Abstract
Fatty acids (FAs) are a major energy source in the body. White adipose tissue (WAT) is a primary site where FAs are stored as triacylglycerols. Brown adipose tissue also stores and recruits FAs as a carbon source for uncoupled β-oxidation during thermogenesis. The deletion of the vitamin D nuclear hormone receptor (VDR) gene in mice (VDRKO) results in a lean WAT phenotype with increased levels of expression of the brown adipose tissue marker Ucp1 in the WAT. However, the impact of vitamin D/VDR on FA composition in WAT has not been explored in detail. To address this question, we examined the FA composition of sc and visceral white adipose depots of VDRKO mice. We found that the levels of a subset of saturated and monounsaturated FAs of C18-C24 are specifically increased in the sc adipose depot in VDRKO mice. We revealed that a specific elongase enzyme (Elovl3), which has an important role in brown fat biology, is directly regulated by VDR and likely contributes to the altered FA composition in VDRKO mice. We also demonstrate that Elovl3 is regulated by vitamin D in vivo and tissue specifically in the sc WAT depot. We discovered that regulation of Elovl3 expression is mediated by ligand-dependent VDR occupancy of a negative-response element in the promoter proximal region of the Elovl3 gene. These data suggest that vitamin D/VDR tissue specifically modulates FA composition in sc WAT through direct regulation of Elovl3 expression.
Vitamin D is the precursor to a potent steroid hormone, calcitriol, that signals via binding to the vitamin D nuclear hormone receptor (VDR) to modulate the expression of target genes. Classically, vitamin D signaling plays an important role in regulating calcium homeostasis (1). However, numerous studies have verified additional functions for vitamin D activity. For example, deletion of the VDR gene in mice results in decreased adipose tissue and altered energy expenditure (2, 3). In addition, overexpression of the human VDR in mouse adipose tissue reduced levels of fatty acid (FA) β-oxidation and lipolysis (4). However, the physiological implications and molecular mechanisms of this effect on adipose tissue have not been elucidated.
FAs have a major role in systemic energy homeostasis (5). When energy is scarce, ie, during starvation, FAs are recruited to the mitochondria for β-oxidation that usually results in the generation of ATP via the electron transport chain (6). In brown and beige adipocytes, the electron transport chain is uncoupled from ATP production and β-oxidation results in energy expenditure via thermogenesis (7). When there is caloric excess, FAs are stored in adipocytes as triacylglycerols (8).
FAs can be obtained from the diet or synthesized endogenously in the liver and adipose tissues from other carbon sources. FA synthesis is performed by the cytosolic enzyme FA synthase (FAS), which produces medium to long-chain FAs up to 16 carbons in length (C16) (8). In addition, FAs can be further elongated into very long-chain FAs (VLCFAs) by 7 distinct elongation of very long chain fatty acid enzymes (Elovl1-Elovl7). These enzymes are substrate-specific with regard to the precursor FA length and saturation and generate a tissue-specific composition of VLCFAs (9, 10). For example, Elovl3 elongates C18-C24 saturated FAs (SFAs) and monounsaturated FAs and is particularly critical to the synthesis of saturated VLCFA and triacylglyceride formation in brown adipocytes (10, 11).
Given the lean phenotype of mice with the VDR gene deleted (VDRKO) mice (2, 3), it was anticipated that the expression level of FAS, which synthesizes long-chain FAs for incorporation into triglycerides in white adipose tissue (WAT), would be low. Intriguingly, and contrary to expectations, the level of FAS in VDRKO WAT was actually observed to be elevated in the WAT of VDRKO mice compared with wild-type controls (2). These results inspired us to hypothesize that vitamin D/VDR signaling confers a specific effect on FA composition, and elucidating this effect would provide further insight into the physiological implications of VDR activity in adipocytes. Therefore, we performed an unbiased global FA analysis comparing VDRKO sc inguinal WAT (iWAT) and visceral perigonadal WAT (gWAT) depots with wild-type controls by gas chromatography. We discovered that VDRKO mice have depot-specific changes in a subset of SFAs and monounsaturated FAs of C18-C24. Our studies identify Elovl3 as a direct and ligand-dependent VDR target gene that can explain the altered FA composition in VDRKO WAT. These findings elucidate a role for VDR in the regulation of FA elongation in WAT and provide additional insight into mechanisms by which VDR might inhibit a brown fat-specific expression profile in WAT.
