Abstract
The mechanism of Western medicine that is commonly used for pain relief is well-known. However, very little is known for oriental herbs, and even less is known for mixture of the two. We investigated the combinational effect of 3 kinds of oriental herbs, usually used for the control of headache, and acetaminophen to relieve headache in microglia cell line, BV2. Lipopolysaccharide (LPS) stimulation induced to produce nitrite and increased the expression of inflammation-related factors like inducible nitric oxide synthase and cyclooxygenase-2 (COX-2) in murine microglia cell line, BV2. Oriental herbs such as Angelica tenuissima, Angelica dahurica, and Scutellaria baicalensis reduced the production of nitric oxide and the expression of COX-2. Moreover, a treatment of acetaminophen combined with oriental herbs was more decreased the COX-2 expression, and its product, prostaglandin E2 production in BV2 cells. Therefore, a combined treatment of oriental herbs such as A. tenuissima, A. dahurica, and S. baicalensis and Western medicine like acetaminophen has a synergistic effect on the decrease of LPS-induced inflammation in microglia.
Keywords: Acetaminophen, Angelica tenuissima, Angelica dahurica, Scutellaria baicalensis, Microglial inflammation
Introduction
Acetaminophen is used worldwide as an antipyretic and analgesic drug. Even though it is used to treat inflammatory pain, it is not generally classified as a non-steroidal anti-inflammatory drug because it exhibits only weak anti-inflammatory activity [1]. The mild anti-oxidant and anti-inflammatory effect of acetaminophen on central nervous system has been studied. For example, acetaminophen protects neurons in hippocampus from amyloid beta peptide-induced oxidative stress by reducing lipid peroxidation and cytoplasmic levels of peroxides, and blunted neuronal apoptosis via reduction of the inflammatory transcription factor nuclear factor κB [2]. It also protects dopamingeric neurons in vitro from oxidative damage evoked by acute exposure to 6-hydroxydopamine or excessive levels of dopamine [3]. In addition, low dose of acetaminophen reduces the release of inflammatory proteins such as tumor necrosis factor α, interleukin 1, macrophage inflammatory protein 1α, and RANTES from cultured brain endothelial cells exposed to oxidant stress [4].
A lot of oriental herbs have been reported to relieve pains and to have anti-inflammatory function. Among them, Scutellaria baicalensis Geogri (S. baicalensis) has long been used as a multi-purpose herb in China, Japan, Korea and other oriental countries because of its anti-pyretic, anti-bacterial, anti-viral, and anti-inflammatory properties. It showed anti-inflammatory effects via decreasing the expression of inducible nitric oxide synthase (iNOS), cyclooxygenase (COX)-2, IκB kinase αβ (IKKαβ) phosphorylation, and IκBα phosphorylation [5,6]. Flavones extracted from S. baicalensis exert potent-anti-inflammatory and anti-oxidant activity for neuroprotective effects [7]. Angelica tenuissima Nakai (A. tenuissima) suppressed nitric oxide production and iNOS expression without any notable cytotoxicity and it also inhibited the expression of inflammatory enzymes like COX-2 in interferon (IFN)-γ/lipopolysaccharide (LPS)-stimulated peritoneal macrophage [8]. Angelica dahurica Bentham et Hooker (A. dahurica) also showed anti-inflammatory activity on macrophages via up-regulation of heme oxygenase-1 (HO-1) [9].
Macrophages play important roles in immune reactions, allergy and inflammation by releasing different types of cytokines [10]. In the brain, the proliferation and degree of activation of microglia and astrocyte determines the level of inflammation [11,12]. The neuro-immune system plays a crucial role in headache and chronic pain. Activated microglia and astrocytes produce and release a variety of neuroexcitatory substances including nitric oxide, excitatory amino acids and pro-inflammatory cytokines. Spinal glial activation and the subsequent release of pro-inflammatory mediators initiate and maintain a range of enhanced pain states [13,14]. Most cells in the brain and spinal cord are neuroglia, not neurons. Because it turns out neuroglia do far more than supporting cells, therapies targeting glia may prove very helpful in headache, as well as depression and other neurologic illnesses.
