Abstract
Context:
DICER1 germline mutation carriers have an increased predisposition to cancer, such as pleuropulmonary blastoma (PPB) and Sertoli-Leydig cell tumor (SLCT), and a high prevalence of multinodular goiter (MNG). Although differentiated thyroid carcinoma (DTC) has been reported in some DICER1 mutation carriers with PPB treated with chemotherapy, the association of DTC with DICER1 mutations is not well established.
Case Description:
We report a family with DICER1 mutation and familial DTC without a history of chemotherapy. A 12-year-old female (patient A) and her 14-year-old sister (patient B) presented with MNG. Family history was notable for a maternal history of DTC and bilateral ovarian SLCT. Both sisters underwent total thyroidectomy. Pathological examination showed nodular hyperplasia and focal papillary thyroid carcinoma within hyperplastic nodules. Subsequently, patient A developed virilization secondary to a unilateral ovarian SLCT. During her evaluation, an incidental cystic nephroma was also found. Three other siblings had MNG on surveillance ultrasound examination; two had thyroidectomies, and one had two microscopic foci of papillary carcinoma. Patient A, her mother, and four affected siblings had a germline heterozygous pathogenic DICER1 mutation c.5441C>T in exon 25, resulting in an amino acid change from p.Ser1814Leu of DICER1. Somatic DICER1 RNase IIIb missense mutations were identified in thyroid nodules from three of the four siblings.
Conclusions:
This family provides novel insight into an emerging phenotype for DICER1 syndrome, with evidence that germline DICER1 mutations are associated with an increased risk of developing familial DTC, even in the absence of prior treatment with chemotherapy.
DICER1 protein is a member of the ribonuclease (RNase) III family of proteins that cleaves noncoding small RNA (microRNA or miRNA) precursors to generate mature miRNAs, which in turn regulate gene expression post-transcriptionally (1, 2). miRNAs are important for many biological processes, including metabolism, morphogenesis, cell proliferation, and apoptosis (1, 2). Heterozygous germline DICER1 mutations, usually in the context of a tissue-specific mutation on the other allele, predispose to various tumors, collectively called DICER1 syndrome or familial pleuropulmonary blastoma (PPB) tumor predisposition syndrome (2–4). PPB and Sertoli-Leydig cell tumor (SLCT) are two of the characteristic tumors of DICER1 syndrome (2, 4).
Multinodular goiter (MNG) appears to be common in individuals with DICER1 mutations (1, 2, 5). Some authors have suggested that treatment with chemotherapy (for PPB) may be a predisposing risk for differentiated thyroid carcinoma (DTC) in individuals with DICER1 germline mutations (6–8).
We report a family with a germline DICER1 mutation (c.5441C>T; p.Ser1814Leu) in six first-degree relatives with MNG, four of whom also had DTC, and none of whom had PPB or prior exposure to chemotherapy. The findings in this family strongly support a direct association of DICER1 mutations with an increased risk for DTC, unrelated to chemotherapy.
Case Description
A 12-year-old female (patient A) and her 14-year-old sister (patient B) presented to the Cincinnati Children's Hospital Medical Center (CCHMC) Endocrinology Clinic for evaluation of palpable thyroid nodules. Both sisters were clinically and biochemically euthyroid. Thyroid ultrasound (US) showed multiple complex nodules. Fine-needle aspiration in both sisters was read as suspicious for follicular neoplasia. Total thyroidectomies were performed.
Two months after thyroid surgery, patient A raised concern about deepening of her voice and increased facial hair, which had developed over several weeks. Laboratory evaluation revealed elevated testosterone. Magnetic resonance imaging showed a large pelvic mass and a right renal cyst. Left oophorectomy and partial right nephrectomy were performed. Pathology confirmed an ovarian SLCT of intermediate differentiation and a cystic nephroma. Chest computerized tomography scan did not show any pulmonary cysts or masses.
