Highlights
-
•
No recurrence of osteofibrous dysplasia at 84 week following a wide extraperiosteal excision.
-
•
The combination of autologous BM-MSCs, HA granules and BMP-2 successfully created new bone tissue.
-
•
The newly formed bone tissue filled in the gap of critical-sized bone defect and was able to improve the patient’s quality of life significantly.
-
•
No neoplastic, immunologic or other side-effects were noted at 84 weeks after autologous MSC transplantation.
Keywords: Autologous mesenchymal stem cells, Critical-sized bone defect, Osteofibrous dysplasia, Wide excision, Bone morphogenetic protein 2, Hydroxyapatite granules
Abstract
Introduction
Osteofibrous dysplasia is a rare non-neoplastic disease that is almost exclusive to pediatric tibial diaphysis. Wide excision of the lesion is recommended to avoid recurrence. However, such radical surgery will results in large segmental bone defects that will require further extensive reconstructive surgery. We report a novel approach of treating bone defect by implementing the diamond concept of bone healing using autologous bone marrow derived mesenchymal stem cells (BM-MSCs).
Presentation of case
An eight-year-old Indonesian male presented with severe bowing deformity of the left lower leg. Radiographic and histological analysis confirmed the diagnosis of osteofibrous dysplasia. A wide excision of the defect was made leaving a critical-sized bone defect. A combination of autologous transplantation of 50 million BM-MSCs, hydroxyapatite (HA) granules, bone morphogenic protein 2 (BMP-2) and Djoko-Zarov hybrid circular external fixator was used to treat the defect. The outcomes measured were subjective complaints, functionality based on LEFS and radiological assessments.
Discussion
Radiographic assessments showed successful new bone tissue formation and integration of implanted HA granules. The external fixator was removed at 42 weeks after adequate callus formation and clinical stability was achieved. The patient underwent progressive functional improvements and reached a near normal functionality of 90% LEFS at 84 week. No therapy side effect or complication was reported.
Conclusion
Osteofibrous dysplasia was successfully excised without signs of recurrence after 84-week follow-up. Autologous transplantation of augmented BM-MSCs has successfully created new normal bone tissue without causing any side effect and had significantly improved the patient’s quality of life.
1. Introduction
Osteofibrous dysplasia is a congenital, non-neoplastic disease of unknown etiology that is exclusive to the pediatric population. The most common presenting symptoms of osteofibrous dysplasia is an painless enlargement of the tibia with varying degree of tibial bowing deformity [1]. Wide excision of the osteofibrous dysplasia is indicated for aggressive lesions and severe deformity [2], [3]. However, such approach poses another challenge by leaving a large segmental defect that requires extensive reconstructive surgery to restore the volume and strength. The condition is classically managed with vascularized bone graft or bone transport coupled with distraction osteogenesis. However, the outcome may not always be satisfactory, and the rate of complications or procedural complexity negates their use [3], [4].
The multipotency of MSCs and its immense potential for treating orthopaedic cases is well known [5], [6]. The osteogenetic capacity of MSCs is theoretically suitable for treating cases such as bone defect, where the absence of osteogenesis is one of the main pathoetiology. Currently, the application of MSCs for bone defect is limited to animal study [7], [8]. In this case report we present a novel approach of treating massive bone defect following a wide excision of osteofibrous dysplasia by using autologus bone marrow derived MSCs (BM-MSCs).
