Abstract
A novel Helicobacter species was identified from the gastrointestinal tract of the Korean striped field mouse (Apodemus agrarius). Biochemical testing, ultrastructure characterization, and 16S rRNA gene sequence analysis suggested that this bacterium represents a distinct taxon. The bacterium was positive for urease activity, susceptible to cephalothin and nalidixic acid, and weakly positive for oxidase and catalase activity. Electron microscopy revealed that the bacterium has spirally curved rod morphology with singular bipolar nonsheathed flagella. Genotypically, the isolated bacterial strains (YMRC 000215, YMRC 000216, and YMRC 000419) were most closely related to a reference strain of Helicobacter mesocricetorum (97.25%, 97.32%, and 97.03% 16S rRNA sequence similarities, respectively). The 16S rRNA sequences of these strains were deposited into GenBank under accession numbers AF284754, AY009129, and AY009130, respectively. We propose the name Helicobacter apodemus for this novel species.
Keywords: 16S rRNA, Apodemus agrarius, Helicobacter apodemus, striped field mouse
Introduction
Since the discovery of Helicobacter (H.) pylori in the human stomach, Helicobacter spp. have become the subject of intensive research and shown to be the causative agents of several gastric diseases, including gastritis, peptic ulcers, and stomach cancer [17,30]. In addition to humans, Helicobacter spp. can be found in various animal species, including horses, pigs, cattle, and birds [2,3,5,6]. These bacteria can colonize the liver, bile, gall bladder, and lining of the gastrointestinal tract [9,25] and can cause damage or reside in the host without causing any clinical signs. To date, 35 formally named Helicobacter species have been identified. However, the majority of these bacteria were isolated from humans and domestic animals; thus, a potentially large number of Helicobacter spp. in wild animals are yet to be identified.
Over the last decade, the presence of Helicobacter spp. has frequently been reported in laboratory facilities housing rodents. To date, more than 10 rodent Helicobacter species have been identified, several of which were shown to be related to pathogenicity in the host. For instance, H. aurati was isolated from adult Syrian hamsters with gastritis [19], and ureasenegative H. typhlonicus was recovered from the cecum and fecal samples of interleukin-10-deficient laboratory mice with typhlocolitis [11]. Furthermore, in an African rodent model, H. mastomyrinus was isolated and shown to possess a cytolethal distending toxin that can cause cell distention; however, the pathogenic potential of this species is unknown [28]. Some Helicobacter spp. may infect the host without causing any disease, such as H. muricola and H. ganmani, which were detected in asymptomatic Korean wild mice [36] and laboratory mice [23], respectively. However, the potential pathogenicity of these bacteria cannot be excluded, as immunocompromised hosts seem to be more susceptible to Helicobacter infections [34]. Moreover, some Helicobacter spp. in humans are believed to have originated from animals [18,30]. These include Helicobacter sp. flexispira, which belongs to the normal flora of the intestinal tract in animals and was found to be associated with bacteremia in an immunocompetent young man [14] and a child with pneumonia [33]. Therefore, the screening and characterization of Helicobacter spp. in rodents may have important benefits for rodent research and human health.
In Korea, the striped field mouse (Apodemus agrarius) is widespread in the wild [37] and is believed to be a natural reservoir of Hantaan virus [20] and intestinal fluke Echinostomas [4]. The presence of Helicobacter species in the striped field mouse is largely unknown; however, because this species has been developed as a new laboratory animal model in Korea, the transmission of these potential zoonotic pathogens, including Helicobacter, is of concern. Furthermore, the presence of Helicobacter may interfere with host immune responses and consequently affect the validity of data in immune-related studies, especially vaccine development [27]. Therefore, screening for the presence of Helicobacter in laboratory animals is important to ensure accuracy and reliability of data in future biomedical studies. Accordingly, this study was conducted to examine the presence of Helicobacter spp. in striped field mice in South Korea and conduct phenotypic and genotypic characterization of the newly identified Helicobacter isolates.
