Abstract
IGF-1 receptor (IGF-1R) signaling is implicated in cardiac hypertrophy and longevity. However, the role of IGF-1R in age-related cardiac remodeling is only partially understood. We therefore sought to determine whether the deletion of the IGF-1R in cardiomyocytes might delay the development of aging-associated myocardial pathologies by examining 2-year-old male cardiomyocyte-specific IGF-1R knockout (CIGF1RKO) mice. Aging was associated with the induction of IGF-1R expression in hearts. Cardiomyocytes hypertrophied with age in wild-type (WT) mice. In contrast, the cardiac hypertrophic response associated with aging was blunted in CIGF1RKO mice. Concomitantly, fibrosis was reduced in aged CIGF1RKO compared with aged WT hearts. Expression of proinflammatory cytokines such as IL-1α, IL-1β, IL-6, and receptor activator of nuclear factor-κB ligand was increased in aged WT hearts, but this increase was attenuated in aged CIGF1RKO hearts. Phosphorylation of Akt was increased in aged WT, but not in aged CIGF1RKO, hearts. In cultured cardiomyocytes, IGF-1 induced senescence as demonstrated by increased senescence-associated β-galactosidase staining, and a phosphoinositide 3-kinase inhibitor inhibited this effect. Furthermore, inhibition of phosphoinositide 3-kinase significantly prevented the increase in IL-1α, IL-1β, receptor activator of nuclear factor-κB ligand, and p21 protein expression by IGF-1. These data reveal an essential role for the IGF-1-IGF-1R-Akt pathway in mediating cardiomyocyte senescence.
The prevalence of cardiovascular disease increases with advancing age. Most developed countries define elderly as individuals with a chronological age of 65 years or older. The oldest old, commonly defined as older than 85 years of age, have a higher prevalence of heart failure and cardiovascular mortality (1–3). Traditional cardiovascular risk factors such as smoking, hypertension, and diabetes are associated with the development of heart failure in the elderly (4). In addition to long-term exposure to risk factors for heart disease, the aged heart exhibits intrinsic structural remodeling, which reduces cardiac functional reserve and predisposes the heart to hemodynamic stress (5, 6). Senescent remodeling includes left ventricular hypertrophy, diastolic dysfunction, interstitial fibrosis, and a reduction in maximal heart rate (7).
Prior studies report that the reduction of IGF-1 signaling leads to longevity in Caenorhabditis elegans (8), Drosophila (9), and dwarf mice (10–12). Overexpression of the Klotho gene, which suppresses tyrosine phosphorylation of insulin and IGF-1 receptors (IGF-1Rs), extends the life span in mice (13). Notably, IGF-1R heterozygous knockout mice live on average 33% longer than their wild-type (WT) controls without differences in food intake, physical activity, or metabolic rate (14, 15). However, it is not known whether decreasing IGF-1R signaling in the heart can retard cardiac aging. Interfering with insulin-IGF-1 signaling in the heart prevents the decline in cardiac performance in aging fruit flies (16). Furthermore, mice with hepatic deficiency of IGF-1 leading to reduced circulating IGF-1 levels resist aging-induced cardiomyocyte contractile dysfunction (17). This contrasts with findings that the inactivation of cardiomyocyte IGF-1R for 6 weeks by a 4-hydroxytamoxifen-inducible Cre recombinase in 11-month-old mice resulted in slightly depressed diastolic cardiac function compared with the age-matched WT animals (18).
Thus, we sought to test the hypothesis that long-term inactivation of IGF-1R in cardiomyocytes delays the development of aging-associated myocardial pathologies using very old cardiomyocyte-specific IGF-1R knockout mice.
Materials and Methods
Animals
Mice with cardiomyocyte-specific IGF-1R gene ablation (CIGF1RKO) were previously generated using Cre recombinase under the control of the α-myosin heavy-chain promoter (19) and backcrossed to C57BL6/J for nine generations. Animals were fed standard chow and housed in temperature-controlled, pathogen-free facilities with a 12-hour light, 12-hour dark cycle. WT (IGF-1Rfl/fl) and CIGF1RKO (IGF-1Rfl/fl;α-myosin heavy-chain-Cre) male mice at 12–14 weeks of age (young), 75 weeks of age (old), and 105–130 weeks of age (very old) were used in this study. The numbers of mice used in the various studies were three to six per group. The survival cohorts consisted of 30 WT and 31 CIGF1RKO mice and deaths were recorded daily. Survival data were assessed at 120 weeks of age. Mice were not subjected to any invasive or metabolic studies other than echocardiography. All animal experiments were conducted in accordance with guidelines approved by the Institutional Animal Care and Use Committee of the Chung-Ang University. The investigation conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health.
Echocardiography
Mice were anesthetized using 1.5% isoflurane and echocardiography was performed using the Vevo 770 System (VisualSonics Inc) with a 30-MHz transducer in the two-dimensional M mode (20).
Histological analysis
After anesthesia with isoflurane, mice were killed by cervical dislocation. Tissue preparation and staining were performed as described previously (20, 21). For hematoxylin and eosin or Sirius red staining, the myocardium was fixed in 4% paraformaldehyde, embedded in paraffin, and sectioned at a thickness of 6 μm. The slides were examined using an Olympus BX51 microscope. For immunostaining, myocardium was fixed in 4% paraformaldehyde and incubated in 15% sucrose solution overnight at 4°C and then transferred to 30% sucrose at 4°C until the tissue sank. Tissue was infiltrated in optimum cutting temperature-filled (Tissue Tek) molds for 30 minutes at room temperature before freezing. The molds were cooled with liquid nitrogen. After the material had frozen, tissue was wrapped in aluminum foil and stored at −70°C. Tissues were then cryosectioned at 8-μm thickness using a cryostat (Leica) and prefixed in acetone for 30 minutes at −70°C and then dried briefly until the acetone was removed. Optimum cutting temperature was removed with water. Sections were incubated in blocking solution for 4 hours at 37°C, followed by overnight incubation in primary antibodies (1:100 anti-IGF-1R, 1:50 anti-IL-1α [Santa Cruz Biotechnology], and 1:200 anti-α-actinin [clone EA-53; Sigma]) at 4°C. After five washes in 0.3% Triton X-100 in PBS for 15 minutes each, the sections were incubated in secondary antibodies (Alexa 488 conjugated goat antirabbit IgG, Alexa 568 conjugated goat antimouse IgG, Alexa 488 conjugated goat antimouse IgG [Invitrogen Corp]) overnight at 4°C. After washing with 0.3% Triton X-100 in PBS, the sections were mounted with or without propidium iodide. For measurement of the myocyte cross-sectional area, sections were stained for membranes with fluorescein isothiocyanate-conjugated wheat germ agglutinin (Invitrogen). The slides were examined using a confocal microscope (LSM 510 META; Carl Zeiss).
