Abstract
Context:
IGF-1 promotes bone growth directly and indirectly through its effects on skeletal muscle. Insulin and IGF-1 share a common cellular signaling process; thus, insulin resistance may influence the IGF-1-muscle-bone relationship.
Objective:
We sought to determine the effect of insulin resistance on the muscle-dependent relationship between IGF-1 and bone mass in premenarcheal girls.
Design, Setting, and Participants:
This was a cross-sectional study conducted at a university research center involving 147 girls ages 9 to 11 years.
Main Outcome Measures:
Glucose, insulin, and IGF-1 were measured from fasting blood samples. Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated from glucose and insulin. Fat-free soft tissue (FFST) mass and bone mineral content (BMC) were measured by dual-energy x-ray absorptiometry. Our primary outcome was BMC/height.
Results:
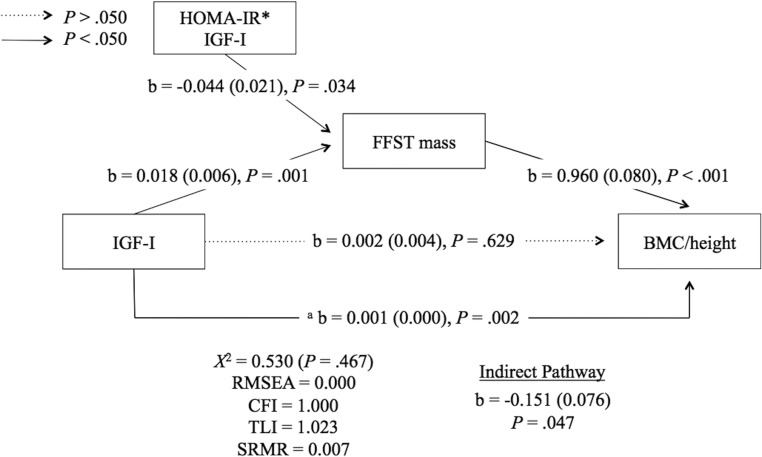
In our path model, IGF-1 predicted FFST mass (b = 0.018; P = .001), which in turn predicted BMC/height (b = 0.960; P < .001). IGF-1 predicted BMC/height (b = 0.001; P = .002), but not after accounting for the mediator of this relationship, FFST mass. The HOMA-IR by IGF-1 interaction negatively predicted FFST mass (b = −0.044; P = .034). HOMA-IR had a significant and negative effect on the muscle-dependent relationship between IGF-1 and BMC/height (b = −0.151; P = .047).
Conclusions:
Lean body mass is an important intermediary factor in the IGF-1-bone relationship. For this reason, bone development may be compromised indirectly via suboptimal IGF-1-dependent muscle development in insulin-resistant children.
Transient fluctuations in insulin sensitivity are a hallmark of pubertal development. These changes occur irrespective of adiposity, are greatest during early adolescence, and reach a nadir at midpuberty (1, 2). Although girls tend to become more insulin resistant than boys due to greater gains in adiposity, these changes are presumed to accompany normal growth patterns (1). However, there is evidence to suggest that insulin resistance beyond that which occurs normally during the pubertal transition may impede optimal musculoskeletal development. Indeed, several investigators have identified metabolic health outcomes associated with insulin action and glucose homeostasis as negative predictors of bone mass and density in children (3–6). Because the osteoblast is an insulin-dependent cell type, impaired insulin signaling within the bone-forming cells may explain the above associations (7). It is also likely that hyperinsulinemia and/or insulin resistance modulates alternative biological processes involved in musculoskeletal development during youth (5).
IGF-1 is an essential hormone in pediatric muscle and bone development. Whereas IGF-1 plays a direct role in bone formation (8, 9), it has also been proposed that the effects of IGF-1 on skeletal muscle precede its effects on bone (10–14). In a transgenic mouse model, mice overexpressing IGF-1 had significantly greater muscle mass and muscle cross-sectional area vs wild-type mice (10). These animals also had increases in tibia and femur cortical bone area and thickness, bone mineral content (BMC), and periosteal circumference, suggesting a muscle-dependent relationship between IGF-1 and cortical bone outcomes. In a cohort of Finnish girls, Xu et al (12) reported positive associations between IGF-1 and cortical bone size and mass that were nullified after adjusting for lower leg muscle cross-sectional area. Furthermore, a muscle-mediated link between IGF-1 and bone mass accrual throughout the pubertal years was also proposed by Breen et al (11).
