Abstract
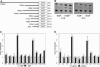
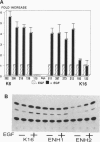
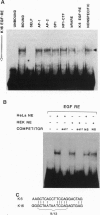
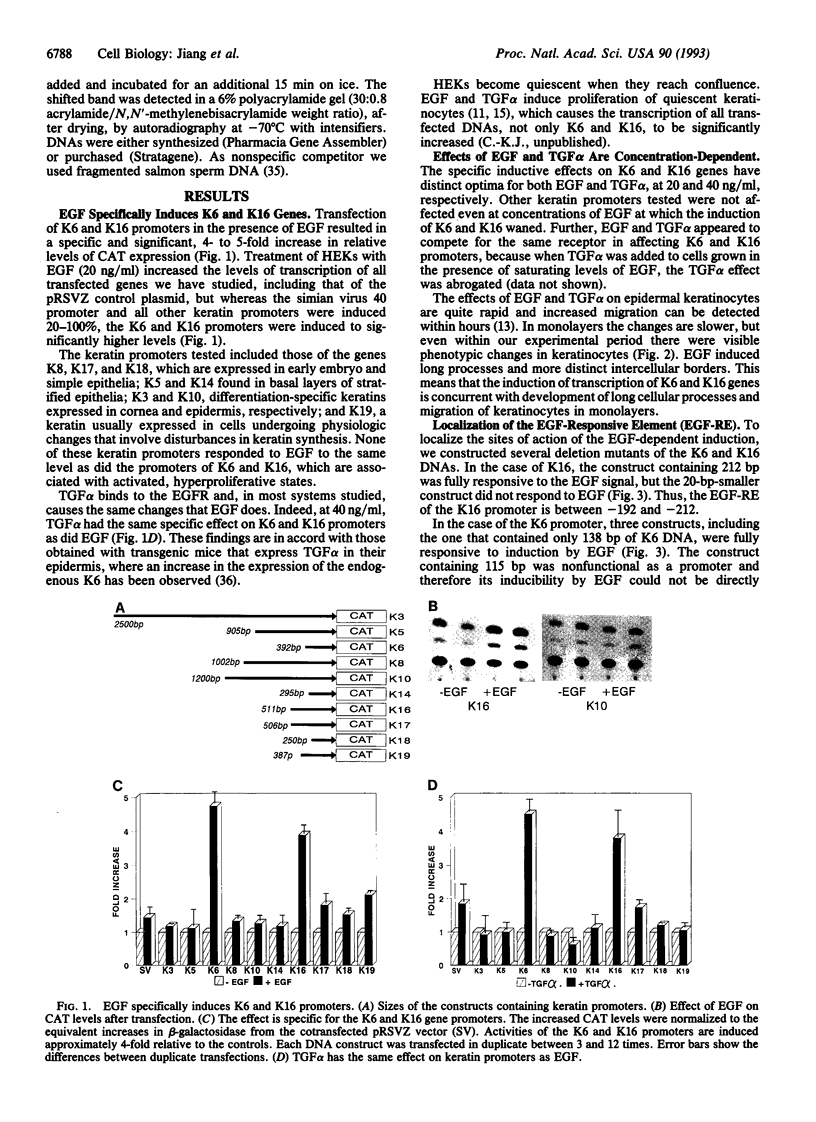
Epidermal injury results in activation of keratinocytes which produce and respond to growth factors and cytokines and become migratory. Activated keratinocytes express a specific pair of keratin proteins, K6 and K16, distinct from the keratins in the healthy epidermis. Keratinocytes can be activated, for example, by binding of the appropriate ligands to the epidermal growth factor receptor (EGFR). We have analyzed the effects of EGFR activation on keratin gene transcription by transfecting DNAs containing keratin promoters linked to a reporter gene into primary cultures of human epidermal keratinocytes in the presence or absence of EGF or transforming growth factor alpha (TGF alpha), two growth factors that activate EGFR. The activation of EGFR had no effect on the promoters of simple epithelial, basal-layer-specific, or differentiation-specific keratins. In contrast, the expression of K6 and K16 was strongly and specifically induced. A 20-bp DNA segment of the K16 gene promoter conveyed the EGF regulation, functioned in a heterologous construct, and therefore constituted an EGF-responsive element. A nuclear protein specifically bound to this element and to the analogous sequence of the K6 promoter. Thus, EGF specifically induces K6 and K16, markers of activated keratinocytes, via nuclear proteins that bind to EGF-responsive elements in the promoters of these keratin genes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackerman P., Glover C. V., Osheroff N. Stimulation of casein kinase II by epidermal growth factor: relationship between the physiological activity of the kinase and the phosphorylation state of its beta subunit. Proc Natl Acad Sci U S A. 1990 Jan;87(2):821–825. doi: 10.1073/pnas.87.2.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bader B. L., Jahn L., Franke W. W. Low level expression of cytokeratins 8, 18 and 19 in vascular smooth muscle cells of human umbilical cord and in cultured cells derived therefrom, with an analysis of the chromosomal locus containing the cytokeratin 19 gene. Eur J Cell Biol. 1988 Dec;47(2):300–319. [PubMed] [Google Scholar]
- Barrandon Y., Green H. Cell migration is essential for sustained growth of keratinocyte colonies: the roles of transforming growth factor-alpha and epidermal growth factor. Cell. 1987 Sep 25;50(7):1131–1137. doi: 10.1016/0092-8674(87)90179-6. [DOI] [PubMed] [Google Scholar]
- Bonifas J. M., Rothman A. L., Epstein E. H., Jr Epidermolysis bullosa simplex: evidence in two families for keratin gene abnormalities. Science. 1991 Nov 22;254(5035):1202–1205. doi: 10.1126/science.1720261. [DOI] [PubMed] [Google Scholar]
- Choi Y., Fuchs E. TGF-beta and retinoic acid: regulators of growth and modifiers of differentiation in human epidermal cells. Cell Regul. 1990 Oct;1(11):791–809. doi: 10.1091/mbc.1.11.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey R. J., Jr, Derynck R., Wilcox J. N., Bringman T. S., Goustin A. S., Moses H. L., Pittelkow M. R. Production and auto-induction of transforming growth factor-alpha in human keratinocytes. 1987 Aug 27-Sep 2Nature. 328(6133):817–820. doi: 10.1038/328817a0. [DOI] [PubMed] [Google Scholar]
- Coulombe P. A., Hutton M. E., Letai A., Hebert A., Paller A. S., Fuchs E. Point mutations in human keratin 14 genes of epidermolysis bullosa simplex patients: genetic and functional analyses. Cell. 1991 Sep 20;66(6):1301–1311. doi: 10.1016/0092-8674(91)90051-y. [DOI] [PubMed] [Google Scholar]
