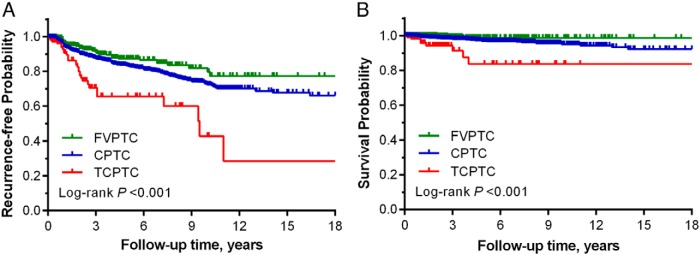
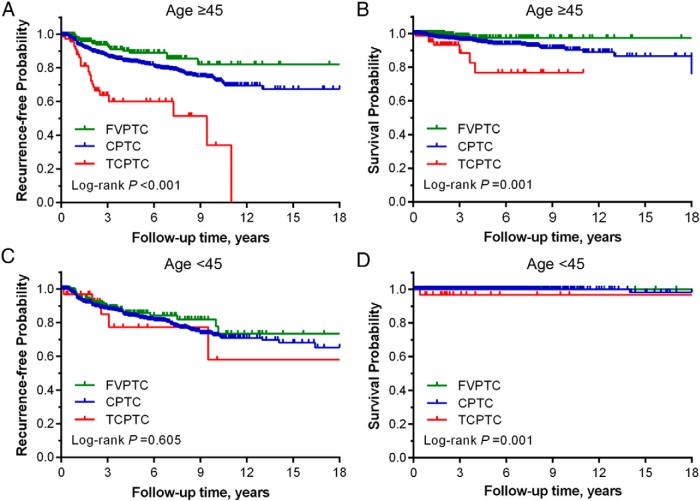
Xiaoguang Shi
Xiaoguang Shi
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,*,
Rengyun Liu
Rengyun Liu
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,*,
Fulvio Basolo
Fulvio Basolo
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
Riccardo Giannini
Riccardo Giannini
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
Xiaopei Shen
Xiaopei Shen
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
Di Teng
Di Teng
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
Haixia Guan
Haixia Guan
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
Zhongyan Shan
Zhongyan Shan
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
Weiping Teng
Weiping Teng
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
Thomas J Musholt
Thomas J Musholt
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
Khawla Al-Kuraya
Khawla Al-Kuraya
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
Laura Fugazzola
Laura Fugazzola
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
Carla Colombo
Carla Colombo
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
Electron Kebebew
Electron Kebebew
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
Barbara Jarzab
Barbara Jarzab
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
Agnieszka Czarniecka
Agnieszka Czarniecka
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
Bela Bendlova
Bela Bendlova
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
Vlasta Sykorova
Vlasta Sykorova
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
Manuel Sobrinho-Simões
Manuel Sobrinho-Simões
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
Paula Soares
Paula Soares
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
Young Kee Shong
Young Kee Shong
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
Tae Yong Kim
Tae Yong Kim
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
Sonia Cheng
Sonia Cheng
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
Sylvia L Asa
Sylvia L Asa
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
David Viola
David Viola
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
Rossella Elisei
Rossella Elisei
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
Linwah Yip
Linwah Yip
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
Caterina Mian
Caterina Mian
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
Federica Vianello
Federica Vianello
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
Yangang Wang
Yangang Wang
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
Shihua Zhao
Shihua Zhao
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
Gisele Oler
Gisele Oler
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
Janete M Cerutti
Janete M Cerutti
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
Efisio Puxeddu
Efisio Puxeddu
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
Shen Qu
Shen Qu
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
Qing Wei
Qing Wei
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
Huixiong Xu
Huixiong Xu
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
Christine J O'Neill
Christine J O'Neill
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
Mark S Sywak
Mark S Sywak
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
Roderick Clifton-Bligh
Roderick Clifton-Bligh
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
Alfred K Lam
Alfred K Lam
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
Garcilaso Riesco-Eizaguirre
Garcilaso Riesco-Eizaguirre
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
Pilar Santisteban
Pilar Santisteban
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
Hongyu Yu
Hongyu Yu
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
Giovanni Tallini
Giovanni Tallini
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
Elizabeth H Holt
Elizabeth H Holt
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
Vasily Vasko