Family history was notable for the patients' mother having had a right hemi-thyroidectomy for a benign thyroid nodule at age 13 years, followed by completion thyroidectomy for follicular thyroid carcinoma in the contralateral lobe at age 18 years (described below). She had also had a left oophorectomy for SLCT of the ovary at age 7 years and a partial right oophorectomy for SLCT at age 18 years. There was no other family history of DTC, SLCT, or PPB in maternal relatives.
The patients' three younger siblings (ages 7 to 11 y) were subsequently found to have MNG on surveillance thyroid US examination. Two siblings with palpable nodules underwent thyroidectomies. The third sibling had a normal thyroid physical examination.
Based on the clinical history, DICER1 syndrome was suspected, and the family was enrolled in the International Ovarian and Testicular Stromal Tumor (OTST) Registry (www.OTSTregistry.org). Patient A, her mother, and four siblings were found to have a germline heterozygous pathogenic DICER1 mutation, c.5441C>T in exon 25, resulting in an amino acid change from p.Ser1814Leu of the DICER1 protein (Table 1).
Table 1.
Pathological Diagnoses and DICER1 Mutation Analyses in Family With Multiple DTCs
| Patient | Pathological Diagnoses | Germline DICER1 Mutation (NM_177438.2) | Somatic DICER1 Mutation in Thyroid (NM_177438.2) | Somatic DICER1 Amino Acid Change | Tissue Diagnosis on Block Selected for Sequencing | Mutant Allele Frequency (No. of Reads) | % “Tumor” in Block Sampled (Crude Visual Estimate of Surface Area Comprised by Tumor) |
|---|---|---|---|---|---|---|---|
| Mother | DTC, SLCT | c.5441C>T | No pathological sample | NA | NA | NA | NA |
| Sister 1 (patient A) | DTC, MNG, SLCT, cystic nephroma | c.5441C>T | c.5126A>G | p.Asp1709Gly | Papillary CA arising in follicular nodule | 19.80% (20/101) | Nodule with papillary CA = 25% of surface area (Fig. 1, C and D) |
| Sister 2 (patient B) | DTC, MNG | c.5441C>T | c.5425G>A | p.Gly1809Arg | Papillary CA arising in follicular nodule | 36.50% (729/1998) | Nodule with papillary CA = 40% of surface area (Fig. 1, A and B) |
| Sister 3 | MNG | c.5441C>T | No hotspot variants | NA | Encapsulated follicular nodule with necrosis | NA | Nodule with necrotic/degenerative change = 40% of surface area |
| Sister 4 | MNG (US) | c.5441C>T | No pathological sample | NA | NA | NA | NA |
| Brother | DTC | c.5441C>T | c.5126A>G | p.Asp1709Gly | Left lobe follicular nodule with papillary hyperplasia and focal papillary CA | 33.70% (34/101) | Nodule = 40% of surface area with papillary CA 1% (Fig. 1, E and F) |
| MNG | c.5428G>C | p.Asp1810His | Right lobe follicular nodule with papillary hyperplasia and focal papillary CA | 30.30% (590/1946) | Nodule = 40% of surface area with papillary CA 5% |
Abbreviations: CA, carcinoma; NA, not applicable.
Pathology findings
Each of the thyroid specimens from the four siblings showed nodular hyperplasia, with multiple discrete, well-circumscribed, and occasionally encapsulated hyperplastic nodules. Thyroid glands in three of four siblings also showed distinct foci of papillary carcinoma developing within encapsulated follicular nodules (Figure 1). No extrathyroidal extension, infiltrative growth, or vascular invasion was identified. Molecular testing for BRAF, NRAS61, HRAS61, and KRAS12/13 mutations and RET/PTC1 and RET/PTC3 rearrangements was negative on tumor tissue from patients A and B.