IGF-1 enzyme-linked immunosorbent assay
Serum IGF-1 levels were measured using a quantikine mouse/rat IGF-I immunoassay kit (R&D Systems) with a sensitivity of 3.5 pg/mL and an intra- and interassay precision of less than 5.7% and 9.2% coefficient of variation, respectively.
Cell culture
Two- to 3-day-old Sprague Dawley rats were killed by decapitation and neonatal rat cardiomyocytes (NRCMs) were prepared as described previously (20). NRCMs were incubated in the absence or presence of various IGF-1 concentrations (10, 100, and 1000 ng/mL) for 72 hours.
In situ staining for senescence-associated β-galactosidase (SA-β-gal) activity
The SA-β-gal assay is one of the best-characterized method to measure senescence and is detected by histochemical staining of tissue or cells using the artificial substrate X-galactosidase (X-gal) (22–25). The presence of the SA-β-gal is independent of DNA synthesis and generally distinguishes senescent cells from quiescent cells. Frozen heart sections (6 μm) or NRCMs were washed in PBS (pH 7.4), fixed with 2% formaldehyde and 0.2% glutaraldehyde, and incubated overnight at 37°C in freshly prepared staining buffer (1 mg mL−1 X-gal [5-bromo-4-chloro-3-indolyl-β-D-galactoside), 5 mm K3Fe[CN]6, 5 mm K4Fe [CN]6, and 2 mm MgCl2 in PBS [pH 6.0], or in citrate-buffered saline [pH 4.5]). At the end of the incubation, tissue or cells were washed with PBS and examined at ×400 magnification. To quantify the SA-β-gal activity in the heart, cryosections were also stained with 4′,6′-diamino-2-phenylindole. SA-β-gal positivity was calculated as the number of cells positive for SA-β-gal divided by the number of nuclei (26).
Western blot analysis
Mouse hearts or NRCMs were lysed with lysis buffer (20 mM Tris-HCl, pH 7.4; 1% Triton X-100; 1 mM EDTA; 30 mM HEPES; 50 mM Na4P2O7; 100 mM NaF) containing 1× protease inhibitor cocktail (Roche Molecular Biochemicals) and phosphatase inhibitors. Proteins were resolved by SDS-PAGE and electrotransferred onto nitrocellulose membranes (GE Healthcare). The antibodies used were IGF-1R, phospho-Akt (Ser473), Akt, phospho-ERK (Thr202/Tyr204), ERK, phospho-p38 MAPK (Thr180/Tyr182), p38 MAPK, phosphorylated c-Jun N-terminal kinase (JNK; Thr183/Tyr185), JNK (Cell Signaling Technology), IL-1α, p21 (Santa Cruz Biotechnology), IL-1β, TNFα (Abbiotec), activator of nuclear factor-κB ligand (RANKL) (Abcam). Immunoblotting was detected by a SuperSignal West femto maximum sensitivity substrate (Thermo Fisher Scientific). Densitometric quantitation was achieved using an AlphaImager 2000 (Alpha Innotech Corp).
RNA isolation and quantitative RT-PCR analysis
Total RNA was obtained from hearts using the RNA-STAT 60 reagent (AMS Biotechnology). To quantify transcripts, the LightCycler system (Roche Molecular Biochemicals) was used. PCRs were performed using SYBR Green I master mix and the following primers: Igf-1 (5′-CACCAGCTCCACCACAGC-3′ and 5′-GGGCATGTCAGTGTGGCG-3′), p16 (5′-CAGATTCGAACTGCGAGGA-3′ and 5′-CAGCGGAACACAAAGAGCA-3′), p19 (5′-GAGAGGGTTTTCTTGGTGA-3′ and 5′-AGAAGAGCTGCTACGTGA-3′), p21 (5′-ATGTCCAATCCTGGTGATGT-3′ and 5′-TGCAGCAGGGCAGAGGAAGT-3′), p53 (5′-GAGCTCCCTCTGAGCCAGGA-3′ and 5′-TGGGCCTTCAAAAAACTCCTCA-3′), IL-1α (5′-CCTACTCGTCGGGAGGAGAC-3′ and 5′-CCCGAAATAAGGCTGCTTTT-3′), IL-1β (5′-AGCCCATCCTCTGTCACTCA-3′ and 5′-TGTCGTTGCTTGGTTCTCCT-3′), IL-6 (5′-CAAGAGACTTCCAGCCAGTTGC-3′ and 5′-TGGCCGAGTAGACCTCATAGTGACC-3′), TNFα (5′-TCCCAGGTTCTCTTCAAGGGA-3′ and 5′-GGTGAGGAGCACGTAGTCGG-3′), and RANKL (5′-CATCGGGTTCCCATAAAG-3′ and 5′-GAAGCAAATGTTGGCGTA-3′). To assess the specificity of the amplified PCR products, a postamplification melting curve analysis was performed and relative quantification was calculated using the comparative cycle threshold method.
Statistical analysis
Data are presented as the mean ± SEM. The Shapiro-Wilk test was used to test for normality of variables. Because some variables did not pass the Shapiro-Wilk test, we additionally checked q-q plot, which did not show marked deviation from linearity. Thus, we decided to apply the normal assumptions for the parametric tests.
