Abstract
Kaempferol exerts cardioprotective actions through incompletely understood mechanisms. This study investigated the molecular mechanisms underlying the cardioprotective effects of kaempferol in sinus node dysfunction (SND) heart. Here, we demonstrate that angiotensin II (Ang II) infusion causes SND through oxidized calmodulin kinase II (CaMKII). In contrast to this, kaempferol protects sinus node against Ang II-induced SND. Ang II evoked apoptosis with caspase-3 activation in sinus nodal cells. However, kaempferol lowered the CaMKII oxidization and the sinus nodal cell death. To block the CaMKII oxidization, gene of p47phox, a cytosolic subunit of NADPH oxidase, was deleted using Cas9 KO plasmid. In the absence of p47phox, sinus nodal cells were highly resistance to Ang II-induced apoptosis, suggesting that oxidized-CaMKII contributed to sinus nodal cell death. In Langendorff heart from Ang II infused mice, kaempferol preserved normal impulse formation at right atrium. These data suggested that kaempferol protects sinus node via inhibition of CaMKII oxidization and may be useful for preventing SND in high risk patients.
Keywords: Kaempferol, Sinus node dysfunction, CaMKII, Angiotensin II
INTRODUCTION
A normal heart beat is started electrically at sinus nodal cells from right atrium [1,2]. The electronic impulse is generated by Ca2+ based signaling pathways [3,4]. Among these, calmodulin kinase II (CaMKII) is a core signaling molecules in pacemaking myocardial cells, because it regulates major Ca2+ homeostatic proteins [4,5,6]. Under oxidative stress conditions, oxidized CaMKII generates its autonomous activity [7] and the excessive activity of CaMKII may lead to an abnormally slow heart rhythm or cardiac death [8,9,10]. In animal experiments, sinus node dysfunction (SND) occurs in conditions of increased oxidative stress [11] and high amounts of circulating angiotensin II (Ang II) with hypertension [12] or structural heart disease [13]. Interestingly, right atrial tissue from patients with heart failure who required artificial pacemakers had more oxidized CaMKII compared to patients with heart failure alone or without heart failure [14].
Previous studies have reported that long-term Ang II induced SND in animal hearts. In this models, Ang II activated NADPH oxidase, leading to oxidation of two methionine residues of CaMKII [7], rendering the protein autonomously active. The excessive CaMKII activity showed sinus pauses, reducing sinus node mass which is source of depolarizing current. In gene-targeting approaches, CaMKII inhibitory peptide expression [15] or NADPH oxidase depletion abrogated the increased sinoatrial cells (SAN) due to Ang II infusion at sinus nodal tissue [14,16]. The results suggested that the selective blockade of NADPH oxidase or CaMKII activity would be a good approach to reducing SND.
Epidemiological studies have demonstrated that some flavonoids may affect treatment for cardiovascular disease [17,18,19]. Kaempferol is a flavonoid which is abundant in a variety of plant derived food and leaves used in traditional medicines [20]. Numerous preclinical studies have documented the pharmacological activities of kaempferol, such as antioxidant and cardioprotection activities [20,21]. One of molecular mechanisms of kaempferol action is to bind p47phox directly, a cytosolic subunit of NADPH oxidase, and significantly inhibits NADPH oxidase activity [11,22].
Our findings describe elucidating mechanisms linking kaempferol action to the control of sinus node function.
MATERIALS AND METHODS
Isolated Langendorff heart and ex vivo electrocardiography (ECG) recording
Mice were anesthetized with avertin i.p., the thoracic cavity opened, and the heart carefully excised. After cannulation of the aorta, hearts were secured by tying below the brachiocephalic artery and perfused retrogradely by the nonrecirculating Langendorff technique with Krebs-Henseleit buffer containing 10 mmol/l glucose (pH 7.4). Perfusion fluid was controlled by a peristaltic pump continuously gassed with 95% O2/5% CO2. ECGs were continuously recorded with Ag+/AgCl electrodes, which were positioned around the hearts in an approximate Einthoven configuration.
Animals and mini-osmotic pump implantation
All in the C57BL/6 background, and corresponding wild type littermate controls, were used in experimental protocols approved by the Ewha Womans University Animal Care Committee. Mice were implanted with osmotic minipumps (ALZET) subcutaneously for delivery of saline (0.9% NaCl), Ang II (490 ng per kg body weight, A9525, Sigma-Aldrich, St. Louis, MO, USA), Ang II+kaempferol (0.5 mmol per kg) for 3 weeks.
Cell culture
Right atria were digested with collagenase II (0.1 g/1 ml, Worthington Inc., CA, USA) for 2 hours. Cells were plated on laminin-coated six-well culture plates with Leibovitz media (to a density of 200,000 cells/well). Cells were maintained using 20% fetal bovine serum (FBS)-Dulbecco's modified Eagle's medium and incubated at 37℃ under an atmosphere of 95% O2/5% CO2 for 5 days. Subsequently, and TBX3 (sinus node marker) and MLCA2 (cardiomyocyte marker) expression were determined using Western blotting.
