Abstract
Removal of an introducer-sheath from a femoral artery after completing transarterial embolization of a patent ductus arteriosus can cause life-threatening hemorrhage in dogs. In the present study, the effectiveness of chitosan acetate dressing in 10 experimental dogs was tested. Under general anesthesia, an introducer-sheath was placed into the femoral artery with percutaneous puncture using Seldinger's technique. The outer diameter of the introducer-sheaths varied from 3.0 to 4.0 mm with an introducer/artery ratio of 80 to 123%. The artery's diameter was measured using ultrasonography. Following removal of the introducer-sheath, a chitosan acetate dressing was applied to the wound and held in place with manual compression for 10 min. Successful hemostasis was reached on 12 arteries. However, on two arteries, hemorrhage was uncontrollable and led to a hypovolemic shock during 10 min of manual compression. Possible causes of the negative outcome in two dogs were their old age and an introducer-sheath with a too large diameter. The chitosan acetate dressing was easy to use and the artery remained patent. Dogs could walk directly after recovery from anesthesia and their femoral arteries were saved. In conclusion, the outer diameter of the introducer-sheath should not exceed 3 mm or the inner diameter of the artery.
Keywords: cardiac catheterization, patent ductus arteriosus
Introduction
One of the most commonly performed minimal invasive cardiac interventions in dogs is embolization of a patent ductus arteriosus. For embolization with an Amplatz Canine Ductal Occluder (Infiniti Medical, USA), the femoral artery is used as an access [10]. Placing an introducer-sheath or a guiding catheter with a hemostatic valve in the femoral artery allows convenient placement of various catheters, guide wires and implant-devices. The introducer-sheath or guiding catheter may be placed in the arterial lumen with either a surgical cut-down or percutaneous puncture of the femoral artery using Seldinger's technique [3]. Although percutaneous puncture is a quicker and less invasive way to create arterial access, removal of the introducer-sheath or guiding catheter at the end of the procedure may cause severe hemorrhage from the arterial puncture site in dogs [12].
Chitosan acetate dressings are commercially available products licensed by the American Food and Drug Administration for hemostasis in humans. The effectiveness of these products in human medicine has been documented for several applications, including controlling hemorrhage from the femoral arterial puncture site after cardiac catheterization [2]. Chitosan acetate dressings have also been investigated in several experimental animals (mostly in swine), but not yet in dogs [6,9,13].
This study was conducted to investigate whether commercially available chitosan acetate dressings (HemCon Medical Technologies, USA) could achieve quick and permanent hemostasis after removal of a percutaneously placed large-bore introducer-sheath from the femoral artery in anesthetized experimental dogs.
Materials and Methods
The Animal Experimental Committee of the Utrecht University has approved this two-staged pilot study (under the following permission no. 2009.II.09.082, 2011.II.03.050 and SO/14/0). The first approval was given for animals that had been subjected to another experimental procedure and were scheduled for euthanasia under general anesthesia. After completion of the terminal experiment on four dogs with successful outcome, permission was granted for a survival experiment on six other dogs.
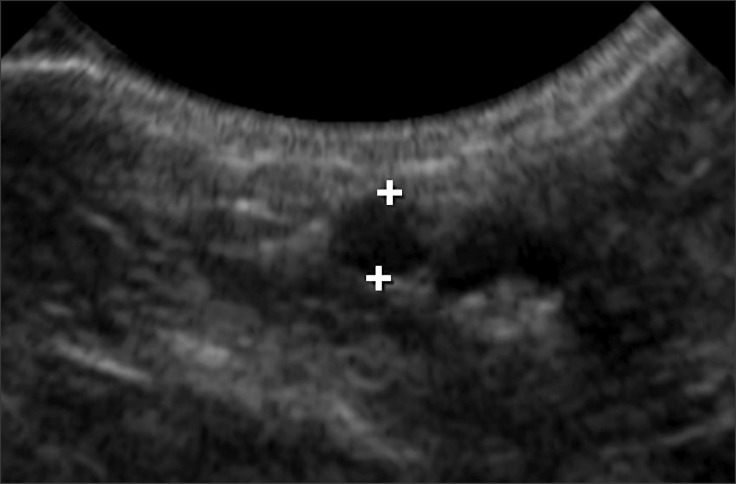
The size of the introducer-sheath was determined based on the diameter of the femoral artery of the individual dogs. The internal diameter of the femoral artery was measured with 2-dimensional grey-scale ultrasonography on cross-sectional images using a phased (5-8 MHz) or linear (5-10 MHz) array transducer (Philips Healthcare, the Netherlands) at the location in which the introducer-sheath was planned to be percutaneously inserted, the medial aspect of the hind limb as proximal as possible (Fig. 1).