The results are analyzed with one-way ANOVA or two-way ANOVA, followed by Tukey's post hoc analysis. A two-way ANOVA was performed with age (young vs very old) and genotype (WT vs CIGF1RKO) as the fixed factors. Main and interaction effects were tested at a critical level of α = .05. The statistical analyses were performed with SPSS version 18.0 (IBM Corp).
Results
Aging induces IGF-1R expression in the heart
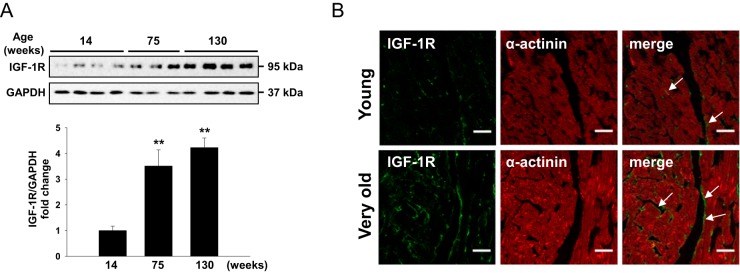
To study the role of IGF-1R in cardiac aging, we first compared IGF-1R levels in young (14 wk old) with old (75 wk old), or very old (130 wk old) WT hearts. Old and very old mice showed increased IGF-1R expression compared with young mice (Figure 1A), suggesting a correlation between IGF-1R signaling and cardiac aging. Immunofluorescence staining of young and very old hearts also confirmed that IGF-1R protein content increased in the aged heart and appeared to be predominantly confined to cardiomyocytes (Figure 1B).
Figure 1.
Aging induces myocardial IGF-1R expression. A, Western blot analysis of IGF-1R protein from young (14 wk old), old (75 wk old), or very old (130 wk old) WT mouse hearts (n = 3–4 per group). Data are presented as the mean ± SEM. Overall value was P < .01 by one-way ANOVA. **, P < .01 vs 14-week-old mice by Tukey's post hoc test. B, Heart cryosections from young and very old WT mice were double stained by immunofluorescence with anti-IGF-1R antibody (green; arrows) and anti-α-actinin antibody (red). Representative images are shown. (magnification, ×40; scale bars, 30 μm). GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
IGF-1R deletion attenuates aging-related cardiac structural remodeling
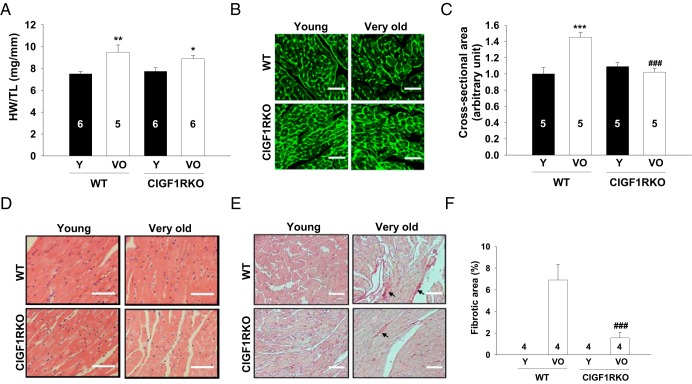
To investigate whether cardiomyocyte IGF-1R is required for the development of aging-associated cardiac remodeling, structure and function in young and aged hearts were analyzed. Heart weight (HW) to tibia length (TL) ratios increased by 26% in very old WT compared with young WT mice. In contrast, very old CIGF1RKO mice showed an attenuated increase in HW/TL ratio relative to young CIGF1RKO mice (Figure 2A). Cardiomyocyte cross-sectional areas measured by wheat germ agglutinin staining significantly increased in very old compared with young WT heart, whereas very old CIGF1RKO mice showed no change relative to corresponding young mice (Figure 2B). The increase in the posterior wall thickness by echocardiography further confirmed ventricular hypertrophy in very old WT mice (Supplemental Table 1). Very old CIGF1RKO mice exhibited preserved cardiac contractile function as demonstrated by comparable fractional shortening despite blunted hypertrophy (Supplemental Table 1). Serum IGF-1 concentration or myocardial IGF-1 expression was not altered in either WT or CIGF1RKO very old mice. Thus changes in endocrine or paracrine IGF-1 production might not be involved in aging-related hypertrophy (Supplemental Figure 1). To determine whether deletion of IGF-1R in cardiomyocytes affects survival rate, we followed up a cohort of WT (n = 30) and CIGF1RKO (n = 31) mice for up to 120 weeks. The survival rates at 120 weeks were similar in WT and CIGF1RKO mice (66% and 68%, respectively) (Supplemental Figure 2). Hematoxylin and eosin staining of very old WT and CIGF1RKO hearts revealed no gross structural differences (Figure 2C). However, aged hearts displayed an increase in interstitial fibrosis (27), as evidenced by Sirius red staining. Very old WT hearts showed an increase in fibrosis compared with young WT hearts, whereas fibrosis was decreased in very old CIGF1RKO mice relative to very old WT mice (Figure 2D).
Figure 2.
IGF-1R deletion attenuates aging-related cardiac hypertrophy and interstitial fibrosis. A, HW to TL ratio. B, Representative immunofluorescence images of ventricular sections stained with fluorescein isothiocyanate-conjugated wheat germ agglutinin (magnification, ×40; scale bars, 30 μm). C, Quantification of cross-sectional area from at least 100 myocytes per ventricle in randomly selected fields of sections from young and very old WT and CIGF1RKO hearts. D, Representative hematoxylin and eosin staining (magnification, ×40; scale bars, 30 μm). E, Representative Sirius red staining (arrows, magnification, ×40; scale bars, 30 μm). F, Quantification of interstitial fibrosis. Numbers of mice are indicated on the bars. Data are presented as the mean ± SEM. Two-way ANOVA was performed to analyze differences by age (young vs very old) and genotype (WT vs CIGF1RKO). **, P < .01 vs young WT; ***, P < .001 vs young WT; *, P < .05 vs young CIGF1RKO; ###, P < .001 vs very old WT. VO, very old; Y, young.
