ABSTRACT
Probiotics and commensal intestinal microbes suppress mammalian cytokine production and intestinal inflammation in various experimental model systems. Limited information exists regarding potential mechanisms of probiotic-mediated immunomodulation in vivo. In this report, we demonstrate that specific probiotic strains of Lactobacillus reuteri suppress intestinal inflammation in a trinitrobenzene sulfonic acid (TNBS)-induced mouse colitis model. Only strains that possess the hdc gene cluster, including the histidine decarboxylase and histidine-histamine antiporter genes, can suppress colitis and mucosal cytokine (interleukin-6 [IL-6] and IL-1β in the colon) gene expression. Suppression of acute colitis in mice was documented by diminished weight loss, colonic injury, serum amyloid A (SAA) protein concentrations, and reduced uptake of [18F]fluorodeoxyglucose ([18F]FDG) in the colon by positron emission tomography (PET). The ability of probiotic L. reuteri to suppress colitis depends on the presence of a bacterial histidine decarboxylase gene(s) in the intestinal microbiome, consumption of a histidine-containing diet, and signaling via the histamine H2 receptor (H2R). Collectively, luminal conversion of l-histidine to histamine by hdc+ L. reuteri activates H2R, and H2R signaling results in suppression of acute inflammation within the mouse colon.
IMPORTANCE
Probiotics are microorganisms that when administered in adequate amounts confer beneficial effects on the host. Supplementation with probiotic strains was shown to suppress intestinal inflammation in patients with inflammatory bowel disease and in rodent colitis models. However, the mechanisms of probiosis are not clear. Our current studies suggest that supplementation with hdc+ L. reuteri, which can convert l-histidine to histamine in the gut, resulted in suppression of colonic inflammation. These findings link luminal conversion of dietary components (amino acid metabolism) by gut microbes and probiotic-mediated suppression of colonic inflammation. The effective combination of diet, gut bacteria, and host receptor-mediated signaling may result in opportunities for therapeutic microbiology and provide clues for discovery and development of next-generation probiotics.
INTRODUCTION
The incidence and prevalence of pediatric and adult inflammatory bowel diseases (IBDs) have steadily increased with time during recent decades (1–3). Immunomodulatory treatment strategies, including corticosteroids, immunosuppressants, and anti-tumor necrosis factor (anti-TNF) medications continue to dominate the approach to amelioration of chronic intestinal inflammation. Established pharmacological agents target activation of immune cells and cytokine-mediated signaling pathways in human cells. As one example, the proinflammatory cytokine TNF has been a primary target in IBD therapeutics for more than a decade, and delineation of druggable targets in the innate immune system has resulted in dramatic improvements in induction and maintenance therapy of these chronic inflammatory diseases. However, insufficient attention to targets within intestinal microbes may limit progress in IBD therapeutics and may limit our understanding of the systems biology of chronic inflammation.
The intestinal microbiota are dominated by the phyla Firmicutes and Bacteroidetes, and differences in bacterial composition among the Firmicutes have been described in patients with IBD (4). Relative deficiencies of specific intestinal microbes may contribute to lack of microbe-derived anti-inflammatory factors in humans. For example, one commensal bacterial species, Faecalibacterium prausnitzii, which can suppress human cytokine production, is present in reduced amounts and often undetectable in patients with Crohn’s disease (5). In addition, manipulation of the gut microbiome might result in new therapeutic strategies. For example, probiotic combinations including lactobacilli and bifidobacteria can suppress inflammation when administered to patients with acute and chronic pouchitis (6). The question remains whether and how host intestinal microbes (e.g., probiotics) contribute to chronic intestinal inflammation.
Lactobacillus reuteri is a commensal intestinal firmicute and probiotic that is widely prevalent in the gastrointestinal tracts of diverse avian and mammalian species (7). L. reuteri is generally recognized as safe (GRAS) and is considered to be a beneficial microbe that has been used globally as a probiotic for approximately 2 decades. L. reuteri has been reported to suppress proinflammatory cytokines in intestinal epithelial cells (8) and monocytes (9) and intestinal inflammation in different rodent models (8, 10–13). However, the underlying mechanisms are still not clear. A recent pangenomic study showed that human-derived clade II L. reuteri strains contained a complete chromosomal hdc gene cluster (genes hdcA, hdcB, and hdcP) and the genetic capacity to convert l-histidine to histamine, and this clade could also suppress human TNF production in vitro. In contrast, clade VI strains, which lacked the hdc gene cluster in their bacterial chromosomes, failed to suppress human TNF production in vitro in the absence of histamine generation (14). Histamine is considered to be a primary candidate immunomodulin, or immunomodulatory compound, produced by this Lactobacillus species. Inactivation of histidine-to-histamine converting capacity by mutagenesis of the histidine decarboxylase gene (hdcA) diminished the ability of hdc-positive L. reuteri strains to suppress production of human TNF in vitro (9). The question whether histidine metabolism, particularly the production of histamine by hdc+ L. reuteri, may contribute to the anti-inflammatory effects of this species in vivo deserves investigation as a possible gateway to deepening our understanding of microbiome-mediated intestinal immunomodulation (15, 16).
In the present study, we investigated the mechanisms of intestinal immunomodulation by probiotics in a mammalian host. Clade II L. reuteri strains can serve as model microbes of the human gut microbiome, and we explored the relative importance of l-histidine metabolism by hdc+ L. reuteri and whether histamine could represent a key signal modulating intestinal immune responses. We demonstrated that microbiome supplementation with hdc+ L. reuteri in a trinitrobenzene sulfonic acid (TNBS)-induced mouse model of colitis resulted in improvement of overall health status, reduction of colonic inflammation, and suppression of proinflammatory cytokine production. Both the histidine-to-histamine converting enzyme, histidine decarboxylase, and dietary l-histidine must be present for probiotic L. reuteri to ameliorate colitis in this mouse model. Luminal conversion of the amino acid l-histidine to histamine and signaling via histamine H2 receptor (H2R) are required for maximal suppression of colitis by L. reuteri.
RESULTS
hdc+ L. reuteri attenuates colonic inflammation in vivo.
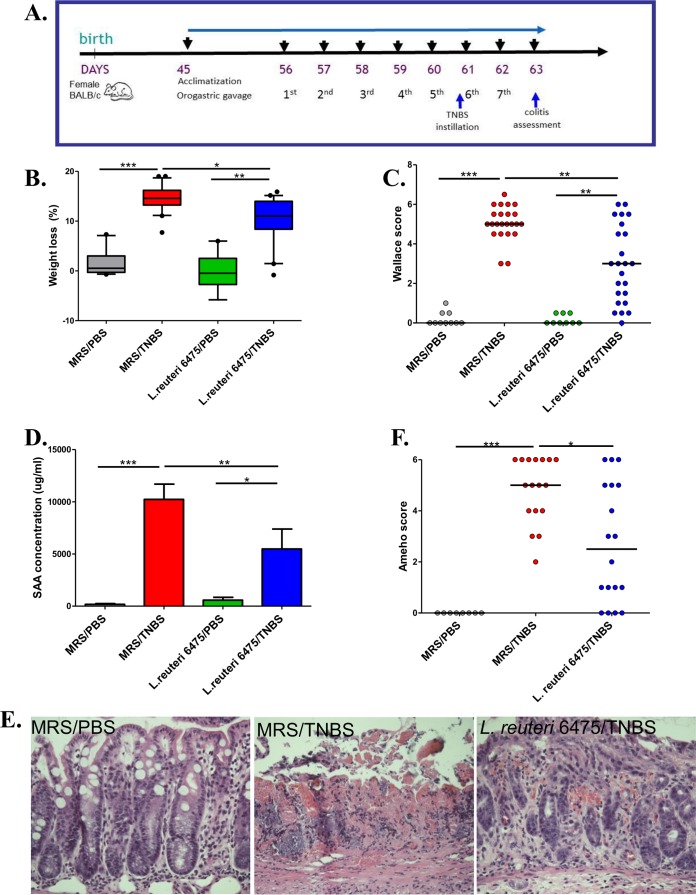
L. reuteri clade II strain 6475 (L. reuteri 6475) was isolated from a Finnish mother’s breast milk sample (8) and has been used commercially as a probiotic. This hdc-positive strain suppresses human TNF production by myeloid cells (9). The TNBS-triggered acute colitis mouse model (17) was selected for evaluation of colitis suppression by intestinal lactobacilli. Adult (8-week-old) female BALB/c mice were fed L. reuteri 6475 (see Fig. S1A in the supplemental material) by daily orogastric gavage following acclimatization and at least 5 days prior to TNBS instillation (Fig. 1A). The severity of colitis was evaluated 2 days after TNBS instillation by weight loss (overall health status), Wallace and Ameho scores (macroscopic and microscopic colonic injury) (18–20) (see Fig. S1B), and serum amyloid A (SAA) protein concentrations (biomarker of mucosal inflammation) (21). In colitis-negative control mice that received phosphate-buffered saline (PBS) only, L. reuteri 6475 maintained a healthy baseline without any evidence of colitis. In colitis-positive control mice that were challenged with TNBS in the absence of L. reuteri, expected results with increased weight loss, Wallace scores, and serum SAA concentrations were observed (Fig. 1B to D), consistent with acute colitis. In contrast, administration of L. reuteri 6475 in late-exponential-phase growth attenuated colitis as indicated by significant reductions in weight loss, Wallace scores, and SAA concentrations compared with colitis-positive controls lacking L. reuteri 6475 (Fig. 1B to D).