Fig. 1. Measurement of the internal diameter of the femoral artery on a cross sectional 2-dimensional ultrasound image.
This study was performed on dogs under general anesthesia according to the same anesthetic protocol used in dogs with patent ductus arteriosus that undergo therapeutic cardiac catheterization at the author's clinic. Intravenous methadone (0.5 mg/kg; Eurovet Animal Health, the Netherlands) and intramuscular atropine sulfate (0.02 mg/kg; Teva Pharmachemie, the Netherlands) were used as premedications, intravenous propofol (2.6-7.1 mg/kg; Abbott Laboratories, UK) was used for induction and inhaled isoflurane (Abbott Laboratories) vaporized in 100% O2 and intravenous fentanyl (5 µg/kg/h in continuous rate infusion; Bipharma, the Netherlands) were used for anesthetic maintenance. Blood pressure was measured with an oscillometric machine (SurgiVet; Smiths Medical, USA) indirectly (in the first two dogs) or directly in the coccygeal (in six dogs) or the femoral artery (in two dogs). The reason for this variation was that the anesthesiologists did not succeed in placing an arterial catheter in the coccygeal or in any artery of each dog. Continuous ECG, pulse-oxymetry, rectal temperature and ETCO2 monitoring were used during anesthesia. In addition, coagulation parameters and hematocrit were measured before intervention in the clinic's laboratory.
The dogs were placed under general anesthesia in dorsal recumbency with the legs abducted. The skin overlying the region of the femoral artery was surgically prepared and a skin incision was made over the femoral artery using a scalpel (number 11). The artery was located with palpation (based on pulsations) and punctured with a needle through the skin incision. The needle was part of an introducer set that also contained a guide-wire, a dilator and the introducer-sheath with a hemostatic valve and a 3-way stopcock (H. Lee SafeSheath; Thomas Medical Products, USA). When pulsating blood appeared in the needle's hub, the guide-wire was inserted through the needle into the artery's lumen. With the guide-wire in place, the needle was removed and the introducer-sheath together with the dilator was placed over the guide-wire into the artery's lumen. Thereafter the dilator and the guide-wire were removed. The correct intra-arterial positioning of the introducer-sheath was confirmed by opening the 3-way stopcock, where pulsating blood was running out.
The differences in the terminal (first) and the survival (second) parts of the experiment are described in the following sections.
Terminal experiment
Four 14-month old beagles (two males and two females, weight 11.3-16.3 kg) that were already part of a terminal (orthopedic) experiment were used. After completion of the present experiment, animals were euthanized while still under general anesthesia.
Instead of repeating the same procedure on each artery under the same circumstances, after each successful hemostasis, the manufacturer's recommendations were modified with the approval of the product's representative, who was present throughout the experiment. In addition to altering the manufacturer's recommendations, introducer-sheaths with an external diameter exceeding the internal diameter of the femoral artery were also tried.
The internal diameter of the femoral artery varied from 2.9 to 3.8 mm, while the outer diameter of the introducer-sheaths ranged from 3.0 to 3.7 mm, with an introducer/artery ratio of 97 to 119%.