Caspase-3 activity
Right atria or sinus nodal cells were homogenized in lysis buffer consisting of (50 mM Tris-HCl pH 7.5, 100 mM KCl, 1 mM ethylenediamine tetraacetic acid, 1 mM ethylene glycol tetraacetic acid, 1 mM dithiothreitol, 0.1 mM phenylmethylsulfonyl fluoride, 0.5 mM benzamidine, 20 mg/l leupeptin, 20 mM sodium pyrophosphate, 50 mM NaF, and 50 mM sodium β-glycerophosphate), and total protein content was determined by the Bradford assay. Caspase-3 activity was determined by EnzChek Caspase-3 Assay Kit (Invitrogen, Carlsbad, CA, USA).
Terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay
Cells were fixed with formalin for 1 hour and washed. Following permeabilization with 0.2% Triton-X 100, DNA fragmentation staining was performed with a TUNEL assay kit from Abcam Inc. (Boston, MA, USA). Apoptotic cells were counted using fluorescence microscopy.
Knockout of p47phox by Cas9 KO plasmid
Cas9 KO plasmid transfection of p47phox in sinus nodal cell was performed using a Lipofectamine 3000 Kit (Life Technologies, CA, USA). Briefly, in six-well culture plates, 0.1×106 cells were plated and subsequently exposed to the Cas9 KO plasmid (or empty vector control, Ctrl) solution for 8 hours at 37℃ in a CO2 incubator. Then, the media was changed to Leibovitz and the cells incubated for another 18 hours. Subsequently, ox-CaMKII, total-CaMKII, and p47phox (using Western blotting) were determined.
Western blot
Right atria (50 mg) or plated sinus nodal cells (0.4×106) were homogenized in ice-cold lysis buffer. After centrifugation at 20,000 ×g for 20 minutes, the protein content of the supernatant was quantified using a Bradford protein assay. Samples were diluted, boiled with sample loading dye, and 100 µg used in sodium dodecyl sulfate polyacrylamide gel electrophoresis. After blotting, membranes were blocked in 5% skim milk in phosphate-buffered saline containing 0.1% Tween-20. Membranes were incubated with rabbit ox-CaMKII, total-CaMKII, and p47phox, TBX3, MLCA2 antibodies and subsequently with secondary goat anti-rabbit horseradish peroxidase-conjugated antibody. Reaction products were visualized using an enhanced chemiluminescence detection kit and quantified by densitometry.
Materials
Total CaMKII, TBX3, and MLCA2 antibodies were obtained from Abcam Inc. Ox-CaMKII was purchased from GeneTex Inc. (Irvine, CA, USA). Beta-actin was purchased from Cell Signaling Technology Inc. (Danvers, Ma, USA). Kaempferol was obtained from Sigma-Aldrich. Cas9 KO plasmid and antibody of p47phox were purchased from Santa Cruz Inc. (Dallas, TX, USA). The enhanced chemiluminescence detection kit was obtained from Amersham (Pittsburgh, PA, USA).
Statistical analysis
Values are means±SEM. Wherever appropriate, one-way ANOVA followed by the Bonferroni test was used to determine differences between group mean values. The level of statistical significance was set at P<0.05.
RESULTS
Ang II infusion causes SND with CaMKII oxidization
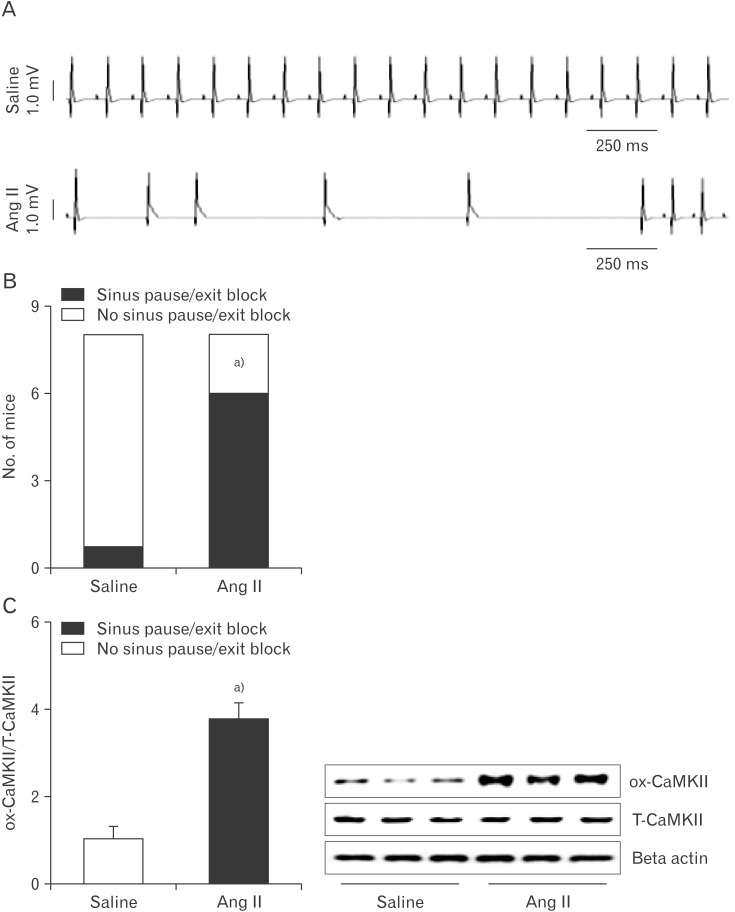
Previous studies have reported that significantly higher oxidized CaMKII promoted sinus dysfunction from heart failure patients and hypertensive animals [14]. In order to determine whether our model of Ang II infusion promoted SND, we measured ECG in Langendorff-perfused hearts isolated from Ang II or saline infused mice for 3 weeks (Fig. 1A). Ang II-infused hearts exhibited frequent sinus pauses, similar to patients who required artificial pacemakers (Fig. 1B). An approximately 3.5-fold increase of cardiac CaMKII oxidization was observed from right atrium following Ang II infusion (Fig. 1C). In contrast to the changes in oxidized CaMKII, total right atrial CaMKII expression was equivalent in Ang II- and saline-infused mice.