Materials and Methods
Animals
Overall, 24 male and 25 female striped field mice (Apodemus agrarius) aged 6 to 12 months were obtained from the Laboratory Animal Resources at the Korea Food and Drug Administration (KFDA) and screened for the presence of Helicobacter spp. The use of these animals and the experimental procedures were designed based on the Guide for Animal Experiments and approved by the Committee for Care and Use of Laboratory Animals of Yonsei University.
Bacterial isolation and culture
The 49 striped field mice were euthanized, and the gastrointestinal tract (stomach, liver, cecum, and colon) was aseptically removed. In addition, 83 freshly pooled fecal samples were collected from the mouse cages. Each tissue and fecal sample was homogenized in 1 mL of phosphate-buffered saline (PBS), after which the suspension was filtered through a 0.8 µm syringe. The filtrates were then inoculated onto moist tryptic soy blood agar plates containing 5% sheep blood and incubated at 37℃ under microaerophilic conditions (BBL GasPak System; BD Diagnostic Systems, USA) for up to 6 days.
PCR amplification
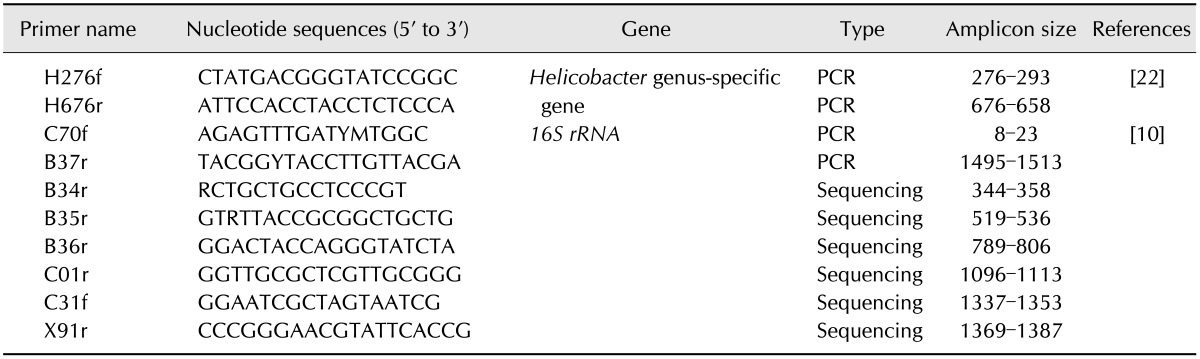
PCR was conducted to identify Helicobacter spp. using the previously designed primers H276f and H676r, after which the 16S rRNA genes of Helicobacter-positive isolates were amplified for further sequencing using primers C70f and B37r (Table 1) [10,22].
Table 1. Oligonucleotide primer used in this study.
Biochemical testing
Biochemical tests commonly used to characterize Helicobacter spp. were performed using an API Campy identification system (VITEK; bioMérieux, USA) for colonies found to be Helicobacter-positive by PCR. The optimum growth temperature, tolerance to 1% glycine and 1.5% NaCl, Gramstaining, motility, urease activity (Hp Kit; Chong Kun Dang Pharmaceutical, Korea), oxidase activity, catalase activity, and hydrolysis of indoxyl acetate were determined as previously described [7,8]. The isolates were tested for susceptibility to cephalothin (30 µg/disc) and nalidixic acid (30 µg/disc) using antibiotic-impregnated discs (Becton, Dickinson and Company, USA).
Electron microscopy
To prepare the isolates for observation by electron microscopy, bacterial colonies were harvested from blood agar plates, suspended in 10 mM Tris buffer (pH 7.4), and centrifuged at 12,000 × g. The pellets were then gently washed three times with deionized water and spread on a carbon-coated grid, after which a small droplet of 4% phosphotungstic acid solution was added. The morphology of the isolates was examined using a Philips CM10 transmission electron microscope (Philips, The Netherlands) at 80 KeV and compared to the morphology of other known Helicobacter species (Table 2).
