Abstract
Context:
Dietary omega-3 fatty acids delay ovarian aging and promote oocyte quality in mice.
Objective:
To test whether dietary supplementation with omega-3 polyunsaturated fatty acids (PUFA) modulates reproductive hormones in reproductive-age women.
Design:
Prospective interventional study.
Setting:
Academic center.
Participants:
Fifteen obese and 12 normal-weight (NW) eumenorrheic women, ages 28–34 years.
Intervention:
Two frequent blood-sampling studies were performed before and after 1 month of omega-3 PUFA supplementation with 4 g of eicosapentaenoic acid and docosahexaenoic acid daily.
Main Outcome Measures:
Serum LH and FSH (basal and after GnRH stimulation).
Results:
The ratio of omega-6 to omega-3 PUFA was significantly reduced in plasma and red blood cell components for both groups after treatment (both P < .01). Omega-3 PUFA supplementation resulted in reduction of FSH and FSH response to GnRH by 17% on average (P = .06 and P = .03, respectively) in NW but not obese women. Serum levels of IL-1β and TNF-α were reduced after omega-3 PUFA supplementation (−72% for IL-1β; −56% for TNF-α; both, P < .05) in obese but not in NW women. This reduction, however, was not associated with a hormonal change in obese women.
Conclusions:
Dietary administration with omega-3 PUFA decreased serum FSH levels in NW but not in obese women with normal ovarian reserve. This effect is intriguing and is directionally consistent with murine data whereby higher dietary omega-3 PUFA extends reproductive lifespan. Our results imply that this nutritional intervention should be tested in women with diminished ovarian reserve in an attempt to delay ovarian aging.
The United States has the highest prevalence of obesity among all countries surveyed in 2012 by the Organization for Economic Cooperation and Development (1). It is estimated that by the end of 2015, 41% of US adults will be obese (2), as defined by a body mass index (BMI) > 30 kg/m2. In women, reproductive morbidity associated with obesity is considerable and involves anovulation, menstrual cycle abnormalities, subfertility, fetal loss, obstetrical complications, and congenital anomalies (3). Obesity has been associated with a state of relative hypogonadotropic hypogonadism, as was shown by animal models (4) and clinical studies in both women and men (5, 6). A gap in knowledge exists because the mechanisms underlying these harmful effects are poorly understood, and no specific treatments exist.
Increasing prevalence of obesity in recent decades has been preceded by dramatic dietary changes in industrialized societies. Over the past 100 years, there has been a considerable shift in the human diet, particularly with respect to the amount and type of consumed fat. Intake of omega-3 polyunsaturated fatty acids (PUFAs), previously consumed in large quantities by humans from vegetable and fish sources, has dwindled (7). The contemporary ratio of dietary omega-6 to omega-3 PUFA is estimated to be as high as 25:1, a drastic change from the 1:1 ratio for previously estimated consumption (8). Dietary fat can have inflammatory or anti-inflammatory properties based upon its composition. Saturated fats increase plasma free fatty acids and promote inflammation, whereas omega-3 PUFAs reduce macrophage-induced cytokine production (9). Recent studies link improved embryo morphology in women (10) and superior oocyte quality in mice (11) with higher dietary intake of or supplementation with omega-3 PUFA. In animal studies, diets enriched with omega-3 PUFA enhance early embryonic development (12) and boost progesterone secretion (13), suggesting that sex steroid metabolism may be affected by modulating PUFA intake. Despite accumulating evidence of potential benefit in animal studies, the impact of omega-3 PUFA supplementation on reproductive hormones is unknown. We sought to test whether dietary supplementation with omega-3 PUFA affects reproductive hormones in women and/or whether this effect is modulated by body mass.
Subjects and Methods
Participants
Twenty-seven regularly menstruating obese and normal-weight (NW) women were recruited from the community through campus-wide advertisement and completed the study. The study was approved by Colorado Multiple Institutional Review Board (13-1420); a signed informed consent was obtained from each participant before participation. Study criteria included: 1) age, 18–42 years; 2) BMI, ≥30 kg/m2 (obese) or 18–25 kg/m2 (NW); 3) history of regular menses every 25–40 days; and 4) normal baseline prolactin, TSH, and blood count. Polycystic ovary syndrome was prospectively ruled out because all participants were required to have regular menstrual cycles and were nonhirsute; we used the National Institutes of Health definition of polycystic ovary syndrome, which includes oligomenorrhea as a central criterion. Participants were excluded if they had allergies to seafood, used medications known to affect reproductive hormones, used exogenous sex steroids within the last 3 months, exercised vigorously more than 4 hours weekly, or were attempting pregnancy. All participants had a baseline physical examination by study personnel and underwent all blood sampling at the Clinical and Translational Research Center of the Colorado Clinical and Translational Sciences Institute. Pregnancy was excluded by a serum pregnancy test at the beginning of each month of testing.
Protocol
All women underwent 8 hours of blood sampling every 10 minutes both at baseline and after approximately 1 month of dietary long chain omega-3 PUFA supplementation (Supplemental Figure 1). Each frequent blood sampling study was completed during the early follicular phase of the menstrual cycle (between d 3 and 6) and included iv administration of a physiological, weight-based (75 ng/kg) bolus of GnRH (Lutrelef; Ferring) given at 6 hours (14). Each participant therefore had a 6-hour window to assess endogenous LH and FSH secretion before the administration of exogenous GnRH.
Starting with day 1 of the next menstrual cycle after baseline, study participants were instructed to take 4 g daily of Lovaza (GlaxoSmithKline). This formulation represents a concentrated and purified PUFA preparation and is approved by the US Food and Drug Administration for treatment of hypertriglyceridemia (15–17). One gram of Lovaza contains approximately 465 mg of eicosapentaenoic acid (EPA) and approximately 375 mg of docosahexaenoic acid (DHA) as ethyl esters. The supplementation continued for two menstrual cycles, and testing started while participants were on their second cycle of the study. On day 1 of the third menses, participants were scheduled for postintervention studies, which were completed with an identical frequent blood sampling session as in month 1. Additionally, daily urinary samples were collected before and after omega-3 supplementation for measurement of estrogen and progesterone metabolites, and this will be the subject of a separate report.