A. tenuissima, A. dahurica, and S. baicalensis are used for the control of headache in oriental medicines. And acetaminophen is commonly used for control of headache in Western medicine. Although acetaminophen is actually taken with the extracts of above 3 kinds of oriental herbs to control of headache in Korea, but it is still largely unknown about their combinational effects on the control of headache. In the present study, we therefore, examined whether they have additive or synergistic effects in the LPS-stimulated microglia cells.
Materials and Methods
Preparation of Oriental herbs
Angelica tenuissima Nakai (A. tenuissima), Angelica dahurica Bentham et Hooker (A. dahurica), and Scutellaria baicalensis Geogri (S. baicalensis) was added to purified water (in volume of 10 times) and extracted at 100℃ for 3 hours. The resulting materials were filtered at temperature lower than 60℃ using 200 µm mesh then decompressed. The dry filtrate was diluted with silicon dioxide at a ratio of 99.975:0.025 (dry filtrate:silicon dioxide), and packed into a vacuum-sealed capsule. Using the transference number, calculated amount of the medicine was dissolved in dimethyl sulfoxide and filtered for treating to cells.
Microglia cell line
Murine microglia cell line, BV2 cell line was obtained from Korea Cell line Bank in Seoul National University College of Medicine. BV2 cells were cultured with RPMI1640 media containing 10% heat-inactivated fetal bovine serum, 2 mM L-glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin.
Reverse transcriptase-polymerase chain reaction (RT-PCR)
Total RNA was extracted from BV2 cells using TRizol Reagent (Invitrogen, Camarillo, CA, USA) according to the manufacturer's instruction. RNA quantity was monitored at 260 nm with NanoDrop (Thermo Scientific, Wilmington, DE, USA). Total RNA (1 µg) was reverse transcribed to cDNA and amplified with the following primers to detect the expression of iNOS on the BV2 cell surfaces. iNOS (sense: 5'-CGG TGC TGT ATT TCC TTA CGA GGC GAA GAA GG-3', antisense: 5'-GGT GCT GTC TGT TAG GAG GTC AAG TAA AGG GC-3'; product, 510 bp), β-actin (sense: 5'-AAG AGC TAT GAG CTG CCT GA-3', antisense: 5'-CAG GAG GAG CAA TGA TCT TG-3'; product, 220 bp). Polymerase chain reaction products were separated on 1.5% agarose gel, and visualized under UV light after ethidium bromide staining.
Measurement of nitrite
BV2 (5×105 cells per well) cells were seeded in 6-well plate, and incubated overnight. Then, BV2 cells were treated with 0.1, 1, and 10 µg/ml concentration of LPS for 24 hours or with 1 µg/ml concentration of LPS for 3, 6, 12, and 24 hours. Then, culture media were collected and nitrite production in the media was measured with Griess assay kit (Intron, Seongnam, Korea) according to the manufacture's instruction.
Immunoblotting
BV2 cells were treated with 1 mg/ml concentration of LPS for 24 hours. Cells were lysed with buffer containing 50 mM Tris-HCl (pH 7.4), 1% NP-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM ethylenediaminetetraacetic acid, and protease inhibitor cocktail. Fifty micrograms of proteins were electrophoresed to 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis gel and transferred to nitrocellulose membrane. Membrane was blocked with 5% skim milk and incubated with anti-COX-2 antibody (Santa Cruz, CA, USA) at 4℃ overnight. Followed by incubation with horseradish peroxidase-conjugated anti-rabbit IgG, bands were detected with ECL detection kit (Amersham, Piscataway, NJ, USA). Detected bands were quantified using a densitometry analysis program (SCION Image Program, SCION Corp., Frederick, MD, USA).