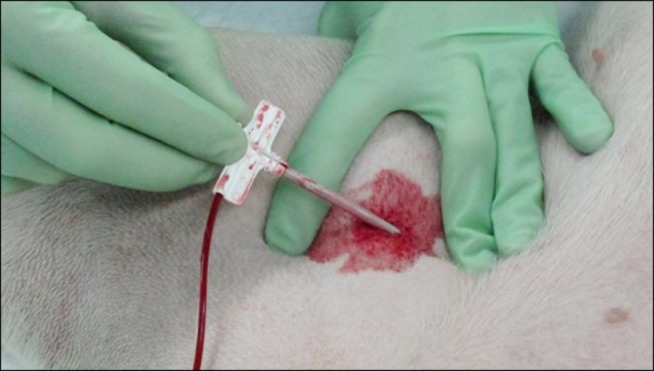
When both femoral arteries had an introducer-sheath in place, the firstly placed introducer-sheath was removed. While the femoral artery was being compressed with the middle finger proximal and the index finger distal to the puncture site to prevent hemorrhage, the introducer-sheath was removed (Fig. 2). According to the manufacturer's recommendations, some external bleeding from the vessel was allowed so that the dressing could stick properly to the wound and the surrounding skin. The dressing was placed on the wound with special attention so that its active surface would not be touched by the operator, then held in place with manual compression for 10 min. The region was subsequently inspected for subcutaneous and external hemorrhage. Blood pressure and heart rate were monitored continuously throughout the procedure.
Fig. 2. Removal of a 10-French introducer-sheath (with 4 mm outer diameter) from the femoral artery of an anesthetized Boerboel dog. The operator is compressing the artery proximally and distally to the puncture site with his fingers to prevent hemorrhage.
In all four dogs, both femoral arteries were catheterized, while in one dog, one femoral vein was also catheterized. The tested chitosan acetate dressing products were the HemCon Patch 1.5" × 1.5" (4 × 4 cm) and the HemCon Bandage 2" × 2" (5 × 5 cm), which have identical effects according to the manufacturer. The only difference between these products (other than their size) is that the bandage is more rigid than the patch. Two wounds were treated with HemCon Bandages and six with HemCon Patches.
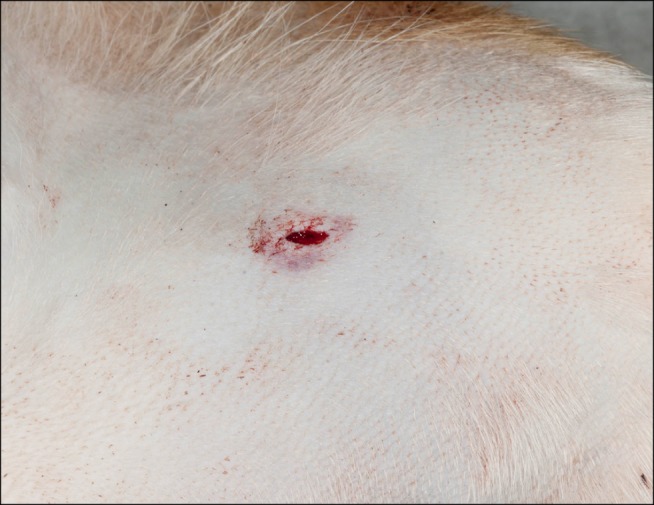
The chitosan acetate dressing (patch or bandage) was left in place for 20 to 80 min, then removed with a stream of sodium chloride 0.9% solution directed between the skin and the dressing using a 10 mL syringe while the dressing was gently pulled with a forceps (Fig. 3). Although the manufacturer's recommendation is to leave the dressing in place for 24 h, we removed it much sooner so that the patency of the artery could be examined with ultrasound. The femoral artery (and vein) was imaged in longitudinal and cross sections with 2-dimensional grey-scale, color and pulsed-wave Doppler ultrasonography. The legs were then moved (abduction, adduction, flexion, extension, endorotation and exorotation) for 1 min every 30 min to imitate walking in an awake animal until euthanasia. Three to five hours after removal of the introducer-sheaths, the dogs were euthanized by intravenous overdose of pentobarbital (Alfasan International, the Netherlands).
Fig. 3. The femoral arterial puncture site in an anesthetized beagle dog after removing the patch that was placed 30 min earlier, i.e., 30 min after removal of the introducer-sheath from the arterial lumen.
In the first two dogs, the manufacturer's recommendations were strictly followed regarding the method of letting the skin around the wound be covered with blood from the puncture site. However, in the third and fourth dogs the protocol was modified because subcutaneous hematoma formation was observed in the first two dogs. Before removing the introducer-sheath, 5 mL of blood was withdrawn from the 3-way stopcock of the introducer-sheath, after which the sheath was removed and this blood was spread on the skin around the puncture site without releasing the manual compression of the punctured artery.