Hormone assays
Serum LH and FSH were measured using a solid-phase, two-site specific immunofluorometric assay (DELFIA; Perkin Elmer) (18). Interassay and intra-assay coefficients of variation were 4.8 and 3.4% for LH, respectively, and 6.6 and 5.0% for FSH, respectively. Ligand Assay and Analysis Core services (University of Virginia) were used to assay inhibin B and anti-Müllerian hormone (AMH) using commercial two-site ELISA assays (Beckman Coulter). Functional sensitivity was determined for each assay (defined as the lowest concentration reliably measured, or the lowest concentration with an interassay coefficient of variation <20%). Functional sensitivity is 10 pg/mL for inhibin B and 0.16 ng/mL for AMH. The intra-assay and interassay coefficients of variation were 4.4 and 5.2%, respectively, for inhibin B, and 3.0 and 7.0%, respectively, for AMH.
Fatty acid composition
Plasma phospholipid and red blood cell (RBC) PUFA composition assessment were obtained for all subjects at baseline and after dietary supplementation. Phospholipid PUFA composition analysis is a validated assessment of dietary PUFA intake over a 2- to 3-week period. Whole blood samples were separated, and plasma was frozen and stored at −80°C for later analysis. Plasma and RBC phospholipids were extracted in methanol and directly methylated with sodium methoxide (25% w/v) using the method of Glaser et al (19). Fatty acid methyl esters (FAMEs) were extracted in hexanes and separated using microcapillary gas liquid chromatography with flame ionization detection. Individual FAMEs were identified by comparison of retention times with known FAME standards. Plasma or RBC PUFA composition was expressed as a percentage of total PUFA.
Cytokines
Serum proinflammatory cytokine levels were determined using a custom-made 10 cytokines array kit (RayBiotech) per the manufacturer's instructions (20). For each membrane, 1 mL of serum from each participant was diluted 1:4 with a blocking buffer and added to the membrane. After incubation and the addition of antibodies, the array was exposed to x-ray film, and signals were detected using a film developer. These images were scanned, and average intensities were measured using Quantity One software (Bio-Rad) after locating signals to antibody array map.
Statistical analysis
The primary outcome measure was the change in the average LH pulse amplitude before and after administration of omega-3 PUFA. With 10 subjects per group, we had 80% power to detect a change of 0.3 IU/L (or a 37.5% reduction in LH pulse amplitude) using a paired t test with unequal variances assuming a moderate correlation of 0.5 between the baseline and follow-up outcomes on the same subject. This change is approximately 40% of the difference between the LH pulse amplitudes of NW and obese patients in cross-sectional studies (18).
For each study period, LH pulsatility was characterized using the first 6 hours of data before GnRH administration. LH pulse frequency and amplitude were computed for each individual before and after omega-3 PUFA using a modified objective pulse detection method that has been previously validated (18, 21). After GnRH administration, for both LH and FSH, mean serum level, peak level, area under the curve (AUC), and maximum response (the arithmetic difference between the nadir before GnRH and the peak post GnRH) were evaluated. Baseline characteristics were compared between obese and NW women for all gonadotropin and PUFA compositions using two-sample t tests, whereas cytokine measures were compared using the Mann-Whitney test due to skewness. A two-sample t test was used to assess whether average change in hormonal and demographic measures differed between obese and NW women. Paired t and Wilcoxon tests were used to assess whether the assessed parameters differed before vs after the omega-3 PUFA intervention. A P < .05 value was used to denote statistical significance. The analysis was repeated on change in each of the other measures listed above. Age adjustment was performed to determine whether a difference in age between NW and obese subjects might have biased the observed associations of the hormonal outcome measures. For all tested comparisons, the association of the reported LH and FSH parameters remained similar, and no changes in the level of significance were detected. When analyzing proinflammatory cytokine levels, no adjustment for multiple comparisons was performed given the exploratory nature of this analysis. All statistical calculations were performed using R software for Windows, version 3.1.2.
Results
Biometric and endocrine characteristics
A total of 27 women (15 obese and 12 NW controls) provided complete data for analysis (Table 1). Although obese women were older (P < .01), there was no significant difference by the main clinical measure of ovarian reserve, serum AMH. All measures of glucose metabolism/insulin sensitivity (fasting glucose, fasting insulin, and homeostatic model assessment [HOMA]) were significantly elevated in the obese group. Inhibin B was significantly lower in the obese women (22% lower on average; P = .049). Omega-3 treatment did not significantly change any ovarian reserve parameters in either group (Tables 2 and 3). All three measures of glucose metabolism decreased in obese women after treatment with omega-3 PUFA, but this improvement was not statistically significant.
Table 1.
Biometric and Endocrine Characteristics in NW and Obese Women at Baseline
| NW Women | Obese Women | Pa | |
|---|---|---|---|
| N | 12 | 15 | |
| Age, y | 28.4 (1.2) | 34.8 (1.2) | <.01 |
| BMI, kg/m2 | 21.8 (0.5) | 37.8 (1.5) | <.01 |
| Weight, kg | 59.3 (1.9) | 103.8 (6.6) | <.01 |
| Waist circumference, cm | 73.6 (2.5) | 109.1 (2.7) | <.01 |
| Waist-hip ratio | 0.89 (0.02) | 0.90 (0.01) | .43 |
| AMH, ng/mL | 5.3 (0.9) | 4.2 (0.7) | .33 |
| Inhibin B, pg/mL | 78.6 (7.2) | 61.3 (4.8) | .0495 |
| Fasting glucose, mg/dL | 79.6 (5.5) | 90.7 (1.9) | .02b |
| Fasting insulin, mIU/L | 8.8 (1.0) | 24.2 (7.7) | <.01b |
| HOMA-IR scorec | 1.7 (0.2) | 5.5 (1.8) | <.01b |
| Plasma N6/N3 ratio | 7.7 (0.8) | 8.6 (0.4) | .36 |
| RBC N6/N3 ratio | 5.1 (0.4) | 5.4 (0.2) | .51 |
| Cycle 1 length, d | 27.3 (0.5) | 27.6 (0.9) | .70 |
| Cycle 2 length, d | 28.8 (0.9) | 28.5 (1.2) | .84 |
| Cycle 3 length, d | 28.8 (0.9) | 28.1 (1.0) | .60 |
Abbreviations: HOMA-IR, HOMA for insulin resistance; N6/N3, omega-6/omega-3. Values represent mean (standard error of the mean).