Measurement of prostaglandin E2 (PGE2)
BV2 cells were treated with 1 µg/ml concentration of LPS, oriental herbs (A. tenuissima, A. dahurica, or S. baicalensis with 1/500 dilution) and acetaminophen (100 µM) for 12 hours. Culture media were collected and the concentration of PGE2 was measured with ELISA kit (SAPPHIRE BIOSCIENCE, Redfern, Australia) according to the manufacture's instruction.
Statistics
Data were expressed as mean±SEM of each group in independent experiments. For comparison of three or more groups, data were analyzed by t test or one-way analysis of variance (ANOVA) followed by Newman-Keuls multiple comparison test. A value of P<0.05 was considered statistically significant. Statistical tests were carried out using GraphPad InStat (GraphPad Software, San Diego, CA, USA).
Results
LPS stimulation increases the inflammation in BV2 cells
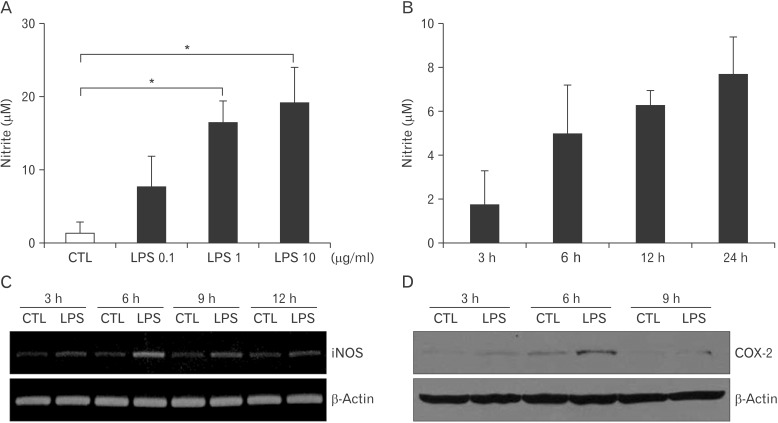
First, we examine the expression of Toll-like receptor 4 (TLR4) on microglia cell line, BV2. Based on RT-PCR, BV2 cells highly expressed TLR4 (Electronic Supplementary Fig. 1). Then, LPS was treated to BV2 cells, and the production of nitric oxide (NO) was examined. Due to the short half-life of NO, the concentration of nitrite as an indicator of NO was measured by Griess assay. As a result, LPS stimulated the release of NO from BV2 cells in a dose-dependent manner and in a time-dependent manner (Fig. 1A, B). A treatment of LPS (1 µg/ml) increased the transcriptional expression of iNOS, which was peak at 6 hours (Fig. 1C). Because COX-2 is usually specific to inflammation, the expression of COX-2 in the BV2 cells was determined after LPS treatment. When 1 µg/ml concentration of LPS was treated to BV2 cells, the expression of COX-2 was increased and its increase was the highest at 6 hours after treatment (Fig. 1D). Thus, BV2 cells express TLR4, and its stimulation by LPS increases the expression of inflammatory mediators.
Fig. 1. Increased nitric oxide production and cyclooxygenase-2 (COX-2) expression by lipopolysaccharide (LPS) treatment in BV2 cells. The concentration of nitrite in the culture supernatants after BV2 cells were treated with 0.1, 1, and 10 µg/ml concentrations of LPS for 24 hours (A) or 1 µg/ml concentrations of LPS for 3, 6, 12, and 24 hr (B), the concentration of nitrite in the culture supernatants was measured by Griess assay. BV2 cells were treated with LPS (1 µg/ml) for 3, 6, 12, and 24 hours. Then, the transcriptional expression of inducible nitric oxide synthase (iNOS) (C) or the translational expression of COX-2 (D) was examined by reverse transcriptase-polymerase chain reaction or immunoblotting, respectively. β-Actin was used as an internal control. CTL, control.
