Highlights
-
•
The use of chemotherapy in our series for pregnancy associated breast cancer was successful with resulting healthy babies.
-
•
Chemotherapy was done from the second trimeater.
-
•
The choice of drugs were doxorubicin and cyclophosphamide administered by a slow infusion protocol.
-
•
The babies did not require ICU care.
-
•
Breast conservation was successfully used in one of the cases who is clinically disease free 5 years post diagnosis.
Keywords: Breast cancer, Pregnancy, Chemotherapy
Abstract
Context
Although breast cancer is a common cancer, Pregnancy associated breast cancer is uncommon. Adjuvant chemotherapy administered intrapartum has been resolved to be safe from the second trimester.
Objective
To review cases of pregnancy associated breast cancer managed with adjuvant intrapartum chemotherapy.
Patients and method
Gravid patients diagnosed with breast cancer had chemotherapy administered by a slow infusion protocol at 3 weekly interval from the second trimester till 4 weeks to expected date of delivery. Obstetric scans were done to monitor fetal growth and development. Requisite surgery was carried out intrapartum and postpartum.
Results
There were three cases of pregnancy associated breast cancer Age range 32–33 years, mean 32.5 years. Two cases presented in the second trimester while one presented in the third trimester. The second case had overt metastatic disease and was grave in respiratory distress. Histology showed invasive lobular carcinoma in two cases and extensive intraductal carcinoma with invasive component in the third. Immunohistochemistry showed triple negative in the first case and hormone positive in the third case. Wide local excision was done for a 3 cm lump in the first case and mastectomy postpartum in the third case. but had no surgery. Doxorubicin and cyclophosphamide was administered three weekly from the second trimester up to 32 weeks and continued postpartum. Taxanes was administered afterwards. The grave clinical state of the second case was markedly improved with the first cycle of chemotherapy instituted. All cases had spontaneous vaginal delivery with good apgar scores. Children had normal developmental milestones. First case with breast conservation is clinically disease free, the second case demised postpartum from disease progression while the third had a mastectomy and is on cue for radiotherapy.
Conclusion
Adjuvant intrapartum chemotherapy had a successful outcome with birth of normal babies with normal developmental milestones in our miniseries.
1. Management of pregnancy associated breast cancer in a developing country. The role of chemotherapy
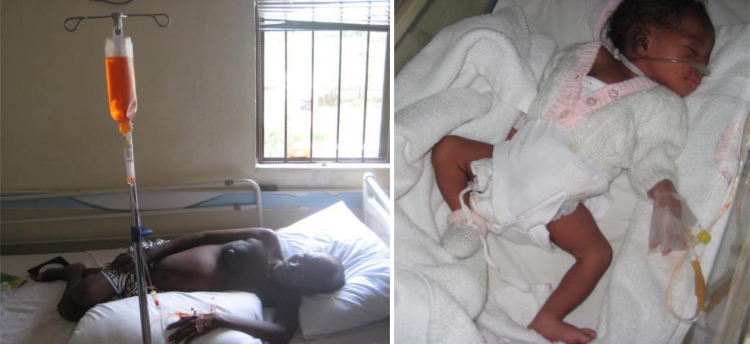
Pregnancy associated breast cancer has been acceptably defined as breast cancer occurring during pregnancy or up to one year postpartum [1]. Although pregnancy associated breast cancer is the second commonest diagnosed malignancy in pregnancy, it is still a rare occurrence at 1 in 3000 pregnant women [1]. The traditional view of a poorly prognostic cancer has transited to survival of the disease being seen at par with nonpregnant cases, multiple studies reporting equivalent survivals in early cases when matched with nonpregnant patients [2], [3], [4]. Nevertheless some studies have dissented reporting advanced cases with lymph node involvement and a poorer prognosis with these patients [5], [6]. With the trend to record improved survival following breast cancer diagnosis, management of intrapartum breast cancer places the health of the mother at conflict with the foetus. This was the classical basis for proponents of termination of pregnancy to allow treatment (Fig. 1) [7], [8].
Fig. 1.
2nd Case on doxorobicin infusion. Healthy baby from case on observation.