P values represent two-sample t test, unless otherwise specified.
P values represent Mann-Whitney test.
HOMA-IR score is a measure of insulin sensitivity, calculated as follows: fasting insulin × fasting glucose/405.
Table 2.
Effect of Omega-3 PUFA Supplementation in NW Women on Reproductive and Metabolic Hormones (n = 12)
| Baseline | After Omega-3 | Δa | Pb | |
|---|---|---|---|---|
| Unstimulated | ||||
| LH | ||||
| Mean level, IU/L | 4.4 (0.6) | 4.1 (0.4) | −0.2 (0.7) | .77 |
| No. of pulses/h | 2.6 (0.3) | 2.8 (0.4) | 0.3 (0.5) | .64 |
| Average pulse amplitude, IU/L | 1.6 (0.2) | 2.0 (0.4) | 0.4 (0.4) | .39 |
| FSH | ||||
| Mean level, IU/L | 4.8 (0.3) | 4.0 (0.3) | −0.8 (0.4) | .06 |
| Peak, IU/L | 5.5 (0.4) | 4.6 (0.4) | −0.9 (0.4) | .06 |
| GnRH-stimulated | ||||
| LH | ||||
| Mean level IU/L | 6.5 (0.6) | 6.5 (0.6) | 0.04 (0.5) | .95 |
| AUC, IU/Lc | 790 (76) | 795 (74) | 4.6 (97) | .96 |
| Peak, IU/L | 9.2 (0.9) | 9.0 (0.9) | −0.2 (1.0) | .84 |
| Maximum response, IU/Ld | 6.1 (0.6) | 6.1 (0.9) | 0.03 (0.7) | .96 |
| FSH | ||||
| Mean level, IU/L | 5.2 (0.4) | 4.3 (0.3) | −0.9 (0.4) | .03 |
| AUC, IU/Lc | 626 (42) | 518 (38) | −109 (45) | .03 |
| Peak, IU/L | 6.0 (0.4) | 4.9 (0.4) | −1.2 (0.4) | .02 |
| Maximum response, IU/Ld | 1.8 (0.2) | 1.4 (0.1) | −0.4 (0.2) | .09 |
| AMH, ng/mL | 5.3 (0.9) | 4.7 (0.8) | −0.6 (0.7) | .36 |
| Inhibin B, pg/mL | 78.6 (7.2) | 76.5 (8.7) | −2.2 (11.3) | .85 |
| Fasting glucose, mg/dL | 79.6 (5.5) | 83.1 (2.2) | 3.5 (5.3) | .87 |
| Fasting insulin, mIU/L | 8.8 (1.0) | 9.2 (1.0) | 0.33 (1.0) | .69 |
| HOMA-IR score | 1.7 (0.2) | 1.9 (0.2) | 0.2 (0.2) | .39 |
| Plasma N6/N3 ratio | 7.7 (0.8) | 3.2 (0.4) | −4.5 (0.7) | <.01 |
| RBC N6/N3 ratio | 5.1 (0.4) | 3.0 (0.2) | −2.0 (0.3) | <.01 |
Abbreviations: HOMA-IR, HOMA for insulin resistance; N6/N3, omega-6/omega-3. Values represent mean (standard error of the mean).
Arithmetic difference between post omega-3 supplement and baseline.
P value for comparing values before and after omega-3 supplement in each group.
AUC is the sum of concentrations for 120 min (GnRH-stimulated) calculated using the trapezoidal rule.
Maximum response to GnRH is defined as the peak level after GnRH bolus minus the lowest value in the hour preceding GnRH.
Table 3.
Effect of Omega-3 PUFA Supplementation in Obese Women on Reproductive and Metabolic Hormones (n = 15)
| Baseline | After Omega-3 | Δa | Pb | |
|---|---|---|---|---|
| Unstimulated | ||||
| LH | ||||
| Mean level, IU/L | 3.3 (0.3) | 3.3 (0.3) | 0.07 (0.3) | .84 |
| No. of pulses/h | 3.1 (0.4) | 2.8 (0.3) | −0.3 (0.6) | .64 |
| Average pulse amplitude, IU/L | 1.3 (0.1) | 1.3 (0.1) | −0.04 (0.2) | .84 |
| FSH | ||||
| Mean level, IU/L | 4.4 (0.4) | 4.2 (0.2) | −0.2 (0.3) | .51 |
| Peak, IU/L | 5.1 (0.4) | 5.0 (0.3) | −0.1 (0.3) | .67 |
| GnRH-stimulated | ||||
| LH | ||||
| Mean level | ||||
| Mean level, IU/L | 6.6 (1.0) | 6.7 (1.2) | 0.2 (0.6) | .82 |
| AUC, IU/Lc | 821 (121) | 836 (150) | 14.7 (79) | .86 |
| Peak, IU/L | 9.7 (1.6) | 9.7 (1.9) | −0.06 (1.0) | .95 |
| Maximum response, IU/Ld | 7.5 (1.3) | 7.3 (1.7) | −0.1 (0.9) | .88 |
| FSH | ||||
| Mean level, IU/L | 5.1 (0.4) | 4.9 (0.3) | −0.2 (0.4) | .71 |
| AUC, IU/Lc | 615 (51) | 598 (38) | −16.9 (48) | .73 |
| Peak, IU/L | 5.8 (0.5) | 5.6 (0.4) | −0.2 (0.4) | .61 |
| Maximum response, IU/Ld | 2.0 (0.2) | 2.0 (0.3) | −0.01 (0.2) | .95 |
| AMH, ng/mL | 4.2 (0.8) | 3.9 (0.9) | −0.3 (0.4) | .43 |
| Inhibin B, pg/mL | 61.3 (5.6) | 65.8 (8.4) | 4.5 (7.9) | .53 |
| Fasting glucose, mg/dL | 90.7 (2.1) | 89.4 (3.1) | −1.2 (2.0) | .50 |
| Fasting insulin, mIU/L | 24.2 (8.6) | 18.9 (3.1) | −5.3 (6.6) | .82 |
| HOMA-IR score | 5.5 (2.2) | 4.1 (0.7) | −1.4 (1.6) | .93 |
| Plasma N6/N3 ratio | 8.6 (0.4) | 3.9 (0.4) | −4.7 (0.7) | <.01 |
| RBC N6/N3 ratio | 5.4 (0.2) | 3.4 (0.2) | −1.9 (0.3) | <.01 |
Abbreviations: HOMA-IR, HOMA for insulin resistance; N6/N3, omega-6/omega-3. Values represent mean (standard error of the mean).