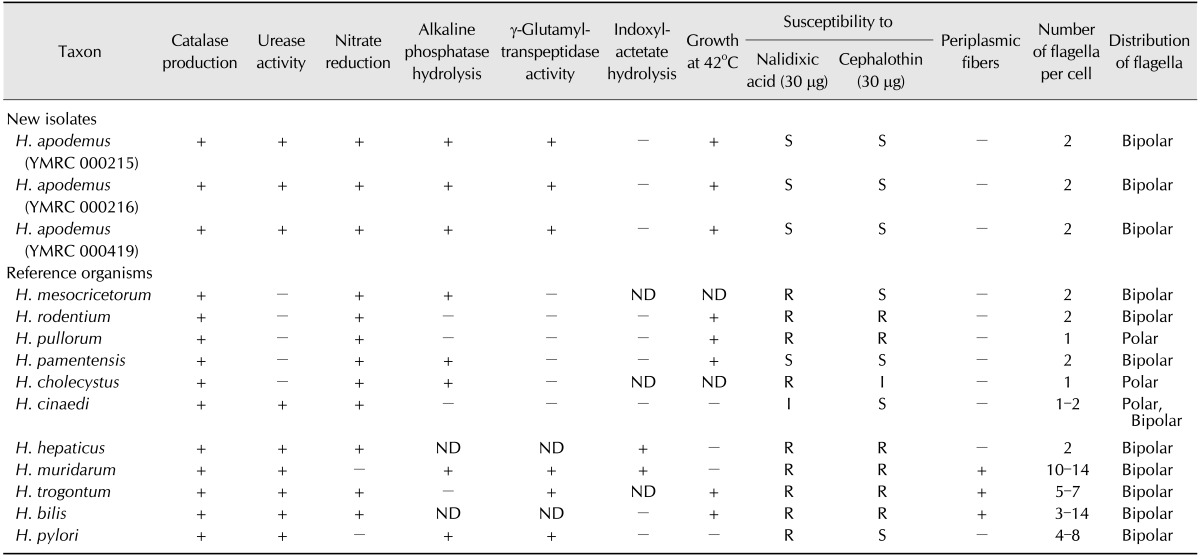
Table 2. Biochemical characteristics of novel Helicobacter spp. in comparison to closely related Helicobacters*.
DNA extraction
Bacterial colonies on blood agar plates were propagated in Brucella broth supplemented with 5% fetal bovine serum and centrifuged at 10,000 × g. The pellets were then washed with PBS, after which the bacterial DNA was extracted using a QIAamp Tissue Kit (Qiagen, USA) according to the manufacturer's instructions. The concentration and purity of the DNA were then measured spectrophotometrically.
DNA sequencing
The products of PCR amplification of the 16S rRNA gene were purified using a JetSorb Kit (Genomed, USA), then sequenced using a Thermo Sequenase Cy 5 Dye Terminator Kit (Amersham Pharmacia Biotech, USA) according to the manufacturer's instructions. The sequencing reactions were performed using the primers B34r, B35r, B36r, C01r, C31f, and X91r as previously described (Table 1) [10]. The sequences of Helicobacter, Wolinella, and Campylobacter spp. obtained from the GenBank database (The National Center for Biotechnology Information, USA) were compared with the 16S rRNA sequences (up to 1446 bp fragments) of our strains. The sequence data were assembled and aligned by ClustalW analysis using MEGA ver. 6.0 [32]. Phylogenetic trees were generated by the neighbor-joining method with bootstrapping based on 1,000 replications [26]. In addition, nucleotide changes at a single position were corrected using Jukes Cantor corrections [16], then analyzed to generate similarity matrices. The 16S rRNA gene sequences for isolates YMRC 000215, YMRC 000216, and YMRC 000419 were deposited into GenBank under accession nos. AF284754, AY009129, and AY009130, respectively.
Results
Bacterial isolation
A Helicobacter-positive band was observed for two (8.3%), five (20.8%), and six (25%) samples of stomach, cecum, and colon, respectively, from 24 male striped field mice and three (12%), two (8%), seven (28%), and 12 (48%) samples of stomach, liver, cecum, and colon, respectively, from 25 female mice. Regardless of sex, 15 (18%) out of 83 fecal samples were positive for Helicobacter.