FIG 1 .
hdc+ L. reuteri attenuates colonic inflammation in vivo. (A) Time line of the mouse experiments. After 10 days of acclimatization, 8-week-old female BALB/c mice received 5 × 109 CFU of bacteria in MRS or MRS medium only by orogastric gavage daily for 7 days. Acute colitis was induced by intrarectal instillation of TNBS-ethanol before the sixth gavage, and colitis severity was evaluated in 2 days. (B to D) Weight loss (B), Wallace scores (C), and SAA concentrations (D) of mice challenged with or without TNBS and gavaged with or without L. reuteri 6475. (E and F) Representative microscopic colonic images (hematoxylin and eosin stained) (E) and Ameho scores (F) from mice in the healthy control group (MRS/PBS), the colitis control group (MRS/TNBS), and the L. reuteri-treated group (L. reuteri 6475/TNBS).
Ameho scores included assessment of histologic inflammation so that a comprehensive evaluation of colitis could be performed. TNBS instillation (sans L. reuteri) caused necrosis extending deeply into the muscularis propria, whereas L. reuteri 6475 administration in TNBS-treated mice yielded mild or prominent mucosal or submucosal inflammation with preservation of intact muscularis mucosae and muscularis propria (Fig. 1E and F). These results were consistent with previous findings (19). Other morphological comparisons yielded reduced colon lengths in mice challenged with TNBS compared with L. reuteri 6475-treated mice (see Fig. S1C and D in the supplemental material) and healthy control mice receiving PBS only, consistent with previous findings (22, 23).
To extend the findings related to colitis suppression by hdc+ L. reuteri, we introduced a second clade II hdc+ L. reuteri strain, ATCC PTA 4659 (L. reuteri 4659), into the same mouse colitis model. Similarly, L. reuteri 4659 diminished the weight loss phenotype, reduced Wallace scores, and decreased SAA concentrations, compared with control mice receiving MRS medium only (see Fig. S2 in the supplemental material).
PET imaging demonstrates the ability of L. reuteri 6475 to suppress intestinal inflammation.
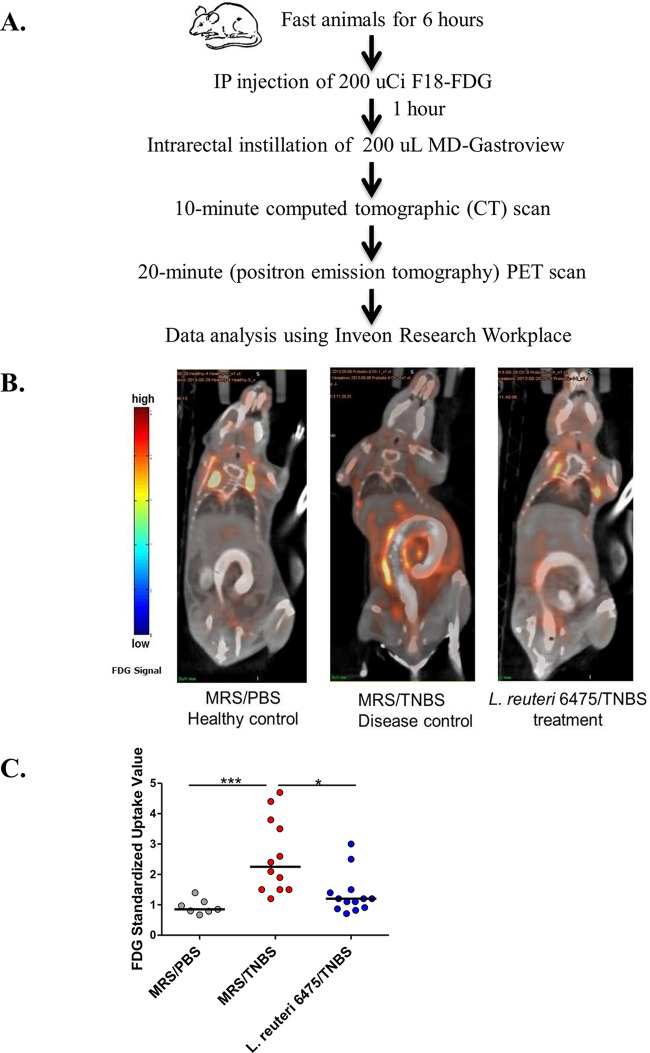
In order to visualize anti-inflammatory effects of L. reuteri 6475, combined computed tomography (CT)-positron emission tomography (PET) imaging was applied to the TNBS colitis model. Healthy control mice, colitic mice, and L. reuteri-treated mice were subjected to live-animal imaging prior to euthanasia (Fig. 2A). [18F]fluorodeoxyglucose ([18F]FDG) has been applied to mouse colitis studies and was used as the tracer compound because immune cells have been shown to increase glucose uptake and phosphorylation after immune activation (24). During colitis, FDG uptake by activated lymphocytes and other immune cells in the colon can be measured by comparing relative intensities of tracer signals by anatomic location. In healthy control mice, FDG signals were mostly detected in the mouse bladder and upper chest region. These sites were proposed to be body sites where increased glucose uptake and metabolism in healthy states have been documented (25). Trace amounts of FDG signals were shown in pericolonic areas in the abdomen, indicating low glucose uptake in the colons of healthy mice (Fig. 2B). In colitic control mice, FDG intensities in the mouse abdomen adjacent to the colon were significantly increased (Fig. 2B), indicating increased glucose uptake surrounding or within the colonic mucosa during colitis. L. reuteri 6475 administration lowered FDG intensity in the mouse colon compared to colitic controls (Fig. 2B). FDG intensity was also shown in three-dimensional images (see Videos S1 to S3 in the supplemental material). FDG signal changes in different mouse groups were further confirmed by the FDG standardized uptake value (SUV) quantified blindly in the region of interest (ROI) (colon) in each mouse using Inveon Research Workplace software (Fig. 2C), indicating attenuation of colonic inflammation by L. reuteri.
FIG 2 .
Detection of colitis attenuation by PET imaging. (A) Time line of the PET imaging experiments. Fasted (for 6 h) mice were anesthetized with isoflurane and received 200 µCi [18F]FDG by intraperitoneal (IP) injection. One hour later, these mice received 200 µl MD-Gastroview rectally immediately before scan initiation. Computed tomography (CT) scanning was performed for 10 min followed by PET scanning for 20 min using the Inveon PET-CT multimodality system. Mice were kept sedated during the scanning process by constant isoflurane inhalation. The images were recorded, and FDG standardized uptake values (SUVs) were analyzed blindly using Inveon Research Workplace software. (B) Representative mouse images captured by PET-CT scanning in each group. The color code bar represents FDG signal intensity. (C) Quantification of FDG signals in mouse colon using SUVs in different groups.
L. reuteri administration increased microbial hdc gene expression in the intestine.
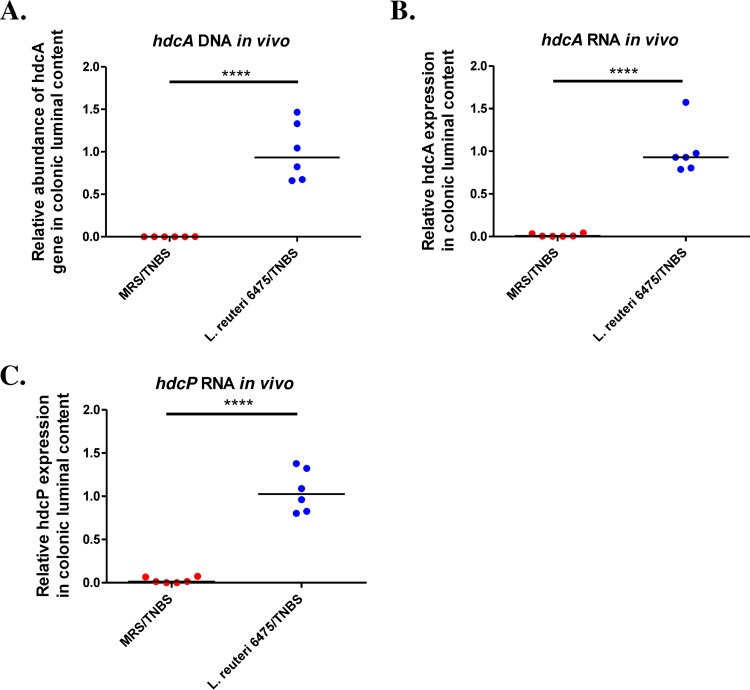
In order to explore whether hdc genes contribute to the anti-inflammatory effects of L. reuteri in vivo, the relative abundances of hdc genes and mRNA were determined by quantitative PCR (qPCR) in mice gavaged with L. reuteri 6475. Our results demonstrated that L. reuteri 6475 administration significantly increased the relative abundance of bacterial hdcA genes in the mouse gut microbiome (Fig. 3A). In terms of mRNA, both hdcA and hdcP gene expression was significantly increased in colonic luminal contents of mice receiving L. reuteri (Fig. 3B and C). These studies indicate a correlation between elevated hdc gene expression via L. reuteri administration and the effect of colitis attenuation. By enhancing the ability of the intestinal microbiome to convert l-histidine to histamine, the gut microbiome was capable of suppressing intestinal inflammation.