The administration of chemotherapy in pregnancy was classically dogged by controversy due to the fear of possible induction of teratogenicity in the foetus. Current evidence showing the safety of chemotherapy has been successfully illustrated in several series [9], [10]. Expectedly, absence of considerably large series in this uncommon presentation as has been obtained with breast cancer makes the presence of standard protocols challenging [11]. We are not aware of any published reports of its utilization in West Africa as the majority of published reports have emerged from the Western world. Hence a report from West Africa where resources are thought to be limited, encourages the routine use of chemotherapy intrapartum for this disease thus avoiding possible spread from intrapartum delay. Additionally, the experience with one of the cases is unique being a gravid woman who presented looking grave with metastatic disease but was ‘resuscitated’ with the first chemotherapy cycle (Table 1). We review the outcome of pregnancy associated breast cancer in the authors practice.
Table 1.
Tumour sizes and treatment modality.
| Age | Size of lump/cm | Histology | Surgery | No of intrapartum chemotherapy cycles | Birth weight/kg |
|---|---|---|---|---|---|
| 32 | 3 | Invasive lobular | Wide local excision | 5 | 3.1 |
| 32 | 14 | Invasive lobular | 4 | 1.8 | |
| 33 | 7 | Intraductal with invasive component | Mastectomy | 1 | 4.1 |
1.1. Patients and methods
The subjects were patients who were presented with a breast lump/mass in pregnancy. Following history and examination, breast biopsy under local anaesthesia was done and specimen sent to the pathologist to make a breast cancer diagnosis. Patients subsequently had abdominopelvic ultrasound scan to date the pregnancy and to ascertain liver involvement. Patients were counselled for chemotherapy administered from the second trimester at three weekly intervals. Subsequent abdominal ultrasound scans were done before each session to monitor fetal growth. Reexcision was done in the case for breast conservation under local anaesthesia. Patients were also receiving antenatal care from the obstetrician until they fell into spontaneous labour and delivery. Following delivery the babies were examined for any discernible malformations. Breastfeeding was omitted in the babies and treatment of the mothers continued.
1.2. Second case
A 32 years old secondary school leaver diagnosed as a T4 tumour which had taken over the whole breast with extensive peau d’orange, nipple retraction and matted axillary lymphadenopathy. Abdominal exam revealed 20 cm hepatomegaly below the costal margin. She appeared grave and in respiratory distress. Histopathology revealed invasive lobular carcinoma. Being in the second trimester, she was commenced on doxorubicin and cyclophosphamide. Her initial grave clinical state had a rapid improvement within days of chemotherapy commencement. Four cycles of doxorubicin was completed intrapartum in the second and third trimester. Spontaneous vaginal delivery occurred at 34 weeks with birthweight 1–8 kg, good apgar scores. Baby did not require intensive care. She had systemic progression of disease postpartum being lost to oncology care and succumbed outside oncology care 3 months postpartum.
1.3. Results
There were three cases of breast cancer diagnosed during pregnancy. Ages ranged between 32–33years. The first case was a T2 tumour (3 cm) with no clinical axillary lymphadenopathy and no systemic involvement. The second case was diagnosed as a T4 tumour which had taken over the whole breast with extensive peau d’orange, nipple retraction and matted axillary lymphadenopathy. Abdominal exam revealed 20 cm hepatomegaly below the costal margin. She was in respiratory distress. The third case had a T3 tumour at 7 cm by 5 cm with axillary lymphadenopathy and no systemic involvement. Histopathology revealed invasive lobular carcinoma in two cases and extensive intraductal carcinoma with an invasive component in the third case. The first two cases were diagnosed during the second trimester while the third was diagnosed at the third trimester. Four cycles of doxorubicin and cyclophosphamide based chemotherapy were administered from the second trimester for the first and second cases and one cycle in the third trimester in the third case. Chemotherapy was continued postpartum following a 3 week break from the expected date of delivery. Paclitaxel was commenced following six cycles of doxorubicin based chemotherapy. All cases had spontaneous vaginal delivery at 37 weeks, 34 weeks and 40 weeks respectively. Birth weights were 3.1 kg, 1.8 kg and 4.1 kg respectively. Apgar scores were good with babies crying spontaneously following birth. All the babies were healthy with no discernible malformations. The low birth weight baby from the second case did not require intensive care. All babies have had normal developmental milestones. The first case had breast conservation and is disease free 5 years post presentation, the second case had systemic progression of disease postpartum and succumbed to the disease while the third one had a mastectomy postpartum and is still on chemotherapy.