Oriental herbs reduce LPS-induced inflammation in BV2 cells
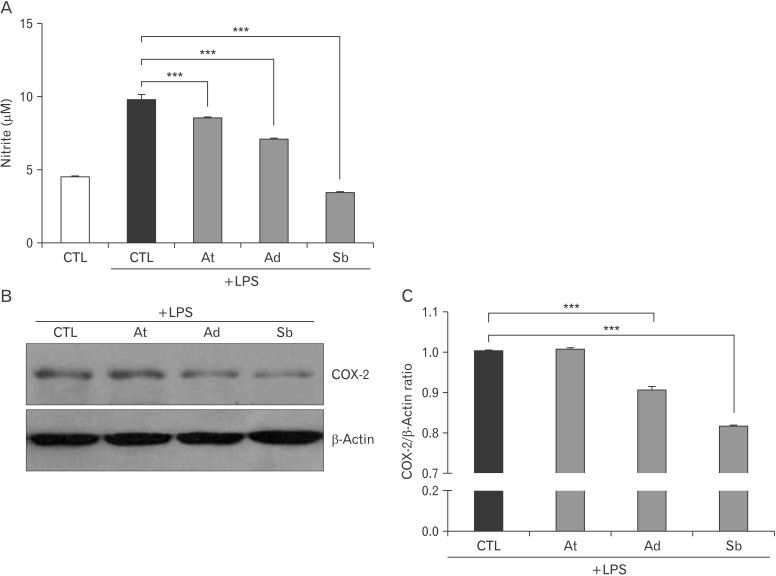
To examine the anti-inflammatory effect of oriental herbs, LPS-stimulated BV2 cells were treated with A. tenuissima, A. dahurica, and S. baicalensis. After 1 µg/ml concentration of LPS was treated with oriental herbs for 6 hours, the production of nitrite was measured by Griess assay. In result, the LPS-induced production of NO was significantly reduced by A. tenuissima, A. dahurica and especially S. baicalensis treatments (Fig. 2A). Moreover, the expression of COX-2 was reduced by treatments of oriental herbs, A. dahurica and S. baicalensis (Fig. 2B, C). Therefore, it seems that A. tenuissima, A. dahurica and especially S. baicalensis are effective on the alleviation of LPS-induced inflammation.
Fig. 2. Decreased nitric oxide production and cyclooxygenase-2 (COX-2) expression by oriental herbs in lipopolysaccharide (LPS)-stimulated BV2 cells. (A) The concentration of nitrite in the culture supernatants after Angelica tenuissima, A. dahurica, and Scutellaria baicalensis treatments with LPS (1 µg/ml) for 24 hours in BV2 cells. (B) BV2 cells were treated with A. tenuissima, A. dahurica, and S. baicalensis combined with 1 µg/ml concentrations of LPS for 24 hours. Then, the expression of COX-2 was examined by immunoblotting. β-Actin was used as an internal control. (C) The relative expression of COX-2 to β-actin was represented after densitometry analysis. At, A. tenuissima; Ad, A. dahurica; Sb, S. baicalensis; CTL, control. ***P<0.001.