FIG 3 .
L. reuteri administration increases hdc gene expression in vivo. (A) Relative abundance of hdcA gene in mouse gut microbiome was significantly increased in mice gavaged with L. reuteri 6475 compared to control mice gavaged with MRS. The relative abundance was determined by qPCR and normalized to the bacterial housekeeping gene rpoB. (B and C) Both hdcA (B) and hdcP (C) gene expression levels were significantly increased in colonic luminal contents of mice receiving L. reuteri compared to control mice receiving MRS. The relative gene expression was determined by qPCR and normalized to the bacterial housekeeping gene rpoB. n = 6 per group. ****, P < 0.0001.
Inactivation of the L. reuteri histidine decarboxylase gene diminished its ability to suppress intestinal inflammation.
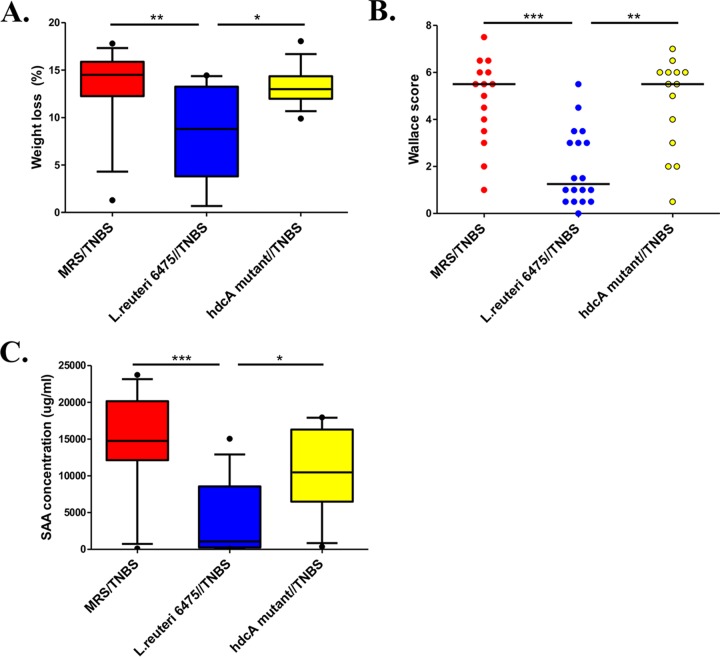
To further investigate the importance of the bacterial histidine decarboxylase gene with respect to intestinal immunomodulation, we explored the anti-inflammatory effects of hdcA within an isogenic mutant and compared with wild-type L. reuteri 6475 using the same TNBS colitis model. The hdcA gene encodes histidine decarboxylase, and the hdcA mutant derived from L. reuteri 6475 does not generate histamine from l-histidine (9). The L. reuteri 6475 hdcA mutant strain was shown to be deficient in terms of colitis suppression in the TNBS colitis mouse model. The hdcA mutant yielded diminished effects in terms of weight loss, Wallace scores, and SAA concentrations (Fig. 4), suggesting that bacterial histidine decarboxylase mediates anti-inflammatory effects via histamine generation. To exclude the possibility that TNBS may adversely affect the function of L. reuteri in the mouse intestine, we performed additional in vitro experiments and found that TNBS did not affect the survival or proliferation of the L. reuteri (wild-type or mutant) strain (see Fig. S3A and B in the supplemental material).
FIG 4 .
Inactivation of the L. reuteri histidine decarboxylase gene diminishes its ability to suppress intestinal inflammation. The anti-inflammatory effects of hdcA within an isogenic mutant which does not produce histamine were compared with those of wild-type L. reuteri 6475 using the TNBS colitis model. The wild-type strain attenuated colitis compared with the medium-control group (MRS/TNBS), whereas the hdcA mutant yielded diminished effects in terms of weight loss (A), Wallace scores (B), and SAA concentrations (C).
Dietary l-histidine enables hdc+ L. reuteri to suppress intestinal inflammation.
In addition to experiments with different L. reuteri strains, the relative importance of dietary l-histidine as the substrate for histamine generation was evaluated by exposing mice to different diets. BALB/c mice were randomly divided into three feeding groups: regular diet containing approximately 0.4% histidine from intact protein sources; defined diet with all essential amino acids, including 0.4% histidine; and a histidine-free diet derived from defined diet with all amino acids except histidine (see Table S1 in the supplemental material).
Mice in each feeding group were gavaged with the same amount of L. reuteri 6475 or MRS medium only as the control group. Mice receiving L. reuteri 6475 and dietary l-histidine (in regular mouse chow or amino acid defined diet) demonstrated the expected amelioration of colitis by reduced Wallace scores and serum SAA concentrations (Fig. 5A and B). In contrast, when the mice received a histidine-free diet, L. reuteri 6475 yielded diminished effects on colitis attenuation, suggesting that histidine intake is important for the anti-inflammatory activity of L. reuteri 6475. Dietary deficiency of l-histidine results in weight loss in mice (see Fig. S4A in the supplemental material), as other studies have shown (26), so weight loss was not used as a primary parameter to evaluate colitis severity in this study. To exclude the possibility that the diets per se contributed to colitis development, groups of mice were fed one of the three diets, and none of these mice developed colitis without TNBS instillation (see Fig. S4B). In summary, l-histidine provides the substrate for intestinal L. reuteri to generate histamine in the presence of active histidine decarboxylase.
FIG 5 .
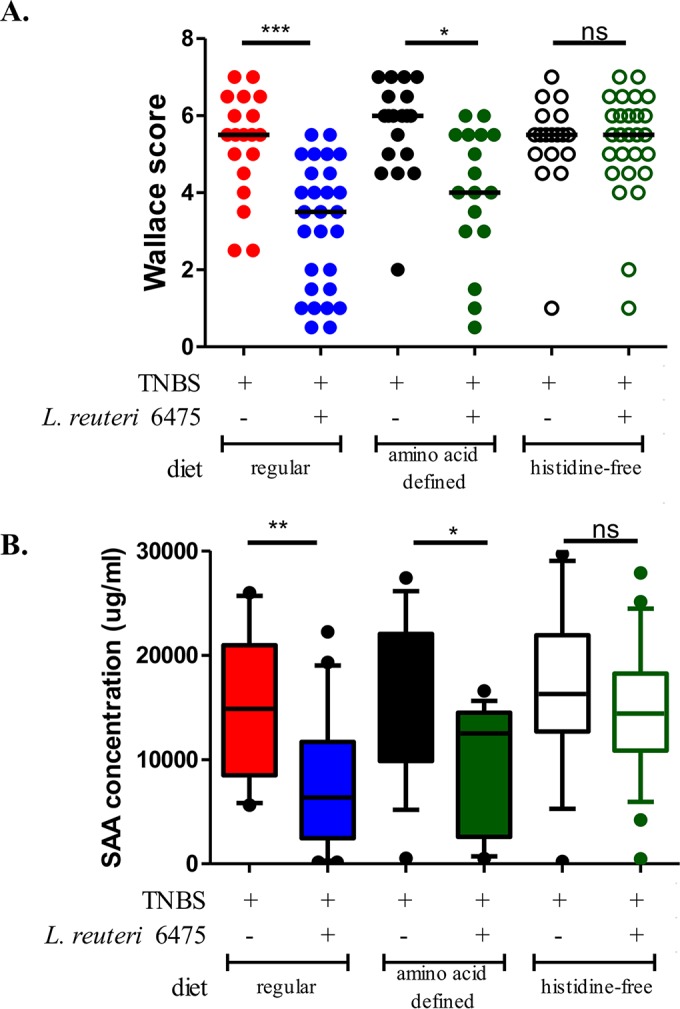
l-histidine deficiency diminishes anti-inflammatory effects. The anti-inflammatory effects of L. reuteri 6475 were compared in mice fed with different diets using the TNBS colitis model. Mice fed a regular diet or an amino acid defined diet showed decreased Wallace scores (A) and plasma SAA concentrations (B) when receiving L. reuteri 6475 compared to the MRS medium control. When mice were fed an l-histidine-deficient diet, L. reuteri 6475 showed diminished anti-inflammatory effects in terms of Wallace scores and SAA concentrations.
Activation of H2R is required for anti-inflammatory effects of microbial histamine.