Biochemical testing and ultrastructure characterization
The bacterial strains that were identified as Helicobacter by PCR were subjected to biochemical tests. Among these, three strains (YMRC 000419, YMRC 000215 and YMRC 000216, isolated from stomach, colon and cecum samples, respectively) that were phenotypically distinct from those of previously reported Helicobacter spp. were selected for further investigation.
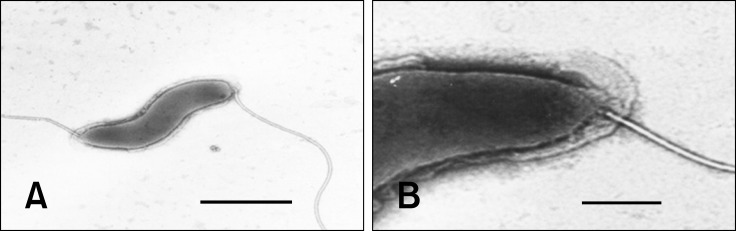
The three strains isolated in this study differed from other Helicobacter spp. in 2 to 7 of the 12 phenotypic traits examined (Table 2). The strains grew well under microaerophilic conditions at 37℃ and showed slight growth at 42℃. However, the isolates did not grow under similar conditions at 25℃ or under aerobic conditions at 37℃. Brucella agar plates containing 1% glycine and 1.5% NaCl did not support their growth. Morphological examination of the bacteria through Gram staining showed various short rods and coccal forms. The strains were positive for urease activity, nitrate reduction, alkaline phosphatase hydrolysis, and γ-glutamyl transpeptidase activity, weakly positive for oxidase and catalase activities, and negative for indoxyl acetate hydrolysis. Antibiotic susceptibility testing showed that the strains were susceptible to cephalothin and nalidixic acid. The bacterial cells were also observed by electron microscopy, which revealed spirally curved rods with singular bipolar nonsheathed flagella and no periplasmic fiber. The cells were 2 to 5 µm in length and 0.4 to 0.6 µm in diameter (Fig. 1).
Fig. 1. Transmission electron micrograph of a negatively stained Helicobacter sp. Nov. cell isolated from the cecum of Apodemus agrarius. (A) YMRC 000215. (B) YMRC 000215. Scale bars = 1 µm (A), or 0.2 µm (B).
Phylogenetic analysis
Approximately 95% of the 1450 bp 16S rRNA gene fragment of the three Helicobacter isolates was sequenced. Two isolates, YMRC 000215 and YMRC 000216, differed by a single base C or U at position 1137 (Escherichia coli numbering), while YMRC 000216 differed by one base from YMRC 000215 and by two bases from YMRC 000419. Thus, the > 99% similarity of the 16S rRNA sequence of the three isolates indicates that they are the same species.