Arithmetic difference between post omega-3 supplement and baseline
P value for comparing values before and post omega-3 supplement treatment in each group
AUC is sum of concentrations for 120 min (GnRH-stimulated) calculated using trapezoidal rule.
Maximum response to GnRH is defined as the peak level after GnRH bolus minus the lowest value in the hour preceding GnRH.
Fatty acid composition
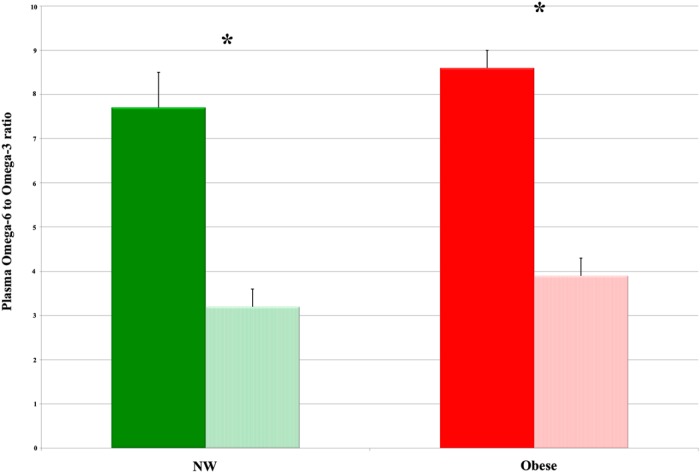
At baseline, the ratio of omega-6 to omega-3 PUFA was similar between the two groups in both plasma and RBC (P = .36 and P = .51, respectively; Table 1). After omega-3 PUFA supplementation, this ratio decreased significantly in both NW and obese women by 58 and 55%, respectively, in plasma (Figure 1); similarly, the ratio in the RBC component decreased by 40 and 36%, respectively (P < .01 for all; Tables 2 and 3). The increase in EPA and DHA (omega-3 PUFA that were directly supplemented) was the greatest. Plasma EPA increased by 557% in NW and by 443% in obese women, whereas plasma DHA increased by 79% in NW and by 130% in obese women (P < .01 for all). Two subjects in the obese group and one in the NW group did not have a measurable change in omega-6 to omega-3 PUFA ratio after supplementation. Of note, these three subjects had the highest and lowest BMI values for our cohort. The two morbidly obese participants had BMI values of 42.2 and 51.2 kg/m2, respectively, and the NW participant had a BMI of 18.9 kg/m2 (lowest BMI in the cohort). When the analysis was restricted to only those who had changes in the omega-6 to omega-3 ratio, similar results were seen (data not shown); thus, all data are presented using the intent-to-treat paradigm for all women who completed the study.
Figure 1.
Impact of omega-3 fatty acid supplementation on plasma omega-6 to omega-3 ratio. Levels in NW and obese women both at baseline (solid columns) and after 1 month of omega-3 supplementation (striped columns) are shown. Data represent mean ± standard error of the mean. *, P < .01.
Gonadotropins
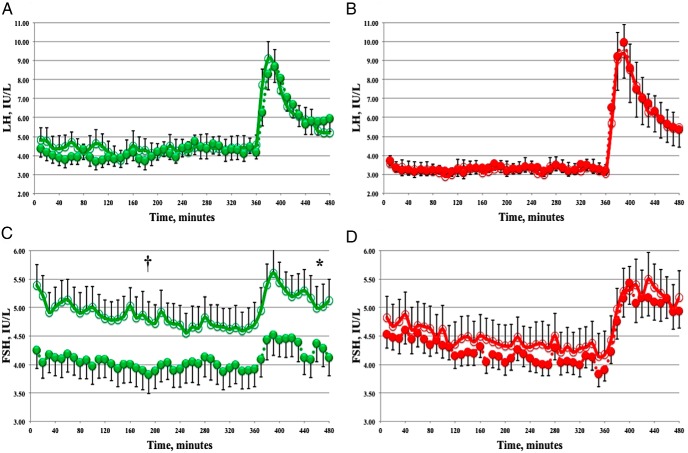
At baseline (month 1), during the first 6 hours of unstimulated testing, mean serum LH was lower but was not significantly different in obese women when compared to NW controls (3.3 ± 0.3 vs 4.4 ± 0.6 IU/L, respectively; P = .12). Similarly, no significant difference was observed for LH pulse amplitude (1.3 ± 0.1 vs 1.6 ± 0.2 IU/L, respectively; P = .28), LH pulse frequency (3.1 ± 0.4 vs 2.6 ± 0.3 pulses/h, respectively; P = .33), or GnRH-stimulated gonadotropin levels. Baseline (month 1) FSH parameters were not statistically different between obese and NW women before or after GnRH.