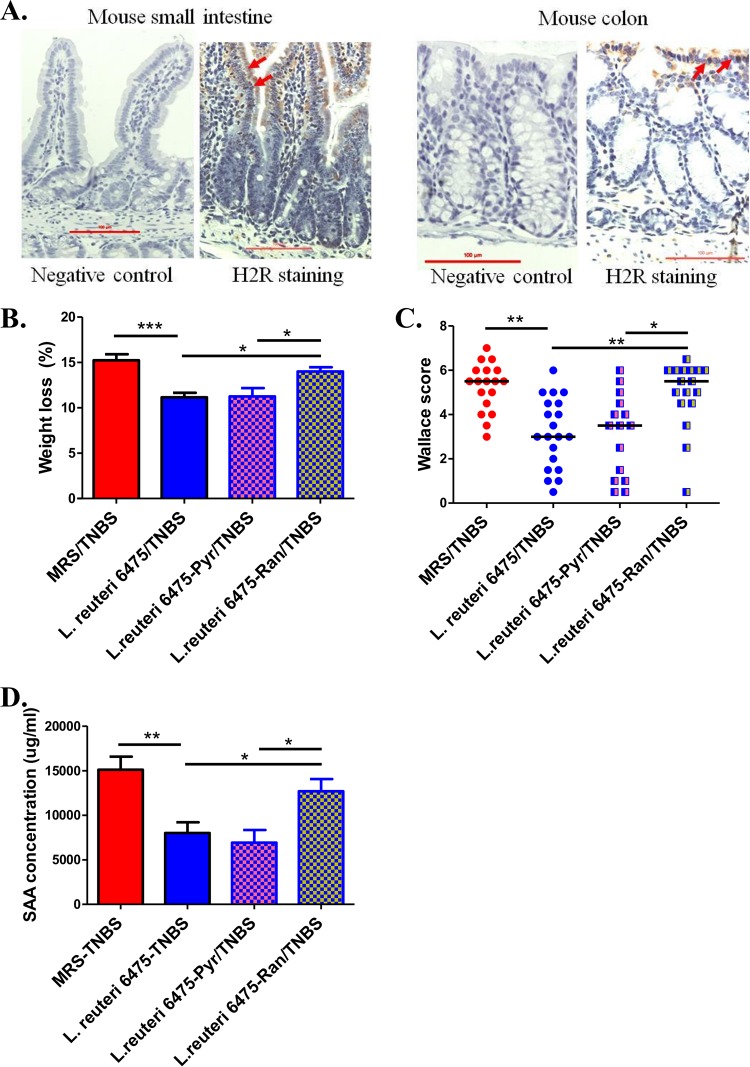
Histamine is a biogenic amine that exerts various pathophysiological functions via four receptors (H1R, H2R, H3R, and H4R) (27). The predominant histamine receptors in the intestinal epithelium are H1R and H2R (28). H2R was shown to be expressed in the intestinal epithelium of humans, simians, and mice (28, 29). Immunohistochemical studies show H2R expression in the BALB/c mouse small intestine and colon, with relatively high intensities in the villi and crypts (Fig. 6A). It has been reported previously that H1R activation results in proinflammatory effects such as interferon (IFN) production and Th1 cell proliferation, while H2R activation appears to suppress inflammation (27, 30, 31). So, we hypothesized that microbe-derived histamine binds and activates H2R in the intestinal epithelium, thereby mediating its anti-inflammatory effects.
FIG 6 .
Activation of H2R is required for the anti-inflammatory effects. (A) Immunohistochemistry studies using H2R-specific antibody showed that H2R was expressed in 9-week-old BALB/c mice, with high intensity in the villi and crypts (red arrows). (B to D) Mice that received pyrilamine (Pyr) did not show an effect on the anti-inflammatory effects of L. reuteri 6475, while mice that received ranitidine (Ran) showed a diminished ability for L. reuteri 6475 to attenuate colitis, as indicated by weight loss (B), Wallace scores (C), and plasma SAA concentrations (D).
To determine whether H2R activation was required for L. reuteri’s capacity to attenuate colitis in the TNBS model, ranitidine, an H2R-specific antagonist, was used to block H2R activation in the mouse gut. In the TNBS colitis experiment, addition of ranitidine (100 mg/kg of body weight) to mice receiving hdc+ L. reuteri by orogastric gavage diminished the anti-inflammatory effects of L. reuteri 6475, whereas blocking H1R with its specific antagonist pyrilamine lacked such effects (Fig. 6B to D). These findings support the proposition that L. reuteri 6475 attenuates colitis via an H2R-dependent signaling mechanism. To exclude the possibility that ranitidine or pyrilamine may have adversely affected the function of L. reuteri, in vitro assays were performed by adding ranitidine or pyrilamine to bacterial cultures, and neither compound affected the survival or proliferation of L. reuteri 6475 (see Fig. S3C and D in the supplemental material).
L. reuteri administration affects cytokine gene expression in the colon.
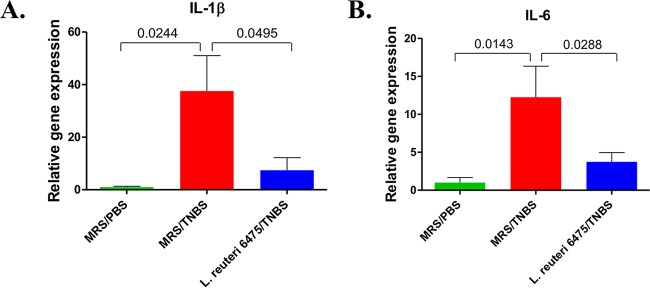
To investigate the consequences of H2R-mediated anti-inflammatory effects by hdc+ L. reuteri, relative patterns of mucosal gene expression of selected cytokines in the colons of colitis-negative, colitis-positive, and L. reuteri 6475-treated mice were evaluated by qPCR using glyceraldehyde-3-phosphate dehydrogenase (GAPDH) as the internal standard (Fig. 7). TNBS instillation significantly increased expression of genes for proinflammatory cytokines interleukin-1β (IL-1β) and IL-6 compared to healthy mice. L. reuteri 6475 treatment of TNBS-challenged mice reduced the relative amounts of mucosal IL-1β and IL-6 gene expression. Other cytokine genes (TNF, IL-10, IL-12, IFN, IL-17, and IL-23) were examined, but no significant differences between colitic mice and L. reuteri-treated mice were detected.
FIG 7 .
L. reuteri administration affects cytokine gene expression in the colon. Gene expression of IL-1β (A) and IL-6 (B) in the colons of healthy mice, colitic mice challenged with TNBS, and L. reuteri 6475-treated colitic mice was measured by reverse transcription-quantitative PCR using GAPDH as the internal standard. n = 10 per group. The P values are indicated in the figure.
DISCUSSION
Probiotic lactobacilli modulate intestinal immune responses by luminal conversion of dietary amino acids into bioactive compounds such as histamine. In the current study, hdc+ L. reuteri strains protected BALB/c mice in a TNBS-induced colitis model, as indicated by improvement in overall health status and amelioration of the colitis phenotype. The colitis phenotype was evaluated by macroscopic and microscopic evaluation of colonic tissue, serum biomarker quantitation, mucosal cytokine gene expression, and [18F]FDG live-animal PET imaging. The importance of dietary histidine and the bacterial enzyme histidine decarboxylase was established by mouse model studies. In the absence of dietary l-histidine or a gut microbiome lacking histidine decarboxylase, colitis suppression by probiotic lactobacilli was reduced significantly. Both the substrate amino acid and the enzymatic machinery, histidine decarboxylase, must be present in the intestinal microbiome in order to generate histamine as the bioactive compound. Histamine H2 receptor signaling in the intestinal epithelium is required for probiotic L. reuteri-mediated immunomodulation and colitis suppression. Previously, L. reuteri was shown to suppress H2R-mediated signaling by increasing cyclic AMP (cAMP) production, protein kinase A (PKA) activation, and suppression of extracellular signal-regulated kinase (ERK) (mitogen-activated protein [MAP] kinase) signaling (9). H2R-mediated signaling via cAMP production and protein kinase A (PKA) activation, followed by inhibition of c-Raf and MEK/ERK MAPK signaling, was described in previous studies (9, 32–34).
The same microbe-derived biochemical compound (histamine) can yield different effects in the host depending on the specific type of histamine receptor. Four different G-protein-coupled histamine receptors have been described, and these receptors differ based on downstream signaling pathways and cell type distributions (27). Histamine receptors are widely distributed in the body, and the histamine type 2 receptor (H2R) appears to be enriched in the mammalian gastrointestinal tract (28, 29). H2R was first characterized in the human stomach as an important target for H2R blockers for treatment of peptic ulcer disease by reduction of acid (HCl) secretion (35). In addition to the importance of H2R in gastric physiology, it is becoming apparent that histamine may have an important immunoregulatory role in the intestine. H2R activation results in cAMP-mediated blockade of c-Raf and suppression of MAP kinase signaling by inhibition of ERK phosphorylation (9, 27). Activation of H2R by histamine suppressed IL-12 production by monocytes (36), IFN-γ production by macrophages (37), TNF secretion by mast cells (38), and IL-12 release by immature dendritic cells (39). In vivo studies showed that histamine suppressed both Th1- and Th2-type responses by H2R (30). Lactobacillus rhamnosus, which secretes histamine significantly, suppressed Peyer patch IL-2, IL-4, IL-5, IL-12, TNF-α, and granulocyte-macrophage colony-stimulating factor (GM-CSF) secretion in wild-type but not H2R-deficient mice (31).
These results indicate that the net effect of luminal histamine may be immunosuppressive and anti-inflammatory in the mammalian gastrointestinal tract. Prior studies of the human metagenome reported that pathways of histidine biosynthesis (histamine precursor) were diminished in patients with IBD relative to healthy controls (40). These studies highlight the potential importance of microbiome-mediated histidine metabolism and histamine generation as a microbial mechanism for intestinal immunomodulation. Recent evidence suggests that blocking H2R signaling pathways in humans may result in adverse effects and severe intestinal inflammation. Retrospective and prospective clinical studies of newborns have documented significantly increased incidence and mortality from necrotizing enterocolitis (NEC) following exposure to H2R antagonists (41–43). In addition to NEC, studies have reported an increased risk of exacerbations in Crohn’s disease secondary to H2R blocker exposure (44). The histamine receptor H2R appears to be the key receptor on the intestinal epithelium, involved in signaling and immunomodulation after binding microbiome-derived histamine.