Markers of senescence and proinflammatory cytokines are reduced in very old CIGF1RKO hearts
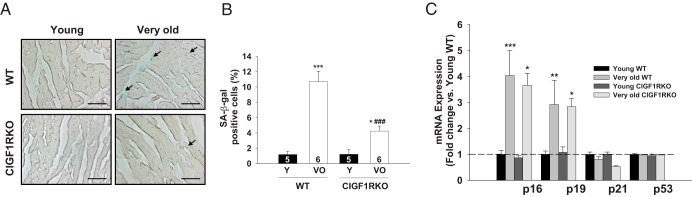
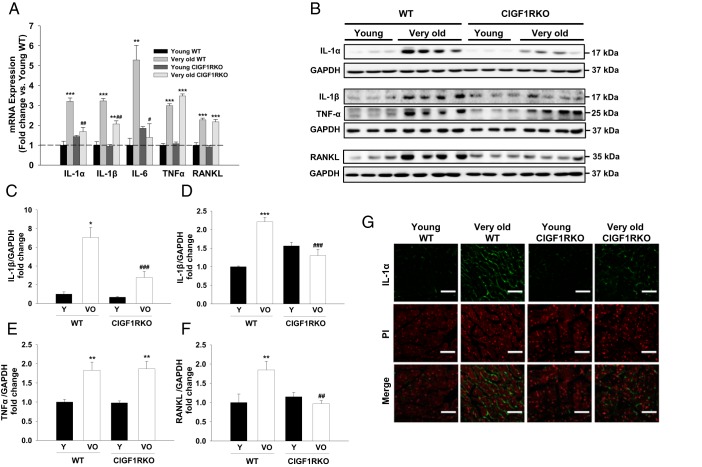
To detect senescence at the cellular level, we evaluated SA-β-gal activity and expression of cell cycle inhibitors, which have been known to be reliable biomarkers (24). SA-β-gal-positive areas were significantly increased in very old WT hearts (9.1-fold vs young WT), but this increase was attenuated in very old CIGF1RKO hearts (3.5-fold vs young CIGF1RKO) (Figure 3, A and B). mRNA expression of cell cycle inhibitors p16 and p19 were significantly increased in both very old WT and very old CIGF1RKO hearts (Figure 3C). Another hallmark of cellular senescence is development of senescence-associated secretory phenotype (SASP), which is characterized by the secretion of inflammatory signals resembling a local immune response (25, 28). Thus, we examined the induction of well-known proinflammatory cytokines in the hearts (20, 29). As shown in Figure 4A, IL-1α, IL-1β, IL-6, TNFα, and RANKL mRNA expressions increased in very old WT hearts (3.2-fold, 3.2-fold, 5.3-fold, 3.0-fold, and 2.3-fold, respectively) relative to young WT hearts, whereas IL-1α, IL-1β, and IL-6 mRNA levels in very old CIGF1RKO hearts were significantly lower than values obtained in very old WT hearts. In addition, levels of IL-1α, IL-1β, TNFα, and RANKL proteins increased in very old WT hearts relative to young WT hearts (Figure 4, B–F). Moreover, IL-1α, IL-1β, and RANKL protein levels in very old CIGF1RKO hearts were significantly lower than those observed in very old WT hearts. In parallel, the amount of positive immunostaining for IL-1α was significantly reduced in very old CIGF1RKO compared with very old WT hearts (Figure 4G). Taken together, these results demonstrate that aging is associated with the induction of proinflammatory cytokines in the heart, and, importantly, this myocardial inflammation is attenuated in the hearts of very old CIGF1RKO mice.
Figure 3.
Senescence markers are decreased in very old CIGF1RKO hearts. A, Sections were stained with SA-β-gal. Representative images are shown (magnification, ×40; scale bars, 30 μm). B, SA-β-gal-positive cells (arrows) were quantified as the number of cells positive for SA-β-gal divided by the number of nuclei. Numbers of mice are indicated on the bars. C, mRNA quantification of cell cycle inhibitors. Results were normalized to actin and mRNA levels in WT hearts were arbitrarily set as 1 (n = 4–6 per group). Data are presented as the mean ± SEM. Two-way ANOVA was performed to analyze differences by age (young vs very old) and genotype (WT vs CIGF1RKO). *, P < .05; **, P < .01; ***, P < .001 vs young WT or young CIGF1RKO; ###, P < .001 vs very old WT. VO, very old; Y, young.
Figure 4.
IGF-1R deletion attenuates aging-related myocardial inflammation. A, mRNA quantification of IL-1α, IL-1β, IL-6, TNFα, and RANKL (n = 4–6 per group). B–F, Western blot analysis and densitometric ratios of IL-1α (C), IL-1 β (D), TNFα (E), and RANKL (F) (n = 3–4 per group). G, Immunostaining for IL-1α (green) is shown overlaid with propidium iodide-stained nuclei (red) (magnification, ×40; scale bars, 30 μm). Data are presented as the mean ± SEM. Two-way ANOVA was performed to analyze differences by age (young vs very old) and genotype (WT vs CIGF1RKO). *, P < .05, **, P < .01, ***, P < .001 vs young WT or young CIGF1RKO; #, P < .05, ##, P < .01, ###, P < .001 vs very old WT. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; VO, very old; Y, young.