Materials and Methods
Animals
Global VDR knockout mice in the C57BL6 background were purchased from The Jackson Laboratory (stock number 006133). All mice were weaned to a high-calcium diet containing 2% calcium, 1.5% phosphorus, and 20% lactose (Harlan) to maintain normal serum calcium levels. Eight- to 10-week old male mice were used in the studies. Wild-type mice (WT) were treated every other day with vehicle (PBS) or calcitriol (D1530; Sigma) dissolved in ethanol, diluted in PBS (final ethanol concentration was 0.5%) by ip injection (200 μL) at 50 ng/mouse over 5 days. Controls were injected with ethanol diluted in PBS. All animal studies were approved by the Institutional Animal Care and Use Committee at Stanford University.
Preparation of stromal vascular fraction (SVF) and adipogenesis
Preparation of SVF was performed as previously described (12). Adipogenesis assays were carried out as previously described (13). Calcitriol was added into the adipocyte culture on day 6 after induction of adipogenesis. Cells were treated for 18 hours before being harvested. Control cells were treated with vehicle at the same time.
RNA isolation and quantitative RT-PCR
Total RNA from adipose and liver tissues was isolated with TRIzol RNA isolation reagents (Life Technologies) according to the manufacturer's protocol. Total RNA from cell culture was isolated with Quick-RNA Miniprep (Zymo Research) according to the manufacturer's protocol.
For quantitative PCR, 500-ng total RNA was reverse transcribed using M-MLV Reverse Transcriptase and random primers (Promega). cDNA samples were diluted 1:5, and quantitative PCR was performed with DyNAmo ColorFlash SYBR Green quantitative PCR kit (Life Technologies) on a CFX384 Real-Time PCR detection system (Bio-Rad). Cycle numbers were normalized to TSTS Binding Protein expression levels. Primer sequences are listed in Supplemental Table 1.
Luciferase reporter constructs and assays
DNA fragments of upstream sequence of the mouse Elovl3 transcription start site were amplified by PCR from a mouse BAC clone (RP23-102B6) and cloned into pGL4.26 luciferase reporter vector (Promega). Deletion of putative VDR-response element (VDRE)4 was generated by PCR using Pfu polymerase (Stratagene). The reporter plasmids were transiently transfected into NIH-3T3 cells using Lipofectamine2000 (Life Technologies). Twenty-four hours after transfection, cells were treated with calcitriol (Sigma) at indicated concentration for another 24 hours. Cells were then collected and luciferase assays were carried out using the Luciferase Assay System (Promega). For more details, see Supplemental Materials and Methods.
Chromatin immunoprecipitation (ChIP) analysis
Ex vivo differentiated white adipocytes were fixed in 1% formaldehyde for 10 minutes. Cells were sonicated in a Bioruptor (Diagenode). Samples were precipitated with anti-VDR C-20 antibody (Table 1) (14) or normal rabbit IgG (Santa Cruz Biotechnology, Inc). Immunoprecipitates were collected using ChIP Grade Protein G Magnetic Beads (Cell Signaling Technology). DNA was purified with Zymo ChIP DNA Clean & Concentrator (Zymogen). Real-time quantitative PCR was performed to determine the ChIP efficiency. Primer sequences are listed in Supplemental Table 1.
Table 1.
Antibody Table
| Peptide/Protein Target | Antigen Sequence (if Known) | Name of Antibody | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody | Species Raised in; Monoclonal or Polyclonal | Dilution Used |
|---|---|---|---|---|---|
| VDR | C-20 X | Santa Cruz Biotechnology, Inc, sc-1008 X | Rabbit; polyclonal | 1:1000 |
Results and Discussion
VDR regulates FA composition in sc adipose tissue
Previously reported adipose tissue phenotype and expression profiling in VDRKO mice suggested to us that VDR could modulate FA composition. In order to test this hypothesis, we performed an unbiased analysis of the total FA composition of sc inguinal adipose (iWAT) and visceral perigonadal adipose (gWAT) from VDRKO compared with WT (Supplemental Table 2). We measured all saturated, monounsaturated, and polyunsaturated C12-C26 FAs, including the geometric isomers. First, we compared the levels of SFAs, monounsaturated, and polyunsaturated FAs in VDRKO and WT. We detected a very small increase of SFAs in the iWAT of VDRKO compared with WT (Figure 1A). However, when we compared the FAs of different lengths in VDRKO vs WT mice, we discovered that levels of stearic acid (18:0), arachidic acid (20:0), eicosenoic acid (20:1), behenic acid (22:0), erucic acid (22:1), tricosanoic acid (23:0), and tetracosenoic acid (24:1) were all significantly increased specifically in the iWAT depot of VDRKO mice (Figure 1, B–E, and Supplemental Table 2). Therefore, VDR specifically affects the level of a subset of saturated and monounsaturated C18-C24 FAs.
