Abstract
Tuberculosis (TB) remains a major public health problem in South Korea. The Joint Committee for the Development of Korean Guidelines for Tuberculosis published the Korean Guidelines for Tuberculosis in 2011 to provide evidence-based practical recommendations to health care workers caring for patients with TB in South Korea. After reviewing recent national and international scientific data on TB, the committee updated the Korean guidelines for TB in 2014. This article presents some practical issues related to the 2014 updated guidelines: namely use of the Mycobacterium tuberculosis - polymerase chain reaction assay and the Xpert MTB/RIF assay in the diagnosis of TB, as well as medical treatment for patients with multidrug-resistant TB.
Keywords: Tuberculosis, Guidelines, Multidrug-Resistant Tuberculosis
Introduction
Tuberculosis (TB) remains a major public health problem in South Korea with the highest incidence of TB among 34 members of Organization for Economic Cooperation and Development (OECD)1.
New scientific informations on TB are published daily worldwide. Thus, it is difficult for health care workers to decide which information should be used in the management of TB patients. Joint Committee for the Development of Korean Guidelines for Tuberculosis, including representatives of the Korea Centers for Disease Control and Prevention and Korean Academy of Tuberculosis and Respiratory Disease, published guidelines for TB in 2011, to provide evidence based practical recommendations to health care workers caring TB patients in South Korea2. This committee updated Korean guidelines for TB in 2014, after reviewing recent national and international scientific informations on TB3. This article reviews some practical issues of the updated guidelines for TB: the use of Mycobacterium tuberculosis -polymerase chain reaction (TBPCR) and Xpert MTB/RIF assay for the diagnosis of TB and medical treatment of multidrug resistant TB (MDR-TB).
Mycobacterium tuberculosis-Polymerase Chain Reaction
Rapid and accurate diagnosis of TB is important for the early initiation of adequate treatment and prevention of transmission of the disease. Conventional acid-fast bacilli (AFB) smear microscopy has been used widely for rapid diagnosis of TB. However, the usefulness of AFB smear is limited by low sensitivity and inability to differentiate M. tuberculosis (MTB) from nontuberculous mycobacteria (NTM). Culture is more sensitive than smear; however, it takes several weeks for the result.
Polymerase chain reaction (PCR) for the detection of MTB specific DNA (TB-PCR) holds great promise for the diagnosis of TB because it is rapid and more sensitive than the conventional AFB smear for the diagnosis of TB. TB-PCR rapidly confirms the presence of MTB in 50%-80% of AFB smearnegative, culture-positive pulmonary TB cases4. Furthermore, TB-PCR can differentiate MTB from NTM.
Recently, the recovery rate of NTM from respiratory specimens is steadily increasing in South Korea5. Thus, the updated guidelines recommend performing TB-PCR in one specimen, preferably the first specimen, from each patient suspected to have TB along with AFB smear and culture. However, TB-PCR should not be ordered routinely when the clinical suspicion of TB is low because the positive predictive value (PPV) of TB-PCR is low in such cases6.
The results of TB-PCR should be interpreted in correlation with the AFB smear results and clinical characteristics.
(1) If the TB-PCR result is positive, presume the patient has TB and begin anti-TB treatment while awaiting culture results.
(2) If the TB-PCR result is negative and the AFB smear result is positive, presume the specimen has NTM and wait culture results without anti-TB treatment.
(3) If the TB-PCR result is negative and the AFB smear result is negative, use clinical judgment to determine whether to begin anti-TB treatment while awaiting results of culture and additional diagnostic tests. Because currently available TB-PCR tests are not sufficiently sensitive to exclude the diagnosis of TB in AFB smear-negative patients suspected of having TB6.
Xpert MTB/RIF Assay
The Xpert MTB/RIF assay (Xpert; Cepheid, Sunnyvale, CA, USA) is an automated diagnostic test system detecting DNA sequences specific for MTB and rifampin resistance within 2 hours using real-time PCR technology7.
