Abstract
Embryonic poly(A)-binding protein (EPAB) is the predominant poly(A)-binding protein in Xenopus, mouse, and human oocytes and early embryos before zygotic genome activation. EPAB is required for translational activation of maternally stored mRNAs in the oocyte and Epab−/− female mice are infertile due to impaired oocyte maturation, cumulus expansion, and ovulation. The aim of this study was to characterize the mechanism of follicular somatic cell dysfunction in Epab−/− mice. Using a coculture system of oocytectomized cumulus oophorus complexes (OOXs) with denuded oocytes, we found that when wild-type OOXs were cocultured with Epab−/− oocytes, or when Epab−/− OOXs were cocultured with WT oocytes, cumulus expansion failed to occur in response to epidermal growth factor (EGF). This finding suggests that oocytes and cumulus cells (CCs) from Epab−/− mice fail to send and receive the necessary signals required for cumulus expansion. The abnormalities in Epab−/− CCs are not due to lower expression of the oocyte-derived factors growth differentiation factor 9 or bone morphogenetic protein 15, because Epab−/− oocytes express these proteins at comparable levels with WT. Epab−/− granulosa cells (GCs) exhibit decreased levels of phosphorylated MEK1/2, ERK1/2, and p90 ribosomal S6 kinase in response to lutenizing hormone and EGF treatment, as well as decreased phosphorylation of the EGF receptor. In conclusion, EPAB, which is oocyte specific, is required for the ability of CCs and GCs to become responsive to LH and EGF signaling. These results emphasize the importance of oocyte-somatic communication for GC and CC function.
In the mammalian ovary, oocytes reside within follicles and are arrested at prophase of the first meiotic division (1). The growth of immature early antral follicles to the preovulatory stage is promoted by the pituitary gonadotropin follicle stimulating hormone (FSH) (2). At the preovulatory stage, a surge of LH triggers the resumption of meiosis, cumulus expansion, and ovulation (3). During this process, cumulus cells (CCs) and granulosa cells (GCs) are reprogrammed to express specific genes required for their terminal differentiation; and CCs produce hyaluronic acid, which expands the space between the cells, embedding them in a mucinous matrix (4, 5). These events are coordinated so that a developmentally competent egg is ovulated into the oviduct to await fertilization (6).
Bidirectional communication between the oocyte and somatic compartment is essential for normal folliculogenesis. CCs and GCs provide nutrients and regulatory signals to the oocyte required for metabolism, oocyte growth, and meiotic and developmental competence (7, 8). In turn, the oocyte controls GC differentiation and function (9, 10). One important way in which the oocyte contributes to the somatic cells is by secreting soluble growth factors, such as growth differentiation factor 9 (GDF9) and bone morphogenetic protein 15 (BMP15). GDF9 and BMP15 belong to the TGF-β superfamily (11, 12), are expressed exclusively in the oocyte (13, 14), and are critical local regulators of ovarian function (15–17). These oocyte-secreted factors lead to activation of SMAD2/3 and MAPK signaling in CCs, which in turn regulate CC gene expression and key cumulus functions (18). GDF9 and BMP15 also enhance hyaluronic acid synthesis in CCs, an essential step in ovulation (19, 20). Therefore, the appropriate cooperation between oocyte-derived growth factors and somatic cells is required for folliculogenesis, cumulus expansion, and ovulation.
In addition, accumulation of epidermal growth factor (EGF)-like growth factors and transactivation of EGF receptor (EGFR) signaling are critical events for LH-induced cumulus expansion and ovulation (21–23). In support of this, the disruption of the EGF signaling network in mice leads to impairment of LH-induced ovulation in vivo (24). EGF-like growth factors amphiregulin (AREG), epiregulin (EREG), and betacellulin (BTC) are rapidly induced by LH, or its analog human chorionic gonadotropin in mural GCs, and function in an autocrine and paracrine manner to transmit LH signals via EGFRs in GCs and CCs (22, 25–27). Cumulus expansion and oocyte meiotic resumption of cumulus oophorus complexes (COCs) can be induced upon LH, EGF, or AREG treatment in vitro, which act in a manner similar to that observed by LH/human chorionic gonadotropin in ovarian follicles in vivo (28–30). The most prominent downstream target of EGFR signaling is the MAPK cascade, which is activated by EGF-like factors, and subsequently elicits distinct biological effects, such as cumulus expansion and ovulation (23). Collectively, these findings support the critical role for the EGF network in integrating the function of GCs/CCs/oocytes within the preovulatory follicle.