- Di Fiore P. P., Falco J., Borrello I., Weissman B., Aaronson S. A. The calcium signal for BALB/MK keratinocyte terminal differentiation counteracts epidermal growth factor (EGF) very early in the EGF-induced proliferative pathway. Mol Cell Biol. 1988 Feb;8(2):557–563. doi: 10.1128/mcb.8.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dignam J. D., Martin P. L., Shastry B. S., Roeder R. G. Eukaryotic gene transcription with purified components. Methods Enzymol. 1983;101:582–598. doi: 10.1016/0076-6879(83)01039-3. [DOI] [PubMed] [Google Scholar]
- Elder J. T., Fisher G. J., Lindquist P. B., Bennett G. L., Pittelkow M. R., Coffey R. J., Jr, Ellingsworth L., Derynck R., Voorhees J. J. Overexpression of transforming growth factor alpha in psoriatic epidermis. Science. 1989 Feb 10;243(4892):811–814. doi: 10.1126/science.2916128. [DOI] [PubMed] [Google Scholar]
- Fisch T. M., Prywes R., Roeder R. G. c-fos sequence necessary for basal expression and induction by epidermal growth factor, 12-O-tetradecanoyl phorbol-13-acetate and the calcium ionophore. Mol Cell Biol. 1987 Oct;7(10):3490–3502. doi: 10.1128/mcb.7.10.3490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs E. Epidermal differentiation: the bare essentials. J Cell Biol. 1990 Dec;111(6 Pt 2):2807–2814. doi: 10.1083/jcb.111.6.2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrilović J., Moens G., Thiery J. P., Jouanneau J. Expression of transfected transforming growth factor alpha induces a motile fibroblast-like phenotype with extracellular matrix-degrading potential in a rat bladder carcinoma cell line. Cell Regul. 1990 Dec;1(13):1003–1014. doi: 10.1091/mbc.1.13.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb A. B., Chang C. K., Posnett D. N., Fanelli B., Tam J. P. Detection of transforming growth factor alpha in normal, malignant, and hyperproliferative human keratinocytes. J Exp Med. 1988 Feb 1;167(2):670–675. doi: 10.1084/jem.167.2.670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han T. H., Lamph W. W., Prywes R. Mapping of epidermal growth factor-, serum-, and phorbol ester-responsive sequence elements in the c-jun promoter. Mol Cell Biol. 1992 Oct;12(10):4472–4477. doi: 10.1128/mcb.12.10.4472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera R. E., Shaw P. E., Nordheim A. Occupation of the c-fos serum response element in vivo by a multi-protein complex is unaltered by growth factor induction. Nature. 1989 Jul 6;340(6228):68–70. doi: 10.1038/340068a0. [DOI] [PubMed] [Google Scholar]
- Herschman H. R. Primary response genes induced by growth factors and tumor promoters. Annu Rev Biochem. 1991;60:281–319. doi: 10.1146/annurev.bi.60.070191.001433. [DOI] [PubMed] [Google Scholar]
- Jiang C. K., Epstein H. S., Tomic M., Freedberg I. M., Blumenberg M. Epithelial-specific keratin gene expression: identification of a 300 base-pair controlling segment. Nucleic Acids Res. 1990 Jan 25;18(2):247–253. doi: 10.1093/nar/18.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang C. K., Epstein H. S., Tomic M., Freedberg I. M., Blumenberg M. Functional comparison of the upstream regulatory DNA sequences of four human epidermal keratin genes. J Invest Dermatol. 1991 Feb;96(2):162–167. doi: 10.1111/1523-1747.ep12460939. [DOI] [PubMed] [Google Scholar]
- Klinge E. M., Sylvestre Y. R., Freedberg I. M., Blumenberg M. Evolution of keratin genes: different protein domains evolve by different pathways. J Mol Evol. 1987;24(4):319–329. doi: 10.1007/BF02134130. [DOI] [PubMed] [Google Scholar]
- Lammers R., Van Obberghen E., Ballotti R., Schlessinger J., Ullrich A. Transphosphorylation as a possible mechanism for insulin and epidermal growth factor receptor activation. J Biol Chem. 1990 Oct 5;265(28):16886–16890. [PubMed] [Google Scholar]