Figure 1.
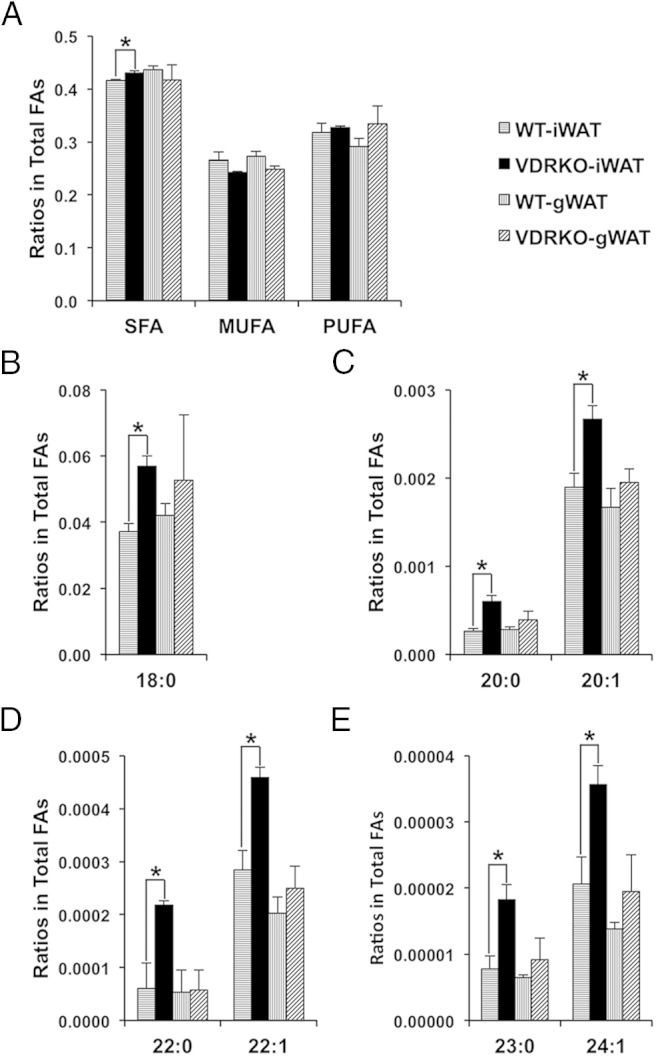
Changes in FA composition in sc inguinal adipose (iWAT) and visceral perigonadal adipose (gWAT) from VDRKO compared with WT. A, Levels of SFAs, monounsaturated FAs (MUFAs), and polyunsaturated FAs (PUFAs) in VDRKO compared with WT. B–E, Levels of specified individual FAs in VDRKO compared with WT; n = 3. All mice were 9 weeks of age. Data are expressed as ratio of FA to total FAs. Error bars represent SEM (*, P < .05).
Elovl3 expression is increased in VDR knockout adipose tissue
The specific dysregulation of C18-C24 SFAs and monounsaturated FAs we identified implicates the process of FA elongation as a target of VDR activity in iWAT. Elongation of FAs greater than C16 requires the activity of specific very long-chain FA elongases (Elovls). There are 7 Elovl enzymes with tissue and substrate-specific elongation activities (9). Elovl1 elongates C18:0-C26:0 acyl-coenzyme A (CoA), with the highest activity towards C22:0-CoA. Elovl2 and Elovl5 act specifically on polyunsaturated acyl-CoAs. Elovl3 and Elovl7 generally exhibit similar substrate specificities towards C16-C22 acyl-CoAs with the highest activity on C18-CoA. Elovl4 elongates C24:0 and C26:0-CoAs. Elovl6 is a potent elongase of C16:0-CoA (10). Based on these substrate specificities of the Elovl enzymes, we reasoned that the increased levels of C18-C24 FAs in the VDRKO iWAT would most likely be caused by altered Elovl1, Elovl3, or Elovl7 activities.