2. Discussion
Cancer in pregnancy is a rare event affecting up to 0.02–0.1% of cases [12]. The age range at 32–33 years with a mean age of 32.5years is in keeping with multiple studies at 32–34 years age range. [3], [10], [13], [14]. Our second case was diagnosed with metastatic breast cancer presenting at the second trimester. Pregnancy associated breast cancer may have a delayed diagnosis as routine breast exam may be skipped during antenatal care. Also, pregnancy induced breast changes of breast enlargement and engorgement may mask the presence of a lump. However our first case had an earlier presentation with a T2 tumour and no axillary lymphadenopathy. This may be attributed to the educational level of the first patient having obtained tertiary education unlike the second case with secondary education. Our third case had an extensive carcinoma in situ with invasive component predating the pregnancy. There are reports that intrapartum pregnancy is often associated with more advanced forms [5], [6]. It remains to be seen whether delayed diagnosis from pregnancy induced breast changes or distraction by the gravid abdomen may account for these advanced cases rather than an inherent aggressive tendency.
Following the current standard of care, chemotherapy was administered from the second trimester for the first two patients and third trimester for the third case. This was critical in reviving the second case that had presented in a grave condition being dyspneic with prostration. However she emerged ambulant with remarkable improvement in general state of well being within days of its commencement. We are of the firm view that delaying the administration of chemotherapy would have resulted in immediate adverse outcome for the second case with consequences for the foetus. Netletton et al. had estimated the daily increased risk of developing metastases in an untreated intrapartum breast cancer at 0.057% [15]. Momah et al. reported the case of a 28 years old that presented with a T1 tumour at first trimester but tragically rapidly progressed to metastases [16]. It is worthy of note that a 30 weeks delay to institution of chemotherapy existed from presentation to administration of chemotherapy postpartum. The experience in our second case may suggest that patients with pregnancy associated breast cancer may not be considered too ill for chemotherapy even when metastatic as it made the difference in achieving a live delivery.
Berry et al. reported an initial 24 patients managed with CAF regimen from the second trimester with no intrapartum events. Good apgar scores, normal birthweights and immediate postpartum health were reported [9]. In the same updated study, Hahn et al. recorded successes in 57 cases with no stillbirths, miscarriages or therapy related perinatal deaths. However three preterm deliveries, one subarachnoid haemorrhage from thrombocytopaenia and one case of Downs syndrome were recorded [10]. Although 10% of neonates in the Hahns series required mechanical ventilation from difficulty with breathing [10], our miniseries did not record any neonatal event as babies only required observation.
Rovera et al. have reported a series of a dozen patients with all healthy babies born following adjuvant and neoadjuvant chemotherapy. Ten of these patients had breast conserving management while mastectomy was done in two. Nine patients were reported to be alive and disease free after a median follow up of 20 months, range 3–52 months [11]. Our case that had breast conservation is alive and disease free. Classically, pregnancy associated breast cancer had been previously thought to be aggressive hence a modified radical mastectomy was considered standard of care. Berry et al. have noted that breast conservation as well as mastectomy could be done for this disease successfully [9]. However breast irradiation, a critical adjuvant for breast conservation would have to be delayed till postpartum given the risks of fetal irradiation [17].
3. Conclusion
Chemotherapy administered from the second trimester was safe in our subjects. It rapidly reversed the grave clinical state of the 2nd case in stage 4 disease with a resulting healthy life birth. An intrapartum adverse outcome with loss of the previable foetus was very likely if chemotherapy was delayed.
Disclosure
The author has not received any grant or funding from any bodies.