On the third and fourth dogs, the following additional tests were performed.
In the third dog, the effects of arterial hypertension were tested by administering intravenous dopamine (20 µg/kg/min; Zambon, the Netherlands) 120 min after removal of the introducer-sheath and 90 min after removal of the patch. A placebo (the wrong side of a HemCon Patch) was used on a femoral artery of the fourth dog.
Survival experiment
Four beagles (two 12 and two 10 year olds, weight 12.4-17.5 kg) and two 8-month-old female Boerboels (weight 47.8 and 51.8 kg) were used. The experimental beagles were to be euthanized shortly after completing the present experiment because of their old age. The young Boerboels, one with a mild congenital double-chambered right ventricle and the other with a severe congenital valvular pulmonic stenosis, were donated to the author's clinic by a breeder for experimental purposes. Special governmental permission was granted for these two dogs so that they could become experimental animals and part of the present study by the Food and Consumer Product Safety Authority, initiated by the Laboratory Animal Welfare Officers of the Utrecht University.
The internal diameter of the femoral artery in these dogs varied from 3.0 to 5.0 mm and the outer diameter of the introducer-sheaths from 3.0 to 4.0 mm. The introducer/artery ratio was 80 to 123%. The same anesthesia protocol was used in the survival as in the terminal part of the experiment. Blood pressure was measured invasively in the coccygeal and in the contralateral femoral artery of one Boerboel. Only one femoral artery of each dog received an introducer-sheath according to the description above.
Successful hemostasis was defined as cessation of external and subcutaneous bleeding within a total of 30 min after removing the introducer-sheath. Subcutaneous hemorrhage was diagnosed by a growing lump around the puncture site, falling arterial blood pressure and rising heart rate.
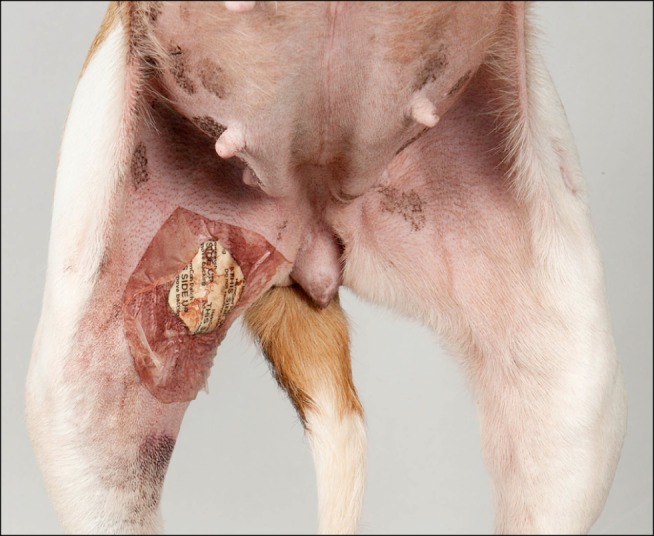
After successful hemostasis, the dogs were immediately allowed to recover from anesthesia (without the recommended 90 min of rest) (Fig. 4). No postoperative restrictions were applied or (sedative) medications were given. Dogs underwent a daily general physical examination and wound control for six consecutive postoperative days, after which they were euthanized, with the exception of a Boerboel with mild congenital cardiac disease that was adopted.
Fig. 4. The puncture site of the right femoral artery is covered with a HemCon patch. The introducer-sheath was removed 2 h before making this photograph.
Dissection of the femoral artery was attempted in all euthanized dogs. Histologic examination of the femoral artery was performed in selected dogs from the group with successful and unsuccessful hemostasis.
Results
The prothrombin time (PT), platelet count and fibrinogen concentration were within the reference range in all dogs before the procedure (PT = 7.2-9.9 sec, platelet count = 144-603 × 109/liter and fibrinogen = 1.0-2.7 g/L). The activated partial thromboplastin time (APTT) was within the reference range (13.2-18.2 sec) in five dogs, slightly prolonged (20.1-23.8 sec) in four dogs and moderately prolonged in one dog (37.9 sec). The hematocrit was within the reference range in eight dogs (42-61%) and mildly reduced in two dogs (34-35%).