Very old CIGF1RKO mice exhibit impaired activation of Akt signaling
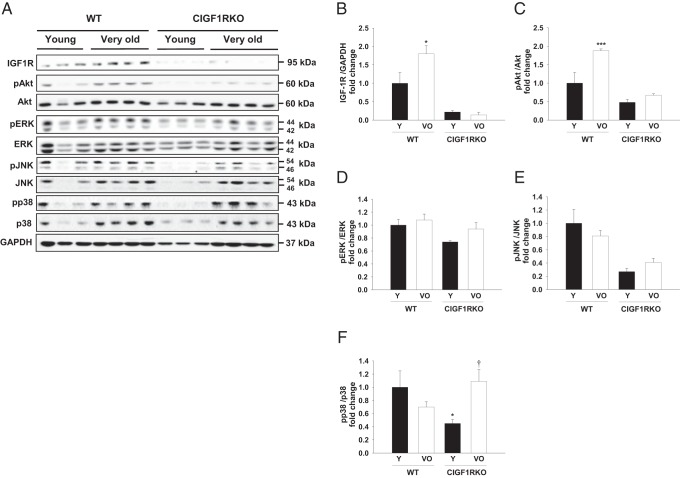
To further investigate potential signal transduction mechanisms that were associated with aging-related cardiac structural remodeling, we measured changes in the phosphoinositide 3-kinases (PI3K) and MAPK pathways, which are effectors of IGF-1R signaling. As expected, IGF-1R expression was nearly absent in both young and very old CIGF1RKO hearts (Figure 5, A and B). Akt signaling was activated in very old WT hearts but not in very old CIGF1RKO hearts (Figure 5, A and C). Whereas the phosphorylation of ERK showed no senescence-associated differences (Figure 5, A and D), a similar pattern was found with respect to phosphorylation of phosphorylated JNK (Figure 5, A and E). The phosphorylation of p38 in young CIGF1RKO hearts was significantly lower than that in young WT hearts but higher with aging (Figure 5, A and F).
Figure 5.
Western blot analysis and densitometric ratios of IGF-1R downstream signaling. A, Immunoblot and densitometric analysis of the following: IGF-1R (B), phosphorylated Akt (C), phosphorylated ERK (D), phosphorylated JNK (E), and phosphorylated p38 (F) in hearts from WT and CIGF1RKO mice (n = 3–4 per group). Data are presented as the mean ± SEM. Two-way ANOVA was performed to analyze differences by age (young vs very old) and genotype (WT vs CIGF1RKO). *, P < .05, ***, P < .001 vs young WT; †, P < .05 vs young CIGF1RKO. GAPDH, glyceraldehyde-3-phosphate dehydrogenase; VO, very old; Y, young.
IGF-1 induces senescence through Akt pathway in cultured NRCMs
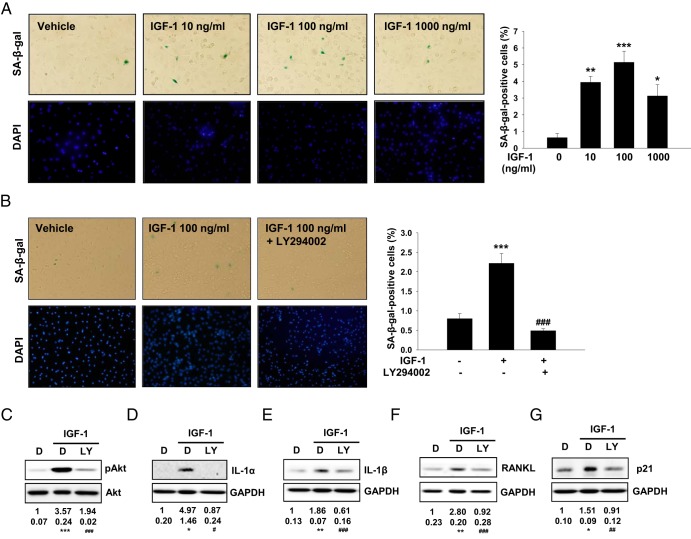
To clarify whether IGF-1-IGF-1R-Akt signaling could regulate markers of cardiomyocyte senescence in a cell-autonomous manner, we treated neonatal ventricular myocytes (NRCMs) with IGF-1. Treatment of NRCMs with IGF-1 (10–1000 ng/mL for 72 hours resulted in cellular senescence as assessed by SA-β-gal-positive cells, reaching a maximum at 100 ng/mL (Figure 6A). To determine whether the IGF-1 modulation of senescence in NRCMs is specifically mediated through Akt, a PI3K inhibitor, LY294002 (30 μm) was added 30 minutes prior to the addition of IGF-1. LY294002 decreased the percentage of SA-β-gal-positive cells (Figure 6, B and C), indicating that Akt signaling is required for IGF-1-induced cellular senescence. Furthermore, inhibition of PI3K significantly prevented the increase in IL-1α, IL-1β, RANKL, and p21 protein expression induced by IGF-1 (Figure 6, D–G). These in vitro data provide additional evidence that IGF-1 signaling promotes cardiomyocyte senescence potentially through the Akt signaling pathway.
Figure 6.
IGF-1 induces senescent-like phenotype in cultured NRCMs. A, Quantitation of SA-β-gal activity after treatment with various concentrations of IGF-1 (10–1000 ng/mL) for 72 hours. SA-β-gal-positive cells were quantified. Representative images are shown (left panels). B, Effect of PI3K inhibition on IGF-1-induced senescence. Cells were pretreated with vehicle (DMSO) or 30 μM PI3K inhibitor (LY294002) for 30 minutes and then incubated with 100 ng/mL IGF-1 for 72 hours. SA-β-gal-positive cells were quantified. Representative images are shown (left panels). Western blot analysis and densitometric ratios of Akt phosphorylation (C), IL-1α (D), IL-1β (E), RANKL (F), p21 (G) after treatment with 100 ng/mL IGF-1 for 72 hours in the absence or presence of 30 μM LY294002 (n = 4–6), in triplicate are shown. Overall P < .05 (D), P < .01 (G), and P < .001 (C, E, and F) were performed by one-way ANOVA. *, P < .05, **, P < .01, ***, P < .001 vs control; ###, P < .001 vs IGF-1 by Tukey's post hoc test. DAPI, 4′,6′-diamino-2-phenylindole; DMSO, dimethylsulfoxide; LY, LY294002.