Footnotes
This research work ‘Management of pregnancy associated breast cancer in a developing country. The role of chemotherapy’ is authentic
References
- 1.Navrozoglou I., Vrekoussis T., Kontostolis E., Dousias V., Zervoudis S. Breast cancer during pregnancy: a mini-review. Eur. J. Surg Oncol. 2008;34:837–843. doi: 10.1016/j.ejso.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 2.Anderson B.O., Petrek J.A., Byrd D.R., Senie R.T., Borgen P.I. Pregnancy influences breast cancer stage at diagnosis in women 30 years of age and younger. Ann. Surg. Oncol. 1996;3(2):204–211. doi: 10.1007/BF02305802. [DOI] [PubMed] [Google Scholar]
- 3.Ishida T., Yokoe T., Kasumi F., Sakamoto G., Makita M., Tominaga T. Clinicopathologic characteristics and prognosis of breast cancer patients associated with pregnancy and lactation: analysis of case-control study in Japan. Jpn. J. Cancer Res. 1992;83:1143–1149. doi: 10.1111/j.1349-7006.1992.tb02737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loibl S., von Minckwitz G., Gwyn K. Breast carcinoma during pregnancy. International recommendations from an expert meeting. Cancer. 2006;106(2):237–246. doi: 10.1002/cncr.21610. [DOI] [PubMed] [Google Scholar]
- 5.Woo J.C., Taechin Y., Hurd T. Breast cancer in pregnancy. a literature review. Arch. Surg. 2003;138:91–99. doi: 10.1001/archsurg.138.1.91. [DOI] [PubMed] [Google Scholar]
- 6.Rodriguez A.O., Chew H., Cress R., Xing G., McElvy S., Danielsen B. Evidence of poorer survival in pregnancy associated breast cancer. Obstet. Gynecol. 2008;112:71–78. doi: 10.1097/AOG.0b013e31817c4ebc. [DOI] [PubMed] [Google Scholar]
- 7.Rugo H.S. Management of breast cancer diagnosed during pregnancy. Curr. Treat. Options Oncol. 2003;4(2):165–173. doi: 10.1007/s11864-003-0018-7. [DOI] [PubMed] [Google Scholar]
- 8.Hoover H.C., Jr. Breast cancer during pregnancy and lactation. Surg. Clin. North. Am. 1990;70(5):1151–1163. doi: 10.1016/s0039-6109(16)45236-9. [DOI] [PubMed] [Google Scholar]
- 9.Berry D.L., Theriault R.L., Holmes F.A. Management of breast cancer during pregnancy using a standardized protocol. J. Clin. Oncol. 1999;17(3):855–861. doi: 10.1200/JCO.1999.17.3.855. [DOI] [PubMed] [Google Scholar]
- 10.Hahn K.M.E., Johnson P.H., Gordon N. Treatment of pregnant breast cancer patients and outcomes of children exposed to chemotherapy in utero. Cancer. 2006;107(6):1219–1226. doi: 10.1002/cncr.22081. [DOI] [PubMed] [Google Scholar]
- 11.Rovera F., Chiappa C., Coglitore A., Baratelli G.M., Fachinetti A., Marelli M. Management of breast cancer during pregnancy. Int. J. Surg. 2013;11:S64–S68. doi: 10.1016/S1743-9191(13)60020-5. [DOI] [PubMed] [Google Scholar]
- 12.Pereg D., Koren G., Lishner M. Cancer in pregnancy: gaps, challenges and solutions. Cancer Treat. Rev. 2008;34:302–312. doi: 10.1016/j.ctrv.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 13.Middleton L., Amin M., Gwyn K. Breast carcinoma in pregnant womendassessment of clinicopathologic and immunohistochemical features. Cancer. 2003;98(5):1055–1060. doi: 10.1002/cncr.11614. [DOI] [PubMed] [Google Scholar]
- 14.Ives A., Saunders C., Semmens J. The Western Australian Gestational Breast Cancer Project: a population-based study of the incidence, management, and outcomes. Breast. 2005;14:276–282. doi: 10.1016/j.breast.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Nettleton J., Long J., Kuban D., Wu R., Shaefffer J., El-Mahdi A. Breast cancer during pregnancy:quantifying the risk of treatment delay. Obstet. Gynecol. 1996 Mar;87(3):414–418. doi: 10.1016/0029-7844(95)00470-x. [DOI] [PubMed] [Google Scholar]
- 16.Momah T., Carryl S., Kondamudi V., Abraham S., Rimpel B., Xiao P. Breast cancer in pregnancy: case report. Pan Afr. Med. J. 2010;5:3. doi: 10.4314/pamj.v5i1.56195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barnes D.M., Newman L.A. Pregnancy associated breast cancer. a literature review. Surg. Clin. N. Am. 2007;87:417–430. doi: 10.1016/j.suc.2007.01.008. [DOI] [PubMed] [Google Scholar]