Terminal experiment
Successful hemostasis was reached in all eight arterial and one venous puncture sites of the four beagles in the terminal part of the experiment using a single HemCon Patch (on six sites) or a HemCon Bandage (on two sites) with 10 min of manual compression.
In every dog, inspection and ultrasonography showed minor subcutaneous hematomas around the puncture site. Color Doppler ultrasonography showed patency of the eight arteries immediately after removal of the dressing. The method used to cover the wound with blood (directly from the puncture site or from a syringe) did not affect the efficacy of the dressing.
In the dog in which the placebo was placed on the arterial puncture site, after 6 min of manual compression a large subcutaneous hematoma developed and uncontrollable external bleeding occurred with blood flowing out between the fingers of the operator, despite continuous manual compression. The blood pressure dropped from 120/52 to 104/50 mm Hg and the heart rate increased from 85 to 90/min. Intravenous colloid was given to restore the blood pressure, a HemCon Bandage (this time with the proper side on the wound) was applied to the wound and manual pressure was applied for 10 min. The hemorrhage stopped and the blood pressure and heart rate remained stable (103/43 mmHg and 84/min respectively) for the rest of the experiment.
In the dog in which hypertension was created, the dressings had already been removed. After 4 min of dopamine infusion, the systolic blood pressure increased from 106 to 170 mm Hg, after which the right wound started to show a minor external and subcutaneous hemorrhage. When the systolic blood pressure reached 138 mmHg (after 3 min) by reducing the intravenous dopamine administration, the bleeding stopped spontaneously without any intervention. The blood pressure and heart rate remained stable after stopping dopamine administration during the rest of the experiment.
Survival experiment
The first two beagles in the survival experiment developed uncontrollable hemorrhage within 8 min of removal of the introducer-sheath (i.e., patch-placement), while manual compression was still applied. First, a large subcutaneous hematoma developed, after which the heart rate increased and the blood pressure dropped quickly. Thereafter, blood was flowing out of the wound between the operator's fingers. The patch was replaced with a new patch and manual compression was applied, but the hemorrhage could not be controlled and subcutaneous as well as external hemorrhage was still ongoing. At this point, the decision was made to euthanize both dogs by intravenous over dosage of pentobarbital. Both dogs, which were 12 years old, were given a 3.7 mm sheath with an introducer/artery ratio of 123%, i.e., sheaths with an external diameter exceeding the internal diameter of the artery. At postmortem dissection, the enormous hematoma did not allow localization of the femoral arterial puncture site.
Successful hemostasis was reached in the other two beagles in the survival portion of the experiment and in the two Boerboels. In one beagle and one Boerboel, the patch reached an immediate hemostasis, whereas in the other surviving beagle the patch had to be replaced after 10 min because of an ongoing minor hemorrhage. In the other Boerboel, a subcutaneous hematoma started to develop immediately after the 10 min of manual compression was stopped. Therefore, the compression time was extended three times by 5 min each (extra 15 min), and definitive hemorrhage control was reached only after a total of 25 min of compression.
The four surviving dogs recovered quickly from anesthesia without any complications and did not show any signs of discomfort. Animals were moving around with ease on the day of the procedure, and the puncture site could not be localized with inspection six days later. Ultrasonography on that day showed a small local aneurysm corresponding to the puncture site in each dog (Fig. 5).