Fig. 1. CaMKII oxidization and sinus node dysfunction in Ang II-infused mice. (A) Representative ECG recordings from Langendorff-perfused hearts isolated from mice infused with Ang II or saline for 3 weeks. (B) Ang II-infused mice have more sinus pauses than saline-infused mice. (C) Western blots shows ox-CaMKII and T-CaMKII from right atrial tissue obtained from infused with Ang II or saline for 3 weeks. Ang II, angiotensin II; CaMKII, calmodulin kinase II; ECG, electrocardiography; ox-CaMKII, oxidized-CaMKII; T-CaMKII, total CaMKII. Data are shown as the means±SEM of 3-6 mice per group. a)Significantly different (P<0.05) from saline infused control.
Ang II induces sinus nodal cell death with CaMKII oxidization
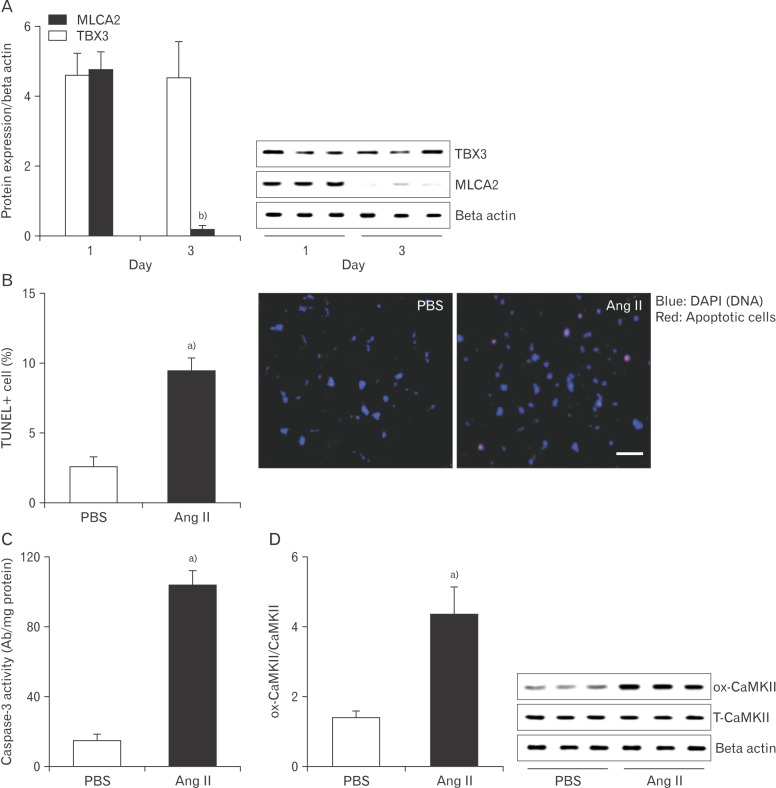
It has been reported that an excessive sinus nodal cell death contributed to SND [23]. Therefore, we hypothesized that elevated Ang II results in sinus nodal cell death. To determine this, we prepared primary cell cultures of the sinus node cells from right atrium. Initially, the isolated right atria cells had both Tbx3 (sinus node marker) [24] and MLC2A (atrial cardiomyocyte marker) (Fig. 2A) [25,26]. Following FBS incubation for 3 days, only Tbx3 expressed cells were occupied and incubated with Ang II or phosphate buffered saline (PBS) for 5 more days. We found that Ang II treated cells had significantly increased DNA fragmentation (Fig. 2B) and caspase-3 activity (Fig. 2C), compared with PBS treated sinus nodal cells. And an approximately 3.5-fold increase of CaMKII oxidization was observed from sinus nodal cells following Ang II (Fig. 2D).
Fig. 2. Effect of Ang II on apoptosis with CaMKII oxidation. Cells were isolated from right atria and incubated with FBS for 1 day or 3 days. (A) Following the indicated days, protein was extracted to determine TBX3 (sinus node marker) and MLCA2 (cardiomyocyte marker) using Western blotting. Cells with dominant TBX3 expression were isolated and maintained using 20% FBS-DMEM. Subsequently, Ang II (20 µM) was added to the culture medium for 5 days. (B) Apoptotic cells were counted using TUNEL assay. Protein was extracted to measure caspase-3 activity (C) and determined ox-CaMKII and T-CaMKII using Western blotting (D). Ang II, angiotensin II; CaMKII, calmodulin kinase II; DMEM, Dulbecco's modified Eagle's medium; FBS, fetal bovine serum; ox-CaMKII, oxidized-CaMKII; PBS, phosphate buffered saline; T-CaMKII, total CaMKII; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling. Data are shown as the means±SEM of 3-6 mice per group. a)Significantly different (P<0.05) from PBS treated control. b)Significantly different (P<0.05) from right atrial cells at 1st day. Scale bar=50 µm.