At month 3 in NW women, both FSH mean level and peak were decreased by 17% vs month 1 studies (4.8 ± 0.3 to 4.0 ± 0.3 IU/L, and 5.5 ± 0.4 to 4.6 ± 0.4 IU/L, respectively), with a trend to statistical significance (P = .06 for both; Table 2 and Figure 2C). In contrast, no observable change was seen in FSH parameters before and after dietary supplementation in obese women (Table 3 and Figure 2D). Upon GnRH stimulation, FSH response has significantly decreased in NW women after omega-3 PUFA treatment (17% for mean serum level, 17% for AUC, and 19% for peak; P < .05 for all; Table 2 and Figure 2C). Again, no significant changes were seen in FSH parameters for obese women (Table 3 and Figure 2D). LH response was not different before or after omega-3 PUFA treatment for either group (Tables 2 and 3 and Figure 2, A and B). This association of the reported LH and FSH parameters remained similar, and no changes in the level of significance were detected after adjusting for age.
Figure 2.
Effect of omega-3 PUFA supplementation on serum gonadotropins. LH (A and B) and FSH (C and D) during unstimulated and GnRH-stimulated portions of frequent blood sampling (GnRH was given after 360 min). Data are from NW women (green; n = 12) and obese women (red; n = 15) at month 1 (open circles) and month 3 (filled circles). Error bars indicate standard error of the mean for group composites. †, P = .06; *, P = .03.
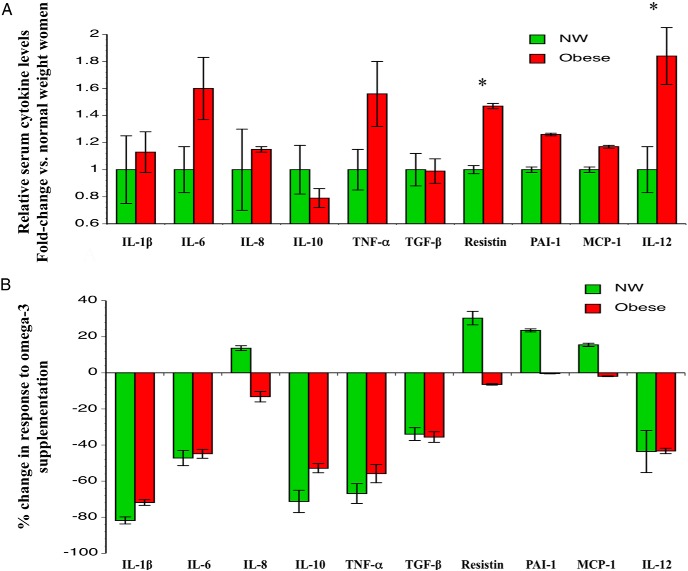
Proinflammatory cytokines
When examining inflammatory cytokines associated with obesity (20, 22), at baseline, two cytokines (resistin and IL-12) were significantly higher in obese women compared to NW controls (Figure 3A). When these measurements were repeated in month 3 of the study after a month of omega-3 supplementation (Figure 3B), all cytokines were decreased in the obese women, with a statistically significant drop in IL-1β and TNF- α (P < .05 for both). No significant changes were seen in cytokines before and after treatment in NW women, despite reductions in levels ranging from 34–82% for some cytokines.
Figure 3.
Impact of omega-3 PUFA supplementation on serum proinflammatory cytokines. A, Relative baseline cytokine levels. Data represent mean ± standard error of the mean at month 1. *, P < .05. B, Change in cytokine levels in response to treatment. Results are expressed as percentage change in cytokine levels after treatment in month 3.
Discussion
In this interventional study, we have shown that supplementing omega-3 PUFA for at least 4 weeks resulted in more than a 50% reduction in the endogenous omega-6 to omega-3 ratio, regardless of body mass. This effect was associated with an improvement in serum IL-1β and TNF-α levels, both proinflammatory cytokines, in obese women. The improvement in the omega-6 to omega-3 ratio in NW women was associated with a reduction of FSH levels, but there was no significant change in any measured gonadotropin parameter for obese women.
We have hypothesized that an adiposity-related proinflammatory environment might be responsible for the relative hypogonadotropic state associated with obesity. Obesity produces a unique inflammatory state that is low grade and chronic (20, 22, 23). TNF-α and IL-1β are known to suppress pituitary LH secretion in animal studies (24). Many studies have shown this possible association between inflammatory cytokines and suppression of the hypothalamic-pituitary-ovarian (HPO) axis (25–27). Total omega-3 PUFAs were observed to be independently associated with lower levels of proinflammatory markers (28, 29). Similar to our study, clinical studies have shown that supplying 4 g of EPA+DHA and using flaxseed oil rich in α-linolenic acid (omega-3 PUFA) in a dietary framework of an omega-6 to omega-3 ratio of 3:1 or 4:1 resulted in substantial inhibition to the production of IL-1β and TNF-α (30, 31). Despite the reduction that was achieved in both of these inflammatory cytokines in obese participants, our study did not reveal any significant changes in reproductive endocrinopathy of obesity after the dietary intervention. However, reducing inflammatory cytokines in obese women could theoretically improve ovarian response to gonadotropins, resulting in lower FSH and better quality oocytes. A large study of assisted reproduction demonstrated that inferior pregnancy rates in obese women are normalized when oocytes from NW donors are transferred into obese recipients (32). Taken together with the rodent data on abnormal oocyte function and structure in obesity by Moley and others (33, 34), this implies that impaired oocyte quality may mediate the link between obesity and reproductive impairment, pregnancy complications, and congenital anomalies. Furthermore, murine diet-induced obesity models demonstrate abnormal oocyte structure and function with a significant elevation in the inner mitochondrial membrane potential and increased mitochondrial respiratory chain activity (35). Thus, an alternative and possibly longer intervention may very well prove effective in improving ovarian responses in obese women and should continue to be studied.
Improvements in insulin sensitivity and glucose metabolism have been shown to improve reproductive function in animal models (36). Measures of glucose metabolism improved in this study, although they did not reach levels of statistical significance, and a larger sample size may be needed to show this effect in women. There is also evidence that insulin can modulate FSH and LH secretion from the pituitary and GnRH secretion (36); this should be the subject of future dedicated studies.