The Xpert is suitable for rapid and accurate diagnosis of TB and rifampin resistance in laboratory resource-poor settings because it is fully automated and requires little technical training to operate it. However, the benefit of Xpert is limited in laboratory resource-rich settings where molecular diagnostic tests like TB-PCR and rapid drug susceptibility test (DST) are readily available. The Xpert cannot replace conventional AFB smear and culture because these tests also have roles in the diagnosis of TB7. Recent reports from resource-rich settings revealed that the Xpert shows poor performance and impact when routinely implemented for all patients with suspected pulmonary TB7,8. Furthermore, the Xpert is expensive compared to other diagnostic tests. Thus, updated guideline recommend Xpert to be used in selected proportion of TB suspects.
Like TB-PCR, the Xpert is more sensitive than AFB smear microscopy for the diagnosis of TB. In a clinical study, the sensitivity of the Xpert was 92.2% for culture-positive pulmonary TB cases (98.2% for smear positive and 72.5% for smearnegative cases), with a specificity of 99.2%9. The Xpert is much more sensitive than AFB smear microscopy in patients who have low numbers of bacilli such as human immunodeficiency virus (HIV) positive patients because sputum AFB smear microscopy is useful only when sputum has sufficient bacillary load10. World Health Organization (WHO) has endorsed Xpert as the initial diagnostic test for TB suspects co-infected with the HIV. Thus, updated guidelines recommend Xpert for the rapid diagnosis of TB and MDR-TB in patients with HIV infection and severe disease because delayed diagnosis of TB and MDR-TB is detrimental in these patients.
The culture based conventional DST requires up to 4 months to provide the results, which delays the detection of drug resistance and risks inappropriate treatment and spread of drug resistant strains. The recently developed rapid DST, which detects mutations associated with drug resistance (rpoB gene for rifampin and katG and inhA gene for isoniazid) using molecular technologies like line probe assay (LPA), significantly reduced diagnostic delays of MDR-TB. LPA is based on reverse hybridization of PCR amplified DNA on the strip, while the Xpert assay is based on real-time PCR to detect mutations associated with drug resistance11. Xpert is faster than LPA but it is limited to rifampin resistance while LPA detect both rifampin and isoniazid resistance. Because MTB that is resistant to rifampin is more likely to have concomitant resistance to isoniazid, rifampin resistance is considered as a surrogate marker of MDR-TB12.
An Xpert result that is positive for rifampin resistance should be carefully interpreted because the PPV of Xpert is low in patient groups in which rifampin resistance is rare. The PPV for rifampin resistance using Xpert exceeds 90% in patient group where the underlying prevalence of rifampin resistance is greater than 15%, but it decreases less than 70% when the prevalence falls below 5%13. Recent data from the Health Insurance Review and Assessment Service of South Korea showed that 2.9% of new case of TB had MDR-TB, while 9.3% of TB patients with prior TB treatment history had MDR-TB14. Thus updated guidelines recommend Xpert to be used to specimens from patients suspected of having MDR-TB like retreatment (treatment failure, relapse) for the rapid diagnosis of MDR-TB. The updated guidelines also recommend positive result of rifampin resistance on Xpert to be confirmed by conventional DST or other rapid DST in patients without risk factors for drug resistance such as new cases.
Even in South Korea, Xpert may have an important role in resource-limited settings like remote and rural areas8. Xpert may also be useful in special settings, where rapid diagnosis of TB is important for the decision of respiratory isolation and early TB treatment is crucial15.
Multi-Drug Resistant TB
MDR-TB, resistant to at least isoniazid and rifampin, the two most effective anti-TB drugs, is a major threat to TB control in South Korea. According to data from the 2008 Health Insurance Review and Assessment Service of South Korea, 4.6% of TB patients (n=2,472) had MDR-TB14. Despite prolonged use of injectable and toxic second-line anti-TB drugs, treatment success rate of MDR-TB is about 62% worldwide16.