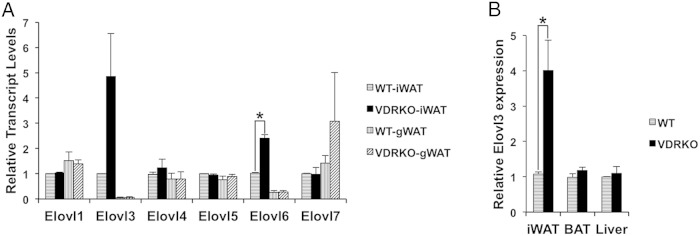
Because VDR regulates the expression of target genes, we surveyed the expression profiles of all 7 Elovl genes in both iWAT and gWAT from VDRKO mice. We discovered that Elovl3 is highly, and Elovl6 is modestly, up-regulated in iWAT in VDRKO mice (Figure 2A). We also revealed that the expression level of Elovl3 is significantly higher in iWAT compared with in gWAT (Figure 2A). The selective increase in Elovl3 expression levels is fully consistent with the specific FA profile of increased SFAs and monounsaturated FAs of C18-C24 we observed in the VDRKO iWAT. We suspect the modest up-regulation of expression of Elovl6 is due to a modest increase in lipogenesis in VDRKO mice (2). As previously reported (15), we found that basal levels of Elovl3 are higher in brown adipose tissue (BAT) and liver compared with WAT. Intriguingly, we found that Elovl3 expression levels are tissue specifically increased in VDRKO iWAT and not in VDRKO gWAT, BAT, or liver (Figure 2, A and B).
Figure 2.
Elovl3 expression is up-regulated in the iWAT of VDRKO mice. A, Expression of Elovl genes in iWAT and gWAT of WT and VDRKO mice. For each Elovl gene, the expression level was normalized to iWAT of WT mice; n = 3. B, Expression of Elovl3 in iWAT, BAT, and liver. Expression levels of Elovl3 in each VDRKO tissue was normalized against WT expression in the same tissue; n = 5. Three biological replicates of age matched VDRKO and WT mice were performed at 8, 8, and 10 weeks of age. Error bars represent SEM (*, P < .05).
Regulation of Elovl3 by VDR is ligand dependent
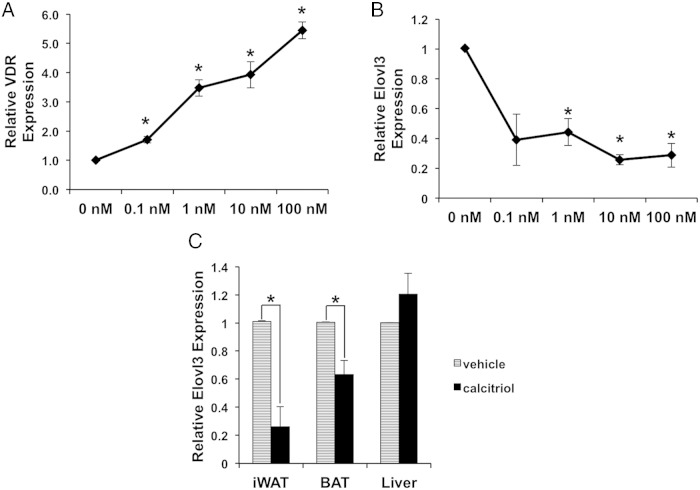
VDR regulates target gene expression by both ligand dependent and independent mechanisms (1, 14). To test whether the regulation of Elovl3 by VDR is ligand dependent, we first generated adipocytes in tissue culture by differentiating the adipose SVF isolated from the iWAT depot of WT. We then treated the cells with a titration of calcitriol. As shown in Figure 3A, VDR is expressed in the ex vivo differentiated white adipocytes and induced by calcitriol treatment, providing a basis for vitamin D/VDR signaling. We discovered that treatment of white adipocytes with calcitriol for 24 hours robustly represses Elovl3 expression (Figure 3B). Taken together, these data suggest that increased Elovl3 expression in VDRKO iWAT results from an adipose tissue autonomous effect of calcitriol acting via the VDR in iWAT that represses Elovl3 expression, rather than from systemic or indirect VDR effects in VDRKO mice. Furthermore, these data demonstrate that the regulation of Elovl3 expression by VDR is ligand-dependent.