CaMK II oxidization requires to Ang II-induced sinus nodal cell apoptosis
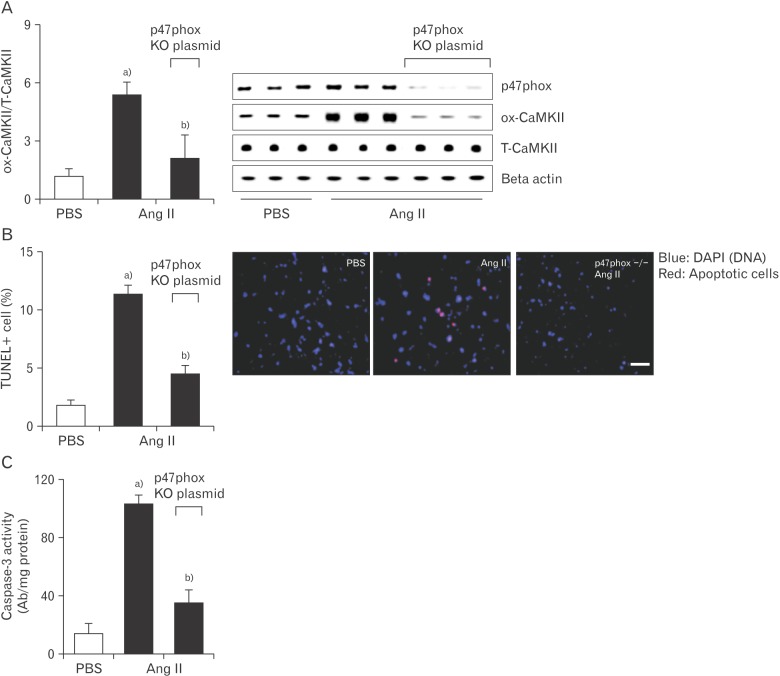
To confirm the relationship between CaMKII oxidization and sinus nodal cell death, we used Cas9 KO plasmid to deplete p47phox expression in isolated sinus nodal cells. In sinus nodal cells in which p47phox was deleted, Ang II had no influence on total p47phox, which remained low and was unable to oxidize CaMKII (Fig. 3A) or increase apoptosis (Fig. 3B, C).
Fig. 3. Knockout of p47phox prevents sinus nodal cell death and CaMKII oxidization observed with Ang II. Cas9 KO plasmid transfection of p47phox in sinus nodal cell was performed using a Lipofectamine 3000. Plated cells were exposed to the Cas9 KO plasmid or empty vector control. After this, Ang II (20 µM) was added to the culture medium for 5 days. From these cells, ox-CaMKII levels (A), apoptotic cells (B), and caspase-3 activity (C) were evaluated. Ang II, angiotensin II; CaMKII, calmodulin kinase II; ox-CaMKII, oxidized-CaMKII; PBS, phosphate buffered saline; T-CaMKII, total CaMKII; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling. Data are shown as the means±SEM of 3-6 mice per group. a)Significantly different (P<0.05) from PBS treated control. b)Significantly different (P<0.05) from Ang II treated control. Scale bar=50 µm.
Kaempferol attenuates CaMKII oxidization and sinus nodal cell death
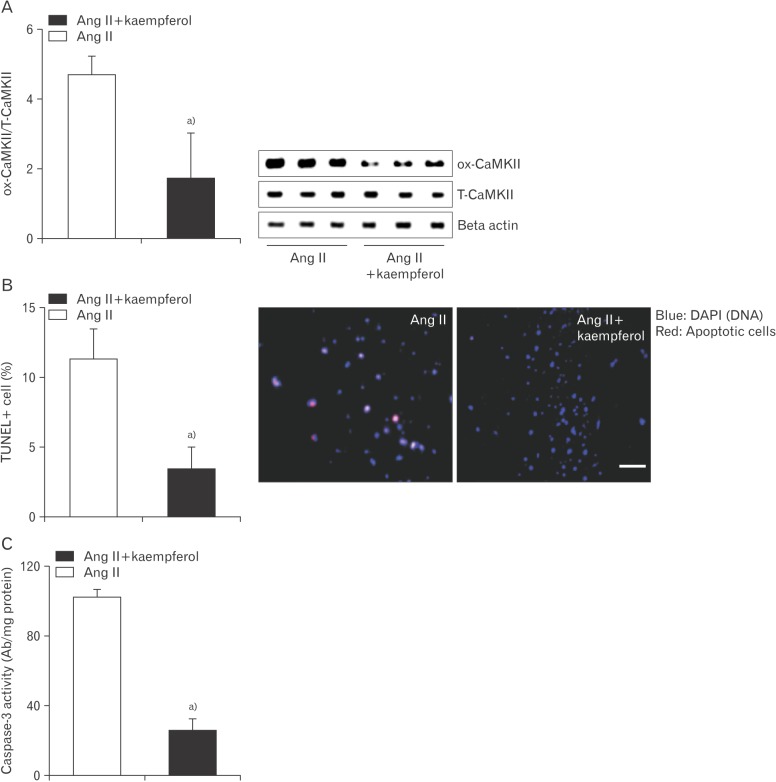
Given the observation that kaempferol decrease reactive oxygen species (ROS) by directly bound NADPH oxidase [22], we hypothesized that kaempferol prevents Ang II-induced sinus nodal cell death by lowering CAMKII oxidization. Indeed, following kaempferol, a drop of CaMKII oxidization was observed in Ang II-treated sinus nodal cells (Fig. 4A). Moreover, sinus nodal cell apoptosis was decreased due to the deoxidized CaMKII by kaempferol (Fig. 4B, C).