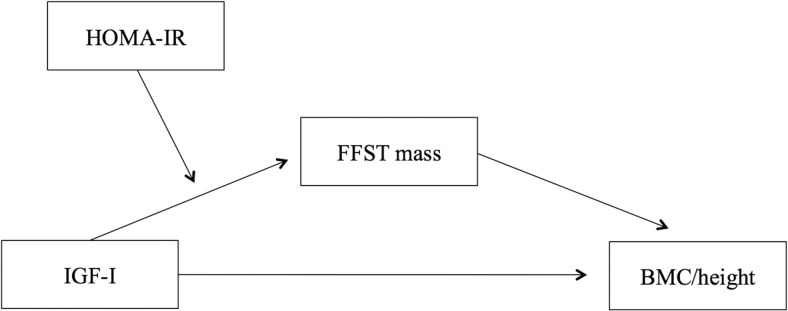
In addition to being an integral link in the IGF-1-bone relationship, skeletal muscle is the primary site of insulin-mediated glucose uptake. Because IGF-1 and the pancreatic β-cell-derived insulin are structurally similar and share a common downstream cellular signaling process (15–17), it is plausible that insulin resistance has an adverse effect on IGF-1-dependent processes. Some researchers have suggested that insulin resistance during puberty may be accompanied by a resistance to IGF-1 (1); however, this was only speculative. The effect of insulin resistance on IGF-1 function, in particular with respect to the IGF-1-muscle and bone relationship, has yet to be examined. The aim of this study was to determine the effect of insulin resistance on the muscle-dependent relationship between IGF-1 and bone mass in girls at the early stages of pubertal maturation (Figure 1).
Figure 1.
Hypothesized effect of insulin resistance (ie, HOMA-IR) on the FFST mass-dependent relationship between IGF-1 and BMC/height. HOMA-IR, homeostasis model assessment of insulin resistance; FFST, fat-free soft tissue mass.
Subjects and Methods
Subjects
This cross-sectional study is an ancillary analysis of a double-blinded, randomized, placebo-controlled zinc sulfate supplementation trial, conducted between 2007 and 2010 (18). This study utilized baseline data from black and white girls, ages 9 to 11 years (n = 147), and in the early stages of puberty. To determine initial study eligibility, potential participants underwent a telephone prescreening. Girls were excluded from this study if they had reached menarche, were previously diagnosed with a chronic disease or growth disorder, or reported the use of medications and/or herbal supplements that are known to influence bone metabolism. Sexual maturation was determined by the criteria set forth by Tanner (19), and all participants were required to have a sexual maturation rating stage of 2 or 3 based on breast development. The parent or guardian of eligible participants was mailed a sexual maturation rating stage self-assessment form, along with photographs and written explanations of each maturation stage, which was completed and mailed back to the laboratory. Once participants met inclusion criteria, an in-lab screening visit was conducted to measure standing height, body weight, sitting height and leg length to calculate maturity offset. Maturity offset, or years relative to peak height velocity (PHV), was calculated as: −9.376 + 0.0001882 × interaction of leg length and sitting height + 0.0022 × interaction of age and leg length + 0.005841 × interaction of age and sitting height − 0.002658 × interaction of age and weight + 0.07693 × ratio of weight to height (20). Study protocols and procedures were approved by The University of Georgia Institutional Review Board for Human Subjects, and each participant and her guardian provided written informed assent and consent, respectively.
Anthropometric measurements
One trained researcher performed all anthropometric measures of participants, who wore light indoor clothing. Weight was measured to the nearest 0.1 kg using an electronic scale (Seca Bella 840). Standing height, sitting height, and leg length were measured to the nearest 0.1 cm using a wall-mounted stadiometer (Novel Products Inc.). Each measure was performed twice and then averaged. In our lab, 10 girls ages 6–10 years were measured twice over a 2-week period to determine measurement reliability, and intraclass correlation coefficients and coefficients of variation (CVs) were computed for standing height (0.99 and 0.4%), body weight (0.99 and 1.4%), and sitting height (0.97 and 0.9%).
Dual-energy x-ray absorptiometry (DXA)
Total body fat-free soft tissue (FFST) mass (in kilograms), fat mass (in kilograms), percentage body fat, and BMC (in grams) were assessed via DXA (Delphi A; Hologic Inc). To ensure quality assurance, the DXA machine was calibrated against a three-step soft tissue wedge (Hologic, Inc), which was composed of different thickness levels of aluminum and Lucite. The same researcher performed and analyzed all DXA scans through instrument-specific software and procedures (Whole Body Analysis software, version 11.2; Hologic Inc). In our lab, 10 girls ages 5 to 8 years were scanned twice over a 7-day period to determine measurement reliability; all intraclass correlation coefficients for bone and body composition outcomes were ≥ 0.98. For all analyses, total body BMC was normalized for height (ie, BMC/height).