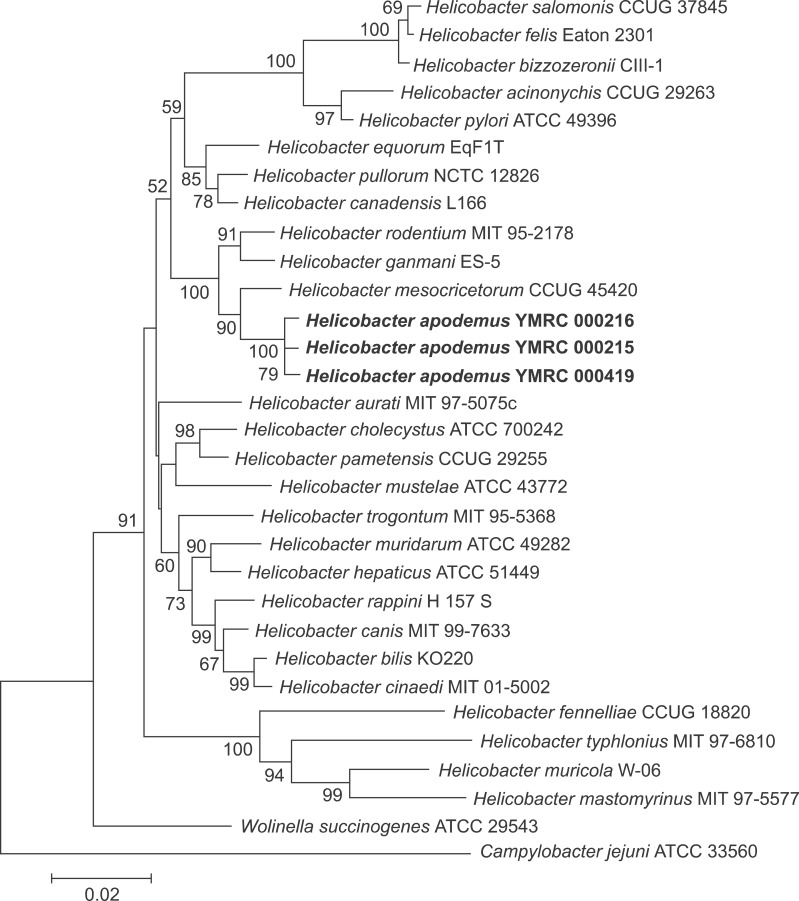
A similarity matrix table and phylogenetic tree were generated to investigate the genetic relatedness of our isolates to other Helicobacter species (Fig. 2). These analyses revealed that YMRC 000215, YMRC 000216, and YMRC 000419 are most closely related to the reference strain of H. mesocricetorum (97.25%, 97.32%, and 97.03% similarity, respectively).
Fig. 2. Phylogenetic tree of strains isolated in this study and members of other genera generated using the neighbor-joining method.
Discussion
In the present study, the majority of presumptive Helicobacter spp. were detected in samples of both male and female striped field mice. To the best of our knowledge, this is the first record of a novel Helicobacter sp. in the striped field mouse. In a previous study in Croatia, 22 wild rodents with gastritis were examined for the presence of Helicobacter using Warthin-Starry staining. However, the results showed that one gastric sample from striped field mice was stained negative for Helicobacter [24]. Several murine Helicobacter species have been reported to commonly colonize in the extragastric region, particularly the large intestine, whereas gastric colonization is more rare [35]. Interestingly, our isolates were detected in stomach samples, as well as extragastric samples. These findings are similar to the reported distribution of H. aurati, which was present in the cecum and stomach samples of adult Syrian hamsters, and H. muridarum, a member of the intestinal flora that was shown to colonize the stomachs of mice [19,21].
The presence of flagella together with urease and catalase activity in our isolates is similar to the properties of the well-known disease-causing species H. pylori, H. bilis, and H. hepaticus [1]. The presence of flagella is believed to aid in the survival of Helicobacter in the stomach by allowing them to burrow into the gastric mucosa, while urease and catalase activities promote survival by neutralizing gastric acid and resisting the host's antioxidant defense mechanism, respectively [13,31]. Thus, the biochemical and morphological traits of our isolates suggest that they are potentially pathogenic.
Our three isolates showed a similar morphology and size to H. mesocricetorum under the electron microscope; however, they differed from H. mesocricetorum phenotypically as they exhibited urease activity, γ-glutamyl transpeptidase activity, and susceptibility to nalidixic acid. Together with H. mesocricetorum, our strains were clustered with H. rodentium and H. ganmani in the phylogenetic tree (Fig. 2). The species in this cluster of Helicobacter were all isolated from the intestinal region and do not possess a sheathed flagella, which is believed to be an important factor for survival under the strongly acidic conditions of the stomach [29,35]. Interestingly, our isolates were able to colonize the stomach despite having non-sheathed flagella.