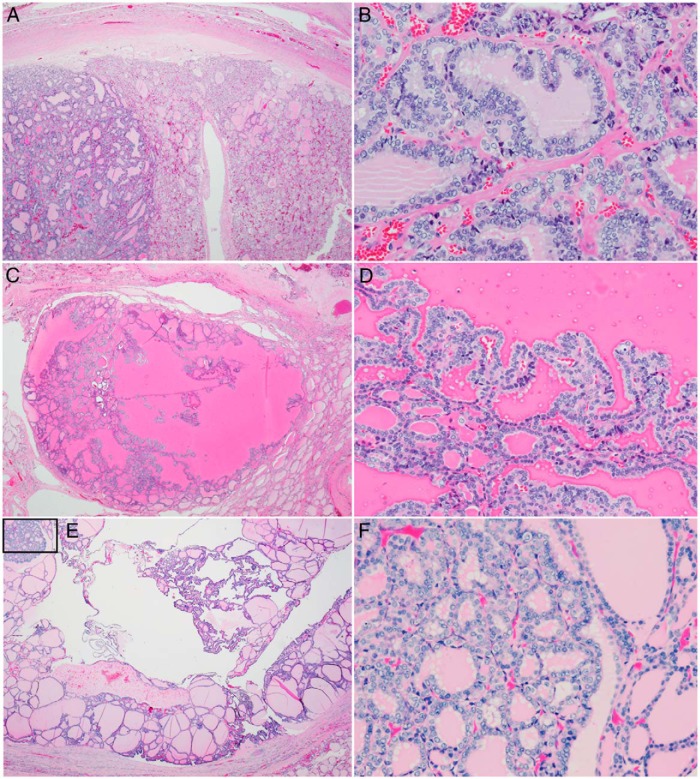
Figure 1.
Pathological features of papillary carcinoma arising in follicular nodules in three siblings with germline DICER1 mutations. A, Low-power view of a 1.3-cm encapsulated follicular nodule in patient B. Note the apparent clonal outgrowth in the left third of the photomicrograph. B, High-power view of this area shows cytological features of papillary carcinoma. C, Low-power view of a section from a 1.6-cm partially encapsulated cystic nodule from patient A. D, Medium-power view shows papillae with overlapping nuclei, nuclear clearing, grooves, and rare pseudoinclusions. E, The brother of these two siblings had two small foci of papillary carcinoma within larger encapsulated follicular nodules with cystic change and papillary hyperplasia. A low-power view of a left lobe follicular nodule with small focus of papillary carcinoma (box) is shown in panel E. F, High-power view of papillary carcinoma shown in panel E.
By report, the mother's thyroid gland showed a 1.5-cm encapsulated, minimally invasive follicular thyroid carcinoma that had a solid and microglandular growth pattern, numerous mitotic figures, and multiple areas of capsular invasion without extracapsular extension.
DICER1 mutation testing was performed on formalin-fixed, paraffin-embedded thyroid tissue using next generation sequencing methods previously described (9). Somatic RNase IIIb missense “hotspot” mutations typical of DICER1 syndrome were identified in three of four patient samples containing discrete thyroid nodules (Table 1).
Discussion
DICER1 syndrome has an emerging phenotype. However, whereas early indications suggest that the incidence of MNG is high (1, 2, 5), actual incidence data are lacking, and there is insufficient data to define the risk of developing DTC. This is a novel report of DTC in a family with a confirmed germline DICER1 mutation, but without PPB, prior history of exposure to chemotherapy, or other known risk factors for developing DTC.