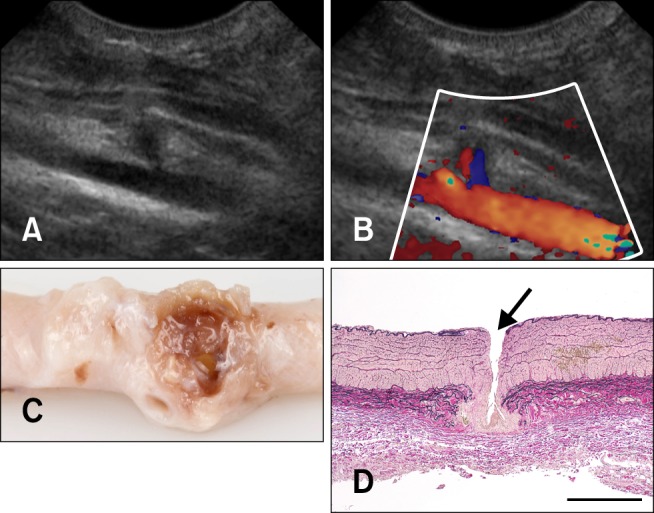
Fig. 5. The femoral artery of a Boerboel dog 6 days after removal of the introducer-sheath. (A) 2-dimensional ultrasonography shows a small local aneurysm at the site of the puncture. (B) Color Doppler ultrasonography shows a patent lumen (the same longitudinal image as on panel A). (C) The dissected femoral artery (stored in formalin). Granulation tissue can be seen around the puncture site. (D) Photomicrogram of the femoral artery around the puncture site (arrow); longitudinal section. Lawson stain, scale bar = 500 µm.
Taken together, the results of the terminal and survival experiments revealed successful hemostasis in 12 (86%) of the 14 arterial puncture sites of the 10 dogs. A second dressing and/or a longer compression time than the recommended 10 min was necessary in two of the 12 successfully treated arteries. In two of the 10 dogs, uncontrollable hemorrhage occurred, which necessitated immediate euthanasia. Once hemorrhage occurred, it took place during the manual compression phase or immediately thereafter, i.e., within 10 min of dressing placement. No hemorrhage occurred hours or days after the procedure.
Histological findings of the arteries (Fig. 5) of the young beagles of the terminal experiment with successful hemostasis and that of an old beagle with unsuccessful hemostasis showed no obvious difference (e.g., in the amount of elastic fibers or signs of atherosclerosis) that could contribute to the different outcomes.
Discussion
At present, veterinary cardiologists lack a simple and effective technique to prevent hemorrhage after completing transarterial cardiac interventions in dogs. However, there are several options that can prevent a potentially fatal arterial hemorrhage. Applying manual compression at the puncture site after completing the intervention has been reported from only one veterinary center [5]. Several days of sedation with a compressing bandage combined with hospitalization has been reported from another veterinary center, but with this approach important benefits of one-day ambulant minimally invasive surgeries are lost [12]. Another possibility is to place an intravascular plug through the puncture site [1,7,11]. However, such plugs have relatively high costs and cannot be used in a small caliber artery (because of obstruction of its lumen) or with a large-gauged introducer-sheath (because of the inability to close the puncture site). The most commonly applied technique in dogs is performing a surgical cut-down with subsequent permanent ligation of the femoral artery. Although hemorrhage can successfully be prevented, the surgery may prolong the procedure and the femoral artery will be lost. Though the loss of a femoral artery does not generally cause noticeable lameness or discomfort to the patient, this approach prevents the artery from being re-used. Because of the shortcomings of the above described procedures, we tested a new product in the setting of percutaneous introducer-placement.
Chitosan is the de-acetylated form of chitin, a poly-N-acetyl glucosamine extracted from marine arthropod shells [4]. When chitosan acetate comes in direct contact with blood, it initiates the formation of a blood clot by attracting erythrocytes. The mechanism of action is thought to be based on ionic interaction between the positively loaded chitosan acetate and negatively loaded red blood cells [14]. Interestingly, possible deficiencies in the coagulation cascade do not seem to affect clot-formation [8]. In addition, chitosan acetate has an antibacterial effect [4]. Commercially available chitosan acetate products are patches and bandages of various sizes containing pressed chitosan acetate.
The present pilot study showed that chitosan acetate dressing (both patch and bandage) can effectively control hemorrhage from the femoral artery in most dogs after removal of a percutaneously placed introducer-sheath as long as the outer diameter of the catheter does not exceed 3 mm and that of the internal arterial diameter. The present experiment was designed to mimic the same situation as a transarterial embolization of a patent ductus arteriosus in dogs.