Combinations of oriental herbs and acetaminophen more ameliorate LPS-induced inflammation in microglia cells
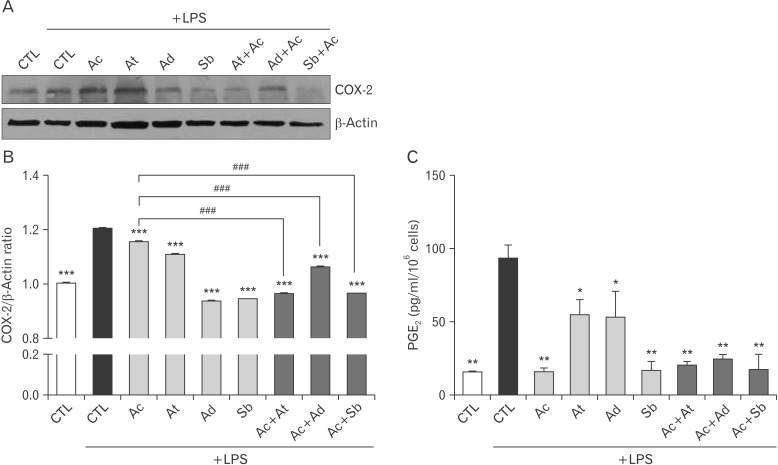
To further investigate the suppressive effect of oriental herbs with Western medicine on inflammation, BV2 cells were stimulated with LPS, and treated with oriental herbs such as A. tenuissima, A. dahurica, and S. baicalensis or Western medicine like acetaminophen or both. Acetaminophen is an well-known COX-2 selective inhibitor [15]. LPS was treated with A. tenuissima, A. dahurica, S. baicalensis or acetaminophen to microglia cells with various combinations, and then the expression of COX-2 was examined by immunoblotting. Although A. tenuissima or acetaminophen treatment did not show great reduction of COX-2 expression, A. dahurica and particulary S. baicalensis significantly decreased the expression of COX-2 in LPS-treated BV2 cells. Moreover, all oriental herb treatments combined with acetaminophen reduced the expression of COX-2 (Fig. 3A, B). PGE2 is produced following the sequential oxidation of arachidonic acid by COX-2 [16]. Increased production of PGE2 by LPS stimulation was markedly decreased by single or combined treatments of oriental herbs and Western medicines in BV2 cells (Fig. 3C). It appears that a combined treatment of A. tenuissima, A. dahurica, or S. baicalensis with acetaminophen is more effective on the reduction of LPS-induced inflammation.
Fig. 3. Decreased cyclooxygenase-2 (COX-2) expression and prostaglandin E2 (PGE2) production by oriental herbs combined with acetaminophen in lipopolysaccharide (LPS)-stimulated BV2 cells. (A-C) Acetaminophen was treated to BV2 cells with a combination with Angelica tenuissima, A. dahurica, or Scutellaria baicalensis after LPS stimulation. (A) The expression of COX-2 was examined by immunoblotting. (B) The relative expression of COX-2 to β-actin was represented after densitometry analysis. (C) And, the concentration of PGE2 in the culture supernatants from BV2 cells were measured by enzyme-linked immunosorbent assay. Ac, acetaminophen; At, A. tenuissima; Ad, A. dahurica; Sb, S. baicalensis. ***P<0.001, **P<0.01, *P<0.05 vs. LPS-treated control (CTL), ###P<0.001.
Discussion
Neuroinflammation is believed to play a crucial role in the development and progress of neurodegenerative diseases such as Alzheimer's disease, Parkinson's disease and Huntinton's disease [7,11]. Furthermore, it is taken part in the migraine resulting from an abnormal dilatation of intracranial blood vessels, leading to mechanical excitation of sensory fibers that innervate theses vessels [17]. A focus of most scientific migraine research has been on drugs that influence the transmission of neural signal. However, a paradigm shift focusing on glial cell activation should be involved [14]. Glial cells are associated in the neuroinflammation. Activated astrocytes and especially microglia produce several neuroexcitatory substances such as reactive oxygen species, NO and inflammatory cytokines [18]. Therefore, we investigated the effect of acetaminophen and three oriental herbs, which are broadly used for alleviating pain, especially headache and migraine, in brain microglia cells.