A complex network of translational activation and repression of dormant maternal mRNAs is required for nuclear and cytoplasmic maturation of the oocyte (31–33). Because fully grown oocytes are transcriptionally silent, oocyte maturation and early developmental processes rely exclusively on maternal mRNAs that are synthesized and stored in advance. Translational activation of stored maternal mRNAs occurs primarily by cytoplasmic polyadenylation (31, 34), which drives the oocyte's reentry into meiosis and controls the gene expression during oocyte maturation, fertilization and early embryo development, until zygotic genome activation (34–37). This process requires cytoplasmic polyadenylation element binding protein 1, which becomes phosphorylated upon stimulation of oocyte maturation, and leads to the elongation of the poly(A) tail of bound transcript by activating a poly(A) polymerase complex (38). It has been demonstrated that several dormant mRNAs in the oocyte have short poly(A) tails, which elongate in response to an exogenous cue (eg, hormonal stimulation or fertilization), and become translated (39–41). The process of translational activation during oocyte maturation is complex (42) and additional pathways independent of cytoplasmic polyadenylation, such as those involving deleted in azoospermia-like (43–45), have also been identified.
Embryonic poly(A)-binding protein (EPAB) is the predominant poly(A) binding protein in Xenopus, mouse, and human germ cells and early embryos until zygotic genome activation, when it is replaced by the somatic cytoplasmic poly(A) binding protein (PABPC1) (42, 46, 47). EPAB is associated with both polyadenylation dependent (cytoplasmic polyadenylation element binding protein 1-/Symplekin/Cleavage and polyadenylation specific factor-Cleavage and polyadenylation specific factor) (48) and independent (deleted in azoospermia-like-Pumilio 2) (44) complexes that mediate translation activation in the oocyte. In Xenopus oocytes, EPAB prevents deadenylation of mRNAs (49), promotes cytoplasmic polyadenylation (48), enhances translation initiation (50), and is required for maturation (51). Epab-deficient female mice are infertile due to impaired translational activation of maternal mRNAs and oocyte maturation (52). In addition, Epab-deficient mice exhibit defective cumulus expansion and ovulation in vivo, suggesting that the LH-mediated preovulatory changes fail to occur in the somatic cells of the follicle (52).
The aim of the current study was to characterize the role of EPAB in regulating GC/CC function. We hypothesized that EPAB is involved in promoting an oocyte-specific signal that is communicated to the surrounding somatic cells and is required for cumulus expansion. We used an established coculture system of denuded oocytes (DOs) and oocytectomized COCs (OOXs) to assess the interactions between the oocyte and the somatic cells in Epab−/− knockout (KO) mice. We found that cumulus expansion failed to occur both in the WT-OOX/KO-oocyte and the KO-OOX/WT-oocyte coculture system, suggesting that not only do Epab−/− oocytes fail to send necessary signals that promote cumulus expansion, but that the Epab−/− CCs fail to become responsive to oocyte-derived signals. EPAB acts through a pathway that is independent of the oocyte-derived GDF9/BMP15 system, as the protein expression of these factors are similar in WT and Epab−/− oocytes. Importantly, the expression of downstream targets are significantly reduced in response to LH, EGF, and AREG in the GCs of Epab−/− mice, which could in part be due to lower phosphorylation levels of the EGFR. Overall, these findings demonstrate that oocyte-specific EPAB is required for the competency of the somatic compartment.
Materials and Methods
Mice
Mice were bred and maintained according to the Yale University animal research requirements. All animal protocols were approved by the Institutional Animal Care and Use Committee (protocol 2011–11027) before the initiation of the studies. The Epab−/− knockout mice were generated as previously described (52).
Isolation and culture of COCs, OOXs, DOs, and evaluation of in vitro cumulus expansion
Denuded oocytes (DOs) and cumulus oophorus complexes (COCs) were collected from the ovaries of 12-week-old WT (Epab+/+) and Epab−/− mice 44–48 hours after ip injection of 5-IU pregnant mare serum gonadotropin (PMSG) (Sigma-Aldrich). Ovaries were punctured with a 26½-gauge needle and COCs were released from the follicles in M2 medium (Sigma-Aldrich). DOs were isolated from COCs by repeat pipetting with a pipette. Oocytectomized complexes (OOXs) were produced using microsurgical technique in order to remove oocytes from the COCs, as previously described (53, 54).
For coculture experiments, 10 WT OOXs or Epab−/− OOXs were cultured with 10 WT or Epab−/− (KO) oocytes in a 20-μL drop of basic medium (α-MEM supplemented with 75-μg/mL penicillin G, 50-μg/mL streptomycin sulfate, 0.23mM pyruvate, and 3-mg/mL BSA [Sigma-Aldrich]) with or without 10-ng/mL EGF (SRP3196; Sigma-Aldrich), covered by liquid paraffin oil (Sigma). WT COCs were used as a positive control. Complexes were incubated for up to 16 hours in a humidified atmosphere at 37°C with 5% CO2 and evaluated for cumulus expansion.