Serum biochemistries
Blood samples were collected by a trained phlebotomist between 7:00 and 10:30 am the morning after an overnight fast. Samples were placed on ice immediately after collection, centrifuged, and stored in a −80°C freezer until analysis for glucose, insulin, and IGF-1. From sera, glucose was measured in triplicate using a microtiter modification of the enzymatic Autokit Glucose method (Wako Chemicals), which has a detection limit of 0–500 mg/dL. The mean intra- and interassay CVs for this analysis were 1.8 and 2.2%, respectively. Insulin was assayed in duplicate from sera using the Human Insulin Specific RIA (HI-14K; Millipore), which has a detection limit of 3.125–100 μU/mL. The mean intra- and interassay CVs for this analysis were 3.5 and 5.3%, respectively. The homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as: fasting insulin (μU/mL) × fasting glucose (mg/dL)/405 (21). From blood collection tubes that were pretreated with EDTA, plasma IGF-1 (ng/mL) concentrations were measured in duplicate using a quantitative sandwich immunoassay technique with recombinant human IGF-1 (R&D Systems). Each plasma sample was pretreated with an acidic solution for the purpose of dissociation before treatment with a buffered protein that contained blue dye and preservatives, then lyophilized to release IGF-1 that was bound to IGF binding proteins (IGFBPs). The inter- and intra-assay CVs were 7.5–8.3 and 3.5–4.3%, respectively.
Statistical analyses
Histograms for all variables were visually inspected for outliers and normal distribution. Skewed or kurtotic distributions were confirmed if > 2.0. All variables of interest followed an approximately normal distribution. Pearson's bivariate and partial correlations were performed. Linear regression was performed to determine the relationship between IGF-1 and BMC/height while controlling for sexual maturation rating stage. These analyses were performed using SPSS version 21 (SPSS Inc).
To test the moderating effect of HOMA-IR on the FFST mass-mediated relationship between IGF-1 and BMC/height, a model 2 moderated mediation analysis was performed as described by Preacher et al (22). Given model convergence issues pertaining to excessive variances in certain model parameters, FFST mass (log), IGF-1 (square root), BMC/height (square root), and the interaction between HOMA-IR and IGF-1 (ie, HOMA-IR*IGF-1; square root) were transformed. Both HOMA-IR and IGF-1 were centered on their respective grand means, and the maximum likelihood estimation was used. We regressed BMC/height on FFST mass (path B1), IGF-1, HOMA-IR, and HOMA-IR*IGF-1. FFST mass was regressed on IGF-1 (path A1), sexual maturation rating stage, HOMA-IR, and HOMA-IR*IGF-1 (path A3). IGF-1 covaried with sexual maturation rating stage and HOMA-IR. HOMA-IR*IGF-1 covaried with HOMA-IR, IGF-1, and sexual maturation rating stage. Sexual maturation covaried with HOMA-IR. The pathway from sexual maturation rating stage to BMC/height was removed from our final model due to the nonsignificant relationship and therefore generated an overidentified model. Overall model goodness of fit was determined through previously set criteria on multiple fit indices (23). Accordingly, the following absolute and relative model fit indices were considered for our model: root mean square error of approximation < 0.08, comparative fit index > 0.90, Tucker-Lewis index > 0.90, and standardized root mean square residual < 0.07. We tested our moderation at a HOMA-IR value of 4.0 through the following equation: (path A1 + path A3 × 4.0) × path B (24). Mplus software (version 7.31) was used for our moderated mediation analysis. The statistical significance level for all analyses was set at P < .050.
Results
Descriptive participant characteristics are presented in Table 1. IGF-1 was positively associated with FFST mass and BMC/height in both unadjusted and sexual maturation-adjusted analyses (all P < .010; Table 2). After additional adjustment for FFST mass, the relationship between IGF-1 and BCM/height was nullified. HOMA-IR was positively associated with both FFST mass and BMC/height in unadjusted and sexual maturation-adjusted analyses (all P < .050). After additional adjustment for FFST mass, HOMA-IR was no longer associated with BMC/height. FFST mass and BMC/height were strongly correlated with one another in unadjusted (r = 0.790; P < .001) and sexual maturation-adjusted (r = 0.758; P < .001) analyses. IGF-1 and HOMA-IR did not correlate with one another in either unadjusted (r = 0.085; P = .305) or sexual maturation-adjusted (r = 0.063; P = .453) analyses.