2. Presentation of case
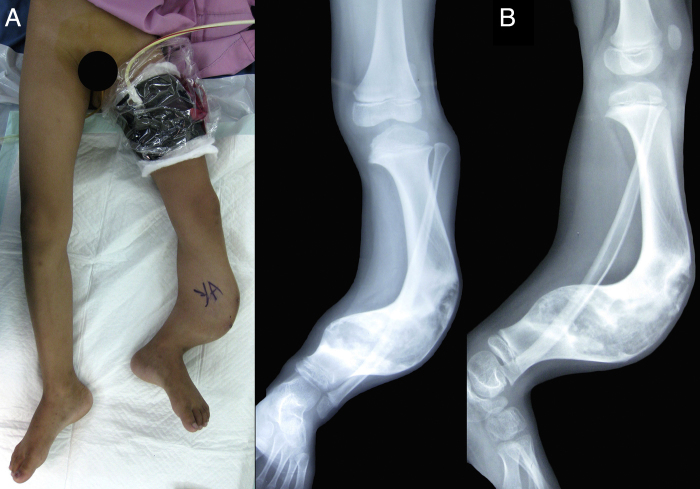
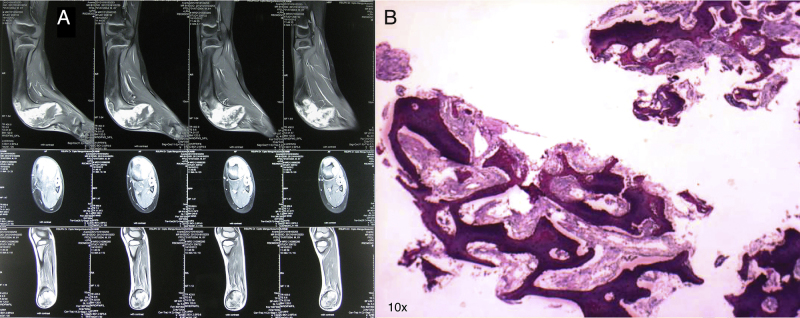
An eight-year-old Indonesian male presented with an obvious bowing deformity of his left lower leg. Physical examination revealed a painless solid mass with anterolateral bowing deformity of the left tibia and 5 cm limb shortening. No sign of inflammation or skin discoloration was noted (Fig. 1A). X-ray showed anterolateral tibial shaft bowing that extends to the distal metaphysis, bubbled appearance of intracortical osteolytic lesions with no periosteal reaction; typical radiographic presentations of osteofibrous dysplasia (Fig. 1B). Magnetic resonance imaging (MRI) with contrast revealed sclerosis of the internal cortical surface with no evidence of soft tissue involvement (Fig. 2A). Histopathological analysis was consistent with osteofibrous dysplasia, showing C-shaped bony spicules with immature bone trabeculae lined with active osteoblasts (Fig. 2B). Daily functionality measured by Lower Extremity Functional Scale (LEFS) [9] was 11.25%. Routine hematology and urine test results were within normal limits.
Fig.1.
Preoperative clinical photograph and plain radiography.
(A) Significant anterolateral bowing of the left tibia and limb shortening. The weight and contour of the tumor subjected the left hip to be externally rotated while in neutral supine position. Left lower leg marked for surgery. (B) Anteroposterior (left) and lateral (right) X-rays showing typical presentations of osteofibrous dysplasia. The lesion was limited to the tibia that extends from the diaphysis to distal metaphysis. Accompanying fibular bowing was evident. No observable periosteal reaction, knee or ankle joint abnormalities.
Fig. 2.
MRI with contrast and histopathology.
(A) Representative preoperative MRI of the sagittal (top), axial (middle), and coronal (bottom) plane. Sclerosis of the internal cortical surface is evident with no soft tissue involvement. (B) Analysis of core needle biopsy showed features consistent with osteofibrous dysplasia (hematoxylin and eosin staining; 10× magnification).
The patient was diagnosed with left tibial osteofibrous dysplasia and planned for a wide excision of the tumor followed by autologous transplantation BM-MSCs in a two-stage procedure. Tumor excision, external fixation, and bone marrow harvest were conducted in the first stage. Auto-transplantation of BM-MSCs and final adjustments of external fixator were performed in the second stage.
A wide excision lesion was performed, leaving a critical-size bone defect. The procedure was followed by fibular osteotomy, soft tissue closure and stabilization using Djoko-Zarov hybrid circular external fixator. The external fixation was maintained for 28 days throughout in vitro BM-MSCs cultivation. Thirty milliliters of bone marrow was aspirated from the posterior iliac crest into a container pre-filled with 5000 U/mL of heparin. The aspirate was diluted with 30 mL of phosphate-buffered saline (PBS) and centrifuged at 3000 rpm for 30 min in room temperature. The buffy coat was washed and transferred into culture flask with Dulbecco’s Modified Eagle Medium (DMEM; Gibco, Grand Island, New York) supplemented with 10% fetal bovine serum. Isolated cells were incubated in 37 °C at 20% O2 and 5% CO2.
