illness. BMC Med. 2013;11:74. doi: 10.1186/1741-7015-11-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Nery FG, Monkul ES, Hatch JP, Fonseca M, Zunta-Soares GB, Frey BN, et al. Celecoxib as an adjunct in the treatment of depressive or mixed episodes of bipolar disorder: a double-blind, randomized, placebo-controlled study. Hum Psychopharmacol. 2008;23:87–94. doi: 10.1002/hup.912. [DOI] [PubMed] [Google Scholar]
- 85.Hlatky MA, Greenland P, Arnett DK, Ballantyne CM, Criqui MH, Elkind MS, et al. Criteria for evaluation of novel markers of cardiovascular risk: a scientific statement from the American Heart Association. Circulation. 2009;119:2408–2416. doi: 10.1161/CIRCULATIONAHA.109.192278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Munkholm K, Vinberg M, Vedel kessing L. Cytokines in bipolar disorder: a systematic review and meta-analysis. J Affect Disord. 2013;144:16–27. doi: 10.1016/j.jad.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 87.Modabbernia A, Taslimi S, Brietzke E, Ashrafi M. Cytokine alterations in bipolar disorder: a meta-analysis of 30 studies. Biol Psychiatry. 2013;74:15–25. doi: 10.1016/j.biopsych.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 88.Klein AB, Williamson R, Santini MA, Clemmensen C, Ettrup A, Rios M, et al. Blood BDNF concentrations reflect brain-tissue BDNF levels across species. Int J Neuropsychopharmacol. 2011;14:347–353. doi: 10.1017/S1461145710000738. [DOI] [PubMed] [Google Scholar]
- 89.Geller B, Badner JA, Tillman R, Christian SL, Bolhofner K, Cook EH., Jr Linkage disequilibrium of the brain-derived neurotrophic factor Val66Met polymorphism in children with a prepubertal and early adolescent bipolar disorder phenotype. Am J Psychiatry. 2004;161:1698–1700. doi: 10.1176/appi.ajp.161.9.1698. [DOI] [PubMed] [Google Scholar]
- 90.Lin PY. State-dependant decrease in levels of brain-derived neurotrophic factor in bipolar disorder: a meta-analytic study. Neurosci Lett. 2009;466:139–143. doi: 10.1016/j.neulet.2009.09.044. [DOI] [PubMed] [Google Scholar]
- 91.Munkholm K, Pedersen BK, Kessing LV, Vinberg M. Elevated levels of plasma brain derived neurotrophic factor in rapid cycling bipolar disorder patients. Psychoneuroendocrinology. 2014;47:199–211. doi: 10.1016/j.psyneuen.2014.05.011. [DOI] [PubMed] [Google Scholar]
- 92.Machado-Vieira R, Dietrich MO, Leke R, Cereser VH, Zanatto V, Kapcszinski F, et al. Decreased plasma brain derived neurotrophic factor levels in unmedicated bipolar patients during manic episode. Biol Psychiatry. 2007;61:142–144. doi: 10.1016/j.biopsych.2006.03.070. [DOI] [PubMed] [Google Scholar]
- 93.Fernandes BS, Gama CS, Kauer-Sant'Anna M, Lobato MI, Belmonte-de-Abreu P, Kapczinski F. Serum brain-derived neurotrophic factor in bipolar and unipolar depression: a potential adjunctive tool for differential diagnosis. J Psychiatr Res. 2009;43:1200–1204. doi: 10.1016/j.jpsychires.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 94.Fernandes BS, Gama CS, Cereser KM, Yatham LY, Fries GR, Colpo G, et al. Brain-derived neurotrophic factor as a state-marker of mood episodes in bipolar disorders: a systematic review and meta-regression analysis. J Psychiatr Res. 2011;45:995–1004. doi: 10.1016/j.jpsychires.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 95.Palomino A, Vallejo-Illarramendi A, Gonzales-Pinto A, Aldama A, Gonzalez-Gomez C, Mosquera F, et al. Decreased levels of plasma BDNF in first-episode schizophrenia and bipolar disorder patients. Schizophr Res. 2006;86:321–322. doi: 10.1016/j.schres.2006.05.028. [DOI] [PubMed] [Google Scholar]
- 96.Grande I, Kapczinski F, Stertz L, Colpo GD, Kunz M, Cereser KM, et al. Peripheral brain-derived neurotrophic factor changes along treatment with extended release quetiapine during acute mood episodes: an open-label trial in drug-free patients with bipolar disorder. J Psychiatr Res. 2012;46:1511–1514. doi: 10.1016/j.jpsychires.2012.08.017. [DOI] [PubMed] [Google Scholar]
- 97.de Sousa RT, van de Bilt MT, Diniz BS, Ladeira RB, Portela LV, Souza DO, et al. Lithium increases plasma brain-derived neurotrophic factor in acute bipolar mania: a preliminary 4 week study. Neurosci Lett. 2011;494:54–56. doi: 10.1016/j.neulet.2011.02.054. [DOI] [PubMed] [Google Scholar]
- 98.Dowlati Y, Herrmann N, Swardfager W, Liu H, Sham L, Reim EK, et al. A meta-analysis of cytokines in major depression. Biol Psychiatry. 2010;67:446–457. doi: 10.1016/j.biopsych.2009.09.033. [DOI] [PubMed] [Google Scholar]
- 99.Liu Y, Ho RC, Mak A. Interleukin (IL)-6, tumour necrosis factor alpha (TNF-alpha) and soluble interleukin-2 receptors (sIL-2R) are elevated in patients with major depressive disorder: a meta-analysis and meta-regression. J Affect Disord. 2012;139:230–239. doi: 10.1016/j.jad.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 100.Hannestad J, DellaGioia N, Bloch M. The effect of antidepressant medication treatment on serum levels of inflammatory cytokines: a meta-analysis. Neuropsychopharmacology. 2011;36:2452–2459. doi: 10.1038/npp.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Young JJ, Bruno D, Pomara N. A review of the relationship between proinflammatory cytokines and major depressive disorder. J Affect Disord. 2014;169:15–20. doi: 10.1016/j.jad.2014.07.032. [DOI] [PubMed] [Google Scholar]
- 102.Bai YM, Chiou WF, Su TP, Li CT, Chen MH. Pro-inflammatory cytokines associated with somatic and pain symptoms in depression. J Affect Disord. 2014;155:28–34. doi: 10.1016/j.jad.2013.10.019. [DOI] [PubMed] [Google Scholar]
- 103.O'Donovan A, Rush G, Hoatam G, Hughes BM, McCrohan A, Kelleher C, et al. Suicidal ideation is associated with elevated inflammation in patients with major depressive disorder. Depress Anxiety. 2013;30:307–314. doi: 10.1002/da.22087. [DOI] [PubMed] [Google Scholar]
- 104.Warner-Schmidt JL, Schmidt EF, Marshall JJ, Rubin AJ, Arango-Lievano M, Kaplitt MG, et al. Cholinergic interneurons in the nucleus accumbens regulate depression-like behavior. Proc Natl Acad Sci U S A. 2012;109:11360–11365. doi: 10.1073/pnas.1209293109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Anisman H, Du L, Palkovits M, Faludi G, Kovacs GG, Szontagh-Kishazi P, et al. Serotonin receptor subtype and p11 mRNA expression in stress-relevant brain regions of suicide and control subjects. J Psychiatry Neurosci. 2008;33:131–141. [PMC free article] [PubMed] [Google Scholar]