Inadequate treatment of MDR-TB results in poor outcome and development of extensively drug resistant TB (XDRTB). WHO updated guidelines for the medical treatment of MDR-TB in 2011, based on evidences from a meta-analysis of individual MDR-TB patient data from 32 observational studies17. The updated Korean guidelines for TB also revised recommendations for the treatment of MDR-TB based on the updated WHO guidelines and recent advances in MDR-TB treatment adapting to the specific situations of the country3.
Each regimen for MDR-TB treatment should be designed individually based on the DST results, history of previously used anti-TB drugs, and DST results of close contact with MDR-TB. The reliability of DST for the second-line anti-TB drugs other than aminoglycosides and fluoroquinolones is low18. Therefore, anti-TB drugs included in the failing regimen should be considered 'probably resistant', even though the result of DST indicate susceptible.
The updated guidelines uses group system for the classification of anti-TB drugs to design treatment regimens for MDR-TB, based on efficacy, experience of use and drug class (Table 1).
Table 1. Groups of anti-tuberculosis (TB) drugs.
| Group | Drug |
|---|---|
| Group 1 (first-line oral anti-TB drugs) | Isoniazid, rifampin, ethambutol, pyrazinamide |
| Group 2 (Injectable anti-TB drugs) | Streptomycin, kanamycin, amikacin, capreomycin |
| Group 3 (fluoroquinolones) | Ofloaxacin, levofloxacin, moxifloxacin, gatifloxacin |
| Group 4 (second-line oral anti-TB drugs) | Prothionamide, cycloserine, para-aminosalicylic acid |
| Group 5 (agents with unclear efficacy) | Clofazimine, linezolid, amoxacillin/clavulanate, clarithromycin, high-dose isoniazid |
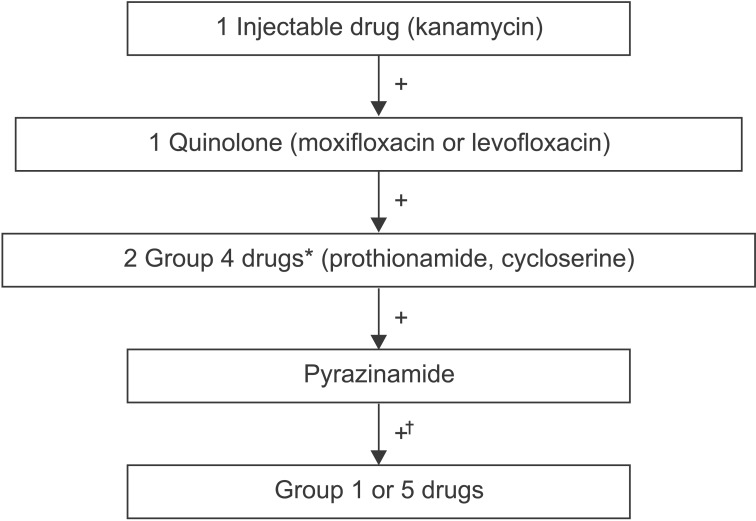
To build a MDR-TB treatment regimen, four second-line anti-TB drugs that are likely to be effective should be selected, beginning with two core drugs (fluoroquinolone and injectable drug) and two group 4 drugs and pyrazinamide. Group 1 and 5 drugs should be added to the regimen if the preceding drugs are not sufficient to make an effective regimen (Figure 1).
Figure 1. Algorithm for anti-tuberculosis (TB) drug selection to build up multidrug resistant TB treatment regimen. *Para-aminosalicylic acid should be selected only if prothionamide and cycloserine cannot be used or are unlikely to be effective and if an additional drug is needed to have at least four effective second-line drugs in the regimen. †Group 1 or 5 drugs may be selected if the preceding drugs are not sufficient to make an effective regimen.
Among fluoroquinolones, later-generation fluoroquinolone like levofloxacin and moxifloxacin should be selected, rather than earlier-generation forms, because later generation fluoroquinolones showed to be significantly associated with cure19.