Figure 3.
Elovl3 expression is repressed by calcitriol treatment. A, Vdr is endogenously expressed in ex vivo differentiated adipocytes and is up-regulated by calcitriol in a dose-dependent manner. B, Elovl3 expression is repressed by calcitriol in ex vivo differentiated adipocytes; n = 3 for A and B. The adipocytes were treated with calcitriol at indicated concentrations (0nM–100nM) for 24 hours. C, Expression of Elovl3 in iWAT, BAT, and liver of WT injected with vehicle (PBS) or calcitriol for 5 days; n = 4. All mice were 9 weeks ± 4 days of age. Error bars represent SEM (*, P < .05).
We also examined whether these findings are relevant in vivo in WT mice. We treated WT mice with calcitriol or vehicle (PBS) control for 5 days and then harvested iWAT, BAT, and liver tissues for analysis. Our results confirm that calcitriol treatment results in a significant repression of Elovl3 expression in vivo in iWAT and BAT but not in the liver (Figure 3C).
Direct regulation of Elovl3 by vitamin D-VDR
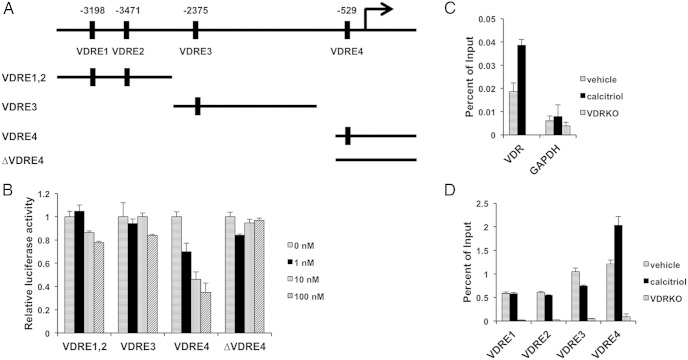
Given our data suggesting that regulation of Elovl3 by VDR is direct, we next sought to identify the VDRE responsible for this regulation. Using the web-based tool, ConSite (16), we identified 4 putative VDREs in the promoter proximal region (−4070 to +1 bp) of mouse Elovl3 gene. To test whether any of these putative VDREs are competent to modulate the expression of Elovl3, we created reporter constructs by subcloning the DNA elements upstream of a minimal promoter and the luciferase gene (Figure 4A). As shown in Figure 4, we found that the construct containing VDRE4 mediates a strong dose-dependent repression of luciferase activity, whereas the constructs containing VDRE1, VDRE2, or VDRE3 did not significantly respond to calcitriol treatment. To verify the function of VDRE4, we mutated VDRE4 in the reporter constructs using site-directed mutagenesis. These experiments confirm that an intact VDRE4 is necessary for the dose-dependent regulation of luciferase activity by calcitriol (Figure 4B). These results indicate that VDRE4 is a critical negative regulatory element modulating Elovl3 expression.
Figure 4.
A VDRE in the Elovl3 promoter sequence negatively regulates Elovl3 expression. A, Diagram showing the locations of 4 putative VDR binding sites (VDRE1–VDRE4) in the region from −4070 to +1 bp from the Elovl3 transcriptional start site identified in silico and luciferase reporter constructs generated from fragments containing the putative VDREs along with a fragment with VDRE4 deleted (ΔVDRE4). B, Relative luciferase activity of respective reporter constructs when treated with a titration of calcitriol concentrations (0nM–100nM); n = 3, error bars represent SEM. C, left, ChIP studies of VDR at a validated VDRE in the first intron of the VDR gene (positive control) (20) and at a GAPDH upstream sequence (negative control). D, ChIP at putative VDREs with ligand-dependent (calcitriol) enrichment of occupancy of VDRE4 with VDR occupancies at the putative VDREs in the upstream regulatory sequence of Elovl3 gene.
To confirm that the endogenous VDRE4 is occupied by VDR, we performed ChIP of VDR in ex vivo differentiated white adipocytes. These studies revealed that the endogenous VDRE4 is occupied by VDR and that this occupancy is ligand dependent (Figure 4, C and D). Together, our data elucidate that ligand-induced VDR occupancy of VDRE4 mediates repression of Elovl3 expression in iWAT.