Cumulus expansion was assessed using a previously described scoring (scores of 0–4) system (55). Score of 0–1 indicates no expansion or minimum expansion; score of 2 indicates that cells in the outer 2 layers began to expand; score of 3 indicates expansion of all layers of the cumulus except corona radiata cells; and score of 4 indicates expansion of the whole cumulus, including corona radiata cells. After scoring, CCs were removed from COCs and OOXs, and the expression of cumulus expansion-related transcripts was determined by real-time PCR.
Isolation and culture of GCs
To test the response of GCs to LH, EGF, and AREG stimulation in vitro, GCs were isolated from ovaries of 12-week-old WT and Epab−/− mice 48 hours after ip injection of 5-IU PMSG (Sigma-Aldrich). Ovaries were punctured using a 26½-gauge needle allowing the release of COCs and GCs from follicles. The COCs were collected and CCs were isolated via mechanical manipulation in HEPES-buffered medium containing 1-mg/mL hyaluronidase (Sigma-Aldrich). The remaining GCs were filtered through a 0.4-μm strainer and centrifuged for 5 minutes at 1500g. For in vitro stimulation experiments, GCs were resuspended in DMEM (Invitrogen) supplemented with 5% fetal bovine serum (Invitrogen) and 1% antimycotic-antibiotics (Gibco). They were cultured in a humidified atmosphere at 37°C with 5% CO2 to allow the cells to adhere to the bottom of the dishes. At 70% confluence, they were serum starved for 12 hours stimulated with either 1-μg/mL LH (39341-83-8; EMD Chemicals, Inc), 10-ng/mL EGF (SRP3196; Sigma-Aldrich Co), or 100-ng/mL AREG (989-AR-100/CF; R&D Systems, Inc).
Western blotting
GCs were washed with PBS and incubated in lysis buffer (20mM Tris-HCL [pH 8.0], 5mM MgCl2, 10mM EGTA [pH 8.0], 1% Triton X-100, 1mM Na3VO4, 50mM NaF, complete protease inhibitor cocktail [Roche Diagnostics[ per 10 mL of buffer) for 15 minutes on ice with shaking. Lysates were cleared by centrifugation at 12 000g for 30 minutes at 4°C. Samples were prepared with 2× sodium dodecyl sulfate sample buffer, separated by 10% sodium dodecyl sulfate-PAGE (Bio-Rad Laboratories), and transferred to polyvinylidene fluoride membrane (Bio-Rad Laboratories) at 85 V for 2 hours. The membrane was blocked with 5% BSA in Tris-buffered saline with Tween-20 for 1 hour at room temperature and incubated with primary antibody diluted in 5% BSA in TBS-T at 4°C overnight. Then membrane was washed 3 times in TBS-T and incubated with horseradish peroxidase-conjugated secondary antibody (1:5000; Chemicon) diluted in TBS-T for 1 hour at room temperature. After washing 3 times in TBS-T, the protein signals were detected using SuperSignal ECL (Pierce) and exposed to film (Kodak). Primary antibodies are listed in Table 1. ImageJ (National Institutes of Health) software (56) was used to measure the intensity of protein bands on the film. Levels of GDF9 and BMP15 were normalized to Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH). Phosphorylated proteins were normalized to total protein and β-actin served as a loading control.
Table 1.
Antibody Table
| Antibody | Isotype | Dilution | Catalog Number | Company |
|---|---|---|---|---|
| (p)-ERK1/2 | Rabbit monoclonal | 1.430555556 | 4370p | Cell Signaling Technology |
| p-MEK 1/2 | Rabbit monoclonal | 0.736111111 | 9154p | Cell Signaling Technology |
| p-p90RSK | Rabbit monoclonal | 0.736111111 | 9335p | Cell Signaling Technology |
| ERK1/2 | Rabbit monoclonal | 0.736111111 | 4695 | Cell Signaling Technology |
| MEK1/2 | Rabbit monoclonal | 0.736111111 | 8727 | Cell Signaling Technology |
| T-90RSK | Rabbit monoclonal | 0.736111111 | 9355 | Cell Signaling Technology |
| β-Actin | Rabbit monoclonal | 3.513888889 | 5125s | Cell Signaling Technology |
| GAPDH | Rabbit monoclonal | 3.513888889 | 3683s | Cell Signaling Technology |
| EGFR | Rabbit monoclonal | 0.736111111 | sc-03 | Santa Cruz Biotechnology, Inc |
| p-EGFR | Rabbit monoclonal | 0.736111111 | 2234s | Cell Signaling Technology |
| LHR | Rabbit monoclonal | 0.736111111 | PA5-21271 | Thermo Fisher Scientific |
| GDF9 | Goat monoclonal | 0.736111111 | sc-12244 | Santa Cruz Biotechnology, Inc |
| BMP15 | Rabbit monoclonal | 0.736111111 | sc-28911 | Santa Cruz Biotechnology, Inc |
| CREB | Rabbit monoclonal | 0.736111111 | 9197 | Cell Signaling Technology |
| p-CREB | Goat monoclonal | 0.736111111 | sc-7978 | Santa Cruz Biotechnology, Inc |
Real-time PCR
Total RNA from GCs, OOXs, or CCs of COCs was extracted using the RNAqueous-Micro kit (Ambion) and reverse transcribed using RETROscript (Ambion). The quantitative reverse transcription PCR (qRT-PCR) was carried out on an iCycler (Bio-Rad Laboratories) and assayed in triplicate. Each 10-μL reaction contained 5 μL of SYBR Green supermix (Bio-Rad Laboratories), 3 μL of H2O, 0.5 μL of each primer, and 1 μL of cDNA. The 2−ΔΔCt (cycle threshold) method was used to calculate relative expression levels after normalization to β-Actin or Rpl19 levels. Results are reported as a fold change in gene expression. The linear dynamic range and PCR efficiency of each primer set was determined using standard curve, and 1 single melting curve analysis was used to exclude nonspecific amplifications. The primers used for real-time PCR are given in Supplemental Table 1.