Migraine is one of the most common complaints seen in primary proactive, and its incidence in the general population is about 8% in men and 12%-15% in women [19,20,21]. In particular, COX mechanisms have been implicated in the migraine, which is in keeping with glial cell activation [14]. COX-2 is strongly induced by pro-inflammatory challenges, whereas COX-1 is constitutively expressed. COX-2 plays a crucial role in inflammation and pathogenesis. Treatments with selective COX-2 inhibitor has been reported to reduce brain inflammation [22]. In our results, LPS stimulation induced to produce NO and increased the expression of COX-2 in microglia, reflecting an increased inflammation (Fig. 1). The increase of inflammatory mediators by LPS stimulation was decreased by a treatment of oriental herbs such as A. tenuissima, A. dahurica, and S. baicalensis (Fig. 2). Acetaminophen and A. tenuissima less reduced the expression of COX-2 after LPS stimulation in BV2 cells. However, their co-treatments more significantly decreased the expression of COX-2 (Fig. 3). COX is an enzyme responsible for synthesize prostanoid including prostaglandin, prostacyclin and thromboxane from arachidonic acid [22]. The production of PGE2 was effectively decreased by acetaminophen and S. baicalensis in BV2 cells. A combined treatment of A. tenuissima or A. dahurica with acetaminophen synergistically diminished the PGE2 production. Acetaminophen considerably reduced the PGE2 production compared with the extent of COX-2 decrease in BV2 cells. The related mechanism is under consideration for further study.
Moreover, it was found that S. baicalensis has a strong anti-inflammatory effect because it solely was enough to inhibit the LPS-induced inflammation in BV2 cells. Study through microarray shows another reason why S. baicalensis can be prescribed by itself. With exception of unknown genes, the expression of 36 genes was decreased after S. baicalensis treatment in LPS-stimulated BV2 cells (Electronic Supplementary Table 1). Most of them were closely associated with the induction of inflammation, including chemokines, cytokines and their transcription factors. IFN and its transcriptional regulator, signal transducer and activator of transcription 1, is associated with the regulation of inflammatory chemokines and mediators [23]. Lymphotoxin A, a member of the tumor necrosis factor family, is a cytokine produced by lymphocytes, and mediates a large variety of inflammatory, immunostimulatory, and anti-viral responses [24]. In addition, the expression of 9 genes was increased by S. baicalensis treatment in LPS-stimulated BV2 cells (Electronic Supplementary Table 2). The elevated genes were related with anti-oxidant activity. For example, glutamate cysteine ligase catalyzes the first and rate-limiting step in the production of the cellular antioxidant glutathione [25]. HO-1 has anti-inflammatory as well as anti-oxidant roles in the brain by reducing iNOS expression and NO release [25]. Sulfiredoxin is a recently identified anti-oxidant protein as a binding partner to peroxiredoxin Tsa1, an anti-oxidant involved in the reduction of H2O2 [23]. Brain is prone to oxidative stress, because it contains high levels of fatty acids, use up to 20% of total body oxygen consumption and has higher level of redox metals primed to catalyze the Fenton reaction of free radical cascade [26]. Since the increased oxidative damage is closely related to the increased inflammation, the increased antioxidant capacity by S. baicalensis treatment might be helpful to reduce the inflammation in the brain. The anti-oxidant and anti-inflammatory effect of S. baicalensis should be further investigated.
Taken together, A. tenuissima and A. dahurica showed a synergistic anti-inflammatory effect when they were treated with acetaminophen, and S. baicalensis remarkably reduced the production of inflammatory mediators single-handed in LPS-stimulated microglia. A combined treatment of A. tenuissima or A. dahurica with acetaminophen seems to decrease the inflammation and relieve the symptom of pain in the brain more effectively than its sole treatment. Also, S. baicalensis is likely to be a greatly useful and effective candidate for pain relief and inflammation-reducing drug.
Acknowledgements
This work is supported by the program "The safety and efficacy of combined usage of herbal and western drugs 2008" (#B082007) of Ministry of Health and Welfare.
Electronic Supplementary Materials
Supplementary data including two tables and one figure can be found with this article online at http://www.acbjournal.org/src/sm/acb-48-244-s001.pdf.
The decreased genes by Scutellaria baicalensis treatment in lipopolysaccharide (LPS)-stimulated BV2 cells
The increased genes by Scutellaria baicalensis treatment in lipopolysaccharide (LPS)-stimulated BV2 cells
The expression of Toll-like receptor 4 (TLR4) in BV2 cells. The transcriptional expression of TLR4 in BV2 cells was examined by reverse transcriptase-polymerase chain reaction. C6, an astrocytoma cell line, was used as a positive control for TLR4. β-Actin was used as an internal control.