Reproductive aging in women is characterized by shortening of menstrual cycles (37), increased oocyte meiotic spindle abnormalities, and aneuploidy (38, 39), along with declining fertility (40). This phenotype is classically associated with elevated levels of serum FSH in murine studies (41) and in humans (42, 43). In an elegant study by Nehra et al (11), it was shown that consumption of a diet rich in omega-3 PUFA prolonged reproductive lifespan in mice over several generations. Notably, dramatic improvement in egg quality and ovarian follicle counts was achieved in the advanced maternal age murine model. To investigate a similar positive effect of an acute dietary treatment with omega-3 PUFA in women, FSH may be used in proxy to study ovarian reserve in women because circulating FSH levels are responsive to the endocrine input from the ovary (44). Thus, our study is the first human investigation, to the best of our knowledge, to test the impact of omega-3 PUFA supplementation on gonadotropins in women. Although serum AMH appears to be a better marker for ovarian reserve than serum FSH, it is also a less dynamic hormone with respect to changes over time and changes due to an intervention (45). Our observed changes in FSH were in concert with animal data (11), in a direction that may indicate a favorable effect on improving reproductive lifespan. This will need to be investigated in a longer duration of supplementation and by examining other markers (eg, ovarian follicle counts) to prove this effect. We have measured AMH before and after supplementation in each group, as reported in Tables 2 and 3. There was no significant difference in serum AHM after 1 month of supplementation used in our study. A future study of longer duration should investigate a potential impact over a longer period of time.
Aside from a possible ovarian effect that may explain our observed results, reduction of FSH response to GnRH suggests a pituitary response. In concert with this argument, animal studies suggest that deteriorated inflammatory milieu results in suppression of HPO hormones (25–27). However, we have observed a combination of baseline serum FSH level reduction along with the FSH response to GnRH, which may indicate a possible reflection of modulated ovarian feedback effect on the central components of the HPO axis.
Although our contention that reduction in serum FSH may be associated with prolonged reproductive lifespan in NW women appears to be supported by our detailed experiments, we acknowledge that there is no evidence that women with hypogonadotropic hypogonadism have a prolonged reproductive lifespan. However, multiple published studies have documented that hypophysectomized animals tend to have a longer reproductive lifespan (46), including studies attempting a “dietary hypophysectomy” (47), a phenomenon that is not fully understood but is directionally consistent with our results.
Our study was limited by the inability to evaluate oocyte markers. This would be advantageous because multiple studies demonstrate that impaired oocyte mitochondrial function and structure are markers of reproductive aging and improved in mouse models (11). This link is thought be partially due to oxidative stress because mouse models demonstrate dramatic abnormalities of inner mitochondrial membrane potential and aberrant mitochondrial respiratory chain activity associated with decreased fertility (48). Omega-3 PUFAs alleviate oxidative stress (49). Thus, the association of improved oocyte quality with modifications of dietary omega-3 PUFA is well supported by animal studies and laid the groundwork to investigate the impact of dietary omega-3 PUFAs in women. Our results suggest that omega-3 PUFAs were associated with consistent reduction of serum FSH in NW women. Elevated serum FSH is a well-known feature of ovarian aging. Thus, we posit that reduction of serum FSH with increased dietary omega-3 PUFA is teleologically, mechanistically, and directionally consistent with data in mice. We further posit that this line of reasoning supports a testable hypothesis that increased dietary omega-3 PUFAs are to be tested in women in an attempt to delay ovarian aging. The relatively modest sample size and the inability to evaluate oocyte markers imply that our study should be regarded as hypothesis generating: Does supplementation with dietary omega-3 PUFA result in delayed ovarian aging? Prospective dedicated studies are needed to test this hypothesis.
Acknowledgments
We acknowledge the University of Virginia, Center for Research in Reproduction Ligand Assay and Analysis Core for hormonal assays. We also gratefully acknowledge invaluable advice and guidance of Nanette Santoro, MD, for proposal preparation and planning. We also gratefully acknowledge invaluable assistance of Jacob Friedman, PhD and Becky de la Houssaye, MS with cytokine measurement and interpretation. GnRH was supplied by Ferring Pharmaceuticals.
This work was supported by the Clinical and Translational Sciences Centers at the University of Colorado School of Medicine 1UL1 RR025780 and by the Eunice Kennedy Shriver National Institute of Child Health and Human Development/National Institutes of Health (Specialized Cooperative Centers Program in Reproduction and Infertility Research; Grant to J.W.P., Pilot grant, PI: A.J.P., U54-HD058155).
The authors acknowledge support for this study from the Center for the Study of Reproductive Biology (Grant U54 HD058155; to A.J.P.). Grant providers had no role in the design, data collection, data analysis, data interpretation, or writing of the report.
ClinicalTrials.gov Identifier: NCT01894581.
Disclosure Summary: Z.A.A., H.L., N.E.C., J.C., M.H., A.P.B., C.R., R.H.E., and A.J.P. have nothing to disclose.
Footnotes
- AMH
- anti-Müllerian hormone
- AUC
- area under the curve
- BMI
- body mass index
- DHA
- docosahexaenoic acid
- EPA
- eicosapentaenoic acid
- FAME
- fatty acid methyl ester
- HOMA
- homeostatic model assessment
- HPO
- hypothalamic-pituitary-ovarian
- NW
- normal-weight
- PUFA
- polyunsaturated fatty acid
- RBC
- red blood cell.