The first medium change was performed on day 5 with thorough washing with PBS to discard floaters and debris. The cells were further cultured with routine medium change every 2–3 days until reaching confluency. Upon reaching confluence, the plastic-adherent fibroblastic cells were trypsinized and characterized with flowcytometry (FACSCalibur™, Franklin Lakes, NJ). More than 99% of the cells expressed CD 73, CD105, while none expressed HLA-DR, CD14, CD19, CD34 and CD45. These findings confirmed that the plastic-adherent cells were of mesenchymal stromal origin. The BM-MSCs were sub-cultured into first passage with routine medium change every 2–3 days.
Subsequent sub-cultures were performed whenever cell confluence was achieved. After 28 days all cultured BM-MSCs were trypsinized, washed, counted, and suspended in freshly obtained patient’s serum on the day of transplantation. Sterility tests were conducted thrice; aspirated bone marrow before passage zero (P0), used medium on day 7 of culture, and the last medium change prior to transplantation. All tissue culture procedures were performed in GMP certified tissue culture laboratory. Approximately fifty million BM-MSCs were transplanted back to fill to the bone defect during the second stage procedure in December 2012. The defect was first filled with 5 g/cm defect of hydroxyapatite granules (HA; Bongros®-HA, Bioalpha, Seungnam, Korea) and 1.66 mg/cm defect of bone morphogenetic protein-2 (BMP-2; Novosis, CGBio, Seoul, Korea). BM-MSCs were carefully introduced evenly throughout the defect and the external fixator was adjusted accordingly. The patient suffered no acute complication and was discharged on the seventh day. The outcomes measured were subjective complaints, functionality based on LEFS, and radiological assessments based on Tiedeman [10] and Lane-Sandhu scoring system [11].
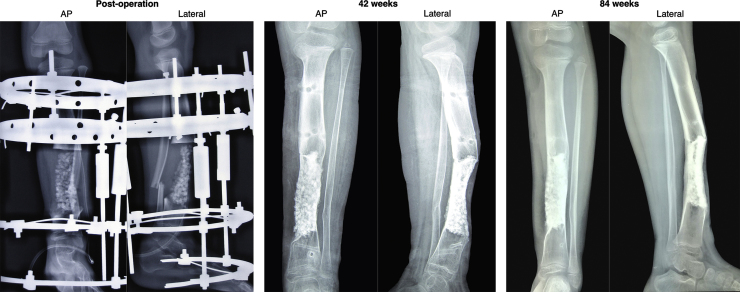
Partial weight bearing was allowed at four weeks and full weight at eight. Limb length discrepancy was corrected and the external frame maintained normal tibial mechanical axis. Thus no frame manipulation was performed on the external hybrid frame throughout the post-operative period. With evident callus and bone stability the external fixator was removed at 42 weeks (Fig. 3).
Fig. 3.
Post-operative radiographic progressions of bone healing.
Progressive new bone tissue formation evidenced by the less noticeable individual HA granules and blurring of the osteotomy lines. Good tibial alignment and adequate callus formation was achieved at 42 week. No radiographic sign of recurrence was found by 84 weeks.