- Madhukar B. V., Oh S. Y., Chang C. C., Wade M., Trosko J. E. Altered regulation of intercellular communication by epidermal growth factor, transforming growth factor-beta and peptide hormones in normal human keratinocytes. Carcinogenesis. 1989 Jan;10(1):13–20. doi: 10.1093/carcin/10.1.13. [DOI] [PubMed] [Google Scholar]
- Merchant J. L., Demediuk B., Brand S. J. A GC-rich element confers epidermal growth factor responsiveness to transcription from the gastrin promoter. Mol Cell Biol. 1991 May;11(5):2686–2696. doi: 10.1128/mcb.11.5.2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moll R., Franke W. W., Schiller D. L., Geiger B., Krepler R. The catalog of human cytokeratins: patterns of expression in normal epithelia, tumors and cultured cells. Cell. 1982 Nov;31(1):11–24. doi: 10.1016/0092-8674(82)90400-7. [DOI] [PubMed] [Google Scholar]
- Moscat J., Molloy C. J., Fleming T. P., Aaronson S. A. Epidermal growth factor activates phosphoinositide turnover and protein kinase C in BALB/MK keratinocytes. Mol Endocrinol. 1988 Sep;2(9):799–805. doi: 10.1210/mend-2-9-799. [DOI] [PubMed] [Google Scholar]
- Nickoloff B. J., Mitra R. S., Riser B. L., Dixit V. M., Varani J. Modulation of keratinocyte motility. Correlation with production of extracellular matrix molecules in response to growth promoting and antiproliferative factors. Am J Pathol. 1988 Sep;132(3):543–551. [PMC free article] [PubMed] [Google Scholar]
- Nunez A. M., Berry M., Imler J. L., Chambon P. The 5' flanking region of the pS2 gene contains a complex enhancer region responsive to oestrogens, epidermal growth factor, a tumour promoter (TPA), the c-Ha-ras oncoprotein and the c-jun protein. EMBO J. 1989 Mar;8(3):823–829. doi: 10.1002/j.1460-2075.1989.tb03443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Partridge M., Green M. R., Langdon J. D., Feldmann M. Production of TGF-alpha and TGF-beta by cultured keratinocytes, skin and oral squamous cell carcinomas--potential autocrine regulation of normal and malignant epithelial cell proliferation. Br J Cancer. 1989 Oct;60(4):542–548. doi: 10.1038/bjc.1989.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savtchenko E. S., Tomic M., Ivker R., Blumenberg M. Three parallel linkage groups of human acidic keratin genes. Genomics. 1990 Jul;7(3):394–407. doi: 10.1016/0888-7543(90)90174-s. [DOI] [PubMed] [Google Scholar]
- Schermer A., Jester J. V., Hardy C., Milano D., Sun T. T. Transient synthesis of K6 and K16 keratins in regenerating rabbit corneal epithelium: keratin markers for an alternative pathway of keratinocyte differentiation. Differentiation. 1989 Dec;42(2):103–110. doi: 10.1111/j.1432-0436.1989.tb00611.x. [DOI] [PubMed] [Google Scholar]
- Snedeker S. M., Brown C. F., DiAugustine R. P. Expression and functional properties of transforming growth factor alpha and epidermal growth factor during mouse mammary gland ductal morphogenesis. Proc Natl Acad Sci U S A. 1991 Jan 1;88(1):276–280. doi: 10.1073/pnas.88.1.276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetzels R. H., Kuijpers H. J., Lane E. B., Leigh I. M., Troyanovsky S. M., Holland R., van Haelst U. J., Ramaekers F. C. Basal cell-specific and hyperproliferation-related keratins in human breast cancer. Am J Pathol. 1991 Mar;138(3):751–763. [PMC free article] [PubMed] [Google Scholar]
- van Zoelen E. J., van Oostwaard T. M., van der Saag P. T., de Laat S. W. Phenotypic transformation of normal rat kidney cells in a growth-factor-defined medium: induction by a neuroblastoma-derived transforming growth factor independently of the EGF receptor. J Cell Physiol. 1985 May;123(2):151–160. doi: 10.1002/jcp.1041230202. [DOI] [PubMed] [Google Scholar]