Table 1.
Descriptive Participant Characteristics
| Mean | SD | |
|---|---|---|
| Age, y | 10.5 | 0.7 |
| Race (black/white), n | 67/80 | |
| Sexual maturation stage (2/3), n | 103/43 | |
| Maturation offset (years to PHV) | −0.84 | 0.67 |
| Weight, kg | 47.0 | 11.3 |
| Height, cm | 148.4 | 6.7 |
| BMI, kg/m2 | 21.3 | 4.4 |
| BMI for age, % | 75.1 | 26.1 |
| FFST mass, kg | 31.1 | 5.1 |
| Fat mass, kg | 15.4 | 7.3 |
| Body fat, % | 30.7 | 8.1 |
| BMC/height, g/cm | 8.86 | 1.20 |
| Insulin, μU/mL | 25.7 | 10.8 |
| Glucose, mg/dL | 86.1 | 7.6 |
| HOMA-IR | 5.51 | 2.47 |
| IGF-1, ng/mL | 407.7 | 244.7 |
Abbreviation: BMI, body mass index.
Table 2.
Bivariate and Partial Correlations Between IGF-1 and HOMA-IR With Total Body FFST Mass and BMC/Height in Premenarcheal Girls
| IGF-1 |
HOMA-IR |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted |
Sexual Maturation-Adjusted |
Sexual Maturation + FFST Mass-Adjusted |
Unadjusted |
Sexual Maturation-Adjusted |
Sexual Maturation + FFST Mass-Adjusted |
|||||||
| r | P | r | P | r | P | r | P | r | P | r | P | |
| FFST mass | 0.294 | <.001 | 0.262 | .002 | 0.381 | <.001 | 0.188 | .024 | ||||
| BMC/height | 0.279 | .001 | 0.253 | .002 | 0.087 | .300 | 0.240 | .004 | 0.342 | <.001 | −0.115 | .170 |
Values in bold are statistically significant at the level of P < .050.
The path model examining the effect of HOMA-IR in the FFST mass-dependent relationship between IGF-1 and BMC/height is depicted in Figure 2. In this model, the paths from IGF-1 to FFST mass (positive), FFST mass to BMC/height (positive), and HOMA-I*IGF-1 to FFST mass (negative) were statistically significant (all P < .050). Testing the moderating effect of HOMA-IR at a cut-point of HOMA-IR = 4.0, the test for an indirect effect was statistically significant and negative (P < .050), meaning that the FFST mass-mediated relationship between IGF-1 and BMC/height was stronger in participants with HOMA-IR < 4.0 vs those with HOMA-IR > 4.0.
Figure 2.
Insulin resistance has a negative effect on the FFST mass-dependent relationship between IGF-1 and bone mass. The interaction between HOMA-IR and IGF-1 was tested at a cut-point of HOMA-IR = 4.0. Unstandardized regression coefficients are presented as b (standard error). Sexual maturation and HOMA-IR were included as covariates in this model but are not displayed. a Relationship between IGF-1 and BMC/height after adjusting for sexual maturation. Broken lines represent nonsignificant relationships. RMSEA, root mean square error of approximation; CFI, comparative fit index; TLI, Tucker-Lewis index; SRMR, standardized root mean square residual. HOMA-IR*IGF-I represents interaction between HOMA-IR and IGF-I.
Discussion
We examined the effect of insulin resistance on the muscle-dependent relationship between IGF-1 and bone mass in children. The rationale for this study is based on the understanding that IGF-1 promotes muscle and bone growth (11, 12, 14), that the effect of IGF-1 on bone is presumed to be mediated through skeletal muscle (10–12), and that insulin and IGF-1 share a common downstream cellular signaling process (15, 17). Thus, it is plausible that insulin resistance may impair IGF-1 action. The main finding from this study was that insulin resistance had a significant negative effect on the FFST mass-dependent relationship between IGF-1 and bone mass in premenarcheal girls. Our data provide a possible explanation of why insulin-resistant children may be at risk for suboptimal bone mass accrual (3, 6, 25) and cortical bone development (5).