References
- 1. Sassi F, Devaux M. Obesity Update 2012. Organization for Economic Cooperation and Development; http://www.oecd.org/health/49716427.pdf. [Google Scholar]
- 2. Wang Y, Beydoun MA. The obesity epidemic in the United States–gender, age, socioeconomic, racial/ethnic, and geographic characteristics: a systematic review and meta-regression analysis. Epidemiol Rev. 2007;29:6–28. [DOI] [PubMed] [Google Scholar]
- 3. Norman JE. The adverse effects of obesity on reproduction. Reproduction. 2010;140:343–345. [DOI] [PubMed] [Google Scholar]
- 4. Todd BJ, Ladyman SR, Grattan DR. Suppression of pulsatile luteinizing hormone secretion but not luteinizing hormone surge in leptin resistant obese Zucker rats. J Neuroendocrinol. 2003;15:61–68. [DOI] [PubMed] [Google Scholar]
- 5. Santoro N, Lasley B, McConnell D, et al. Body size and ethnicity are associated with menstrual cycle alterations in women in the early menopausal transition: The Study of Women's Health across the Nation (SWAN) Daily Hormone Study. J Clin Endocrinol Metab. 2004;89:2622–2631. [DOI] [PubMed] [Google Scholar]
- 6. Vermeulen A, Kaufman JM, Deslypere JP, Thomas G. Attenuated luteinizing hormone (LH) pulse amplitude but normal LH pulse frequency, and its relation to plasma androgens in hypogonadism of obese men. J Clin Endocrinol Metab. 1993;76:1140–1146. [DOI] [PubMed] [Google Scholar]
- 7. Simopoulos AP. Essential fatty acids in health and chronic disease. Am J Clin Nutr. 1999;70:560S–569S [DOI] [PubMed] [Google Scholar]
- 8. Simopoulos AP. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed Pharmacother. 2002;56:365–379. [DOI] [PubMed] [Google Scholar]
- 9. Machtinger R, Combelles CM, Missmer SA, Correia KF, Fox JH, Racowsky C. The association between severe obesity and characteristics of failed fertilized oocytes. Hum Reprod. 2012;27:3198–3207. [DOI] [PubMed] [Google Scholar]
- 10. Hammiche F, Vujkovic M, Wijburg W, et al. Increased preconception omega-3 polyunsaturated fatty acid intake improves embryo morphology. Fertil Steril. 2011;95:1820–1823. [DOI] [PubMed] [Google Scholar]
- 11. Nehra D, Le HD, Fallon EM, et al. Prolonging the female reproductive lifespan and improving egg quality with dietary omega-3 fatty acids. Aging Cell. 2012;11:1046–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Thangavelu G, Colazo MG, Ambrose DJ, Oba M, Okine EK, Dyck MK. Diets enriched in unsaturated fatty acids enhance early embryonic development in lactating Holstein cows. Theriogenology. 2007;68:949–957. [DOI] [PubMed] [Google Scholar]
- 13. Wonnacott KE, Kwong WY, Hughes J, et al. Dietary omega-3 and -6 polyunsaturated fatty acids affect the composition and development of sheep granulosa cells, oocytes and embryos. Reproduction. 2010;139:57–69. [DOI] [PubMed] [Google Scholar]
- 14. Martin K, Santoro N, Hall J, Filicori M, Wierman M, Crowley WF., Jr Clinical review 15: management of ovulatory disorders with pulsatile gonadotropin-releasing hormone. J Clin Endocrinol Metab. 1990;71:1081A–1081G. [DOI] [PubMed] [Google Scholar]
- 15. Davidson MH, Stein EA, Bays HE, et al. Efficacy and tolerability of adding prescription omega-3 fatty acids 4 g/d to simvastatin 40 mg/d in hypertriglyceridemic patients: an 8-week, randomized, double-blind, placebo-controlled study. Clin Ther. 2007;29:1354–1367. [DOI] [PubMed] [Google Scholar]
- 16. Bays HE, Maki KC, McKenney J, et al. Long-term up to 24-month efficacy and safety of concomitant prescription omega-3-acid ethyl esters and simvastatin in hypertriglyceridemic patients. Curr Med Res Opin. 2010;26:907–915. [DOI] [PubMed] [Google Scholar]
- 17. Bays HE, Tighe AP, Sadovsky R, Davidson MH. Prescription omega-3 fatty acids and their lipid effects: physiologic mechanisms of action and clinical implications. Expert Rev Cardiovasc Ther. 2008;6:391–409. [DOI] [PubMed] [Google Scholar]
- 18. Jain A, Polotsky AJ, Rochester D, et al. Pulsatile luteinizing hormone amplitude and progesterone metabolite excretion are reduced in obese women. J Clin Endocrinol Metab. 2007;92:2468–2473. [DOI] [PubMed] [Google Scholar]
- 19. Glaser C, Demmelmair H, Koletzko B. High-throughput analysis of fatty acid composition of plasma glycerophospholipids. J Lipid Res. 2010;51:216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Heerwagen MJ, Stewart MS, de la Houssaye BA, Janssen RC, Friedman JE. Transgenic increase in N-3/n-6 fatty acid ratio reduces maternal obesity-associated inflammation and limits adverse developmental programming in mice. PLoS One. 2013;8:e67791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rochester D, Jain A, Polotsky AJ, et al. Partial recovery of luteal function after bariatric surgery in obese women. Fertil Steril. 2009;92:1410–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gregor MF, Hotamisligil GS. Inflammatory mechanisms in obesity. Annu Rev Immunol. 2011;29:415–445. [DOI] [PubMed] [Google Scholar]
- 23. Bastard JP, Maachi M, Lagathu C, et al. Recent advances in the relationship between obesity, inflammation, and insulin resistance. Eur Cytokine Netw. 2006;17:4–12. [PubMed] [Google Scholar]
- 24. Rivier C, Vale W. Cytokines act within the brain to inhibit luteinizing hormone secretion and ovulation in the rat. Endocrinology. 1990;127:849–856. [DOI] [PubMed] [Google Scholar]