To our knowledge, this predisposing missense mutation NM177438.2:c.5441C>T; Ser1814Leu has not been described previously in other individuals, including 120 individuals with germline DICER1 mutations in our PPB and OTST cohorts, 82 individuals in the Leiden open variation database (https://grenada.lumc.nl/LOVD2/mendelian_genes/home.php?select_db=DICER1), or the broader mutation databases dbSNP (http://www.ncbi.nlm.nih.gov/SNP/) or 1000 genomes (http://www.1000genomes.org/). Although no functional testing was performed, the germline missense mutation described in these family members is predicted to be deleterious based on several factors. It is unlikely that a polar amino acid can be replaced with a nonpolar amino acid without consequence, particularly the critically important RNase IIIb cleavage domain of the DICER1 protein. The SIFT algorithm also predicts this p.Ser1814Leu in the RNase IIIb domain to be deleterious (SIFT weight = 0) (10). The 1814 Serine is conserved all the way back to Caenorhabditis elegans. Finally, this mutation segregates with disease in six first-degree relatives. Pathogenic germline missense variants are somewhat unusual in DICER1 syndrome. In our recent publication, we describe only five individuals with germline deleterious missense mutations compared to 33 individuals with stop codons, 44 individuals with small indels with frameshift, seven splice site mutations, and one exon deletion (11).
Sequencing of tissue obtained from selected blocks of thyroid tissue from four siblings showed somatic RNase IIIb DICER1 missense mutations in each of the three children with carcinoma arising within a follicular nodule. Each of these somatic missense mutations involved one of the five amino acid “hotspots” in the RNase IIIb domains, suggesting that thyroid disease in DICER1 patients has a similar genetic pathogenesis to other DICER1 syndrome tumors, namely PPB, SLCT, cystic nephroma, and nasal chondromesenchymal hamartoma (4, 8, 9, 12). However, many questions regarding the stepwise pathogenesis from normal thyroid to follicular neoplasia to papillary cancer remain and cannot be answered by this mutation analysis alone. None of these cancers showed features of aggressive biological behavior. No lymphatic spread or invasion beyond the thyroid gland was seen. The presence of DICER1 hotspot mutations in a subset of these thyroid nodules provides further evidence that development of these nodules and subsequently differentiated cancer can be linked to genetics, as opposed to DNA damage related to prior chemotherapy, as has been suggested in previous observations (8).
Guidelines for baseline and routine surveillance by thyroid US screening in patients with germline DICER1 mutations have not been defined. Recommendations from the International PPB and OTST Registries suggest that thyroid physical examination should be performed annually (4). Thyroid US is recommended if thyroid gland asymmetry and/or a nodule is detected on physical examination, or if the patient has previously received, or is anticipated to receive, chemotherapy or repeated upper-body radiological imaging for PPB or other DICER1-related malignancy (4). A thyroid US could be repeated every 3 to 5 years if no nodule is detected (4). Our findings suggest that patients with DICER1 mutations are at increased risk of not only MNG, but also DTC, even in the absence of chemotherapy or radiation, and screening with both physical examination and US should be carefully considered. DICER1 families who have a first-degree relative with DTC may warrant more frequent screening. Because the relative risk for development of DTC in a nodule of a DICER1 carrier is unknown, nodules confirmed by US should be managed in accordance with guidelines from the American Thyroid Association (www.thyroid.org) or other organizations.
Additional case collection, with enrollment in patient registries and natural history studies (www.PPBregistry.org, www.OTSTregistry.org, and www.ppb.cancer.gov), is needed to better understand the frequency and prognosis of DTC in DICER1 syndrome. At present, patients with germline DICER1 mutations appear to have an increased risk of developing DTC at a young age, even in the absence of exposure to chemotherapy.
Acknowledgments
We thank Dr Yuri Nikiforov at the University of Pittsburgh Medical Center for reviewing the thyroid pathology slides of patient A and three siblings; Dr Kathryn Wikenheiser-Brokamp and the Department of Pathology at CCHMC for preparing and shipping the thyroid specimens for mutation testing; and Scott Harding in the Department of Pathology at Children's National Medical Center for performing the mutation testing on the thyroid specimens. We also thank the family described in this report for their consent to publish this manuscript and for their support of DICER1-related research. In addition, we are grateful to the many patients, families, genetic counselors, and treating physicians who participate in the International PPB Registry and International Ovarian and Testicular Stromal Tumor Registry, and to the Pine Tree Apple Tennis Classic and St Baldrick's Foundation for their ongoing support of rare tumor research.