Fig. 4. Kaempferol reduces cell death by lowering CaMKII oxidization. Sinus nodal cells were incubated with Ang II (20 µM) in the absence or presence of a kaempferol (15 mM) for 5 days. Protein was extracted to determine ox-CaMKII using Western blotting (A) and measure caspase-3 activity (C). Apoptotic cells were counted using TUNEL assay (B). Ang II, angiotensin II; CaMKII, calmodulin kinase II; ox-CaMKII, oxidized-CaMKII; T-CaMKII, total CaMKII; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling. Data are shown as the means±SEM of 3-6 mice per group. a)Significantly different (P<0.05) from Ang II treated control. Scale bar=50 µm.
Kaempferol protects against SND
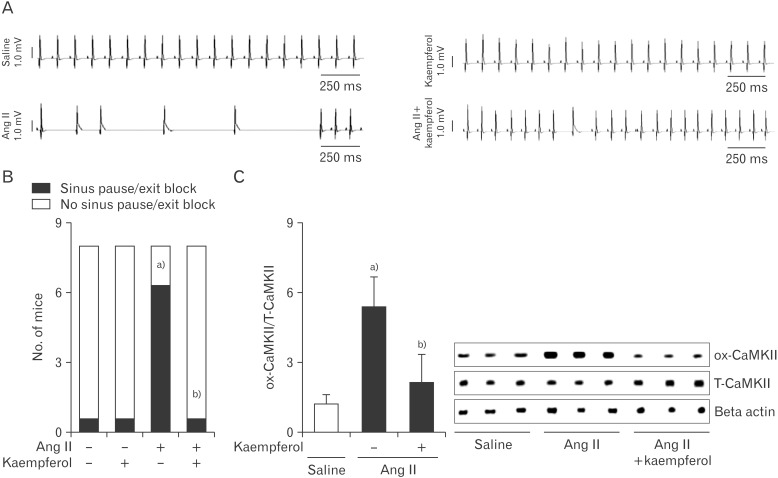
As normal sinus node function requires a critical mass of sinus nodal cells to form impulses and electrical signals, we hypothesized that kaempferol improves the recovery of Ang II-induced SND by increasing cell survival. To confirm this, we infused Ang II or Ang II with kaempferol subcutaneously for 3 week and measured ECG and Langendorff-perfused hearts. Ang II-infused mice exhibited frequent sinus pauses (Fig. 5B) and the P-P wave interval was greater than the baseline sinus cycle length (Fig. 5A), compared with saline-infused control mice. In contrast, kaempferol produced a significant reduction of sinus pauses and turned back into normal sinus cycle length from Ang II-induced abnormal wave (Fig. 5A). In addition to this, kaempferol had no effect on saline-infused sinus node function. Moreover, kaempferol significantly decreased the Ang II-induced CaMK II oxidization (Fig. 5C).
Fig. 5. Kaempferol improves the recovery of Ang II-induced sinus nodal dysfunction. (A) ECG recordings from Langendorff-perfused hearts isolated from mice infused with Ang II in the absence or presence of a kaempferol for 3 weeks. (B) Together with kaempferol, Ang II infused mice decreased sinus pauses than Ang II-infused alone. (C) Western blots demonstrates ox-CaMKII and T-CaMKII from right atrial tissue obtained from infused with Ang II, Ang II+kaempferol, or saline for 3 weeks. Ang II, angiotensin II; CaMKII, calmodulin kinase II; ECG, electrocardiography; ox-CaMKII, oxidized-CaMKII; T-CaMKII, total CaMKII. Data are shown as the means±SEM of 3-6 mice per group. a)Significantly different (P<0.05) from Saline treated control. b)Significantly different (P<0.05) from Ang II treated control.
DISCUSSION
A large portion of mortality in hospitalized heart failure patients (~40%) may be secondary to SND [27,28]. Patients with SND may due to genetic syndromes. For instance, defects of HCN4 or ankyrins promotes SND [29,30]. However, these genetic deficiency is rare in SND and it is very little known about the common molecular pathways leading to SND. In severe SND human hearts, ROS is elevated [7,31] and then CaMKII is highly oxidized with an increase in plasma Ang II levels [7,14]. ROS causes damage to the cell membranes, organelles, proteins in heart, subsequently causes structural failure and cell death [15]. As sinus node requires a critical size to maintain stable rhythm and adapt its beating rate, ROS can initiate SND.