In recent years, there have been few reports of the presence of H. apodemus based on 16S rRNA sequences in GenBank. The presence of H. apodemus in wild rodents (Dipus sagitta, Euchoreutes naso, Meriones meridianus, and Nesokia indica) in different regions of China indicates the epidemic nature of H. apodemus infection in the wild [12]. A study in Sweden also demonstrated the presence of H. apodemus with a 98.3% sequence similarity to 16S rDNA in laboratory mice [15]. Taken together, these results indicate that H. apodemus may have multiple hosts.
In summary, a novel Helicobacter sp. isolated from the gastrointestinal tract of the striped field mouse (Apodemus agrarius) was described. The results of biochemical and phenotypic characterization and sequence analysis of the 16S rRNA gene strongly suggest that this isolate represents a distinct taxon; thus, we propose the name Helicobacter apodemus for this species. Further studies may be needed to investigate the pathogenic genes and possible pathogenesis of H. apodemus.
Acknowledgments
This research was supported by Korea Mouse Phenotyping Project (2013M3A9D5072550) of the Ministry of Science, ICT and Future Planning through the National Research Foundation to Je Kyung Seong.
Footnotes
Conflict of Interest: There is no conflict of interest.
References
- 1.Andersen LP, Wadström T. Basic bacteriology and culture. In: Mobley HLT, Mendz GL, Hazell SL, editors. Helicobacter pylori: Physiology and Genetics. Chapt. 4. Washington, D.C.: ASM press; 2001. [Google Scholar]
- 2.Baele M, Decostere A, Vandamme P, Ceelen L, Hellemans A, Mast J, Chiers K, Ducatelle R, Haesebrouck F. Isolation and characterization of Helicobacter suis sp. nov. from pig stomachs. Int J Syst Evol Microbiol. 2008;58:1350–1358. doi: 10.1099/ijs.0.65133-0. [DOI] [PubMed] [Google Scholar]
- 3.Bezdekova B, Futas J. Helicobacter species and gastric ulceration in horses: a clinical study. Vet Med (Praha) 2009;54:577–582. [Google Scholar]
- 4.Chai JY, Park JH, Jung BK, Guk SM, Kim JL, Shin EH, Klein TA, Kim HC, Chong ST, Baek LJ, Song JW. Echinostome infections in the striped-field mouse, Apodemus agrarius, and the Ussuri white-toothed shrew, Crocidura lasiura, caught near the demilitarized zone, Gyeonggi-do (Province), Republic of Korea. Korean J Parasitol. 2009;47:311–314. doi: 10.3347/kjp.2009.47.3.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Groote D, van Doorn LJ, Ducatelle R, Verschuuren A, Tilmant K, Quint WGV, Haesebrouck F, Vandamme P. Phylogenetic characterization of 'Candidatus Helicobacter bovis', a new gastric Helicobacter in cattle. Int J Syst Bacteriol. 1999;49:1707–1715. doi: 10.1099/00207713-49-4-1707. [DOI] [PubMed] [Google Scholar]
- 6.Dewhirst FE, Seymour C, Fraser GJ, Paster BJ, Fox JG. Phylogeny of Helicobacter isolates from bird and swine feces and description of Helicobacter pametensis sp. nov. Int J Syst Bacteriol. 1994;44:553–560. doi: 10.1099/00207713-44-3-553. [DOI] [PubMed] [Google Scholar]
- 7.Finegold SM, Martin WJ, Scott EG. Baily and Scott's Diagnostic Microbiology. 6th ed. St. Louis: Mosby; 1982. pp. 663–673. [Google Scholar]