Among injectable drugs, kanamycin should be selected because streptomycin showed greater likelihood of ototoxicity and higher drug resistance rate.
Among the group 4 drugs, the association with cure was highest with prothionamide followed by cycloserine and paraaminosalicylic acid (PAS). Thus, prothionamide should be selected unless there is a particular contraindication. PAS should be selected only if prothionamide and cycloserine cannot be used or are unlikely to be effective and if an additional drug is needed to have at least four effective second-line drugs in the regimen.
Pyrazinamide should be added to the MDR-TB treatment regimen even if the strain shows resistance to pyrazinamide because it has several benefits including potent sterilizing activity and the reliability of DST for pyrazinamide is low18.
In contrast to pyrazinamide, ethambutol is not routinely added to MDR-TB treatment regimens because the contribution of ethambutol in MDR-TB treatment remains unclear17. Ethambutol may be selected to treat MDR-TB, but it should not be counted among the effective drugs making up the MDR-TB treatment regimen.
Group 5 drugs may be added to the MDR-TB treatment regimen if regimens with other anti-TB drugs are not likely to be effective for the treatment of MDR-TB. Among group 5 drugs, linezolid may be the first option because a recent metaanalysis showed that only linezolid was independently associated with favorable outcomes in the treatment of XDR-TB or fluoroquinolone-resistant MDR-TB20.
Injectable drug should be administered at least 8 months. The duration can be modified based on severity of disease, prior therapy, drug resistance pattern, and response to therapy. Total duration of treatment should be at least 20 months for MDR-TB patients who had no previous MDR-TB treatment. The duration may be adjusted according to clinical and bacteriologic response to treatment.
Acknowledgements
The present research was conducted by the research fund of Dankook University in 2014.
Footnotes
Conflicts of Interest: No potential conflict of interest relevant to this article was reported.
References
- 1.Kim JH, Yim JJ. Achievements in and challenges of tuberculosis control in South Korea. Emerg Infect Dis. 2015;21:1913–1920. doi: 10.3201/eid2111.141894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Joint Committee for the Development of Korean Guidelines for Tuberculosis; Korea Centers for Disease Control and Prevention. Korean guidelines for tuberculosis. 1st ed. Seoul and Cheongwon: Joint Committee for the Development of Korean Guidelines for Tuberculosis, Korea Centers for Disease Control and Prevention; 2011. [Google Scholar]
- 3.Joint Committee for the Revision of Korean Guidelines for Tuberculosis; Korea Centers for Disease Control and Prevention. Korean guidelines for tuberculosis. 2nd ed. Seoul and Cheongwon: Joint Committee for the Revision of Korean Guidelines for Tuberculosis, Korea Centers for Disease Control and Prevention; 2014. [Google Scholar]
- 4.Singh A, Kashyap VK. Specific and rapid detection of Mycobacterium tuberculosis complex in clinical samples by polymerase chain reaction. Interdiscip Perspect Infect Dis. 2012;2012:654694. doi: 10.1155/2012/654694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koh WJ, Chang B, Jeong BH, Jeon K, Kim SY, Lee NY, et al. Increasing recovery of nontuberculous mycobacteria from respiratory specimens over a 10-year period in a tertiary referral hospital in South Korea. Tuberc Respir Dis. 2013;75:199–204. doi: 10.4046/trd.2013.75.5.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dinnes J, Deeks J, Kunst H, Gibson A, Cummins E, Waugh N, et al. A systematic review of rapid diagnostic tests for the detection of tuberculosis infection. Health Technol Assess. 2007;11:1–196. doi: 10.3310/hta11030. [DOI] [PubMed] [Google Scholar]