Discussion
The present study demonstrates that deletion of IGF-1R in cardiomyocytes attenuated aging-related cardiac pathologies, including ventricular hypertrophy, interstitial fibrosis, and inflammation. Mechanistically we showed that IGF-IGF-1R-Akt signaling may be an essential regulator of cardiomyocyte senescence in vivo and in vitro.
IGF-1 is primarily produced in the liver after the stimulation by pituitary-derived GH and acts by binding to IGF-1Rs to promote organ growth. The mRNA expression of GH in the pituitary declines with age in mice, as they do in humans (30). In parallel, serum IGF-1 levels decline progressively in healthy people from early adulthood to older age (31). In the Framingham Heart Study, serum IGF-1 concentrations were inversely related to the risk of congestive heart failure in elderly people without a previous myocardial infarction (32). However, both low- and high-serum concentrations of IGF-I have been reported to correlate with increased risk of cardiovascular mortality (33). We did not find decreased serum levels of IGF-1 in aged WT mice, which might represent interspecies differences. Of note, IGF-1 is also produced and released by cardiac fibroblasts and promotes collagen synthesis by fibroblasts in an autocrine manner and cardiomyocyte hypertrophy via paracrine signaling (34, 35). It has been reported that continuous myocardial expression of IGF-1 in transgenic mice initially induced physiological hypertrophy, but later in life this hypertrophy progressed to a pathological condition characterized by decreased systolic performance and increased interstitial fibrosis (36).
Our data provide novel evidence that physiological IGF-1R signaling promotes cardiomyocyte senescence and that long-term deletion of IGF-1R in cardiomyocytes prevents structural deterioration in aged hearts. It seems paradoxical that IGF-1R expression increased in aged hearts, given that local IGF-1 or IGF-1R levels may decline with aging in mice. For example, in cerebral vasculature, expression of IGF-1 significantly decreases with age (37). Although the underlying mechanism is not understood, we speculate that induction of IGF-1R but not IGF-1 in aging hearts could represent a compensatory mechanism that promotes aging-associated cardiac remodeling. Indeed, we showed previously that IGF-1R expression is increased in cardiomyocytes in response to exercise training and that genetic deletion of IGF-1R significantly attenuated exercise-induced cardiac hypertrophy (19). Thus, it could be anticipated that the very old CIGF1RKO mice would display blunted cardiac hypertrophy under natural aging circumstances. Furthermore, aging-related fibrosis was diminished in CIGF1RKO hearts. Interestingly, despite substantial structural remodeling in very old WT hearts, systolic function as measured by M-mode echocardiography remained in the normal range. The effect of aging on systolic function in mice remains controversial (26, 27, 38, 39), but age-related cardiac structural changes have been known to parallel the development of diastolic dysfunction in mice (40). Thus, this study is limited by that lack of Doppler echocardiography or invasive hemodynamic measurements that would provide information about diastolic function. Despite equivalent levels of systolic cardiac function, the decreased fibrosis in CIGF1RKO could be associated with an increased ability to adapt to hemodynamic stress. The capacity for adaptation to hemodynamic stress and ischemia is diminished in aged myocardium (41). Therefore, future studies using models of pressure overload or ischemia will further elucidate the protective role of IGF-1R in very old hearts.
Cellular senescence is defined as an essentially irreversible arrest of cell proliferation or growth that is initiated by genomic or epigenomic damage (42). In many tissues, characteristics of cellular senescence include SA-β-gal activity, telomere shortening, DNA damage, proinflammatory properties, and increased reactive oxygen species (24). Our data showed that SA-β-gal activity was increased in very old WT hearts, which is consistent with previous reports (26, 43). Inuzuka et al (26) reported that the expression of p16 and p19 mRNA, but not p21 and p53 mRNA, was increased in aged FVB mice hearts. However, aged mice on a mixed genetic background did not show any increase in cell cycle inhibitor expression (43). In the present study, of the four cell cycle inhibitors examined, only p16 and p19 mRNA levels were increased in very old hearts, suggesting differences that could potentially be attributable to differences in the mouse strain used. Although expression of p16 and p19 mRNA was not altered in very old CIGF1RKO hearts, it is interesting that p21 mRNA expression trended lower in very old CIGF1RKO compared with young CIGF1RKO hearts. Further detailed studies will be needed to investigate the mechanisms and consequences for these differences in cell cycle regulation in murine hearts.
The heart produces proinflammatory cytokines such as TNFα, IL-1α, IL-1β, IL-6, and RANKL in pathological states, and these cytokines may promote cardiac remodeling by facilitating hypertrophy and fibrosis (20, 44, 45). Moreover, chronic inflammation is a characteristic of aging, and senescent cells secrete components of the SASP, including proinflammatory cytokines, chemokines, and proteases (25). Although the role of SASP in aging phenotypes has been extensively investigated, the association of cardiac inflammation with aging is not clearly defined. One of the salient features of our findings is that aging induces proinflammatory cytokines in the heart mediated by, at least in part, the IGF-1R system.
Phosphorylation of Akt was increased in very old WT hearts but not in very old CIGF1RKO hearts, suggesting IGF-1R-Akt signaling pathways may promote cardiac aging. Previous studies demonstrated that IGF-1 enhances cellular senescence in rat vascular smooth muscle cells and human fibroblasts (46). This prompted us to perform in vitro experiments to determine whether IGF-1 could induce senescence-associated proinflammatory cytokine induction in primary cultured cardiomyocytes. Pretreatment with the PI3K inhibitor LY294002 attenuated IGF-1-induced senescence in NRCMs. Suppression of PI3K prevented cardiac aging, whereas cardiac-specific Akt overexpression accentuated aging-induced cardiac hypertrophy and promoted mitochondrial and myocardial contractile dysfunction (26, 47, 48). These data support the hypothesis that a major mechanism by which disruption of IGF-1R signaling attenuates senescence cardiomyocytes is by inhibition of Akt signaling.
In summary, we have provided direct evidence that long-term deletion of myocardial IGF-1R in mice rescues aging-related cardiac pathologies and may provide the rationale for exploring the possibility of antagonizing myocardial IGF-1R signaling to delay aging-related adverse cardiac remodeling that may predispose to hemodynamic stress.