- 25. Breen KM, Thackray VG, Hsu T, Mak-McCully RA, Coss D, Mellon PL. Stress levels of glucocorticoids inhibit LHβ-subunit gene expression in gonadotrope cells. Mol Endocrinol. 2012;26:1716–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Turnbull AV, Rivier C. Inhibition of gonadotropin-induced testosterone secretion by the intracerebroventricular injection of interleukin-1 β in the male rat. Endocrinology. 1997;138:1008–1013. [DOI] [PubMed] [Google Scholar]
- 27. Ma XC, Chen LT, Oliver J, Horvath E, Phelps CP. Cytokine and adrenal axis responses to endotoxin. Brain Res. 2000;861:135–142. [DOI] [PubMed] [Google Scholar]
- 28. Ferrucci L, Cherubini A, Bandinelli S, et al. Relationship of plasma polyunsaturated fatty acids to circulating inflammatory markers. J Clin Endocrinol Metab. 2006;91:439–446. [DOI] [PubMed] [Google Scholar]
- 29. Zhao G, Etherton TD, Martin KR, Gillies PJ, West SG, Kris-Etherton PM. Dietary α-linolenic acid inhibits proinflammatory cytokine production by peripheral blood mononuclear cells in hypercholesterolemic subjects. Am J Clin Nutr. 2007;85:385–391. [DOI] [PubMed] [Google Scholar]
- 30. James MJ, Cleland LG. Dietary n-3 fatty acids and therapy for rheumatoid arthritis. Semin Arthritis Rheum. 1997;27:85–97. [DOI] [PubMed] [Google Scholar]
- 31. Broughton KS, Johnson CS, Pace BK, Liebman M, Kleppinger KM. Reduced asthma symptoms with n-3 fatty acid ingestion are related to 5-series leukotriene production. Am J Clin Nutr. 1997;65:1011–1017. [DOI] [PubMed] [Google Scholar]
- 32. Luke B, Brown MB, Missmer SA, Bukulmez O, Leach R, Stern JE. The effect of increasing obesity on the response to and outcome of assisted reproductive technology: a national study. Fertil Steril. 2011;96:820–825. [DOI] [PubMed] [Google Scholar]
- 33. Marquard KL, Stephens SM, Jungheim ES, et al. Polycystic ovary syndrome and maternal obesity affect oocyte size in in vitro fertilization/intracytoplasmic sperm injection cycles. Fertil Steril. 2011;95:2146–2149, 2149.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jungheim ES, Schoeller EL, Marquard KL, Louden ED, Schaffer JE, Moley KH. Diet-induced obesity model: abnormal oocytes and persistent growth abnormalities in the offspring. Endocrinology. 2010;151:4039–4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Igosheva N, Abramov AY, Poston L, et al. Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PLoS One. 2010;5:e10074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Louden ED, Luzzo KM, Jimenez PT, Chi T, Chi M, Moley KH. TallyHO obese female mice experience poor reproductive outcomes and abnormal blastocyst metabolism that is reversed by metformin. Reprod Fertil Dev. 2014;27:31–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lenton EA, Landgren BM, Sexton L, Harper R. Normal variation in the length of the follicular phase of the menstrual cycle: effect of chronological age. Br J Obstet Gynaecol. 1984;91:681–684. [DOI] [PubMed] [Google Scholar]
- 38. Kuliev A, Zlatopolsky Z, Kirillova I, Spivakova J, Cieslak Janzen J. Meiosis errors in over 20,000 oocytes studied in the practice of preimplantation aneuploidy testing. Reprod Biomed Online. 2011;22:2–8. [DOI] [PubMed] [Google Scholar]
- 39. Jones KT. Meiosis in oocytes: predisposition to aneuploidy and its increased incidence with age. Hum Reprod Update. 2008;14:143–158. [DOI] [PubMed] [Google Scholar]
- 40. Heffner LJ. Advanced maternal age–how old is too old? N Engl J Med. 2004;351:1927–1929. [DOI] [PubMed] [Google Scholar]
- 41. Bernstein LR, Mackenzie AC, Kraemer DC, et al. Shortened estrous cycle length, increased FSH levels, FSH variance, oocyte spindle aberrations, and early declining fertility in aging senescence-accelerated mouse prone-8 (SAMP8) mice: concomitant characteristics of human midlife female reproductive aging. Endocrinology. 2014;155:2287–2300. [DOI] [PubMed] [Google Scholar]
- 42. Klein NA, Battaglia DE, Fujimoto VY, Davis GS, Bremner WJ, Soules MR. Reproductive aging: accelerated ovarian follicular development associated with a monotropic follicle-stimulating hormone rise in normal older women. J Clin Endocrinol Metab. 1996;81:1038–1045. [DOI] [PubMed] [Google Scholar]
- 43. Lee SJ, Lenton EA, Sexton L, Cooke ID. The effect of age on the cyclical patterns of plasma LH, FSH, oestradiol and progesterone in women with regular menstrual cycles. Hum Reprod. 1988;3:851–855. [DOI] [PubMed] [Google Scholar]
- 44. Elvin JA, Matzuk MM. Mouse models of ovarian failure. Rev Reprod. 1998;3:183–195. [DOI] [PubMed] [Google Scholar]
- 45. Dewailly D, Andersen CY, Balen A, et al. The physiology and clinical utility of anti-Mullerian hormone in women. Hum Reprod Update. 2014;20:370–385. [DOI] [PubMed] [Google Scholar]
- 46. Jones EC, Krohn PL. The effect of hypophysectomy on age changes in the ovaries of mice. J Endocrinol. 1961;21:497–509. [DOI] [PubMed] [Google Scholar]
- 47. Nelson JF, Gosden RG, Felicio LS. Effect of dietary restriction on estrous cyclicity and follicular reserves in aging C57BL/6J mice. Biol Reprod. 1985;32:515–522. [DOI] [PubMed] [Google Scholar]
- 48. Ben-Meir A, Burstein E, Borrego-Alvarez A, et al. Coenzyme Q10 restores oocyte mitochondrial function and fertility during reproductive aging. Aging Cell. 2015;14:887–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wu A, Ying Z, Gomez-Pinilla F. Dietary omega-3 fatty acids normalize BDNF levels, reduce oxidative damage, and counteract learning disability after traumatic brain injury in rats. J Neurotrauma. 2004;21:1457–1467. [DOI] [PubMed] [Google Scholar]