- 8.Fox JG, Dewhirst FE, Tully JG, Paster BJ, Yan L, Taylor NS, Collins MJ, Jr, Gorelick PL, Ward JM. Helicobacter hepaticus sp. nov., a microaerophilic bacterium isolated from livers and intestinal mucosal scrapings from mice. J Clin Microbiol. 1994;32:1238–1245. doi: 10.1128/jcm.32.5.1238-1245.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fox JG, Ge Z, Whary MT, Erdman SE, Horwitz BH. Helicobacter hepaticus infection in mice: models for understanding lower bowel inflammation and cancer. Mucosal Immunol. 2011;4:22–30. doi: 10.1038/mi.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fox JG, Yan LL, Dewhirst FE, Paster BJ, Shames B, Murphy JC, Hayward A, Belcher JC, Mendes EN. Helicobacter bilis sp. nov., a novel Helicobacter species isolated from bile, livers, and intestines of aged, inbred mice. J Clin Microbiol. 1995;33:445–454. doi: 10.1128/jcm.33.2.445-454.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franklin CL, Gorelick PL, Riley LK, Dewhirst FE, Livingston RS, Ward JM, Beckwith CS, Fox JG. Helicobacter typhlonius sp. nov., a novel murine urease-negative Helicobacter species. J Clin Microbiol. 2001;39:3920–3926. doi: 10.1128/JCM.39.11.3920-3926.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goto K, Jiang W, Zheng Q, Oku Y, Kamiya H, Itoh T, Ito M. Epidemiology of Helicobacter infection in wild rodents in the Xinjiang-Uygur autonomous region of China. Curr Microbiol. 2004;49:221–223. doi: 10.1007/s00284-004-4287-6. [DOI] [PubMed] [Google Scholar]
- 13.Harris AG, Hinds FE, Beckhouse AG, Kolesnikow T, Hazell SL. Resistance to hydrogen peroxide in Helicobacter pylori: role of catalase (KatA) and Fur, and functional analysis of a novel gene product designated 'KatA-associated protein', KapA (HP0874) Microbiology. 2002;148:3813–3825. doi: 10.1099/00221287-148-12-3813. [DOI] [PubMed] [Google Scholar]
- 14.Iten A, Graf S, Egger M, Täuber M, Graf J. Helicobacter sp. flexispira bacteremia in an immunocompetent young adult. J Clin Microbiol. 2001;39:1716–1720. doi: 10.1128/JCM.39.5.1716-1720.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johansson SK, Feinstein RE, Johansson KE, Lindberg AV. Occurrence of Helicobacter species other than H. hepaticus in laboratory mice and rats in Sweden. Comp Med. 2006;56:110–113. [PubMed] [Google Scholar]
- 16.Jukes TH, Cantor CR. Evolution of protein molecules. In: Munro HN, Allison JB, editors. Mammalian Protein Metabolism. New York: Academic Press; 1969. pp. 22–132. [Google Scholar]
- 17.Marshall B, Warren JR. Unidentified curved bacilli in the stomach of patients with gastritis and peptic ulceration. Lancet. 1984;323:1311–1315. doi: 10.1016/s0140-6736(84)91816-6. [DOI] [PubMed] [Google Scholar]
- 18.O'Rourke JL, Grehan M, Lee A. Non-pylori Helicobacter species in humans. Gut. 2001;49:601–606. doi: 10.1136/gut.49.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patterson MM, Schrenzel MD, Feng Y, Fox JG. Gastritis and intestinal metaplasia in Syrian hamsters infected with Helicobacter aurati and two other microaerobes. Vet Pathol. 2000;37:589–596. doi: 10.1354/vp.37-6-589. [DOI] [PubMed] [Google Scholar]
- 20.Peters CJ, Simpson GL, Levy H. Spectrum of hantavirus infection: hemorrhagic fever with renal syndrome and hantavirus pulmonary syndrome. Annu Rev Med. 1999;50:531–545. doi: 10.1146/annurev.med.50.1.531. [DOI] [PubMed] [Google Scholar]
- 21.Queiroz DM, Contigli C, Coimbra RS, Nogueira AM, Mendes EN, Rocha GA, Moura SB. Spiral bacterium associated with gastric, ileal and caecal mucosa of mice. Lab Anim. 1992;26:288–294. doi: 10.1258/002367792780745760. [DOI] [PubMed] [Google Scholar]