Through path analysis, we showed that the muscle-dependent link between IGF-1 and bone mass was attenuated in our insulin-resistant girls. These findings suggest that despite having similar IGF-1 concentrations, the myotrophic effects of IGF-1 may differ between insulin-resistant vs insulin-sensitive children. Based on the hypothesis that IGF-1 cellular signaling processes are compromised secondary to insulin resistance, particularly within muscle tissue, this explanation is plausible (1). Indeed, attenuated IGF-1 cellular signaling in the muscle and/or bone has been shown in animal models of aging (8) and physical unloading (9). Additionally, in a rat model of diet-induced type 2 diabetes, Li et al (26) showed significant reductions in mRNA and protein expression of muscle and bone cell insulin receptor substrate, an essential component of insulin/IGF-1-mediated AKT/mTOR signaling processes (17). The concept of “IGF-1 resistance” has been proposed as one explanation for the skeletal phenotype that is characteristic of idiopathic osteoporosis; however, to our knowledge, this has not yet been studied in the context of insulin resistance in humans (27).
It is important to consider that insulin resistance may negatively influence IGF-1-dependent muscle growth because lean body mass is a strong determinant of pediatric bone development and is presumed to facilitate the IGF-1-bone relationship (12, 28). As an example, Xu et al (12) showed that the positive relationship between IGF-1 and midtibia cortical BMC was nullified after controlling for muscle cross-sectional area in a prospective study of girls in the early to later stages of maturation. This relationship was also evident in mice overexpressing IGF-1, demonstrating a muscle-dependent effect of IGF-1 on cortical bone mass and size (10). In the current study, the significant and positive correlation between IGF-1 and bone mass was attenuated after adjusting for FFST mass. This finding was replicated in our path model, ie, the nonsignificant direct pathway from IGF-1 to bone mass represents the relationship between IGF-1 and BMC/height while controlling for the mediator (ie, FFST mass). The rationale for these findings is that IGF-1 promotes muscle growth, which in turn leads to bone mass accrual and cortical bone areal expansion (11, 12, 14). Although these data provide valuable insight regarding the contribution of skeletal muscle in the IGF-1-bone relationship, stating that the link between IGF-1 and bone is mediated by muscle mass is premature. In our causal path model, however, we show for the first time that FFST mass is a true mediator in the pathway from IGF-1 to bone mass in our sample of early pubertal girls. We do not dispute that IGF-1 is involved in bone development through direct processes. Circulatory IGF-1 concentrations most closely mirror hepatic production. Moreover, both the muscle and bone produce IGF-1, acting in an autocrine and/or paracrine fashion (29, 30), and may further explain why the direct path from IGF-1 to BMC/height was not significant in the current study.
Because insulin promotes hepatic IGF-1 production, it would be expected that the insulin-resistant girls in the current study with higher fasting insulin concentrations would also have higher IGF-1 concentrations (31). However, we showed only marginal associations between HOMA-IR and IGF-1. A number of explanations may account for this null finding, including a nonlinear relationship between insulin resistance and circulatory IGF-1 (32) as well as the relatively high mean HOMA-IR (approximately 5.5) in our sample. We used a conservative cut-point of 4.0 to test the interaction between HOMA-IR and IGF-1 in our causal model (24); however, more liberal values of HOMA-IR to denote “insulin resistance,” eg, 3.16, have been published previously (33). Despite having similar total IGF-1 concentrations, insulin-resistant and hyperinsulinemic children may have higher biologically active IGF-1 than their healthier counterparts due to modulations in IGFBPs (34). These differences in IGF-1 availability in favor of the insulin-resistant children further support the hypothesized modulation of IGF-1 action secondary to insulin resistance.
Most of the girls in the current study were proximal to the estimated age of PHV, which precedes peak lean mass velocity and peak bone mass velocity. We speculate that the 12% of study participants who had surpassed the estimated age of PHV had not yet reached peak lean mass velocity or peak bone mass velocity because these pubertal milestones are not typically achieved until approximately 13 years of age (35). Furthermore, plateaus in peak lean mass precede plateaus in peak bone mass, neither of which are typically achieved until young adulthood (36). Because impaired IGF-1-dependent muscle accretion may result in discrepancies in the attainment of peak bone mass in insulin-resistant children, this should be of particular concern since nearly one-fourth of adult bone mass is accrued during the 2 years surrounding PHV (37). Our participants were on average 10.5 years of age and nearly 1 year away from estimated PHV. Thus, one could argue that there is substantial time to mitigate the trajectory of insulin resistance-related muscle and bone developmental inadequacies by optimizing insulin sensitivity during youth. Prospective studies are needed to confirm whether the effect of insulin resistance on IGF-1-dependent muscle development, and subsequently bone development, is hindered throughout maturation.