Statistical analysis
Each result represents at least 3 independent experiments. Values were analyzed either by Student's t test, one-way ANOVA, or two-way ANOVA, as described in each figure legend. All statistical analyses were performed using GraphPad Prism software, and significance was assessed at P ≤ .05.
Results
Epab−/− oocytes fail to promote expansion of WT CCs and Epab−/− CCs fail to expand in response to stimuli from WT oocytes
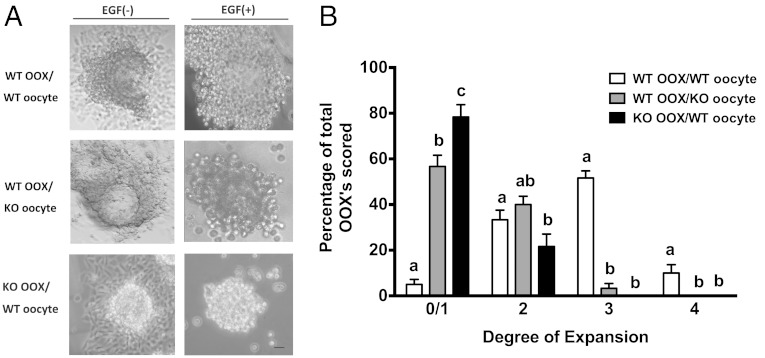
To determine whether the defect in cumulus expansion in Epab−/− mice is due to factors inherent to the oocyte, we cultured 3 different groups: 1) WT-OOX/WT-oocyte group (WT OOXs cocultured with WT DOs 2) WT-OOX/KO-oocyte group (WT OOXs cocultured with Epab−/− DOs), and 3) KO-OOX/WT-oocyte group (KO OOXs cocultured with WT DOs. After 16 hours of coculture in the absence of EGF (control), the OOXs adhered to the tissue culture plate and assumed a fibroblastic appearance and cumulus expansion was not observed in any of the 3 groups (Figure 1A). When cocultured in the presence of EGF, over 50% of the OOXs from the WT-OOX/WT-oocyte group maintained a spherical appearance and expanded into a 3-dimensional, gelatinous sphere, which comprised CCs reaching to almost full expansion (level 3) (Figure 1, A and B) (55). These results are similar to COCs with intact oocytes cultured in EGF-containing media (data not shown). However, the degree of OOX expansion in the WT-OOX/KO-oocyte group and the KO-OOX/WT-oocyte group was significantly reduced (Figure 1, A and B). Only 3.33% of WT-OOXs cultured with KO oocytes reached a score of 3, 56.67% and 40% of WT-OOXs cultured with KO oocytes remained at a score of 0/1 and a score of 2, respectively. Furthermore, 78.33% and 21.67% of KO-OOXs cultured with WT oocytes displayed a score of 0/1 and a score of 2, respectively. Level 3 expansion was not observed in the KO-OOX/WT-oocyte group.
Figure 1.