Vasily Vasko
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,
Mingzhao Xing
Mingzhao Xing
1Laboratory for Cellular and Molecular Thyroid Research, Division of Endocrinology, Diabetes & Metabolism, Department of Medicine (X.Shi., R.L., X.Shen., D.T., M.X.), Johns Hopkins University School of Medicine, Baltimore, MD 21287; Department of Surgery, Division of Pathology (F.B., R.G.), 56126 Pisa, Italy; The Endocrine Institute and The Liaoning Provincial Key Laboratory of Endocrine Diseases, Department of Endocrinology and Metabolism (H.G., Z.S., W.T.), The First Hospital of China Medical University, Shenyang, Liaoning Province 110001, China; Endocrine Surgery (T.J.M.), University Medical Center, Johannes Gutenberg University Mainz, 55101 Mainz, Germany; Human Cancer Genomic Research (K.A.K.), Research Centre, King Faisal Specialist Hospital and Research Centre, Riyadh 12713, Kingdom of Saudi Arabia; Fondazione Instituto di Ricovero e Cura a Carattere Scientifico (IRCCS) Ca' Granda Policlinico, Milan, and Department of Pathophysiology and Transplantation (L.E., C.C.), University of Milan, 20122 Milan Italy; Endocrine Oncology Branch, Center for Cancer Research (E.K.), National Cancer Institute, National Institutes of Health, Bethesda, Maryland 20892; Maria Sklodowska-Curie Memorial Cancer Center and Institute of Oncology (A.C., B.J.), 44-101 Gliwice, Poland; Department of Molecular Endocrinology (B.B., V.S.), Institute of Endocrinology, Prague 11694, Czech Republic; Institute of Molecular Pathology and Immunology (Manuel Sobrinho-Simões, M.S.S.; Paula Soares, P.So.), University of Porto (Ipatimup) and Medical Faculty of the University of Porto, 4200-319 Porto, Portugal; College of Medicine (Y.K.S., T.Y.K.), University of Ulsan College of Medicine, Seoul, South Korea; Department of Pathology (S.C.; S.L.A.), University Health Network, Toronto, ON M5G 2C4 Canada; Endocrine Unit, Department of Clinical and Experimental Medicine (D.V., R.E.), World Health Organization, Collaborating Center for the Study and Treatment of Thyroid Diseases and Other Endocrine and Metabolic Disorders, University of Pisa, 56124 Pisa, Italy; University of Pittsburgh School of Medicine (L.Y.), Pittsburgh, Pennsylvania 15213; Department of Medicine, Endocrinology Unit (C.M.), University of Padua, Padua 35128, Italy; Veneto Institute of Oncology (F.V.), IRCCS, Padua 35128, Italy; Department of Endocrinology (Y.W., S.Z.), The Affiliated Hospital of Qingdao University, Qingdao 266003, China; Genetic Bases of Thyroid Tumor Laboratory, Division of Genetics (G.O., J.M.C.), Federal University of São Paulo, São Paulo 04039-032, Brazil; Department of Internal Medicine (E.P.), University of Perugia, 06100 Perugia, Italy; Department of Endocrinology (S.Q.), Department of Medical Ultrasound (H.X.), Shanghai Tenth People's Hospital, Thyroid Institute, Tongji University School of Medicine, Shanghai, 200072, China; Endocrine Surgical Unit (C.J.O., M.S.S., R.C.B.), The University of Sydney, Sydney 2052, Australia; Cancer Molecular Pathology of Menzies Health Institute Queensland (A.K.L.), Griffith University–Gold Coast, Southport 4215, Australia; Hospital La Paz, Health Research Institute, and Hospital Universitario de Móstoles (G.R.-E.), and Biomedical Research Institute (G.R.-E., P.Sa.), “Alberto Sols,” Spanish Council of Research Consejo Superior de Investigaciones Científicas, and Autonomous University of Madrid, Madrid, 28029 Madrid, Spain; Department of Pathology (H.Y.), Changzheng Hospital, Second Military Medical University, Shanghai 200003, China; Department of Medicine, Anatomic Pathology Unit (G.T.), Ospedale Bellaria, University of Bologna, 40139 Bologna, Italy; Endocrine Section, Department of Internal Medicine (E.H.H.), Yale University School of Medicine, New Haven, Connecticut 06520; and Department of Pediatrics (V.V.), Uniformed Services University of the Health Sciences, Bethesda, Maryland 20814
1,✉