- 7.Gupta RK, Lawn SD, Booth H, Morris-Jones S. What is the role for Xpert(R) MTB/RIF in high-resource settings? Experience from a central London hospital. Int J Tuberc Lung Dis. 2014;18:1323–1326. doi: 10.5588/ijtld.14.0259. [DOI] [PubMed] [Google Scholar]
- 8.Sohn H, Aero AD, Menzies D, Behr M, Schwartzman K, Alvarez GG, et al. Xpert MTB/RIF testing in a low tuberculosis incidence, high-resource setting: limitations in accuracy and clinical impact. Clin Infect Dis. 2014;58:970–976. doi: 10.1093/cid/ciu022. [DOI] [PubMed] [Google Scholar]
- 9.Boehme CC, Nabeta P, Hillemann D, Nicol MP, Shenai S, Krapp F, et al. Rapid molecular detection of tuberculosis and rifampin resistance. N Engl J Med. 2010;363:1005–1015. doi: 10.1056/NEJMoa0907847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barnes PF, Bloch AB, Davidson PT, Snider DE., Jr Tuberculosis in patients with human immunodeficiency virus infection. N Engl J Med. 1991;324:1644–1650. doi: 10.1056/NEJM199106063242307. [DOI] [PubMed] [Google Scholar]
- 11.Lawn SD, Mwaba P, Bates M, Piatek A, Alexander H, Marais BJ, et al. Advances in tuberculosis diagnostics: the Xpert MTB/RIF assay and future prospects for a point-of-care test. Lancet Infect Dis. 2013;13:349–361. doi: 10.1016/S1473-3099(13)70008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Somoskovi A, Parsons LM, Salfinger M. The molecular basis of resistance to isoniazid, rifampin, and pyrazinamide in Mycobacterium tuberculosis. Respir Res. 2001;2:164–168. doi: 10.1186/rr54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weyer K, Mirzayev F, Migliori GB, Van Gemert W, D'Ambrosio L, Zignol M, et al. Rapid molecular TB diagnosis: evidence, policy making and global implementation of Xpert MTB/RIF. Eur Respir J. 2013;42:252–271. doi: 10.1183/09031936.00157212. [DOI] [PubMed] [Google Scholar]
- 14.Park YS, Hong SJ, Boo YK, Hwang ES, Kim HJ, Cho SH, et al. The national status of tuberculosis using nationwide medical records survey of patients with tuberculosis in Korea. Tuberc Respir Dis. 2012;73:48–55. doi: 10.4046/trd.2012.73.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Millman AJ, Dowdy DW, Miller CR, Brownell R, Metcalfe JZ, Cattamanchi A, et al. Rapid molecular testing for TB to guide respiratory isolation in the U.S.: a cost-benefit analysis. PLoS One. 2013;8:e79669. doi: 10.1371/journal.pone.0079669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orenstein EW, Basu S, Shah NS, Andrews JR, Friedland GH, Moll AP, et al. Treatment outcomes among patients with multidrug-resistant tuberculosis: systematic review and metaanalysis. Lancet Infect Dis. 2009;9:153–161. doi: 10.1016/S1473-3099(09)70041-6. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. Guidelines for the programmatic management of drug-resistant tuberculosis, 2011 update. Geneva: World Health Organization; 2011. [PubMed] [Google Scholar]
- 18.World Health Organization. Policy guidance on drug susceptibility testing (DST) of second-line anti-tuberculosis drugs. Geneva: World Health Organization; 2008. [PubMed] [Google Scholar]
- 19.Ahuja SD, Ashkin D, Avendano M, Banerjee R, Bauer M, Bayona JN, et al. Multidrug resistant pulmonary tuberculosis treatment regimens and patient outcomes: an individual patient data meta-analysis of 9,153 patients. PLoS Med. 2012;9:e1001300. doi: 10.1371/journal.pmed.1001300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang KC, Yew WW, Tam CM, Leung CC. WHO group 5 drugs and difficult multidrug-resistant tuberculosis: a systematic review with cohort analysis and meta-analysis. Antimicrob Agents Chemother. 2013;57:4097–4104. doi: 10.1128/AAC.00120-13. [DOI] [PMC free article] [PubMed] [Google Scholar]