Epab−/− oocytes fail to promote cumulus expansion and Epab−/− CCs fail to undergo expansion in the presence of WT oocytes. Oocytes and OOXs were obtained from COCs of 12-week-old WT or Epab−/− mice and cultured in combinations, as indicated, for 16 hours in the presence (+) or absence (−) of 10-ng/mL EGF. A, Representative images of OOX/oocyte coculture combinations in the presence or absence of 10-ng/mL EGF. WT OOXs cultured with WT oocytes showed cumulus expansion in the presence of EGF. Conversely, WT OOXs cultured with KO oocytes or KO OOXs cultured with WT oocytes did not undergo expansion despite EGF treatment. Scale bar, 10 μm. B, Cumulus expansion of OOXs was scored after 16 hours of coculture with WT or Epab−/− DOs in the presence of EGF. The grade of cumulus expansion was assessed as previously described (55). A score of 0/1 indicates no detectable response (0) or the minimum observable response (1). A score of 4 indicates the maximum degree of expansion in which the cumulus oophorus and corona radiata have undergone expansion. A score of 2 or 3 indicate intermediate levels of expansion between 0/1 and 4. Data are presented as mean ± SEM from 3 independent experiments. Bars with different letters are significantly different (P < .05). Significance was determined by one-way ANOVA.
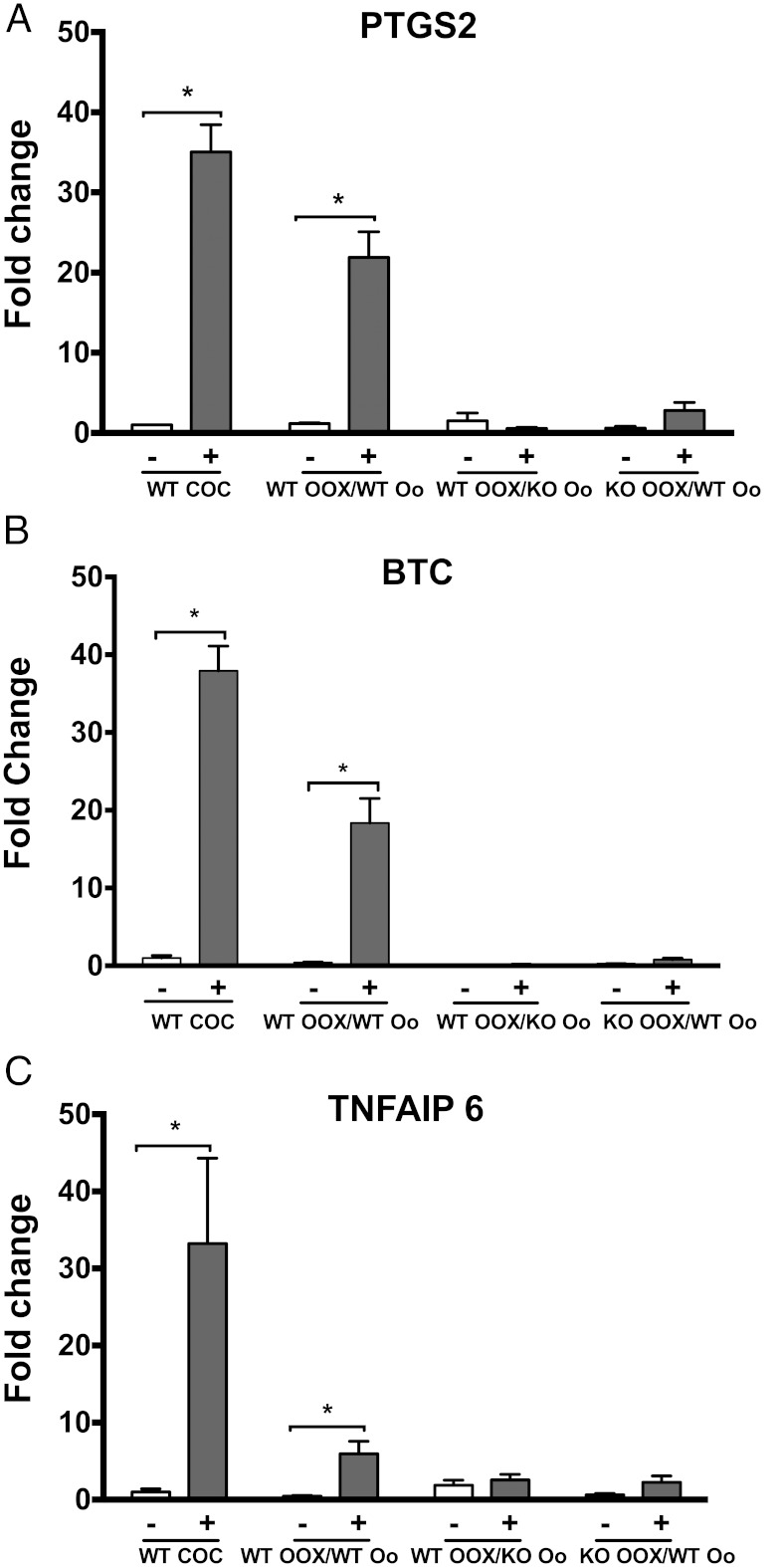
We also tested whether the expression of genes known to regulate cumulus expansion is altered in Epab−/− mice. After 16 hours of the coculture, OOXs were collected for qRT-PCR to assess the levels of Ptgs2, Btc, and Tnfaip6. Compared with control group (no EGF treatment), CC expression of transcripts encoding Prostaglandin-Endoperoxide Synthase 2 (Figure 2A), BTC (Figure 2B), and Tumor Necrosis Factor, Alpha-Induced Protein 6 (Figure 2C), showed significant increase in the WT OOX/WT-oocyte group in response to EGF treatment, but there was no change in the WT-OOX/KO-oocyte or KO-OOX/WT-oocyte groups (Figure 2, A–C). These observations demonstrate that the oocytes of Epab−/− mice fail to send the necessary signals to WT CCs that promote cumulus expansion. In addition, CCs from Epab−/− mice are unable to respond to oocyte-derived signals from WT oocytes.