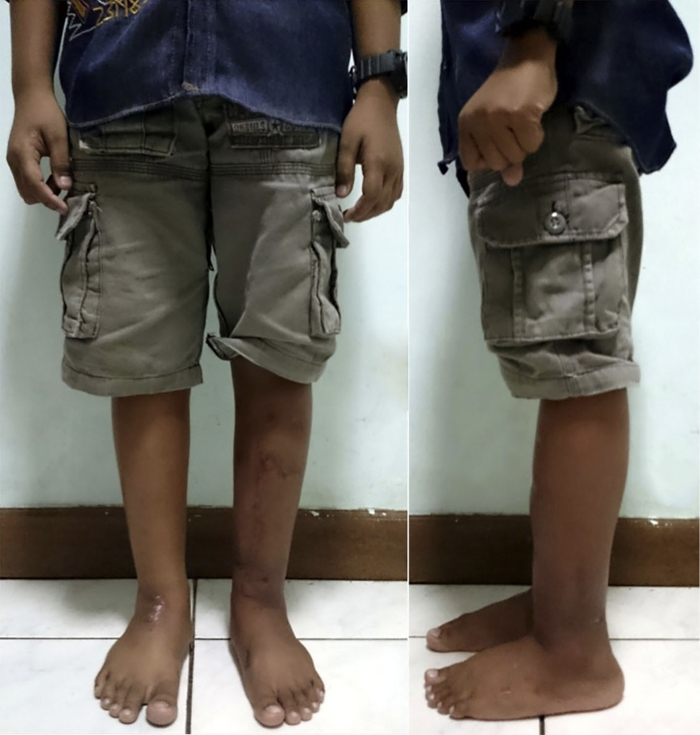
Pin tract wounds were managed with regular gauze change and topical antibiotic. No significant complaints or complications were reported during the 84 weeks follow-up (Fig. 4). The patient’s LEFS gradually improved, especially after the external frame removal. Since then, the patient was able to carry out daily activities with little to no problem and regularly participated in high-intensity sport. Progressions of post-operative outcomes are summarized in Table 1.
Fig. 4.
Clinical appearance at 84 weeks.
Straight tibial mechanical axis was achieved and allowed normal weight distribution on the left ankle. With no leg length discrepancy or joint abnormality, the patient was able to stand erect for an extended period.
Table 1.
Summary of post-operative follow-up.
| Time | Clinical complaints | VAS | LEFS | Tiedeman/Lane-Sandhu |
|---|---|---|---|---|
| Week 4 | Slight pain upon active movement | 1 | 42.5% | 6/2 |
| Week 8 | Slight pain upon active movement | 1 | 42.25% | 6/2 |
| Week 12 | Slight pain upon active movement | 1 | 42.25% | 6/2 |
| Week 16 | None | 0 | 50% | 6/2 |
| Week 36 | None | 0 | 66.25% | 9/3 |
| Week 42 | None | 0 | 66.25% | 9/3 |
| Week 84 | None | 0 | 90% | 12/4 |
VAS–visual analog scale.
LEFS–lower extremity functional scale.
3. Discussion
A widely acceptable view for managing osteofibrous dysplasia is to postpone surgical therapy until skeletal maturity is reached and to avoid extensive surgery to avoid complications. We discuss several reasons why wide excision was performed in our patient. The general recommendation to avoid operation before the skeletal maturity was due to the high recurrence rate of osteofibrous dysplasia following surgical intervention [1], [12], [13]. An Italian study reported a 64% rate of recurrence (16 out of 25) in patients who underwent curettage or subperiosteal tangential resection [1]. Seven of out those 16 patients had second recurrence after non-wide excision reoperation was done. In contrast, those who underwent wide extraperiosteal resection did not have any recurring osteolytic lesions. A similar trend was also observed in a retrospective study conducted in England [3].
Additionally, there is a small chance but high risks association between osteofibrous dysplasia and the malignant adamantinoma. Hazelbag et al. reported 3 patients with classical adamantinoma were previously diagnosed with osteofibrous dysplasia-like adamantinoma [14]. In a review of 16 patients, Lee et al. reported 2 cases of definitive adamantinoma that was previously diagnosed with osteofibrous dysplasia by the same pathologist [3]. In another patient, histopathological diagnoses of both fibrous dysplasia and adamantinoma were made from the same curettage specimen. The author advocated the use of radical extraperiosteal excision for all cases of osteofibrous dysplasia, contrasting previous recommendations. Another basis for a wide excision in our patient was the severity of his bowing deformity.
Corrective osteotomy may not satisfactorily correct the mechanical axis deviation, and carries the risks for recurrence unless en blocexcision was made. Circular external fixator was chosen for superior mechanical stability and the versatility to be easily manipulated if adjustments are needed during the post-operative period. We believed prompt correction of the deformed limb is of a great importance to restore the patient's quality of life, irrespective of skeletal maturity.