Given the observation that sinus node cell pacemaker activity is depressed by the CaMKII inhibitor in a dose-dependent manner, CaMKII is major molecule in Ca2+ based signaling pathways to regulate sinus node function [3,8]. Interestingly, following its oxidization, over-activated CaMKII promotes cell death, it may contributes to arrhythmia, heart failure, and sudden death [14,16]. Indeed, Ang II infusion accelerated SND in wild type mice but failed to induce SND in CaMKII inhibitory peptide treated mice or transgenic mice [14]. Taken together, the results suggest that selective CaMKII inhibitors will protect sinus node and prevent SND under oxidative stress. However, at present there are no biological therapeutics to arrest or prevent SND in high-risk patients.
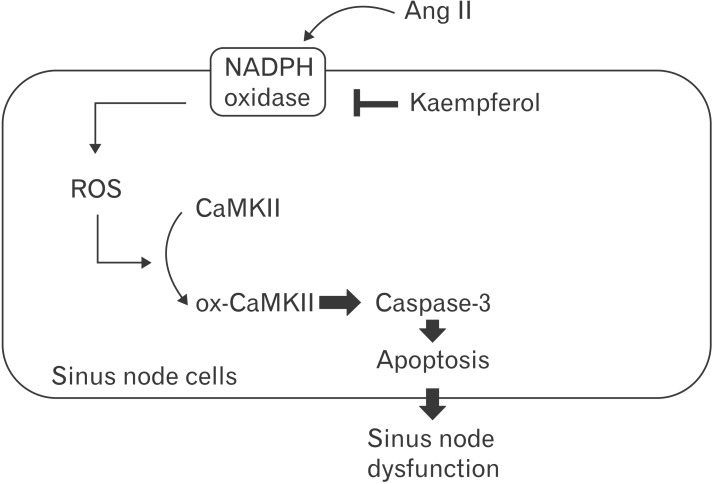
Among antioxidants, it has been known that kaempferol is protective against common causes of cardiomyopathy, including myocardial infarction and ischemia [32]. But kaempferol has not previously been considered as a clinical molecule in SND. In this study, using in vitro and ex vivo models, we investigated whether kaempferol required to protect the heart against sinus nodal cell death and subsequently prevent severe SND. To mimic SND animal models, sinus nodal cells were isolated and incubated with Ang II for 5 days. Although Ang II evoked cell death with CaMKII oxidation, kaempferol decreased the CaMKII oxidation and apoptotic cell death. It has been known that CaMKII oxidation is accelerated by NADPH oxidase in vivo and kaempferol inhibits NADPH oxidase activity through directly binding in the subunit (p47phox) [11,14,22]. In vitro, for the first time, sinus nodal cells were isolated and p47phox gene was deleted. Ang II was not capable to induce apoptosis in the p47phox knock out cells. Furthermore, Ang II induced frequent sinus pauses and abnormal electronic impulse cycles were normalized by kaempferol in Langendorff heart (Fig. 5). These isolated SAN cells and ex vivo models are suggesting that Ang II-induced SND is primarily due to a loss of SAN cell density and kaempferol protects sinus node function by reducing the apoptotic cell death (Fig. 6). Therefore, we speculated that kaempferol is a good antioxidant to protect sinus node.
Fig. 6. Schematic mechanism for kaempferol regulation of sinus node protection. After activation of sinus nodal NADPH oxidase with Ang II, increased amounts of ROS promote CaMKII oxidization which then mediates apoptosis through caspase-3. Following the apoptosis, reducing the sinus node volume causes sinus node dysfunction. Inhibition of NADPH oxidase by kaempferol attenuates CaMKII oxidization and apoptosis of sinus nodal cells, leading to a reduction of sinus node dysfunction. Ang II, angiotensin II; CaMKII, calmodulin kinase II; ox-CaMKII, oxidized-CaMKII; ROS, reactive oxygen species.
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education (2014R1A1A2064 810). And also the work was supported by the Ewha Womans University Research Grant of 2014.