Figure 2.
The expression of transcripts encoding proteins that mediate cumulus expansion do not increase in response to EGF in CCs from WT OOX/KO oocyte and KO OOX/WT oocyte groups. Oocytes (Oo) and OOX's were obtained from COCs of 12-week-old WT or Epab−/− mice 44–48 hours after PMSG injection. The expression of Ptgs2 (A), Btc (B), and Tnfaip6 (C) in CCs after 16 hours of coculture in the presence or absence of 10-ng/mL EGF was assessed using qRT-PCR. Expression of target genes was normalized to β-actin levels, and results are shown as the fold change in gene expression between EGF stimulation (+) and no EGF stimulation (−). Data are presented as mean ± SEM from 3 independent experiments. *, significance between transcript levels with or without EGF treatment (P < .05). Significance was determined by t test.
To confirm that the CCs from Epab−/− ovaries are properly differentiated, mRNA levels for CC markers (Amh, Slc38a3, and Ar) (57) was determined by qRT-PCR (Supplemental Figure 1). The expression of these CC transcripts in Epab−/− CCs was comparable with WT.
EPAB deficiency has no effect on the expression of GDF9 and BMP15
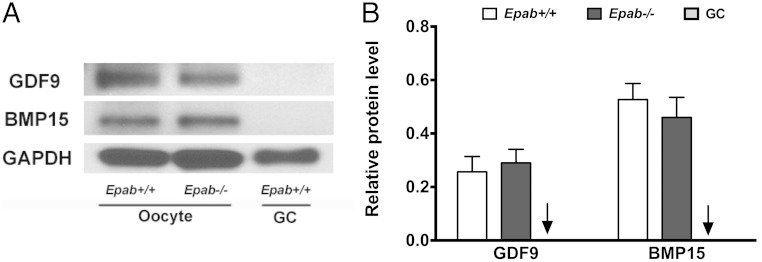
Because Epab−/− oocytes fail to communicate with the surrounding CCs, it is possible that the expression of oocyte-derived factors is altered in Epab−/− oocytes. GDF9 and BMP15 are 2 key mediators of oocyte-CC interactions (58, 59), and decreased expression could explain the abnormalities observed in Epab−/− ovaries. To examine this, 300 GV oocytes were collected from WT and Epab−/− mice at 12 weeks of age. GCs were collected as a negative control. We found that the expression of GDF9 and BMP15 were not altered in Epab−/− oocytes (Figure 3, A and B). As expected, the GCs did not display any immunoreactive bands. Because follicle growth and cumulus expansion requires timely expression of oocyte factors GDF9 and BMP15, it is possible that EPAB's effect is downstream of these factors, or that EPAB is required for the activation of an independent signaling pathway that regulates folliculogenesis.
Figure 3.
The expression of GDF9 and BMP15 is not altered in the oocytes of Epab−/− mice. A, GDF9 and BMP15 expression was detected by Western blot analysis using 300 GV oocytes from WT or Epab−/− mice. GCs from WT mice were used as a negative control. B, Band intensities were analyzed using densitometry and normalized to GAPDH. Data are represented as the mean ± SEM from 3 independent experiments. There was no significant difference in GDF9 or BMP15 expression between WT and Epab−/− oocytes. Arrow designates WT GC bar.
GCs of Epab−/− mice exhibit an impaired response to LH, EGF, and AREG stimulation
Next, we tested whether failed cumulus expansion could also be due to a defect in downstream signaling events in GCs in response to LH. The LH surge causes dramatic functional and structural changes in COCs that lead to mucification with a hyaluronan-rich matrix. During this process, LH induces the rapid expression of the EGF-like factors AREG, EREG, and BTC that act on the EGFR expressed by GCs and CCs (6–8). MEK1/2, ERK1/2, and p90RSK are downstream targets of the EGF network and regulate the expression of genes involved in hyaluronan synthesis and accumulation (60).