The absolute and relative size of the introducer-sheath (compared to the arterial lumen), the blood pressure and the age of the dog seem to be important factors influencing successful hemorrhage control. Our goal was to place as thick an introducer-sheath as possible, i.e., the outer diameter of the introducer close to the internal arterial diameter, measured with 2D-ultrasound. A thicker catheter than the inner diameter of the femoral artery (measured preoperatively with ultrasound) cannot be placed in the femoral artery, if a surgical cut-down is used (author's unpublished observation). An introducer with a 3 mm external diameter was selected because a 9-French guiding catheter (with an outer diameter of 3 mm) is necessary to place the largest available sized Amplatz Canine Ductal Occluder for closure of a patent ductus arteriosus. If the operator decides to use an additional introducer-sheath, then this catheter needs a 9-French introducer-sheath with an outer diameter of 3.7 mm. That is why introducer-sheaths with an outer diameter of 3.0 to 4.0 mm were tested.
It should be noted that this study had several limitations. Based on the results of this pilot study, a second study should be carried out under uniform conditions and on more dogs so that statistically sound conclusions can be drawn. Embolization of patent ductus arteriosus in dogs with Amplatz Canine Duct Occluder has routinely been performed using percutaneous puncture of the femoral artery with Seldinger's technique at the Faculty of Veterinary Medicine in Perugia, Italy [5]. The canine patients of this clinic would offer the best possibility to test chitosan acetate dressings on the target population, in which only manual compression has been applied to date [5].
The manufacturer's recommendations were altered (at least 10 min of manual compression; resting the leg for at least 90 min; patch- or bandage-removal after 24 h) because dogs may start walking much earlier than humans after a cardiac catheterization, and they might remove the patch themselves by licking the wound.
The major advantages of chitosan acetate patches and bandages are that: 1) their use requires only a few minutes of training and no specific expertise is needed; 2) the artery remains patent immediately after hemorrhage-control has been achieved; 3) the dressings can be used with any size introducer-sheath and on any size artery; and 4) the costs of the dressings are low.
The most likely reason why the results of similar human studies showed no failure of the same product is the small size of the sheath relative to the vessel's diameter [2]. A 6-French sheath (2.7 mm external diameter) in an adult human being is quite small compared to the same sized sheath in a 15 kg beagle dog.
Chitosan acetate dressing may be a possible alternative to replace surgical cut-down with percutaneous placement of an introducer-sheath or guiding catheter in dogs that undergo transarterial embolization of a patent ductus arteriosus. Successful hemostasis was reached on 12 of the 14 femoral arteries in the present study. A major risk is that, in the case of an unsuccessful hemostasis, surgical intervention (i.e., ligating the artery) is probably very challenging, if not impossible, because of the ongoing arterial hemorrhage and rapid development of a large subcutaneous hematoma. More studies are required before routine application can be safely recommended for routine veterinary patient care.
Acknowledgments
The HemCon Bandages and Patches used in this study were provided free of charge by Dr. Rene Smit, HemCon Benelux. The costs of the experiments were financed by the author's clinic (project Advances in Veterinary Medicine). Rianne Paardekooper, a veterinary master's student, assisted with most of the experiments, performed the literature study and wrote the first draft of this manuscript. Several anesthesiologists of the author's clinic were responsible for anesthesia of the dogs, among others Rob Sap, Ies Akkerdaas, Loes van Gennip, Ron van Wandelen and Joost Uilenreef. Histological evaluation of the femoral artery was conducted by Dr. Guy Grinwis, Department of Pathobiology of the author's institution. Macroscopic photographs were made by Mr. Joop Fama (Multimedia of the Faculty of Veterinary Medicine) and Rene Smit.
This study was presented as a research abstract (oral communication) at the 22nd ECVIM-CA congress in Maastricht, the Netherlands on 7 September 2012 with the title "Chitosan (HemCon) patch effectively controls hemorrhage from femoral arterial puncture site in dogs after removal of a large-bore introducer-sheath."
Footnotes
Conflict of Interest: There is no conflict of interest.