Limb salvage procedures often results in large bone defect. Several options for reconstruction includes arthrodesis, bone graft, bone transfer, bone transport or mesenchymal stem cell implantation. Arthrodesis creates a stable, painless and durable limb, however it immobilizes joints and cause limb inequality. Osteoarticular or bulk allografts are subjected to high rates of early complications such as infection, fracture, nonunion, immunologic complications or disease transmission [15]. Vascularized bone grafts are suitable for large skeletal defects. With rapid incorporation, vascularized bone graft creates stronger construct secondary to graft hypertrophy and does not cause adverse immunologic response. However it requires longer operation, donor and surgical site morbidity. Bone transfer has little to no osteoinductive properties and there are limited reports on this technique [16]. Distraction osteogenesis or bone transport requires extended period of treatment and high patient compliance level. It causes more complications and frequently results in poor functional outcome [17].
Our patient suffered from critical-sized bone defect following the segmental bone resection. Defined as the loss of bone length 2–2.5 times the diameter of affected bone, which will not heal spontaneously during the lifetime of the patient [18]. Thus, we utilized the osteogenic potency of MSCs to treat the patient. The combination of allogeneic or autologous MSCs with synthetic bone substitute (hydroxyapatite-tricalcium phosphate) was found to enhance bone healing in canines with critical-sized bone defect [7]. Greater osteogenic activity was found in rat calvarial defects treated with BM-MSCs, platelet-rich plasma (PRP) and biphasic calcium phosphate (BCP) bone ceramic, compared to PRP and BCP alone [8]. Data from animal experiments exploring MSCs for treating bone defect were encouraging. The well-known diamond concept emphasized importance of osteogenic, osteoconductive, osteoinductive and stable mechanical environment for fracture healing [19]. In this study, each component was represented by BM-MSCs, HA granules, BMP-2 and Djoko-Zarov hybrid frame respectively. HA granules will served as the main platform for osteogenic BM-MSCs expansion, that is enhanced by the BMP-2 [20]. We hypothesized that such combination is key for a successful management of large bone defects.
During the first three post-operative weeks the patient was unable to squat, climb stairs, run, make sharp turns or hop. With less pain and adaptation to the external frame, the patient’s LEFS gradually improved within the next 12 weeks. The circular frame installed distal from the knee restricted full knee flexion. Thus the patient was unable to perform full squat until the external frame removal. Radiographic improvements were signified by the presence of thicker callus forming within the more radiopaque HA granules. Less distinction of individual HA granules and blurring of the two horizontal osteotomy lines were progressively more evident with subsequent radiography. The external frame was removed at week 42 based on sufficient callus formation; 75% or 3 based on Lane-Sandhu scoring system, no clinical complaint and ability for full weight bearing. Significant improvements of LEFS were observed after the external frame removal. The patient quickly gained confidence and engaged brisk walking. Near-normal function of 90% LEFS was achieved at 84 week with improved radiographic findings. At this stage, the patient routinely played football with no complaints of bone pain or joint stiffness. Series of X-rays showed no evidence of recurrent osteolytic lesions. This finding supports the wide extraperiosteal excision approach for treating osteofibrous dysplasia. No osteomyelitis, immunogenic, or other significant side effects has occurred.
This report lacks histological results from the newly formed bone tissue. Such information is valuable to assess the quality and to detect any subtle morphogenic differences between normal bone and bone made by the MSC, HA and BMP-2 combination. Such study was not performed due to the newly formed tibia that has not remodeled into normal shaft diameter and shape. Additionally, obtaining histological sample at this stage may risk is structural compromise or infection. We expect to observe continuous anatomical improvements through bone remodeling in the course of several years. There were several limitations to the technique used. Although distraction osteogenesis was not carried out, the application of cumbersome circular external fixation was still required. Additionally, the risk of side effect from MSC therapy has not been fully described. Thus, our patient was subjected to risks of secondary malignancy or unfavorable immunologic response.
To our knowledge, this is the first report for combined therapy of BM-MSCs, HA, and BMP-2 for treating bone defect in human. Results presented in this report are in favor of MSCs-based therapy for critical-sized bone defect. Routine autologous MSCs therapy may avoid the need of bone graft or bone transport surgery that requires exceptional patient compliance throughout distraction osteogenesis and rehabilitation period. It is important to recognize that the applications of this novel approach may potentially be extended for other orthopaedic complications such as nonunion. However, results from larger controlled clinical trial are required to validate our findings before it can be considered as a standard treatment option.