Acknowledgments
Present address for J.A.: Korea Medical Institute, 142–35 Samsung dong, Gangnamgu, Seoul, Korea.
This work was supported by grants (to J.K. in 2008; to J.A. in 2012) from the Korean Diabetes Association. E.D.A. was supported by National Institutes of Health Grant R01DK092065.
Disclosure Summary: The authors have nothing to disclose.
For News & Views see page 44
- CIGF1RKO
- cardiomyocyte-specific IGF-1R knockout
- HW
- heart weight
- IGF-1R
- IGF-1 receptor
- JNK
- c-Jun N-terminal kinase
- NRCM
- neonatal rat cardiomyocyte
- PI3K
- phosphoinositide 3-kinase
- RANKL
- receptor activator of nuclear factor-κB ligand
- SA-β-gal
- senescence-associated β-galactosidase
- SASP
- senescence-associated secretory phenotype
- TL
- tibia length
- WT
- wild type
- X-gal
- X-galactosidase.
References
- 1. Suzman RM. Introducing the oldest old. In: Suzman RM, Willis DP, Manton KG, eds. The Oldest Old. New York: Oxford University Press; 1992:3–16. [Google Scholar]
- 2. Heidenreich PA, Trogdon JG, Khavjou OA, et al. Forecasting the future of cardiovascular disease in the United States: a policy statement from the American Heart Association. Circulation. 2011;123:933–944. [DOI] [PubMed] [Google Scholar]
- 3. Vigen R, Maddox TM, Allen LA. Aging of the United States population: impact on heart failure. Curr Heart Fail Rep. 2012;9:369–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen YT, Vaccarino V, Williams CS, Butler J, Berkman LF, Krumholz HM. Risk factors for heart failure in the elderly: a prospective community-based study. Am J Med. 1999;106:605–612. [DOI] [PubMed] [Google Scholar]
- 5. Oxenham H, Sharpe N. Cardiovascular aging and heart failure. Eur J Heart Fail. 2003;5:427–434. [DOI] [PubMed] [Google Scholar]
- 6. Dai DF, Rabinovitch PS. Cardiac aging in mice and humans: the role of mitochondrial oxidative stress. Trends Cardiovasc Med. 2009;19:213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Fontana L, Vinciguerra M, Longo VD. Growth factors, nutrient signaling, and cardiovascular aging. Circ Res. 2012;110:1139–1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kimura KD, Tissenbaum HA, Liu Y, Ruvkun G. daf-2, an insulin receptor-like gene that regulates longevity and diapause in Caenorhabditis elegans. Science. 1997;277:942–946. [DOI] [PubMed] [Google Scholar]
- 9. Clancy DJ, Gems D, Harshman LG, et al. Extension of life-span by loss of CHICO, a Drosophila insulin receptor substrate protein. Science. 2001;292:104–106. [DOI] [PubMed] [Google Scholar]
- 10. Brown-Borg HM, Borg KE, Meliska CJ, Bartke A. Dwarf mice and the ageing process. Nature. 1996;384:33. [DOI] [PubMed] [Google Scholar]
- 11. Flurkey K, Papaconstantinou J, Miller RA, Harrison DE. Lifespan extension and delayed immune and collagen aging in mutant mice with defects in growth hormone production. Proc Natl Acad Sci USA. 2001;98:6736–6741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Coschigano KT, Clemmons D, Bellush LL, Kopchick JJ. Assessment of growth parameters and life span of GHR/BP gene-disrupted mice. Endocrinology. 2000;141:2608–2613. [DOI] [PubMed] [Google Scholar]
- 13. Kurosu H, Yamamoto M, Clark JD, et al. Suppression of aging in mice by the hormone Klotho. Science. 2005;309:1829–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Holzenberger M, Dupont J, Ducos B, et al. IGF-1 receptor regulates lifespan and resistance to oxidative stress in mice. Nature. 2003;421:182–187. [DOI] [PubMed] [Google Scholar]
- 15. Xu J, Gontier G, Chaker Z, Lacube P, Dupont J, Holzenberger M. Longevity effect of IGF-1R(+/−) mutation depends on genetic background-specific receptor activation. Aging Cell. 2014;13:19–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wessells RJ, Fitzgerald E, Cypser JR, Tatar M, Bodmer R. Insulin regulation of heart function in aging fruit flies. Nat Genet. 2004;36:1275–1281. [DOI] [PubMed] [Google Scholar]
- 17. Li Q, Ceylan-Isik AF, Li J, Ren J. Deficiency of insulin-like growth factor 1 reduces sensitivity to aging-associated cardiomyocyte dysfunction. Rejuvenation Res. 2008;11:725–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moellendorf S, Kessels C, Peiseler L, et al. IGF-IR signaling attenuates the age-related decline of diastolic cardiac function. Am J Physiol Endocrinol Metab. 2012;303:E213–E222. [DOI] [PubMed] [Google Scholar]
- 19. Kim J, Wende AR, Sena S, et al. Insulin-like growth factor I receptor signaling is required for exercise-induced cardiac hypertrophy. Mol Endocrinol. 2008;22:2531–2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ock S, Ahn J, Lee SH, et al. Receptor activator of nuclear factor-κB ligand is a novel inducer of myocardial inflammation. Cardiovasc Res. 2012;94:105–114. [DOI] [PubMed] [Google Scholar]
- 21. Satoh M, Ogita H, Takeshita K, Mukai Y, Kwiatkowski DJ, Liao JK. Requirement of Rac1 in the development of cardiac hypertrophy. Proc Natl Acad Sci USA. 2006;103:7432–7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lee BY, Han JA, Im JS, et al. Senescence-associated β-galactosidase is lysosomal beta-galactosidase. Aging Cell. 2006;5:187–195. [DOI] [PubMed] [Google Scholar]
- 23. Itahana K, Campisi J, Dimri GP. Methods to detect biomarkers of cellular senescence: the senescence-associated β-galactosidase assay. Methods Mol Biol. 2007;371:21–31. [DOI] [PubMed] [Google Scholar]