References
- 1.Liu J, Xin L, Benson VL, Allen DG, Ju YK. Store-operated calcium entry and the localization of STIM1 and Orai1 proteins in isolated mouse sinoatrial node cells. Front Physiol. 2015;6:69. doi: 10.3389/fphys.2015.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Milanesi R, Bucchi A, Baruscotti M. The genetic basis for inherited forms of sinoatrial dysfunction and atrioventricular node dysfunction. J Interv Card Electrophysiol. 2015;43:121–134. doi: 10.1007/s10840-015-9998-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Swulius MT, Waxham MN. Ca(2+)/calmodulin-dependent protein kinases. Cell Mol Life Sci. 2008;65:2637–2657. doi: 10.1007/s00018-008-8086-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang LZ, Kockskämper J, Khan S, Suarez J, Walther S, Doleschal B, Unterer G, Khafaga M, Mächler H, Heinzel FR, Dillmann WH, Pieske B, Spiess J. cAMP- and Ca(2)(+) /calmodulin-dependent protein kinases mediate inotropic, lusitropic and arrhythmogenic effects of urocortin 2 in mouse ventricular myocytes. Br J Pharmacol. 2011;162:544–556. doi: 10.1111/j.1476-5381.2010.01067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y, Cui H, Wang W, Zhao B, Lai J. The region-specific activation of Ca2+/calmodulin dependent protein kinase II and extracellular signal-regulated kinases in hippocampus following chronic alcohol exposure. Brain Res Bull. 2012;89:191–196. doi: 10.1016/j.brainresbull.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 6.Huynh QK, Pagratis N. Kinetic mechanisms of Ca++/calmodulin dependent protein kinases. Arch Biochem Biophys. 2011;506:130–136. doi: 10.1016/j.abb.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 7.Erickson JR, Joiner ML, Guan X, Kutschke W, Yang J, Oddis CV, Bartlett RK, Lowe JS, O'Donnell SE, Aykin-Burns N, Zimmerman MC, Zimmerman K, Ham AJ, Weiss RM, Spitz DR, Shea MA, Colbran RJ, Mohler PJ, Anderson ME. A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell. 2008;133:462–474. doi: 10.1016/j.cell.2008.02.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santalla M, Valverde CA, Harnichar E, Lacunza E, Aguilar-Fuentes J, Mattiazzi A, Ferrero P. Aging and CaMKII alter intracellular Ca2+ transients and heart rhythm in Drosophila melanogaster. PLoS One. 2014;9:e101871. doi: 10.1371/journal.pone.0101871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Carlo MN, Said M, Ling H, Valverde CA, De Giusti VC, Sommese L, Palomeque J, Aiello EA, Skapura DG, Rinaldi G, Respress JL, Brown JH, Wehrens XH, Salas MA, Mattiazzi A. CaMKII-dependent phosphorylation of cardiac ryanodine receptors regulates cell death in cardiac ischemia/reperfusion injury. J Mol Cell Cardiol. 2014;74:274–283. doi: 10.1016/j.yjmcc.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szobi A, Rajtik T, Carnicka S, Ravingerova T, Adameova A. Mitigation of postischemic cardiac contractile dysfunction by CaMKII inhibition: effects on programmed necrotic and apoptotic cell death. Mol Cell Biochem. 2014;388:269–276. doi: 10.1007/s11010-013-1918-x. [DOI] [PubMed] [Google Scholar]
- 11.Zhao HR, Jiang T, Tian YY, Gao Q, Li Z, Pan Y, Wu L, Lu J, Zhang YD. Angiotensin II triggers apoptosis via enhancement of NADPH oxidase-dependent oxidative stress in a dopaminergic neuronal cell line. Neurochem Res. 2015;40:854–863. doi: 10.1007/s11064-015-1536-y. [DOI] [PubMed] [Google Scholar]
- 12.Carney EF. Hypertension: vascular type 1A angiotensin II receptors regulate renal blood flow and natriuresis. Nat Rev Nephrol. 2015;11:318. doi: 10.1038/nrneph.2015.69. [DOI] [PubMed] [Google Scholar]
- 13.Rincon J, Correia D, Arcaya JL, Finol E, Fernández A, Pérez M, Yaguas K, Talavera E, Chávez M, Summer R, Romero F. Role of angiotensin II type 1 receptor on renal NAD(P)H oxidase, oxidative stress and inflammation in nitric oxide inhibition induced-hypertension. Life Sci. 2015;124:81–90. doi: 10.1016/j.lfs.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swaminathan PD, Purohit A, Soni S, Voigt N, Singh MV, Glukhov AV, Gao Z, He BJ, Luczak ED, Joiner ML, Kutschke W, Yang J, Donahue JK, Weiss RM, Grumbach IM, Ogawa M, Chen PS, Efimov I, Dobrev D, Mohler PJ, Hund TJ, Anderson ME. Oxidized CaMKII causes cardiac sinus node dysfunction in mice. J Clin Invest. 2011;121:3277–3288. doi: 10.1172/JCI57833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang R, Khoo MS, Wu Y, Yang Y, Grueter CE, Ni G, Price EE, Jr, Thiel W, Guatimosim S, Song LS, Madu EC, Shah AN, Vishnivetskaya TA, Atkinson JB, Gurevich VV, Salama G, Lederer WJ, Colbran RJ, Anderson ME. Calmodulin kinase II inhibition protects against structural heart disease. Nat Med. 2005;11:409–417. doi: 10.1038/nm1215. [DOI] [PubMed] [Google Scholar]