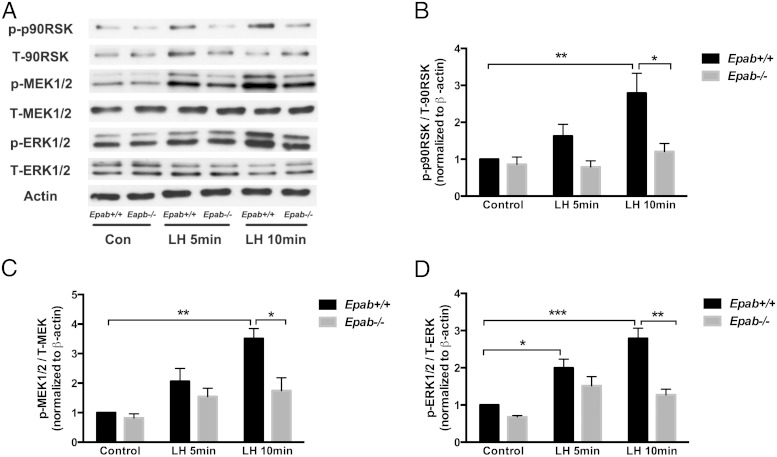
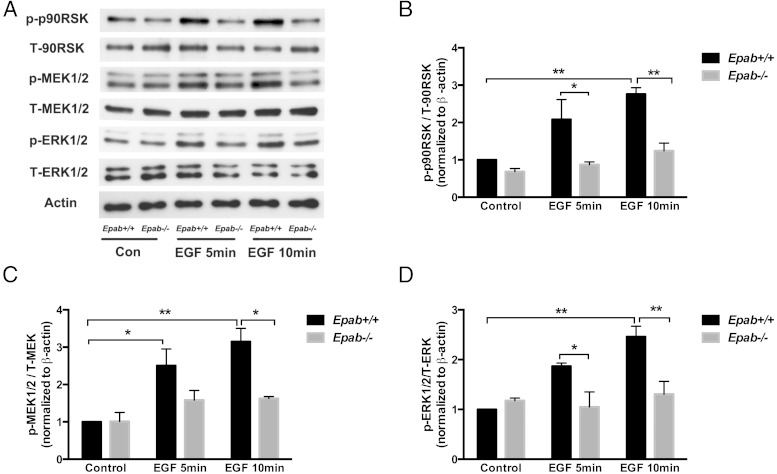
To determine whether the LH signaling pathway is affected in GCs of Epab−/− mice, GCs were collected from the ovaries of PMSG-primed WT and Epab−/− mice at 12 weeks of age, cultured to preconfluence, serum starved for 12 hours, and treated with LH (1 μg/mL) for 5 and 10 minutes. The expression of downstream mediators was then examined by Western blot analysis. An immunopositive signal for total and phosphorylated (p) MEK1/2, ERK1/2, and p90RSK, was observed in GCs from WT and Epab−/− mice (Figure 4A). In WT GCs, LH strongly activated ERK signaling such that phosphorylation of MEK1/2 (p-MEK1/2) and p90RSK (p-p90RSK) (Figure 4, B and D) was significantly increased after 10 minutes of LH treatment, and phosphorylation of ERK1/2 (p-ERK1/2) (Figure 4C) was significantly increased after 5 and 10 minutes of LH treatment. However, the phosphorylation of MEK1/2, ERK1/2, and p90RSK did not increase significantly in Epab−/− GCs in response to LH. Although there was a small increase in p-MEK1/2 and p-ERK1/2 in Epab−/− GCs, it was not significantly different from the control. Furthermore, when compared with WT, the levels of p-MEK1/2, p-ERK1/2, and p-p90RSK were significantly reduced in the GCs of Epab−/− mice after 10 minutes of LH treatment (Figure 4, B–D).
Figure 4.
The response to LH stimulation is impaired in GCs of Epab−/− mice. A–D, Western blot analysis was performed to compare LH-induced phosphorylation of MEK1/2, ERK1/2, and p90RSK in GCs from WT and Epab−/− mice. GCs were collected from WT and Epab−/− mice at 12 weeks of age 44–48 hours after 5-IU PMSG injection, cultured, serum starved, and treated with LH (1 μg/mL) for 5 and 10 minutes. Representative Western blottings of total (T) and phosphorylated (p) proteins are shown in A. The intensity of Western blot bands was analyzed using densitometry. The ratio of p-MEK1/2 (B), p-ERK1/2 (C), and p-p90RSK (D) to total protein was normalized to untreated WT control. Data are presented as mean ± SEM from 3 separate experiments. *, significant difference between groups (*, P < .05; **, P < .01; ***, P < .001). Significance was determined by two-way ANOVA followed by Tukey's multiple comparisons test.