References
- 1.Aksoy M, Becquemin JP, Desgranges P, Allaire E, Kobeiter H. The safety and efficacy of angioseal in therapeutic endovascular interventions. Eur J Vasc Endovasc Surg. 2006;32:90–93. doi: 10.1016/j.ejvs.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 2.Arbel J, Rozenbaum E, Reges O, Neuman Y, Levi A, Erel J, Haskia AR, Caneti M, Sherf M, Mosseri M. Usage of chitosan for femoral (USF) haemostasis after percutaneous procedures: a comparative open label study. EuroIntervention. 2011;6:1104–1109. doi: 10.4244/EIJV6I9A192. [DOI] [PubMed] [Google Scholar]
- 3.Beal MW, Hughes D. Vascular access: theory and techniques in the small animal emergency patient. Clin Tech Small Anim Pract. 2000;15:101–109. doi: 10.1053/svms.2000.6802. [DOI] [PubMed] [Google Scholar]
- 4.Burkatovskaya M, Castano AP, Demidova-Rice TN, Tegos GP, Hamblin MR. Effect of chitosan acetate bandage on wound healing in infected and noninfected wounds in mice. Wound Repair Regen. 2008;16:425–431. doi: 10.1111/j.1524-475X.2008.00382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Caivano D, Birettoni F, Fruganti A, Rishniw M, Knafelz P, Moïse NS, Porciello F. Transthoracic echocardiographically-guided interventional cardiac procedures in the dog. J Vet Cardiol. 2012;14:431–444. doi: 10.1016/j.jvc.2012.02.010. [DOI] [PubMed] [Google Scholar]
- 6.Devlin JJ, Kircher S, Kozen BG, Littlejohn LF, Johnson AS. Comparison of ChitoFlex, CELOX, and QuikClot in control of hemorrhage. J Emerg Med. 2011;41:237–245. doi: 10.1016/j.jemermed.2009.02.017. [DOI] [PubMed] [Google Scholar]
- 7.Gargiulo NJ, 3rd, Veith FJ, Ohki T, Scher LA, Berdejo GL, Lipsitz EC, Menegus M, Greenberg M. Histologic and duplex comparison of the perclose and angio-seal percutaneous closure devices. Vascular. 2007;15:24–29. doi: 10.2310/6670.2007.00004. [DOI] [PubMed] [Google Scholar]
- 8.Klokkevold PR, Fukayama H, Sung EC, Bertolami CN. The effect of chitosan (poly-N-acetyl glucosamine) on lingual hemostasis in heparinized rabbits. J Oral Maxillofac Surg. 1999;57:49–52. doi: 10.1016/s0278-2391(99)90632-8. [DOI] [PubMed] [Google Scholar]
- 9.MacIntyre AD, Quick JA, Barnes SL. Hemostatic dressings reduce tourniquet time while maintaining hemorrhage control. Am Surg. 2011;77:162–165. [PubMed] [Google Scholar]
- 10.Nguyenba TP, Tobias AH. The Amplatz canine duct occluder: a novel device for patent ductus arteriosus occlusion. J Vet Cardiol. 2007;9:109–117. doi: 10.1016/j.jvc.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 11.Sanghi P, Virmani R, Do D, Erikson J, Elliott J, Cilingiroglu M, Matthews H, Kazi M, Ricker R, Bailey SR. A comparative evaluation of arterial blood flow and the healing response after femoral artery closure using angioseal STS Plus and StarClose in a porcine model. J Interv Cardiol. 2008;21:329–336. doi: 10.1111/j.1540-8183.2008.00367.x. [DOI] [PubMed] [Google Scholar]
- 12.Schneider M, Schneider I, Hildebrandt N, Wehner M. Percutaneous angiography of Patent Ductus Arteriosus in dogs: techniques, results and implications for intravascular occlusion. J Vet Cardiol. 2003;5:21–27. doi: 10.1016/S1760-2734(06)70048-0. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz RB, Reynolds BZ, Shiver SA, Lerner EB, Greenfield EM, Solis RA, Kimpel NA, Coule PL, McManus JG. Comparison of two packable hemostatic Gauze dressings in a porcine hemorrhage model. Prehosp Emerg Care. 2011;15:477–482. doi: 10.3109/10903127.2011.598615. [DOI] [PubMed] [Google Scholar]
- 14.Thatte HS, Zagarins SE, Amiji M, Khuri SF. Poly-N-acetyl glucosamine-mediated red blood cell interactions. J Trauma. 2004;57(Suppl):S7–S12. doi: 10.1097/01.ta.0000136742.04816.38. [DOI] [PubMed] [Google Scholar]