The consumption of hdc gene cluster-positive probiotics in the presence of dietary histidine may maximize the potential benefits by resulting in histamine generation only in intestinal regions enriched for histamine H2 receptors. Oral administration of histamine may cause adverse outcomes, but the careful selection of microbes that colonize specific areas of the small or large intestine may maximize histamine H2 receptor signaling in the intestinal epithelium. Conversely, provision of l-histidine in the diet enables luminal conversion and luminal histamine generation by hdc gene cluster-positive microbes. Another consideration is the relative instability of histamine in vivo. Histamine is unstable in vivo and could be quickly metabolized by histamine N-methyltransferase or diamine oxidase (45). Lower concentrations of histamine might be protective, whereas higher concentrations might be detrimental to epithelial protection from infection (27). The continuous production of small amounts of histamine by the gut microbiome may result in suppression of intestinal inflammation. With respect to bacterial genetics of histamine production, the current study did not include an hdcA complementation strain because antibiotic consumption by mice to maintain antibiotic resistance plasmids may profoundly affect gut microbial composition. Future generation of hdcA complementation strains by recombineering (46) may remove the requirement for antibiotic selection. Such future genetic strategies would be helpful to confirm whether histidine decarboxylase is essential for suppression of intestinal inflammation by probiotic L. reuteri.
Adverse effects of histamine are likely due to the preponderance of the H1 receptor in the airways (47) and upper gastrointestinal tract. When ingested orally, histamine may cause adverse reactions and symptoms such as pruritus, bronchoconstriction, airway inflammation, and allergic symptoms (48). For this reason, the food and beverage industry has developed active programs to screen for histamine in foodstuffs and contamination by histamine-generating bacteria. H1R is coupled to Gq/11 family proteins, triggering downstream calcium mobilization with proinflammatory effects. In addition to H1R, H4R also appears to contribute to respiratory disease symptoms. Classical antihistamines relieve respiratory tract symptoms by antagonizing H1R and, more recently, H4R (49). H3R is primarily neuronal, highlighting histamine’s role as a potential neurotransmitter. The characterization of the relative distribution of histamine receptors in the gastrointestinal tract and other body sites will facilitate a more complete understanding of the biology of histamine in vivo.
This report highlights the potential importance of luminal conversion and amino acid metabolism in the biology of the intestinal microbiome and host-microbe mutualism. Luminal conversion of amino acids and different classes of nutrients effectively links diet, the gut bacteria, and mammalian intestinal biology. Bacterial amino acid decarboxylases have been reported to convert glutamate to the neurotransmitter gamma-aminobutyric acid (GABA) (50) and tyrosine to tyramine and phenylalanine to the neuromodulatory compound phenethylamine (51). Differences in the relative abundances of pathways involved in amino acid metabolism present in the gut microbiome may contribute to different disease phenotypes in individuals genetically predisposed to IBD or other immune-mediated conditions. Dietary amino acids are potential substrates for a variety of microbial amino acid decarboxylases, and diverse compounds, including biogenic amines, may be produced. These compounds, such as histamine, may have important consequences for mucosal immunity or functioning of the nervous system. Our study indicates that luminal conversion of an amino acid, l-histidine, to histamine by hdc+ L. reuteri activates H2R and yields anti-inflammatory effects in the mouse colon. This study combined specific cellular elements of the intestinal microbiome, the genes and enzymatic machinery involved in luminal conversion, and the specific receptors involved in receiving microbial signals. These studies could foster the development of new probiotic therapies by facilitating the selection of natural hdc gene cluster-positive strains (or strains with any defined genetic feature contributing to immunomodulation) combined with dietary elements (e.g., amino acids) or enabling genetic engineering of next-generation probiotics by defining specific microbial genes involved in mitigation of intestinal inflammation. By defining mechanisms of microbiome-mediated immunomodulation in the mammalian intestine, bacterial strains and microbial gene databases can be leveraged to identify next-generation probiotics and microbe-derived medicinal compounds for the treatment of chronic inflammatory diseases.
MATERIALS AND METHODS
Bacterial strains and microbiological culture conditions.
L. reuteri ATCC PTA 6475 and its hdcA mutant as described previously (9) were used to colonize the mice. L. reuteri ATCC PTA 4659, isolated from the breast milk of healthy Finnish women, was a gift from BioGaia AB (Stockholm, Sweden). All L. reuteri strains were cultured at 37°C in deMan, Rogosa, Sharpe (MRS) medium (Difco, Franklin Lakes, NJ) in an anaerobic workstation (MACS MG-500; Microbiology International, Frederick, MD) supplied with a mixture of 10% CO2, 10% H2, and 80% N2. Quantitative analysis of bacteria was performed by counting bacterial CFU on an MRS agar plate per milliliter of bacterial culture relative to optical density at 600 nm (OD600) measured by a SmartSpec Plus spectrophotometer (Bio-Rad Laboratories, CA).
Animals.
Female BALB/c mice (45 days old) were purchased from Harland Laboratories (Houston, TX) and maintained under specific-pathogen-free (SPF) conditions. Mice were kept under filter-top cages (5 mice per cage) and had free access to distilled water and Harlan rodent chow 2918 (default diet) or other diets as described in Table S1 in the supplemental material. All mouse experiments were performed in an SPF animal facility according to an Institutional Animal Care and Use Committee (IACUC)-approved mouse protocol at Baylor College of Medicine, Houston, TX.
Preparation of bacteria and administration to mice.
L. reuteri strains and culture conditions were as described above. Bacteria were harvested at exponential phase (5.5 h in MRS medium with an initial OD600 of 0.03) and centrifuged at 2,500 × g for 4 min, and bacterial pellets were resuspended in sterile MRS medium for animal feeding. All L. reuteri strains were prepared freshly before administration to mice. Each mouse received 5 × 109 CFU of bacteria in 0.2 ml MRS or MRS medium alone (medium control) by orogastric gavage at a frequency of once per day for 7 days. In selected experiments, pyrilamine or ranitidine at a dose of 100 mg/kg body weight was added in the bacterial medium to feed mice by orogastric gavage.
Induction of colitis by intrarectal instillation of TNBS.
At 6 h before the six orogastric gavages mentioned above, mice preanesthetized by constant isoflurane inhalation were challenged with 5% (wt/vol) TNBS in H2O (Sigma-Aldrich, St. Louis, MO) diluted with an equal volume of absolute ethanol intrarectally (4 cm distal to the anus) via catheter (Braintree Scientific, USA) at a dose of 100 mg/kg of body weight. Mice were kept head down in a vertical position for 2 min after enema to ensure complete retention of enema in the colon. Mice from respective control groups received PBS at a dose of 100 mg/kg of body weight instead of TNBS.
Tissue preparations.
Mice were euthanized 48 h after colitis induction. Blood samples were collected from mice via cardiac puncture in blood sample collection tubes with K2-EDTA (Becton, Dickinson and Company, Franklin Lakes, NJ), centrifuged at 17,000 × g for 10 min at 4°C to isolate plasma, and stored at −80°C until use. The gastrointestinal tract was carefully removed. Colons and ceca were excised, and colon lengths were measured. Luminal contents in the colon were collected, flash frozen immediately in liquid nitrogen, and stored at −80°C until use.
Mouse intestines were fixed in 10% formalin, embedded with paraffin, and sectioned with a microtome at 5 µm. The sectioned tissues were used for immunohistochemistry targeting H2R expression using specific antibody (Alomone Labs, Jerusalem, Israel). In specific experiments, the colon tissue samples located precisely 2 cm above the anal canal were cut into two parts. One part was fixed overnight in 10% formalin and embedded in paraffin. After hematoxylin and eosin staining, slides were examined microscopically and scored blindly by two pathologists according to Ameho criteria (20). Another tissue fragment was stored in RNAlater (Ambion, Austin, TX) and flash frozen immediately in liquid nitrogen for cytokine gene expression analysis.
Colitis assessment.
Colitis severity was assessed by weight loss, Wallace score, and serum amyloid protein A (SAA) concentrations as described below. Weight loss is calculated by the formula weight loss = [(w1 − w2)/w1] × 100%, where w1 represents mouse body weight before TNBS instillation and w2 represents mouse body weight at 48 h after colitis induction. Wallace score (see Fig. S1B in the supplemental material), which was used to quantify colonic injury macroscopically on the excised and longitudinally opened colons, was given blindly by two trained technicians as described before (18). SAA concentrations in plasma were measured using enzyme-linked immunosorbent assay (ELISA) kits from Alpco (Salem, NH), according to the manufacturer’s instructions.
PET imaging.
PET-CT scanning was performed 48 h after colitis induction in selected experiments as described previously (24) with minor modifications. Briefly, fasted (for 6 h) mice were anesthetized with isoflurane and received 200 µCi [18F]FDG by intraperitoneal (i.p.) injection. One hour later, these mice received 200 µl MD-Gastroview (Mallinckrodt Inc., MO) rectally via a 3.5 French (F) catheter immediately before scan initiation. Computed tomography (CT) scanning was performed for 10 min followed by PET scanning for 20 min using the Inveon PET-CT multimodality system (Siemens). Mice were kept sedated during the scanning process by constant isoflurane inhalation. The images were recorded, and FDG standardized uptake values (SUVs) were analyzed blindly using Inveon Research Workplace software. The MD-Gastroview appears as radiopaque in the CT image, thus highlighting the colon clearly. A region of interest (ROI) comprising the colon was extracted from the CT scan and transferred to the space of the PET scan. Tissues/organs (such as the bladder) other than the colon were excluded from the measurements, and the averages of SUVs of the remaining voxels were calculated to represent the severity of the inflammation.