In this study, we demonstrate that VDR directly represses Elovl3 expression tissue specifically in iWAT in mice. This newly identified regulation may contribute to the effect of vitamin D signaling on adiposity and lipid metabolism. Intriguingly, an earlier study by Oda et al (17) revealed that VDR induces, rather than represses, Elovl3 and Elovl4 expression in skin cells, although a direct regulation of Elovl3 by VDR was not examined. Collectively with our studies, these results indicate that VDR regulation of VLCFA elongation is responsive to context and tissue-specific requirements.
The fact that we observed VDR regulation of Elovl3 expression specifically in the sc depot (iWAT) and not in the visceral gWAT depot in VDRKO mice is intriguing. For reasons that are still not fully understood, there is strong indication that sc and visceral adipose tissue have some distinct physiological roles (18). Indeed, excess sc adipose tissue is associated with a favorable metabolic profile, whereas excess visceral adipose tissue is thought to confer adverse systemic metabolic processes, including insulin resistance (18). Although the full spectrum and mechanisms of these differences are still being defined, and are likely multifactorial, 1 unique property of sc adipose tissue that has recently been demonstrated is its propensity to take on some properties of brown fat under certain conditions in a process often referred to as “beiging” (7). For reasons that also have yet to be fully elucidated, the sc adipose depot is significantly more susceptible to beiging than the visceral depots, for example, in response to cold exposure (7). There is considerable excitement about the medical implications of this beiging process for developing therapies to treat obesity and metabolic disease as the thermogenic beige adipocytes can increase energy expenditure through the uncoupling of the electron transport chain in β-oxidation that diverts energy from ATP production to thermogenesis (7). This process is facilitated by the expression of the quintessential and functionally relevant brown fat-specific protein UCP1, that enables protons to “leak” down their gradient independently from the ATP synthase machinery (7). It is, therefore, particularly intriguing that VDRKO mice were reported to have increased levels of beige, Ucp1-positive, adipocytes in their sc depots (2, 3). In addition, we have previously demonstrated that the regulation of UCP1 by VDR is conserved in humans (14). In this context, it is notable that the process of β-oxidation that is uncoupled by Ucp1 in beige and brown adipocytes relies on the recruitment of FAs as the carbon source. Furthermore, Elovl3 is an elongase that is particularly highly expressed in brown and beige adipocytes and induced over 200-fold in BAT in response to exposure to cold, suggesting functional context specificity (11). In support of this, mice with a deleted Elovl3 gene are unable to hyperrecruit brown fat during acute cold exposure (11). Therefore, we suggest that VDR serves as a modulator of thermogenesis in sc WAT. Intriguingly, even though Elovl3 is not overexpressed in VDRKO BAT (Figure 2B), administration of calcitriol was able to reduce Elovl3 expression in the BAT depot of WT (Figure 3C). These results suggest that the modulation of Elovl3 by vitamin D/VDR is restricted to iWAT under physiological conditions, but in BAT, Elovl3 expression is susceptible to pharmacological inhibition by calcitriol. As under physiological conditions, Elovl3 is highly expressed in BAT, and low in WAT (19), we propose that the context-specific regulation of Elovl3 might enable a restriction of VDR modulation of thermogenesis to iWAT but also raises the potential that the pharmacologic use of vitamin D may have additional effects on brown adipocytes. In sum, our data elucidate a previously unrecognized role for vitamin D/VDR in tissue-specific FA homeostasis that suggest mechanisms by which different adipose depots achieve specific metabolic properties.
Acknowledgments
We thank members of the Feldman laboratory for helpful discussions. B.J.F. is the Bechtel Endowed Faculty Scholar in Pediatric Translational Medicine.
This work was supported by the National Institutes of Health Director's New Innovator Award DP2 OD006740 and Stanford SPARK Translational Research Program at Stanford (B.J.F.). M.G. was supported by a medical student fellowship from the Howard Hughes Medical Institute.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BAT
- brown adipose tissue
- CoA
- coenzyme A
- ChIP
- chromatin immunoprecipitation
- Elovl
- elongation of very long chain fatty acids enzyme
- FA
- fatty acid
- FAS
- FA synthase
- gWAT
- perigonadal WAT
- iWAT
- inguinal WAT
- SFA
- saturated FA
- SVF
- stromal vascular fraction
- VDR
- vitamin D nuclear hormone receptor
- VDRE
- VDR-response element
- VLCFA
- very long-chain FA
- WAT
- white adipose tissue
- WT
- wild-type mice.