- 24. Bernardes de Jesus B, Blasco MA. Assessing cell and organ senescence biomarkers. Circ Res. 2012;111:97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest. 2013;123:966–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Inuzuka Y, Okuda J, Kawashima T, et al. Suppression of phosphoinositide 3-kinase prevents cardiac aging in mice. Circulation. 2009;120:1695–1703. [DOI] [PubMed] [Google Scholar]
- 27. Boyle AJ, Shih H, Hwang J, et al. Cardiomyopathy of aging in the mammalian heart is characterized by myocardial hypertrophy, fibrosis and a predisposition towards cardiomyocyte apoptosis and autophagy. Exp Gerontol. 2011;46:549–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Freund A, Orjalo AV, Desprez PY, Campisi J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol Med. 2010;16:238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Abe J. Role of PKCs and NF-κB activation in myocardial inflammation: enemy or ally? J Mol Cell Cardiol. 2007;43:404–408. [DOI] [PubMed] [Google Scholar]
- 30. Crew MD, Spindler SR, Walford RL, Koizumi A. Age-related decrease of growth hormone and prolactin gene expression in the mouse pituitary. Endocrinology. 1987;121:1251–1255. [DOI] [PubMed] [Google Scholar]
- 31. Leifke E, Gorenoi V, Wichers C, Von Zur Muhlen A, Von Buren E, Brabant G. Age-related changes of serum sex hormones, insulin-like growth factor-1 and sex-hormone binding globulin levels in men: cross-sectional data from a healthy male cohort. Clin Endocrinol (Oxf). 2000;53:689–695. [DOI] [PubMed] [Google Scholar]
- 32. Vasan RS, Sullivan LM, D'Agostino RB, et al. Serum insulin-like growth factor I and risk for heart failure in elderly individuals without a previous myocardial infarction: the Framingham Heart Study. Ann Intern Med. 2003;139:642–648. [DOI] [PubMed] [Google Scholar]
- 33. van Bunderen CC, van Nieuwpoort IC, van Schoor NM, Deeg DJ, Lips P, Drent ML. The association of serum insulin-like growth factor-I with mortality, cardiovascular disease, and cancer in the elderly: a population-based study. J Clin Endocrinol Metab. 2010;95:4616–4624. [DOI] [PubMed] [Google Scholar]
- 34. Horio T, Maki T, Kishimoto I, et al. Production and autocrine/paracrine effects of endogenous insulin-like growth factor-1 in rat cardiac fibroblasts. Regul Pept. 2005;124:65–72. [DOI] [PubMed] [Google Scholar]
- 35. Takeda N, Manabe I, Uchino Y, et al. Cardiac fibroblasts are essential for the adaptive response of the murine heart to pressure overload. J Clin Invest. 2010;120:254–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Delaughter MC, Taffet GE, Fiorotto ML, Entman ML, Schwartz RJ. Local insulin-like growth factor I expression induces physiologic, then pathologic, cardiac hypertrophy in transgenic mice. FASEB J. 1999;13:1923–1929. [DOI] [PubMed] [Google Scholar]
- 37. Ungvari Z, Csiszar A. The emerging role of IGF-1 deficiency in cardiovascular aging: recent advances. J Gerontol A Biol Sci Med Sci. 2012;67:599–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Bujak M, Kweon HJ, Chatila K, Li N, Taffet G, Frangogiannis NG. Aging-related defects are associated with adverse cardiac remodeling in a mouse model of reperfused myocardial infarction. J Am Coll Cardiol. 2008;51:1384–1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ma H, Wang J, Thomas DP, et al. Impaired macrophage migration inhibitory factor-AMP-activated protein kinase activation and ischemic recovery in the senescent heart. Circulation. 2010;122:282–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dai DF, Chen T, Johnson SC, Szeto H, Rabinovitch PS. Cardiac aging: from molecular mechanisms to significance in human health and disease. Antioxid Redox Signal. 2012;16:1492–1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Isoyama S, Nitta-Komatsubara Y. Acute and chronic adaptation to hemodynamic overload and ischemia in the aged heart. Heart Fail Rev. 2002;7:63–69. [DOI] [PubMed] [Google Scholar]
- 42. Campisi J. Aging, cellular senescence, and cancer. Annu Rev Physiol. 2013;75:685–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Yuan Q, Chen Z, Santulli G, et al. Functional role of calstabin2 in age-related cardiac alterations. Sci Rep. 2014;4:7425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kleinbongard P, Schulz R, Heusch G. TNFα in myocardial ischemia/reperfusion, remodeling and heart failure. Heart Fail Rev. 2011;16:49–69. [DOI] [PubMed] [Google Scholar]
- 45. Shimano M, Ouchi N, Walsh K. Cardiokines: recent progress in elucidating the cardiac secretome. Circulation. 2012;126:e327–e332. [DOI] [PubMed] [Google Scholar]
- 46. Handayaningsih AE, Takahashi M, Fukuoka H, et al. IGF-I enhances cellular senescence via the reactive oxygen species-p53 pathway. Biochem Biophys Res Commun. 2012;425:478–484. [DOI] [PubMed] [Google Scholar]
- 47. Hua Y, Zhang Y, Ceylan-Isik AF, Wold LE, Nunn JM, Ren J. Chronic Akt activation accentuates aging-induced cardiac hypertrophy and myocardial contractile dysfunction: role of autophagy. Basic Res Cardiol. 2011;106:1173–1191. [DOI] [PubMed] [Google Scholar]
- 48. Wende AR, O'Neill BT, Bugger H, et al. Enhanced cardiac Akt/protein kinase B signaling contributes to pathological cardiac hypertrophy in part by impairing mitochondrial function via transcriptional repression of mitochondrion-targeted nuclear genes. Mol Cell Biol. 2015;35:831–846. [DOI] [PMC free article] [PubMed] [Google Scholar]