The major strength of this study was our utilization of path analysis statistical techniques while exploring a novel mechanism through which insulin resistance may negatively influence pediatric muscle and bone development. From our path analysis, the criterion for each of our model fit indices was met, highlighting the merit of our hypothesized model despite our relatively small sample size. Given that our study used cross-sectional data, we cannot be certain that insulin resistance has a direct effect on the IGF-1-muscle-bone relationship. Our inclusion of only two-dimensional (ie, DXA-derived) musculoskeletal outcomes limits the interpretation of our findings because we are unable to ascertain whether these relationships apply to cortical and/or trabecular bone structural indices. Future studies should prospectively examine cortical and trabecular bone at appendicular skeletal sites because IGF-1 promotes cortical bone areal expansion (38) and insulin resistance is a negative determinant of cortical bone size in adolescents (5). Because others have shown agreement between total body BMC with tibia diaphyseal cortical bone area in children (39), our findings may be attributed to cortical bone characteristics that we did not measure. The current study included only female participants; thus, we cannot draw conclusions on whether these relationships are consistent across sexes. We suspect that these relationships would differ in boys vs girls, given the sex-related differences in the strength of the muscle-bone relationship (40).
Conclusions
We are the first to report that insulin resistance is an important consideration in the context of the IGF-1-muscle and bone relationships. Through path analysis we showed that the muscle-dependent relationship between IGF-1 and bone mass was compromised in insulin-resistant children. This is of particular importance during the years surrounding PHV, given the rapid muscle and bone mass accrual that occurs during this specific developmental period (37, 41). Based on our results, it is premature to state that excessively insulin-resistant children are also resistant to IGF-1; however, as discussed previously, this is one possible explanation for our results. Although in vivo and in vitro data are lacking, the concept of IGF-1 resistance has been proposed in pubertal children (1) and is suspected to contribute to the skeletal inadequacies that are characteristic of idiopathic osteoporosis (27). Nearly 25% of US youth are currently considered prediabetic (42). These children may be prone to suboptimal IGF-1-dependent muscle and bone mass accrual because insulin resistance is a seminal characteristic of type 2 diabetes progression. Prospective studies including both boys and girls, along with measures of appendicular cortical and trabecular bone structure and IGFBPs, are warranted to enhance our understanding of IGF-1, insulin resistance, and muscle and bone development during youth.
Acknowledgments
This work was supported by National Institutes of Health Grant R03 HD54630 and National Institute of Food and Agriculture, HATCH Project GEO00647.
Clinical Trial Registration No. NCT01892098.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMC
- bone mineral content
- CV
- coefficient of variation
- DXA
- dual-energy x-ray absorptiometry
- FFST
- fat-free soft tissue
- HOMA-IR
- homeostasis model assessment of insulin resistance
- IGFBP
- IGF binding protein
- PHV
- peak height velocity.
References
- 1. Jeffery AN, Metcalf BS, Hosking J, Streeter AJ, Voss LD, Wilkin TJ. Age before stage: insulin resistance rises before the onset of puberty: a 9-year longitudinal study (EarlyBird 26). Diabetes Care. 2012;35(3):536–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Moran A, Jacobs DR, Jr, Steinberger J, et al. Changes in insulin resistance and cardiovascular risk during adolescence: establishment of differential risk in males and females. Circulation. 2008;117(18):2361–2368. [DOI] [PubMed] [Google Scholar]