This work is supported by National Institutes of Health Grant NCI R01CA143167 (to D.A.H.). The International Ovarian and Testicular Stromal Tumor Registry is supported by St Baldrick's Foundation, the Pine Tree Apple Tennis Classic, Hyundai Hope on Wheels, and the Randy Shaver Community Cancer Fund.
Disclosure Summary: The authors each state that they have no conflicts of interest to disclose.
Footnotes
- DTC
- differentiated thyroid carcinoma
- MNG
- multinodular goiter
- PPB
- pleuropulmonary blastoma
- RNase
- ribonuclease
- SLCT
- Sertoli-Leydig cell tumor
- US
- ultrasound.
References
- 1. Rio Frio T, Bahubeshi A, Kanellopoulou C, et al. DICER1 mutations in familial multinodular goiter with and without ovarian Sertoli-Leydig cell tumors. JAMA. 2011;305:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Slade I, Bacchelli C, Davies H, et al. DICER1 syndrome: clarifying the diagnosis, clinical features and management implications of a pleiotropic tumour predisposition syndrome. J Med Genet. 2011;48:273–278. [DOI] [PubMed] [Google Scholar]
- 3. Hill DA, Ivanovich J, Priest JR, et al. DICER1 mutations in familial pleuropulmonary blastoma. Science. 2009;325:965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Doros L, Schultz KA, Stewart DR, et al. DICER1-related disorders. In: Pagon RA, Adam MP, Ardinger HH, eds. GeneReviews. http://www.ncbi.nlm.nih.gov/books/NBK196157/. Published April 24, 2014. [Google Scholar]
- 5. Rath SR, Bartley A, Charles A, et al. Multinodular goiter in children: an important pointer to a germline DICER1 mutation. J Clin Endocrinol Metab. 2014;99:1947–1948. [DOI] [PubMed] [Google Scholar]
- 6. Rome A, Gentet JC, Coze C, André N. Pediatric thyroid cancer arising as a fourth cancer in a child with pleuropulmonary blastoma. Pediatr Blood Cancer. 2008;50:1081. [DOI] [PubMed] [Google Scholar]
- 7. Oue T, Inoue M, Kubota A, Kuwae Y, Kawa K. Pediatric thyroid cancer arising after treatment for pleuropulmonary blastoma. Pediatr Blood Cancer. 2008;50:901–902. [DOI] [PubMed] [Google Scholar]
- 8. de Kock L, Sabbaghian N, Soglio DB, et al. Exploring the association between DICER1 mutations and differentiated thyroid carcinoma. J Clin Endocrinol Metab. 2014;99:E1072–E1077. [DOI] [PubMed] [Google Scholar]
- 9. Pugh TJ, Yu W, Yang J, et al. Exome sequencing of pleuropulmonary blastoma reveals frequent biallelic loss of TP53 and two hits in DICER1 resulting in retention of 5p-derived miRNA hairpin loop sequences. Oncogene. 2014;33:5295–5302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sim NL, Kumar P, Hu J, Henikoff S, Schneider G, Ng PC. SIFT web server: predicting effects of amino acid substitutions on proteins. Nucleic Acids Res. 2012;40:W452–W457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brenneman M, Field A, Yang J, et al. Temporal order of RNase IIIb and loss-of-function mutations during development determines phenotype in DICER1 syndrome: a unique variant of the two-hit tumor suppression model [version 1; referees: 2 approved with reservations]. F1000 Research. 2015;4:214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heravi-Moussavi A, Anglesio MS, Cheng SW, et al. Recurrent somatic DICER1 mutations in nonepithelial ovarian cancers. N Engl J Med. 2012;366:234–242. [DOI] [PubMed] [Google Scholar]