References
- 1.Graham GG, Davies MJ, Day RO, Mohamudally A, Scott KF. The modern pharmacology of paracetamol: therapeutic actions, mechanism of action, metabolism, toxicity and recent pharmacological findings. Inflammopharmacology. 2013;21:201–232. doi: 10.1007/s10787-013-0172-x. [DOI] [PubMed] [Google Scholar]
- 2.Bisaglia M, Venezia V, Piccioli P, Stanzione S, Porcile C, Russo C, Mancini F, Milanese C, Schettini G. Acetaminophen protects hippocampal neurons and PC12 cultures from amyloid beta-peptides induced oxidative stress and reduces NF-kappaB activation. Neurochem Int. 2002;41:43–54. doi: 10.1016/s0197-0186(01)00136-x. [DOI] [PubMed] [Google Scholar]
- 3.Locke CJ, Fox SA, Caldwell GA, Caldwell KA. Acetaminophen attenuates dopamine neuron degeneration in animal models of Parkinson's disease. Neurosci Lett. 2008;439:129–133. doi: 10.1016/j.neulet.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 4.Tripathy D, Grammas P. Acetaminophen protects brain endothelial cells against oxidative stress. Microvasc Res. 2009;77:289–296. doi: 10.1016/j.mvr.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kim EH, Shim B, Kang S, Jeong G, Lee JS, Yu YB, Chun M. Anti-inflammatory effects of Scutellaria baicalensis extract via suppression of immune modulators and MAP kinase signaling molecules. J Ethnopharmacol. 2009;126:320–331. doi: 10.1016/j.jep.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 6.Yoon SB, Lee YJ, Park SK, Kim HC, Bae H, Kim HM, Ko SG, Choi HY, Oh MS, Park W. Anti-inflammatory effects of Scutellaria baicalensis water extract on LPS-activated RAW 264.7 macrophages. J Ethnopharmacol. 2009;125:286–290. doi: 10.1016/j.jep.2009.06.027. [DOI] [PubMed] [Google Scholar]
- 7.Gasiorowski K, Lamer-Zarawska E, Leszek J, Parvathaneni K, Yendluri BB, Blach-Olszewska Z, Aliev G. Flavones from root of Scutellaria baicalensis Georgi: drugs of the future in neurodegeneration? CNS Neurol Disord Drug Targets. 2011;10:184–191. doi: 10.2174/187152711794480384. [DOI] [PubMed] [Google Scholar]
- 8.Lee SY, Park HJ, Cha DS, Shin TY, Na HJ, Moon WS, Kang YG, Jeon H. Antioxidant and anti-inflammatory effect of Angelica tenuissima in IFN-γ/LPS-stimulated peritoneal macrophage. Korean J Orient Physiol Pathol. 2008;22:1549–1556. [Google Scholar]
- 9.Lee MY, Lee JA, Seo CS, Ha H, Lee H, Son JK, Shin HK. Anti-inflammatory activity of Angelica dahurica ethanolic extract on RAW264.7 cells via upregulation of heme oxygenase-1. Food Chem Toxicol. 2011;49:1047–1055. doi: 10.1016/j.fct.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 10.Ross J, Auger M, Burke B, Lewis C. The biology of the macrophage. Macrophage. 2002;2:16–23. [Google Scholar]
- 11.Gao HM, Hong JS. Why neurodegenerative diseases are progressive: uncontrolled inflammation drives disease progression. Trends Immunol. 2008;29:357–365. doi: 10.1016/j.it.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Krause DL, Müller N. Neuroinflammation, microglia and implications for anti-inflammatory treatment in Alzheimer's disease. Int J Alzheimers Dis. 2010;2010:732806. doi: 10.4061/2010/732806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hutchinson MR, Bland ST, Johnson KW, Rice KC, Maier SF, Watkins LR. Opioid-induced glial activation: mechanisms of activation and implications for opioid analgesia, dependence, and reward. ScientificWorldJournal. 2007;7:98–111. doi: 10.1100/tsw.2007.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bartley J. Could glial activation be a factor in migraine? Med Hypotheses. 2009;72:255–257. doi: 10.1016/j.mehy.2008.09.048. [DOI] [PubMed] [Google Scholar]