4. Conclusion
In conclusion, osteofibrous dysplasia in an eight-year-old patient was successfully excised without recurrence after 84 week. This finding supports newer recommendations to widely excise any case of osteofibrous dysplasia. Autologous transplantation of BM-MSCs combined with HA, BMP-2 and external fixation has successfully created new normal bone tissue and returned normal functionality of the patient. This novel approach has satisfactorily managed the critical-sized bone defect with no significant side effects or complications.
Conflict of interest
We declare no conflict of interest in this research. Bifarma Adiluhung Ltd. was not involved in the conception of study design, data analysis, interpretation or writing of the manuscript.
Funding
This research is funded by a collaborative research grant between Universitas Indonesia and National University of Singapore.
Ethical approval
Ethical approval for this study was granted by the Ethics Committee of the Faculty of Medicine, University of Indonesia (No: 567/PT02.FK/ETIK/2012).
Author contribution
-
1.
Ismail Hadisoebroto Dilogo: Conception of study design, supervised the project, manuscript writing, revision, and approved article submission.
-
2.
Achmad Fauzi Kamal: Orthopaedic oncology surgical team; patient diagnosis, performed wide excision, manuscript revision, and approved article submission.
-
3.
Bambang Gunawan: Orthopaedic reconstructive surgery team; performed external fixation and maintenance, manuscript revision, and approved article for submission.
-
4.
Rangga Valentino Rawung: Conducted patient follow-ups, data acquisition, interpretation, analysis, manuscript drafting and finalization.
Consent
The patient’s assent and signed informed consent from his parents were obtained. The original written informed consent was provided in Bahasa Indonesia. The possibility of publication was mentioned within the informed consent by the first paragraph of the second page that the patient’s father has agreed and signed upon. Copy of the written consent is available by request.
Guarantor
Ismail Hadisoebroto Dilogo is the corresponding author and guarantor.
Acknowledgements
We would like to thank Laboratory of Regenerative and Cellular Therapy (ReGeniC), Bifarma Adiluhung limited Company (Ltd.) for the facilities and technical assistance in performing BM-MSCs culture.
References
- 1.Campanacci M., Laus M. Osteofibrous dysplasia of the tibia and fibula. J. Bone Joint Surg. Am. 1981;63(3):367–375. [PubMed] [Google Scholar]
- 2.Mankin H.J., Trahan C.A., Fondren G., Mankin C.J. Non-ossifying fibroma, fibrous cortical defect and Jaffe-Campanacci syndrome: a biologic and clinical review. Chir. Organi Mov. 2009;93(1):1–7. doi: 10.1007/s12306-009-0016-4. [DOI] [PubMed] [Google Scholar]
- 3.Lee R.S., Weitzel S., Eastwood D.M., Monsell F., Pringle J., Cannon S.R. Osteofibrous dysplasia of the tibia. Is there a need for a radical surgical approach? J. Bone Joint Surg. Br. 2006;88(5):658–664. doi: 10.1302/0301-620X.88B5.17358. [DOI] [PubMed] [Google Scholar]
- 4.Wang J.W., Shih C.H., Chen W.J. Osteofibrous dysplasia (ossifying fibroma of long bones). A report of four cases and review of the literature. Clin. Orthop. Relat. Res. 1992;278:235–243. [PubMed] [Google Scholar]
- 5.Arthur A., Zannettino A., Gronthos S. The therapeutic applications of multipotential mesenchymal/stromal stem cells in skeletal tissue repair. J. Cell Physiol. 2009;218(2):237–245. doi: 10.1002/jcp.21592. [DOI] [PubMed] [Google Scholar]