- 3. Lawlor DA, Sattar N, Sayers A, Tobias JH. The association of fasting insulin, glucose, and lipids with bone mass in adolescents: findings from a cross-sectional study. J Clin Endocrinol Metab. 2012;97(6):2068–2076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pollock NK. Childhood obesity, bone development, and cardiometabolic risk factors. Mol Cell Endocrinol. 2015;410:52–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sayers A, Lawlor DA, Sattar N, Tobias JH. The association between insulin levels and cortical bone: findings from a cross-sectional analysis of pQCT parameters in adolescents. J Bone Miner Res. 2012;27(3):610–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Afghani A, Cruz ML, Goran MI. Impaired glucose tolerance and bone mineral content in overweight Latino children with a family history of type 2 diabetes. Diabetes Care. 2005;28(2):372–378. [DOI] [PubMed] [Google Scholar]
- 7. Pramojanee SN, Phimphilai M, Chattipakorn N, Chattipakorn SC. Possible roles of insulin signaling in osteoblasts. Endocr Res. 2014;39(4):144–151. [DOI] [PubMed] [Google Scholar]
- 8. Cao JJ, Kurimoto P, Boudignon B, Rosen C, Lima F, Halloran BP. Aging impairs IGF-I receptor activation and induces skeletal resistance to IGF-I. J Bone Miner Res. 2007;22(8):1271–1279. [DOI] [PubMed] [Google Scholar]
- 9. Sakata T, Wang Y, Halloran BP, Elalieh HZ, Cao J, Bikle DD. Skeletal unloading induces resistance to insulin-like growth factor-I (IGF-I) by inhibiting activation of the IGF-I signaling pathways. J Bone Miner Res. 2004;19(3):436–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Banu J, Wang L, Kalu DN. Effects of increased muscle mass on bone in male mice overexpressing IGF-I in skeletal muscles. Calcif Tissue Int. 2003;73(2):196–201. [DOI] [PubMed] [Google Scholar]
- 11. Breen ME, Laing EM, Hall DB, et al. 25-Hydroxyvitamin D, insulin-like growth factor-I, and bone mineral accrual during growth. J Clin Endocrinol Metab. 2011;96(1):E89–E98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Xu L, Wang Q, Wang Q, et al. Concerted actions of insulin-like growth factor 1, testosterone, and estradiol on peripubertal bone growth: a 7-year longitudinal study. J Bone Miner Res. 2011;26(9):2204–2211. [DOI] [PubMed] [Google Scholar]
- 13. Kindler JM, Lewis RD, Hamrick MW. Skeletal muscle and pediatric bone development. Curr Opin Endocrinol Diabetes Obes. 2015;22(6):467–474. [DOI] [PubMed] [Google Scholar]
- 14. Garnett SP, Högler W, Blades B, et al. Relation between hormones and body composition, including bone, in prepubertal children. Am J Clin Nutr. 2004;80(4):966–972. [DOI] [PubMed] [Google Scholar]
- 15. Entingh-Pearsall A, Kahn CR. Differential roles of the insulin and insulin-like growth factor-I (IGF-I) receptors in response to insulin and IGF-I. J Biol Chem. 2004;279(36):38016–38024. [DOI] [PubMed] [Google Scholar]
- 16. Rinderknecht E, Humbel RE. The amino acid sequence of human insulin-like growth factor I and its structural homology with proinsulin. J Biol Chem. 1978;253(8):2769–2776. [PubMed] [Google Scholar]
- 17. Duan C, Ren H, Gao S. Insulin-like growth factors (IGFs), IGF receptors, and IGF-binding proteins: roles in skeletal muscle growth and differentiation. Gen Comp Endocrinol. 2010;167(3):344–351. [DOI] [PubMed] [Google Scholar]
- 18. Berger PK, Ppllock NK, Laing EM, et al. Zinc supplementation increases procollagen type 1 amino-terminal propeptide in premenarcheal girls: a randomized controlled trial [published online October 21, 2015]. J Nutr. doi:10.3945/jn.115.218792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tanner JM. Growth and adolescence. 2nd ed Oxford, UK: Blackwell Scientific Publications; 1962. [Google Scholar]
- 20. Mirwald RL, Baxter-Jones AD, Bailey DA, Beunen GP. An assessment of maturity from anthropometric measurements. Med Sci Sports Exerc. 2002;34(4):689–694. [DOI] [PubMed] [Google Scholar]
- 21. Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. [DOI] [PubMed] [Google Scholar]
- 22. Preacher KJ, Rucker DD, Hayes AF. Addressing Moderated Mediation Hypotheses: Theory, Methods, and Prescriptions. Multivar Behav Res. 2007;42(1):185–227. [DOI] [PubMed] [Google Scholar]
- 23. Hu L, Bentler PM. Cutoff criteria for fit indexes in covariance structure analysis: conventional criteria versus new alternatives. Struct Equ Modeling. 1999;6(1):1–55. [Google Scholar]