- 15.Hinz B, Cheremina O, Brune K. Acetaminophen (paracetamol) is a selective cyclooxygenase-2 inhibitor in man. FASEB J. 2008;22:383–390. doi: 10.1096/fj.07-8506com. [DOI] [PubMed] [Google Scholar]
- 16.Giuliano F, Warner TD. Origins of prostaglandin E2: involvements of cyclooxygenase (COX)-1 and COX-2 in human and rat systems. J Pharmacol Exp Ther. 2002;303:1001–1006. doi: 10.1124/jpet.102.041244. [DOI] [PubMed] [Google Scholar]
- 17.Waeber C, Moskowitz MA. Migraine as an inflammatory disorder. Neurology. 2005;64(10 Suppl 2):S9–S15. doi: 10.1212/wnl.64.10_suppl_2.s9. [DOI] [PubMed] [Google Scholar]
- 18.Watkins LR, Milligan ED, Maier SF. Glial proinflammatory cytokines mediate exaggerated pain states: implications for clinical pain. Adv Exp Med Biol. 2003;521:1–21. [PubMed] [Google Scholar]
- 19.Diener HC, Katsarava Z, Limmroth V. Current diagnosis and treatment of migraine. Ophthalmologe. 2008;105:501–508. doi: 10.1007/s00347-008-1747-6. [DOI] [PubMed] [Google Scholar]
- 20.Rasmussen BK, Jensen R, Schroll M, Olesen J. Epidemiology of headache in a general population: a prevalence study. J Clin Epidemiol. 1991;44:1147–1157. doi: 10.1016/0895-4356(91)90147-2. [DOI] [PubMed] [Google Scholar]
- 21.Mett A, Tfelt-Hansen P. Acute migraine therapy: recent evidence from randomized comparative trials. Curr Opin Neurol. 2008;21:331–337. doi: 10.1097/WCO.0b013e3282fee843. [DOI] [PubMed] [Google Scholar]
- 22.Tzeng SF, Hsiao HY, Mak OT. Prostaglandins and cyclooxygenases in glial cells during brain inflammation. Curr Drug Targets Inflamm Allergy. 2005;4:335–340. doi: 10.2174/1568010054022051. [DOI] [PubMed] [Google Scholar]
- 23.Findlay VJ, Tapiero H, Townsend DM. Sulfiredoxin: a potential therapeutic agent? Biomed Pharmacother. 2005;59:374–379. doi: 10.1016/j.biopha.2005.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buhrmann C, Shayan P, Aggarwal BB, Shakibaei M. Evidence that TNF-beta (lymphotoxin alpha) can activate the inflammatory environment in human chondrocytes. Arthritis Res Ther. 2013;15:R202. doi: 10.1186/ar4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Franklin CC, Backos DS, Mohar I, White CC, Forman HJ, Kavanagh TJ. Structure, function, and post-translational regulation of the catalytic and modifier subunits of glutamate cysteine ligase. Mol Aspects Med. 2009;30:86–98. doi: 10.1016/j.mam.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uttara B, Singh AV, Zamboni P, Mahajan RT. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The decreased genes by Scutellaria baicalensis treatment in lipopolysaccharide (LPS)-stimulated BV2 cells
The increased genes by Scutellaria baicalensis treatment in lipopolysaccharide (LPS)-stimulated BV2 cells
The expression of Toll-like receptor 4 (TLR4) in BV2 cells. The transcriptional expression of TLR4 in BV2 cells was examined by reverse transcriptase-polymerase chain reaction. C6, an astrocytoma cell line, was used as a positive control for TLR4. β-Actin was used as an internal control.