References
- 1. Feldman D, Krishnan AV, Swami S, et al. , eds. Osteoporosis. 4th ed San Diego, CA: Elsevier Academic Press; 2013:283–329. [Google Scholar]
- 2. Narvaez CJ, Matthews D, Broun E, Chan M, Welsh J. Lean phenotype and resistance to diet-induced obesity in vitamin D receptor knockout mice correlates with induction of uncoupling protein-1 in white adipose tissue. Endocrinology. 2009;150(2):651–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wong KE, Szeto FL, Zhang W, et al. Involvement of the vitamin D receptor in energy metabolism: regulation of uncoupling proteins. Am J Physiol Endocrinol Metab. 2009;296(4):E820–E828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wong KE, Kong J, Zhang W, et al. Targeted expression of human vitamin D receptor in adipocytes decreases energy expenditure and induces obesity in mice. J Biol Chem. 2011;286(39):33804–33810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ichimura A, Hirasawa A, Hara T, Tsujimoto G. Free fatty acid receptors act as nutrient sensors to regulate energy homeostasis. Prostaglandins Other Lipid Mediat. 2009;89(3–4):82–88. [DOI] [PubMed] [Google Scholar]
- 6. Houten SM, Wanders RJ. A general introduction to the biochemistry of mitochondrial fatty acid β-oxidation. J Inherit Metab Dis. 2010;33(5):469–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19(10):1252–1263. [DOI] [PubMed] [Google Scholar]
- 8. Strable MS, Ntambi JM. Genetic control of de novo lipogenesis: role in diet-induced obesity. Crit Rev Biochem Mol Biol. 2010;45(3):199–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jump DB. Mammalian fatty acid elongases. Methods Mol Biol. 2009;579:375–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ohno Y, Suto S, Yamanaka M, et al. Elovl1 production of C24 acyl-coas is linked to C24 sphingolipid synthesis. Proc Natl Acad Sci USA. 2010;107(43):18439–18444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Westerberg R, Månsson JE, Golozoubova V, et al. Elovl3 Is an important component for early onset of lipid recruitment in brown adipose tissue. J Biol Chem. 2006;281(8):4958–4968. [DOI] [PubMed] [Google Scholar]
- 12. Krueger KC, Costa MJ, Du H, Feldman BJ. Characterization of Cre recombinase activity for in vivo targeting of adipocyte precursor cells. Stem Cell Rep. 2014;3(6):1147–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Costa MJ, So AY, Kaasik K, et al. Circadian rhythm gene period 3 is an inhibitor of the adipocyte cell fate. J Biol Chem. 2011;286(11):9063–9070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Malloy PJ, Feldman BJ. Cell-autonomous regulation of brown fat identity gene Ucp1 by unliganded vitamin D receptor. Mol Endocrinol. 2013;27(10):1632–1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tvrdik P, Asadi A, Kozak LP, Nedergaard J, Cannon B, Jacobsson A. Cig30, a mouse member of a novel membrane protein gene family, is involved in the recruitment of brown adipose tissue. J Biol Chem. 1997;272(50):31738–31746. [DOI] [PubMed] [Google Scholar]
- 16. Sandelin A, Wasserman WW, Lenhard B. Consite: web-based prediction of regulatory elements using cross-species comparison. Nucleic Acids Res. 2004;32(Web Server issue):W249–W252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Oda Y, Uchida Y, Moradian S, Crumrine D, Elias PM, Bikle DD. Vitamin D receptor and coactivators Src2 and 3 regulate epidermis-specific sphingolipid production and permeability barrier formation. J Invest Dermatol. 2009;129(6):1367–1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wajchenberg BL. Subcutaneous and visceral adipose tissue: their relation to the metabolic syndrome. Endocr Rev. 2000;21(6):697–738. [DOI] [PubMed] [Google Scholar]
- 19. Tvrdik P, Westerberg R, Silve S, et al. Role of a new mammalian gene family in the biosynthesis of very long chain fatty acids and sphingolipids. J Cell Biol. 2000;149(3):707–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zella LA, Kim S, Shevde NK, Pike JW. Enhancers located within two introns of the vitamin D receptor gene mediate transcriptional autoregulation by 1,25-dihydroxyvitamin D3. Mol Endocrinol. 2006;20(6):1231–1247. [DOI] [PubMed] [Google Scholar]