Toxicity of TNBS, pyrilamine, or ranitidine against L. reuteri 6475.
In growth curve assays, different concentrations (0 µg/ml, 2.5 µg/ml, 25 µg/ml, or 250 µg/ml) of TNBS, pyrilamine, or ranitidine were added to MRS medium inoculated with wild-type L. reuteri 6475 or hdcA mutant strains. The OD600s were measured at different time points. In bacterial killing assays, L. reuteri 6475 or hdcA mutant cultures were normalized to an OD600 of 1 and then treated for 1 h with 250 µg/ml of TNBS, pyrilamine, or ranitidine. The cultures were then diluted in sterile MRS medium and plated on MRS agar to count CFU.
Determination of the relative abundances of hdc genes and mRNA in vivo.
Total DNA from luminal contents in the colon was isolated using ZR Fecal DNA MiniPrep (Zymo Research, Irvine, CA) according to the manufacturer’s instructions. For total RNA extraction, luminal contents in the colon were resuspended in RNAlater (Ambion, Austin, TX) and transferred to 2.0-ml FastPrep tubes (MP Biomedicals, CA) prefilled with 200 µl 0.1-mm lysing beads. The samples were homogenized with a FastPrep-24 instrument (MP Biomedicals, CA) at 4.0 m/s for 20 s twice, and the supernatants were collected for RNA isolation using the RNeasy minikit (Qiagen, USA). Methods for cDNA synthesis and qPCR are described below. Relative DNA and mRNA quantities of hdc genes were normalized to the housekeeping gene rpoB (β subunit of bacterial RNA polymerase).
Determination of cytokine gene expression in the mouse colon.
To quantify the relative mRNA levels of IL-1β, IL-6, IL-10, IL-12, IL-17, IL-23, tumor necrosis factor (TNF), and interferon (IFN), RNA was extracted from the colon samples mentioned above using the RNeasy minikit (Qiagen, USA). One microgram of RNA was reverse transcribed to single-stranded cDNA using the stated protocol for SuperScript III reverse transcriptase (Invitrogen, Carlsbad, CA). Real-time PCR was performed using the real-time PCR system (Stratagene). The PCR mixture (adjusted with H2O to a total volume of 20 µl) contained 1 µl template DNA, 10 µl 2× FastStart Universal Probe Master (Rox) (Roche Applied Science), and 0.5 µl of the respective primers (10 µM each). All primers used in this study were designed using the Universal ProbeLibrary Assay Design Center (Roche Applied Science, Indianapolis, IN) and are described in Table S2 in the supplemental material. Relative mRNA levels of target genes were normalized to the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH).
Statistical analysis.
Biostatistical analyses were performed using GraphPad Prism (version 5) software (GraphPad Inc., La Jolla, CA). For numeric variables that fit normal distribution (determined using the Kolmogorov-Smirnov test), data were presented as means with standard errors, and different groups were compared with the t test (two groups) or one-way analysis of variance (ANOVA) (more than two groups). Otherwise, data were presented as box-and-whisker plots showing the median and 10th and 90th percentiles or scatter plots, and different groups were compared with the nonparametric Mann-Whitney U test (two groups) or the Kruskal-Wallis test (more than two groups). Differences between the groups were considered significant at P < 0.05 (*), P < 0.01 (**), P < 0.001 (***), and P < 0.0001 (****).
SUPPLEMENTAL MATERIAL
MRS-PBS healthy control. Representative three-dimensional reconstruction of FDG signal in healthy mice that received MRS medium and were challenged with PBS. The video is shown from the abdominal side to the back side, 15 slices per second. Download
MRS-TNBS colitis control. Representative three-dimensional reconstruction of FDG signal in colitic mice that received MRS medium and were challenged with TNBS. The video is shown from the abdominal side to the back side, 15 slices per second. Download
L. reuteri 6475-TNBS treated. Representative three-dimensional reconstruction of FDG signal in L. reuteri 6475-treated mice that received L. reuteri 6475 and were challenged with TNBS. The video is shown from the abdominal side to the back side, 15 slices per second. Download
(A) L. reuteri 6475 growth curve in MRS medium. The bacterial harvest time is indicated by an arrow (5.5 h, late exponential phase). (B) Representative colon figures (view from the luminal side of the colon opened longitudinally) indicating the Wallace score criteria: grade 0, healthy appearance; grade 1, focal hyperemia, slight thickening, no ulcers; grade 2, hyperemia, prominent thickening, no ulcers; grade 3, ulceration with inflammation at one site; grade 4, ulceration with inflammation at two or more sites; grade 5, major sites of damage extending >1 cm; grade 6 to 10, when area of damage extends beyond 2 cm, and the score is increased by each additional centimeter of tissue involvement. (C and D) Representative colonic images (C) and colon lengths (D) in each group: healthy control (left), colitic control (middle), and L. reuteri 6475-treated mice (right). Download
L. reuteri ATCC PTA 4659, another clade II L. reuteri strain that is able to produce histamine, attenuated TNBS-induced colitis as indicated by significantly reduced weight loss (A), Wallace score (B), and SAA concentration (C) compared with controls receiving MRS medium only. Download
(A) TNBS did not affect the proliferation of L. reuteri as indicated by the growth curve of wild-type L. reuteri 6475 or the isogenic hdcA mutant strain in the presence of different concentrations of TNBS. (B) Addition of TNBS (250 µg/ml) to bacterial culture did not affect the viability of either wild-type L. reuteri 6475 or the isogenic hdcA mutant strain as indicated by the number of colonies. (C and D) Addition of pyrilamine or ranitidine (up to 250 µg/ml) to L. reuteri 6475 culture did not affect the proliferation (C) or viability (D) of L. reuteri 6475. Download
(A) Weight change (percent) in mice fed regular (green), amino acid defined (blue), or histidine-free (red) diets with or without L. reuteri 6475 gavage. (B) Feeding mice a regular diet, an amino acid defined diet, or a histidine-free diet did not lead to development of colitis as indicated by low Wallace scores when the mice did not receive TNBS instillation. Download
Comparison of regular, amino acid defined, and histidine-free diets that were used to feed mice.
Primers and probes used for the gene expression studies.
ACKNOWLEDGMENTS
This work was supported by the National Institutes of Health (R01 AT004326, UH3 DK083990, and U01 CA170930) and the National Institutes of Health (National Institute for Diabetes and Digestive and Kidney Diseases)-funded Texas Medical Center Digestive Diseases Center (DK56338) (J.V.).
We thank Eamonn Connolly (BioGaia AB, Stockholm) for providing the L. reuteri strains, Toni-Ann Mistretta and Bhanu Priya Ganesh for assistance with data plotting and statistical analysis, and Coreen Johnson for assistance with bacterial DNA and RNA extractions from luminal contents. We thank Texas Children’s Hospital for the use of the Small Animal Imaging Facility and especially Caterina Kaffes and. M. Waleed Gaber for PET imaging.
We disclose the following: J.V. receives unrestricted research support from BioGaia AB. The remaining authors disclose no conflicts.
C.G. designed and performed all the experiments, ran the analysis, and wrote the manuscript. A.M. performed all the staining. D.R. performed PET imaging and PET analysis. V.J. and M.L. helped collect mouse samples. Z.S. and Y.M.-A. performed histological analyses. J.V. provided guidance, designed the experiments, and wrote the manuscript.
Footnotes
Citation Gao C, Major A, Rendon D, Lugo M, Jackson V, Shi Z, Mori-Akiyama Y, Versalovic J. 2015. Histamine H2 receptor-mediated suppression of intestinal inflammation by probiotic Lactobacillus reuteri. mBio 6(6):e01358-15. doi:10.1128/mBio.01358-15.