- 6.Brooke G., Cook M., Blair C., Han R., Heazlewood C., Jones B. Therapeutic applications of mesenchymal stromal cells. Semin. Cell Dev. Biol. 2007;18(6):846–858. doi: 10.1016/j.semcdb.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 7.Arinzeh T.L., Peter S.J., Archambault M.P., van den Bos C., Gordon S., Kraus K. Allogeneic mesenchymal stem cells regenerate bone in a critical-sized canine segmental defect. J. Bone Joint Surg. Am. 2003;85-A(10):1927–1935. doi: 10.2106/00004623-200310000-00010. [DOI] [PubMed] [Google Scholar]
- 8.Agacayak S., Gulsun B., Ucan M.C., Karaoz E., Nergiz Y. Effects of mesenchymal stem cells in critical size bone defect. Eur. Rev. Med. Pharmacol. Sci. 2012;16(5):679–686. [PubMed] [Google Scholar]
- 9.Binkley J.M., Stratford P.W., Lott S.A., Riddle D.L. The Lower Extremity Functional Scale (LEFS): scale development, measurement properties, and clinical application. North American Orthopaedic Rehabilitation Research Network. Phys. Ther. 1999;79(4):371–383. [PubMed] [Google Scholar]
- 10.Tiedeman J.J., Lippiello L., Connolly J.F., Strates B.S. Quantitative roentgenographic densitometry for assessing fracture healing. Clin. Orthop. Relat. Res. 1990;253:279–286. [PubMed] [Google Scholar]
- 11.Lane J.M., Sandhu H.S. Current approaches to experimental bone grafting. Orthop. Clin. North Am. 1987;18(2):213–225. [PubMed] [Google Scholar]
- 12.Ozaki T., Hamada M., Sugihara S., Kunisada T., Mitani S., Inoue H. Treatment outcome of osteofibrous dysplasia. J. Pediatr. Orthop. B. 1998;7(3):199–202. doi: 10.1097/01202412-199807000-00004. [DOI] [PubMed] [Google Scholar]
- 13.Hahn S.B., Kim S.H., Cho N.H., Choi C.J., Kim B.S., Kang H.J. Treatment of osteofibrous dysplasia and associated lesions. Yonsei Med. J. 2007;48(3):502–510. doi: 10.3349/ymj.2007.48.3.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hazelbag H.M., Taminiau A.H., Fleuren G.J., Hogendoorn P.C. Adamantinoma of the long bones. A clinicopathological study of thirty-two patients with emphasis on histological subtype, precursor lesion, and biological behavior. J. Bone Joint Surg. Am. 1994;76(10):1482–1499. doi: 10.2106/00004623-199410000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Nandi S.K., Roy S., Mukherjee P., Kundu B., De D.K., Basu D. Orthopaedic applications of bone graft & graft substitutes: a review. Indian J. Med. Res. 2010;132:15–30. [PubMed] [Google Scholar]
- 16.Enneking W.F., Eady J.L., Burchardt H. Autogenous cortical bone grafts in the reconstruction of segmental skeletal defects. J. Bone Joint Surg. Am. 1980;62(7):1039–1058. [PubMed] [Google Scholar]
- 17.Ozaki T., Nakatsuka Y., Kunisada T., Kawai A., Dan’ura T., Naito N. High complication rate of reconstruction using Ilizarov bone transport method in patients with bone sarcomas. Arch. Orthop. Trauma Surg. 1998;118(3):136–139. doi: 10.1007/s004020050333. [DOI] [PubMed] [Google Scholar]
- 18.Lindsey R.W., Gugala Z., Milne E., Sun M., Gannon F.H., Latta L.L. The efficacy of cylindrical titanium mesh cage for the reconstruction of a critical-size canine segmental femoral diaphyseal defect. J. Orthop. Res. 2006;24(7):1438–1453. doi: 10.1002/jor.20154. [DOI] [PubMed] [Google Scholar]
- 19.Giannoudis P.V., Einhorn T.A., Marsh D. Fracture healing: the diamond concept. Injury. 2007;38(Suppl 4):S3–S6. doi: 10.1016/s0020-1383(08)70003-2. [DOI] [PubMed] [Google Scholar]
- 20.Watanabe K., Tsuchiya H., Sakurakichi K., Tomita K. Bone transport using hydroxyapatite loaded with bone morphogenetic protein in rabbits. J. Bone Joint Surg. Br. 2007;89(8):1122–1129. doi: 10.1302/0301-620X.89B8.19003. [DOI] [PubMed] [Google Scholar]