- 16.Luo M, Guan X, Luczak ED, Lang D, Kutschke W, Gao Z, Yang J, Glynn P, Sossalla S, Swaminathan PD, Weiss RM, Yang B, Rokita AG, Maier LS, Efimov IR, Hund TJ, Anderson ME. Diabetes increases mortality after myocardial infarction by oxidizing CaMKII. J Clin Invest. 2013;123:1262–1274. doi: 10.1172/JCI65268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCullough ML, Peterson JJ, Patel R, Jacques PF, Shah R, Dwyer JT. Flavonoid intake and cardiovascular disease mortality in a prospective cohort of US adults. Am J Clin Nutr. 2012;95:454–464. doi: 10.3945/ajcn.111.016634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Esmaillzadeh A, Azadbakht L. Dietary flavonoid intake and cardiovascular mortality. Br J Nutr. 2008;100:695–697. doi: 10.1017/S0007114508945700. [DOI] [PubMed] [Google Scholar]
- 19.Chong MF, George TW, Alimbetov D, Jin Y, Weech M, Macready AL, Spencer JP, Kennedy OB, Minihane AM, Gordon MH, Lovegrove JA. Impact of the quantity and flavonoid content of fruits and vegetables on markers of intake in adults with an increased risk of cardiovascular disease: the FLAVURS trial. Eur J Nutr. 2013;52:361–378. doi: 10.1007/s00394-012-0343-3. [DOI] [PubMed] [Google Scholar]
- 20.Barbosa E, Calzada F, Campos R. In vivo antigiardial activity of three flavonoids isolated of some medicinal plants used in Mexican traditional medicine for the treatment of diarrhea. J Ethnopharmacol. 2007;109:552–554. doi: 10.1016/j.jep.2006.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Xiao HB, Jun F, Lu XY, Chen XJ, Chao T, Sun ZL. Protective effects of kaempferol against endothelial damage by an improvement in nitric oxide production and a decrease in asymmetric dimethylarginine level. Eur J Pharmacol. 2009;616:213–222. doi: 10.1016/j.ejphar.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 22.Wang SB, Jang JY, Chae YH, Min JH, Baek JY, Kim M, Park Y, Hwang GS, Ryu JS, Chang TS. Kaempferol suppresses collagen-induced platelet activation by inhibiting NADPH oxidase and protecting SHP-2 from oxidative inactivation. Free Radic Biol Med. 2015;83:41–53. doi: 10.1016/j.freeradbiomed.2015.01.018. [DOI] [PubMed] [Google Scholar]
- 23.James TN, St Martin E, Willis PW, 3rd, Lohr TO. Apoptosis as a possible cause of gradual development of complete heart block and fatal arrhythmias associated with absence of the AV node, sinus node, and internodal pathways. Circulation. 1996;93:1424–1438. doi: 10.1161/01.cir.93.7.1424. [DOI] [PubMed] [Google Scholar]
- 24.Hoogaars WM, Engel A, Brons JF, Verkerk AO, de Lange FJ, Wong LY, Bakker ML, Clout DE, Wakker V, Barnett P, Ravesloot JH, Moorman AF, Verheijck EE, Christoffels VM. Tbx3 controls the sinoatrial node gene program and imposes pacemaker function on the atria. Genes Dev. 2007;21:1098–1112. doi: 10.1101/gad.416007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Myers FB, Silver JS, Zhuge Y, Beygui RE, Zarins CK, Lee LP, Abilez OJ. Robust pluripotent stem cell expansion and cardiomyocyte differentiation via geometric patterning. Integr Biol (Camb) 2013;5:1495–1506. doi: 10.1039/c2ib20191g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lian X, Hsiao C, Wilson G, Zhu K, Hazeltine LB, Azarin SM, Raval KK, Zhang J, Kamp TJ, Palecek SP. Robust cardiomyocyte differentiation from human pluripotent stem cells via temporal modulation of canonical Wnt signaling. Proc Natl Acad Sci U S A. 2012;109:E1848–E1857. doi: 10.1073/pnas.1200250109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jordan JL, Yamaguchi I, Mandel WJ. Studies on the mechanism of sinus node dysfunction in the sick sinus syndrome. Circulation. 1978;57:217–223. doi: 10.1161/01.cir.57.2.217. [DOI] [PubMed] [Google Scholar]
- 28.Nitardy A, Langreck H, Dietz R, Stockburger M. Reduction of right ventricular pacing in patients with sinus node dysfunction through programming a long atrioventricular delay along with the DDIR mode. Clin Res Cardiol. 2009;98:25–32. doi: 10.1007/s00392-008-0716-z. [DOI] [PubMed] [Google Scholar]
- 29.Le Scouarnec S, Bhasin N, Vieyres C, Hund TJ, Cunha SR, Koval O, Marionneau C, Chen B, Wu Y, Demolombe S, Song LS, Le Marec H, Probst V, Schott JJ, Anderson ME, Mohler PJ. Dysfunction in ankyrin-B-dependent ion channel and transporter targeting causes human sinus node disease. Proc Natl Acad Sci U S A. 2008;105:15617–15622. doi: 10.1073/pnas.0805500105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nof E, Antzelevitch C, Glikson M. The contribution of HCN4 to normal sinus node function in humans and animal models. Pacing Clin Electrophysiol. 2010;33:100–106. doi: 10.1111/j.1540-8159.2009.02563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JM, Kalman JM. Sinus node dysfunction and atrial fibrillation: two sides of the same coin? Europace. 2013;15:161–162. doi: 10.1093/europace/eus223. [DOI] [PubMed] [Google Scholar]
- 32.Kim DS, Ha KC, Kwon DY, Kim MS, Kim HR, Chae SW, Chae HJ. Kaempferol protects ischemia/reperfusion-induced cardiac damage through the regulation of endoplasmic reticulum stress. Immunopharmacol Immunotoxicol. 2008;30:257–270. doi: 10.1080/08923970701812530. [DOI] [PubMed] [Google Scholar]