REFERENCES
- 1.Molodecky NA, Soon IS, Rabi DM, Ghali WA, Ferris M, Chernoff G, Benchimol EI, Panaccione R, Ghosh S, Barkema HW, Kaplan GG. 2012. Increasing incidence and prevalence of the inflammatory bowel diseases with time, based on systematic review. Gastroenterology 142:46–54. doi: 10.1053/j.gastro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Moradkhani A, Beckman LJ, Tabibian JH. 2013. Health-related quality of life in inflammatory bowel disease: psychosocial, clinical, socioeconomic, and demographic predictors. J Crohns Colitis 7:467–473. doi: 10.1016/j.crohns.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Benchimol EI, To T, Griffiths AM, Rabeneck L, Guttmann A. 2011. Outcomes of pediatric inflammatory bowel disease: socioeconomic status disparity in a universal-access healthcare system. J Pediatr 158:960–967. doi: 10.1016/j.jpeds.2010.11.039. [DOI] [PubMed] [Google Scholar]
- 4.Knights D, Lassen KG, Xavier RJ. 2013. Advances in inflammatory bowel disease pathogenesis: linking host genetics and the microbiome. Gut 62:1505–1510. doi: 10.1136/gutjnl-2012-303954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sokol H, Pigneur B, Watterlot L, Lakhdari O, Bermudez-Humaran LG, Gratadoux JJ, Blugeon S, Bridonneau C, Furet JP, Corthier G, Grangette C, Vasquez N, Pochart P, Trugnan G, Thomas G, Blottiere HM, Dore J, Marteau P, Seksik P, Langella P. 2008. Faecalibacterium prausnitzii is an anti-inflammatory commensal bacterium identified by gut microbiota analysis of Crohn disease patients. Proc Natl Acad Sci U S A 105:16731–16736. doi: 10.1073/pnas.0804812105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gionchetti P, Rizzello F, Venturi A, Brigidi P, Matteuzzi D, Bazzocchi G, Poggioli G, Miglioli M, Campieri M. 2000. Oral bacteriotherapy as maintenance treatment in patients with chronic pouchitis: a double-blind, placebo-controlled trial. Gastroenterology 119:305–309. doi: 10.1053/gast.2000.9370. [DOI] [PubMed] [Google Scholar]
- 7.Casas IA, Dobrogosz WJ. 2000. Validation of the probiotic concept: Lactobacillus reuteri confers broad-spectrum protection against disease in humans and animals. Microb Ecol Health Dis 12:247–285. doi: 10.1080/08910600050216246-1. [DOI] [Google Scholar]
- 8.Liu Y, Fatheree NY, Mangalat N, Rhoads JM. 2010. Human-derived probiotic Lactobacillus reuteri strains differentially reduce intestinal inflammation. Am J Physiol Gastrointest Liver Physiol 299:G1087–G1096. doi: 10.1152/ajpgi.00124.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas CM, Hong T, van Pijkeren JP, Hemarajata P, Trinh DV, Hu W, Britton RA, Kalkum M, Versalovic J. 2012. Histamine derived from probiotic Lactobacillus reuteri suppresses TNF via modulation of PKA and ERK signaling. PLoS One 7:e31951. doi: 10.1371/journal.pone.0031951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Madsen KL, Doyle JS, Jewell LD, Tavernini MM, Fedorak RN. 1999. Lactobacillus species prevents colitis in interleukin 10 gene-deficient mice. Gastroenterology 116:1107–1114. doi: 10.1016/S0016-5085(99)70013-2. [DOI] [PubMed] [Google Scholar]
- 11.Pena JA, Li SY, Wilson PH, Thibodeau SA, Szary AJ, Versalovic J. 2004. Genotypic and phenotypic studies of murine intestinal lactobacilli: species differences in mice with and without colitis. Appl Environ Microbiol 70:558–568. doi: 10.1128/AEM.70.1.558-568.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schreiber O, Petersson J, Phillipson M, Perry M, Roos S, Holm L. 2009. Lactobacillus reuteri prevents colitis by reducing P-selectin-associated leukocyte- and platelet-endothelial cell interactions. Am J Physiol Gastrointest Liver Physiol 296:G534–G542. doi: 10.1152/ajpgi.90470.2008. [DOI] [PubMed] [Google Scholar]
- 13.Preidis GA, Saulnier DM, Blutt SE, Mistretta T, Riehle KP, Major AM, Venable SF, Barrish JP, Finegold MJ, Petrosino JF, Guerrant RL, Conner ME, Versalovic J. 2012. Host response to probiotics determined by nutritional status of rotavirus-infected neonatal mice. J Pediatr Gastroenterol Nutr 55:299–307. doi: 10.1097/MPG.0b013e31824d2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Spinler JK, Sontakke A, Hollister EB, Venable SF, Oh PL, Balderas MA, Saulnier DMA, Mistretta TA, Devaraj S, Walter J, Versalovic J, Highlander SK. 2014. From prediction to function using evolutionary genomics: human-specific ecotypes of Lactobacillus reuteri have diverse probiotic functions. Genome Biol Evol 6:1772–1789. doi: 10.1093/gbe/evu137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Preidis GA, Versalovic J. 2009. Targeting the human microbiome with antibiotics, probiotics, and prebiotics: gastroenterology enters the metagenomics era. Gastroenterology 136:2015–2031. doi: 10.1053/j.gastro.2009.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hemarajata P, Gao C, Pflughoeft KJ, Thomas CM, Saulnier DM, Spinler JK, Versalovic J. 2013. Lactobacillus reuteri-specific immunoregulatory gene rsiR modulates histamine production and immunomodulation by Lactobacillus reuteri. J Bacteriol 195:5567–5576. doi: 10.1128/JB.00261-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scheiffele F, Fuss IJ. 2002. Induction of TNBS colitis in mice. Curr Protoc Immunol Chapter 15:Unit 15.19. doi: 10.1002/0471142735.im1519s49. [DOI] [PubMed] [Google Scholar]
- 18.Wallace JL, MacNaughton WK, Morris GP, Beck PL. 1989. Inhibition of leukotriene synthesis markedly accelerates healing in a rat model of inflammatory bowel disease. Gastroenterology 96:29–36. [DOI] [PubMed] [Google Scholar]
- 19.Foligné B, Nutten S, Steidler L, Dennin V, Goudercourt D, Mercenier A, Pot B. 2006. Recommendations for improved use of the murine TNBS-induced colitis model in evaluating anti-inflammatory properties of lactic acid bacteria: technical and microbiological aspects. Dig Dis Sci 51:390–400. doi: 10.1007/s10620-006-3143-x. [DOI] [PubMed] [Google Scholar]
- 20.Ameho CK, Adjei AA, Harrison EK, Takeshita K, Morioka T, Arakaki Y, Ito E, Suzuki I, Kulkarni AD, Kawajiri A, Yamamoto S. 1997. Prophylactic effect of dietary glutamine supplementation on interleukin 8 and tumour necrosis factor alpha production in trinitrobenzene sulphonic acid induced colitis. Gut 41:487–493. doi: 10.1136/gut.41.4.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Villiers WJS, Varilek GW, de Beer FC, Guo J, Kindy MS. 2000. Increased serum amyloid a levels reflect colitis severity and precede amyloid formation in IL-2 knockout mice. Cytokine 12:1337–1347. doi: 10.1006/cyto.2000.0716. [DOI] [PubMed] [Google Scholar]
- 22.Sasaoka T, Ito M, Yamashita J, Nakajima K, Tanaka I, Narita M, Hara Y, Hada K, Takahashi M, Ohno Y, Matsuo T, Kaneshiro Y, Tanaka H, Kaneko K. 2011. Treatment with IL-27 attenuates experimental colitis through the suppression of the development of IL-17-producing T helper cells. Am J Physiol Gastrointest Liver Physiol 300:G568–G576. doi: 10.1152/ajpgi.00329.2010. [DOI] [PubMed] [Google Scholar]
- 23.Dutra RC, Claudino RF, Bento AF, Marcon R, Schmidt EC, Bouzon ZL, Pianowski LF, Calixto JB. 2011. Preventive and therapeutic euphol treatment attenuates experimental colitis in mice. PLoS One 6:e27122. doi: 10.1371/journal.pone.0027122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brewer S, McPherson M, Fujiwara D, Turovskaya O, Ziring D, Chen L, Takedatsu H, Targan SR, Wei B, Braun J. 2008. Molecular imaging of murine intestinal inflammation with 2-deoxy-2-[18F]fluoro-d-glucose and positron emission tomography. Gastroenterology 135:744–755. doi: 10.1053/j.gastro.2008.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Galitovskiy V, Kuruvilla S, Sevriokov E, Corches A, Pan M, Kalantari-Dehaghi M, Chernyavsky A, Mukherjee J, Grando S. 2013. Development of novel approach to diagnostic imaging of lung cancer with 18F-Nifene PET/CT using A/J mice treated with NNK. J Cancer Res Ther 1:128–137. doi: 10.14312/2052-4994.2013-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shibata K, Fukuwatari T, Zushi S, Sugimoto E. 2001. Effect of dietary histidine content on the change in content of skin urocanic acid isomers in hairless mice irradiated with ultraviolet B. Biosci Biotechnol Biochem 65:1415–1418. doi: 10.1271/bbb.65.1415. [DOI] [PubMed] [Google Scholar]
- 27.O’Mahony L, Akdis M, Akdis CA. 2011. Regulation of the immune response and inflammation by histamine and histamine receptors. J Allergy Clin Immunol 128:1153–1162. doi: 10.1016/j.jaci.2011.06.051. [DOI] [PubMed] [Google Scholar]