- 22.Riley LK, Franklin CL, Hook RR, Jr, Besch-Williford C. Identification of murine helicobacters by PCR and restriction enzyme analyses. J Clin Microbiol. 1996;34:942–946. doi: 10.1128/jcm.34.4.942-946.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Robertson BR, O'Rourke JL, Vandamme P, On S, Lee A. Helicobacter ganmani sp. nov., a urease-negative anaerobe isolated from the intestines of laboratory mice. Int J Syst Evol Microbiol. 2001;51:1881–1889. doi: 10.1099/00207713-51-5-1881. [DOI] [PubMed] [Google Scholar]
- 24.Robić M, Artuković B, Beck A, Turk R, Belić M, Svetina A, Grabarević Ž. Histopathological changes in the stomachs of wild rodents in Croatia and the first finding of the Helicobacter species. Vet Arh. 2011;81:415–421. [Google Scholar]
- 25.Sabbaghian MS, Ranaudo J, Zeng L, Alongi AP, Perez-Perez G, Shamamian P. Identification of Helicobacter spp. in bile and gallbladder tissue of patients with symptomatic gallbladder disease. HPB (Oxford) 2010;12:129–133. doi: 10.1111/j.1477-2574.2009.00148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 27.Shen Z, Fox JG, Dewhirst FE, Paster BJ, Foltz CJ, Yan L, Shames B, Perry L. Helicobacter rodentium sp. nov., a urease-negative Helicobacter species isolated from laboratory mice. Int J Syst Bacteriol. 1997;47:627–634. doi: 10.1099/00207713-47-3-627. [DOI] [PubMed] [Google Scholar]
- 28.Shen Z, Xu S, Dewhirst FE, Paster BJ, Pena JA, Modlin IM, Kidd M, Fox JG. A novel enterohepatic Helicobacter species 'Helicobacter mastomyrinus' isolated from the liver and intestine of rodents. Helicobacter. 2005;10:59–70. doi: 10.1111/j.1523-5378.2005.00292.x. [DOI] [PubMed] [Google Scholar]
- 29.Simmons JH, Riley LK, Besch-Williford CL, Franklin CL. Helicobacter mesocricetorum sp. nov., a novel Helicobacter isolated from the feces of Syrian hamsters. J Clin Microbiol. 2000;38:1811–1817. doi: 10.1128/jcm.38.5.1811-1817.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Solnick JV. Clinical significance of Helicobacter species other than Helicobacter pylori. Clin Infect Dis. 2003;36:349–354. doi: 10.1086/346038. [DOI] [PubMed] [Google Scholar]
- 31.Strugatsky D, McNulty R, Munson K, Chen CK, Soltis SM, Sachs G, Luecke H. Structure of the proton-gated urea channel from the gastric pathogen Helicobacter pylori. Nature. 2013;493:255–258. doi: 10.1038/nature11684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tee W, Leder K, Karroum E, Dyall-Smith M. "Flexispira rappini" bacteremia in a child with pneumonia. J Clin Microbiol. 1998;36:1679–1682. doi: 10.1128/jcm.36.6.1679-1682.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ward JM, Anver MR, Haines DC, Melhorn JM, Gorelick P, Yan L, Fox JG. Inflammatory large bowel disease in immunodeficient mice naturally infected with Helicobacter hepaticus. Lab Anim Sci. 1996;46:15–20. [PubMed] [Google Scholar]
- 35.Whary MT, Fox JG. Natural and experimental Helicobacter infections. Comp Med. 2004;54:128–158. [PubMed] [Google Scholar]
- 36.Won YS, Yoon JH, Lee CH, Kim BH, Hyun BH, Choi YK. Helicobacter muricola sp. nov., a novel Helicobacter species isolated from the ceca and feces of Korean wild mouse (Mus musculus molossinus) FEMS Microbiol Lett. 2002;209:45–51. doi: 10.1111/j.1574-6968.2002.tb11107.x. [DOI] [PubMed] [Google Scholar]
- 37.Yoon MH, Jung SJ, Oh HS. Population structure and reproductive pattern of the Korean striped field mouse, apodemus agrarius. Korean J Biol Sci. 1997;1:53–61. [Google Scholar]