- 24. Reinehr T, Andler W. Changes in the atherogenic risk factor profile according to degree of weight loss. Arch Dis Child. 2004;89(5):419–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pollock NK, Bernard PJ, Gutin B, Davis CL, Zhu H, Dong Y. Adolescent obesity, bone mass, and cardiometabolic risk factors. J Pediatr. 2011;158(5):727–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Li B, Wang Y, Liu Y, Ma J, Li Y. Altered gene expression involved in insulin signaling pathway in type II diabetic osteoporosis rats model. Endocrine. 2013;43(1):136–146. [DOI] [PubMed] [Google Scholar]
- 27. Cohen A, Dempster DW, Recker RR, et al. Abnormal bone microarchitecture and evidence of osteoblast dysfunction in premenopausal women with idiopathic osteoporosis. J Clin Endocrinol Metab. 2011;96(10):3095–3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Xu L, Nicholson P, Wang Q, Alén M, Cheng S. Bone and muscle development during puberty in girls: a seven-year longitudinal study. J Bone Miner Res. 2009;24(10):1693–1698. [DOI] [PubMed] [Google Scholar]
- 29. Hamrick MW, McNeil PL, Patterson SL. Role of muscle-derived growth factors in bone formation. J Musculoskelet Neuronal Interact. 2010;10(1):64–70. [PMC free article] [PubMed] [Google Scholar]
- 30. Song YH, Song JL, Delafontaine P, Godard MP. The therapeutic potential of IGF-I in skeletal muscle repair. Trends Endocrinol Metab. 2013;24(6):310–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Böni-Schnetzler M, Schmid C, Meier PJ, Froesch ER. Insulin regulates insulin-like growth factor I mRNA in rat hepatocytes. Am J Physiol. 1991;260(6):E846–E851. [DOI] [PubMed] [Google Scholar]
- 32. Friedrich N, Thuesen B, Jørgensen T, et al. The association between IGF-I and insulin resistance: a general population study in Danish adults. Diabetes Care. 2012;35(4):768–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Keskin M, Kurtoglu S, Kendirci M, Atabek ME, Yazici C. Homeostasis model assessment is more reliable than the fasting glucose/insulin ratio and quantitative insulin sensitivity check index for assessing insulin resistance among obese children and adolescents. Pediatrics. 2005;115(4):e500–e503. [DOI] [PubMed] [Google Scholar]
- 34. Nam SY, Lee EJ, Kim KR, et al. Effect of obesity on total and free insulin-like growth factor (IGF)-1, and their relationship to IGF-binding protein (BP)-1, IGFBP-2, IGFBP-3, insulin, and growth hormone. Int J Obes Relat Metab Disord. 1997;21(5):355–359. [DOI] [PubMed] [Google Scholar]
- 35. Rauch F, Bailey DA, Baxter-Jones A, Mirwald R, Faulkner R. The 'muscle-bone unit' during the pubertal growth spurt. Bone. 2004;34(5):771–775. [DOI] [PubMed] [Google Scholar]
- 36. Boot AM, de Ridder MA, van der Sluis IM, van Slobbe I, Krenning EP, Keizer-Schrama SM. Peak bone mineral density, lean body mass and fractures. Bone. 2010;46(2):336–341. [DOI] [PubMed] [Google Scholar]
- 37. Baxter-Jones AD, Faulkner RA, Forwood MR, Mirwald RL, Bailey DA. Bone mineral accrual from 8 to 30 years of age: an estimation of peak bone mass. J Bone Miner Res. 2011;26(8):1729–1739. [DOI] [PubMed] [Google Scholar]
- 38. Yakar S, Canalis E, Sun H, et al. Serum IGF-1 determines skeletal strength by regulating subperiosteal expansion and trait interactions. J Bone Miner Res. 2009;24(8):1481–1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Leonard MB, Shults J, Elliott DM, Stallings VA, Zemel BS. Interpretation of whole body dual energy X-ray absorptiometry measures in children: comparison with peripheral quantitative computed tomography. Bone. 2004;34(6):1044–1052. [DOI] [PubMed] [Google Scholar]
- 40. Macdonald HM, Kontulainen SA, Mackelvie-O'Brien KJ, et al. Maturity- and sex-related changes in tibial bone geometry, strength and bone-muscle strength indices during growth: a 20-month pQCT study. Bone. 2005;36(6):1003–1011. [DOI] [PubMed] [Google Scholar]
- 41. Jackowski SA, Faulkner RA, Farthing JP, Kontulainen SA, Beck TJ, Baxter-Jones AD. Peak lean tissue mass accrual precedes changes in bone strength indices at the proximal femur during the pubertal growth spurt. Bone. 2009;44(6):1186–1190. [DOI] [PubMed] [Google Scholar]
- 42. May AL, Kuklina EV, Yoon PW. Prevalence of cardiovascular disease risk factors among US adolescents, 1999–2008. Pediatrics. 2012;129(6):1035–1041. [DOI] [PubMed] [Google Scholar]