- 28.Sander LE, Lorentz A, Sellge G, Coeffier M, Neipp M, Veres T, Frieling T, Meier PN, Manns MP, Bischoff SC. 2006. Selective expression of histamine receptors H1R, H2R, and H4R, but not H3R, in the human intestinal tract. Gut 55:498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim H, Dwyer L, Song JH, Martin-Cano FE, Bahney J, Peri L, Britton FC, Sanders KM, Koh SD. 2011. Identification of histamine receptors and effects of histamine on murine and simian colonic excitability. Neurogastroenterol Motil 23:949–e409. doi: 10.1111/j.1365-2982.2011.01760.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jutel M, Watanabe T, Klunker S, Akdis M, Thomet OAR, Malolepszy J, Zak-Nejmark T, Koga R, Kobayashi T, Blaser K, Akdis CA. 2001. Histamine regulates T-cell and antibody responses by differential expression of H1 and H2 receptors. Nature 413:420–425. doi: 10.1038/35096564. [DOI] [PubMed] [Google Scholar]
- 31.Frei R, Ferstl R, Konieczna P, Ziegler M, Simon T, Rugeles TM, Mailand S, Watanabe T, Lauener R, Akdis CA, O’Mahony L. 2013. Histamine receptor 2 modifies dendritic cell responses to microbial ligands. J Allergy Clin Immunol 132:194–204. doi: 10.1016/j.jaci.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 32.Funaki C, Hodges RR, Dartt DA. 2010. Identification of the Raf-1 signaling pathway used by cAMP to inhibit p42/p44 MAPK in rat lacrimal gland acini: role in potentiation of protein secretion. Invest Ophthalmol Vis Sci 51:6321–6328. doi: 10.1167/iovs.10-5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hershenson MB, Chao TS, Abe MK, Gomes I, Kelleher MD, Solway J, Rosner MR. 1995. Histamine antagonizes serotonin and growth factor-induced mitogen-activated protein kinase activation in bovine tracheal smooth muscle cells. J Biol Chem 270:19908–19913. doi: 10.1074/jbc.270.34.19908. [DOI] [PubMed] [Google Scholar]
- 34.Waltereit R, Weller M. 2003. Signaling from cAMP/PKA to MAPK and synaptic plasticity. Mol Neurobiol 27:99–106. doi: 10.1385/MN:27:1:99. [DOI] [PubMed] [Google Scholar]
- 35.Garnett WR. 2003. History of acid suppression: focus on the hospital setting. Pharmacotherapy 23:56S–60S. doi: 10.1592/phco.23.13.56S.31932. [DOI] [PubMed] [Google Scholar]
- 36.Elenkov IJ, Webster E, Papanicolaou DA, Fleisher TA, Chrousos GP, Wilder RL. 1998. Histamine potently suppresses human IL-12 and stimulates IL-10 production via H2 receptors. J Immunol 161:2586–2593. [PubMed] [Google Scholar]
- 37.Horvath B, Szalai C, Mandi Y, Laszlo V, Radvany Z, Darvas Z, Falus A. 1999. Histamine and histamine-receptor antagonists modify gene expression and biosynthesis of interferon gamma in peripheral human blood mononuclear cells and in CD19-depleted cell subsets. Immunol Lett 70:95–99. doi: 10.1016/S0165-2478(99)00126-1. [DOI] [PubMed] [Google Scholar]
- 38.Bissonnette EY. 1996. Histamine inhibits tumor necrosis factor alpha release by mast cells through H2 and H3 receptors. Am J Respir Cell Mol Biol 14:620–626. doi: 10.1165/ajrcmb.14.6.8652190. [DOI] [PubMed] [Google Scholar]
- 39.Mazzoni A, Young HA, Spitzer JH, Visintin A, Segal DM. 2001. Histamine regulates cytokine production in maturing dendritic cells, resulting in altered T cell polarization. J Clin Invest 108:1865–1873. doi: 10.1172/JCI200113930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morgan XC, Tickle TL, Sokol H, Gevers D, Devaney KL, Ward DV, Reyes JA, Shah SA, LeLeiko N, Snapper SB, Bousvaros A, Korzenik J, Sands BE, Xavier RJ, Huttenhower C. 2012. Dysfunction of the intestinal microbiome in inflammatory bowel disease and treatment. Genome Biol 13:R79. doi: 10.1186/gb-2012-13-9-r79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gupta RW, Tran L, Norori J, Ferris MJ, Eren AM, Taylor CM, Dowd SE, Penn D. 2013. Histamine-2 receptor blockers alter the fecal microbiota in premature infants. J Pediatr Gastroenterol Nutr 56:397–400. doi: 10.1097/MPG.0b013e318282a8c2. [DOI] [PubMed] [Google Scholar]
- 42.Terrin G, Passariello A, De Curtis M, Manguso F, Salvia G, Lega L, Messina F, Paludetto R, Canani RB. 2012. Ranitidine is associated with infections, necrotizing enterocolitis, and fatal outcome in newborns. Pediatrics 129:e40–e45. doi: 10.1542/peds.2011-0796. [DOI] [PubMed] [Google Scholar]
- 43.Guillet R, Stoll BJ, Cotten CM, Gantz M, McDonald S, Poole WK, Phelps DL. 2006. Association of H2-blocker therapy and higher incidence of necrotizing enterocolitis in very low birth weight infants. Pediatrics 117:e137–e142. doi: 10.1542/peds.2005-1543. [DOI] [PubMed] [Google Scholar]
- 44.Juillerat P, Schneeweiss S, Cook EF, Ananthakrishnan AN, Mogun H, Korzenik JR. 2012. Drugs that inhibit gastric acid secretion may alter the course of inflammatory bowel disease. Aliment Pharmacol Ther 36:239–247. doi: 10.1111/j.1365-2036.2012.05173.x. [DOI] [PubMed] [Google Scholar]
- 45.Akdis CA, Blaser K. 2003. Histamine in the immune regulation of allergic inflammation. J Allergy Clin Immunol 112:15–22. doi: 10.1067/mai.2003.1585. [DOI] [PubMed] [Google Scholar]
- 46.Van Pijkeren JP, Britton RA. 2012. High efficiency recombineering in lactic acid bacteria. Nucleic Acids Res 40:e76. doi: 10.1093/nar/gks147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Togias A. 2003. H1-receptors: localization and role in airway physiology and in immune functions. J Allergy Clin Immunol 112:S60–S68. doi: 10.1016/S0091-6749(03)01878-5. [DOI] [PubMed] [Google Scholar]
- 48.Naila A, Flint S, Fletcher G, Bremer P, Meerdink G. 2010. Control of biogenic amines in food—existing and emerging approaches. J Food Sci 75:R139–R150. doi: 10.1111/j.1750-3841.2010.01774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thurmond RL, Gelfand EW, Dunford PJ. 2008. The role of histamine H1 and H4 receptors in allergic inflammation: the search for new antihistamines. Nat Rev Drug Discov 7:41–53. doi: 10.1038/nrd2465. [DOI] [PubMed] [Google Scholar]
- 50.Li H, Cao Y. 2010. Lactic acid bacterial cell factories for gamma-aminobutyric acid. Amino Acids 39:1107–1116. doi: 10.1007/s00726-010-0582-7. [DOI] [PubMed] [Google Scholar]
- 51.Marcobal A, De las Rivas B, Landete JM, Tabera L, Muñoz R. 2012. Tyramine and phenylethylamine biosynthesis by food bacteria. Crit Rev Food Sci Nutr 52:448–467. doi: 10.1080/10408398.2010.500545. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
MRS-PBS healthy control. Representative three-dimensional reconstruction of FDG signal in healthy mice that received MRS medium and were challenged with PBS. The video is shown from the abdominal side to the back side, 15 slices per second. Download
MRS-TNBS colitis control. Representative three-dimensional reconstruction of FDG signal in colitic mice that received MRS medium and were challenged with TNBS. The video is shown from the abdominal side to the back side, 15 slices per second. Download
L. reuteri 6475-TNBS treated. Representative three-dimensional reconstruction of FDG signal in L. reuteri 6475-treated mice that received L. reuteri 6475 and were challenged with TNBS. The video is shown from the abdominal side to the back side, 15 slices per second. Download
(A) L. reuteri 6475 growth curve in MRS medium. The bacterial harvest time is indicated by an arrow (5.5 h, late exponential phase). (B) Representative colon figures (view from the luminal side of the colon opened longitudinally) indicating the Wallace score criteria: grade 0, healthy appearance; grade 1, focal hyperemia, slight thickening, no ulcers; grade 2, hyperemia, prominent thickening, no ulcers; grade 3, ulceration with inflammation at one site; grade 4, ulceration with inflammation at two or more sites; grade 5, major sites of damage extending >1 cm; grade 6 to 10, when area of damage extends beyond 2 cm, and the score is increased by each additional centimeter of tissue involvement. (C and D) Representative colonic images (C) and colon lengths (D) in each group: healthy control (left), colitic control (middle), and L. reuteri 6475-treated mice (right). Download
L. reuteri ATCC PTA 4659, another clade II L. reuteri strain that is able to produce histamine, attenuated TNBS-induced colitis as indicated by significantly reduced weight loss (A), Wallace score (B), and SAA concentration (C) compared with controls receiving MRS medium only. Download
(A) TNBS did not affect the proliferation of L. reuteri as indicated by the growth curve of wild-type L. reuteri 6475 or the isogenic hdcA mutant strain in the presence of different concentrations of TNBS. (B) Addition of TNBS (250 µg/ml) to bacterial culture did not affect the viability of either wild-type L. reuteri 6475 or the isogenic hdcA mutant strain as indicated by the number of colonies. (C and D) Addition of pyrilamine or ranitidine (up to 250 µg/ml) to L. reuteri 6475 culture did not affect the proliferation (C) or viability (D) of L. reuteri 6475. Download
(A) Weight change (percent) in mice fed regular (green), amino acid defined (blue), or histidine-free (red) diets with or without L. reuteri 6475 gavage. (B) Feeding mice a regular diet, an amino acid defined diet, or a histidine-free diet did not lead to development of colitis as indicated by low Wallace scores when the mice did not receive TNBS instillation. Download
Comparison of regular, amino acid defined, and histidine-free diets that were used to feed mice.
Primers and probes used for the gene expression studies.