Because the ERK cascade is an important downstream mediator of EGF pathways, we tested whether MEK/ERK/p90RSK activation in response to EGF stimulation is also affected by EPAB deficiency. GCs were collected as stated above and stimulated with 10-ng/mL EGF for 5 and 10 minutes (Figure 5A). EGF-stimulated phosphorylation of p90RSK (Figure 5B), MEK1/2 (Figure 5C), and ERK1/2 (Figure 5D) was significantly increased in WT GCs but not in Epab−/− GCs after 10 minutes of treatment. These results demonstrate that the GCs of Epab−/− mice are incapable of activating the ERK cascade in response to LH and EGF.
Figure 5.
The response to EGF stimulation is impaired in GCs of Epab−/− mice. A–D, Western blotting was performed to compare EGF-induced phosphorylation of MEK1/2, ERK1/2, and p90RSK in GCs from WT and Epab−/− mice. GCs were collected from WT and Epab−/− mice at 12 weeks of age 44–48 hours after 5-IU PMSG injection, cultured, serum starved, and treated with EGF (10 ng/mL) for 5 and 10 minutes. Representative Western blottings of total (T) and phosphorylated (p) proteins are shown in A. The intensity of Western blot bands was analyzed using densitometry. The ratio of p-MEK1/2 (B), p-ERK1/2 (C), and p-p90RSK (D) to total protein was normalized to untreated WT control. Data are presented as mean ± SEM from 3 separate experiments. *, significant difference between groups (*, P < .05; **, P < .01; ***, P < .001). Significance was determined by two-way ANOVA followed by Tukey's multiple comparisons test.
The response to AREG was also evaluated, because it is a member of the EGF-like growth factor family and a likely mediator of LH action in vitro (22, 26). Cultured GCs were treated with AREG (100 ng/mL) for 5 and 10 minutes. Western blot analyses showed that AREG strongly phosphorylated MEK1/2, ERK1/2, and p90RSK in GCs of WT mice (Supplemental Figure 2). However, phosphorylation of MEK1/2, ERK1/2, and p90RSK were significantly reduced in GCs of Epab−/− compared with WT within 5 or 10 minutes of AREG stimulation (Supplemental Figure 2).
If the differentiation or maturation of GCs from Epab−/− mice was affected, it may explain the impaired signaling response to LH and EGF. The expression of GC markers (Lhcgr, Cyp11a1, and Cd34) was evaluated by real-time PCR and was similar between WT and Epab−/− GCs. However, Cyp19a1 was significantly lower in Epab−/− GCs (Supplemental Figure 1). Although Epab−/− GCs express markers of GC differentiation, lower expression of Cyp19a1 suggests that some aspect of their maturation has been affected.
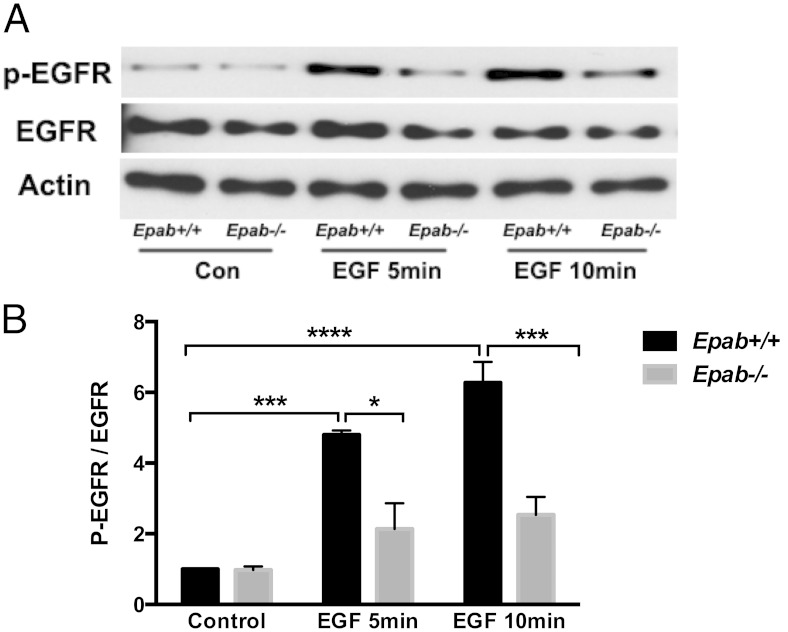
GCs of Epab−/− mice exhibit impaired EGFR phosphorylation in response to EGF
LH induced oocyte maturation and ovulation requires transactivation of EGFR in the somatic cells (61), and it is possible that impaired MEK/ERK/p90RSK activation observed in Epab−/− GCs is due to abnormalities in EGFR activation. We examined the expression of total EGFR and p-EGFR in the GCs of PMSG-primed WT and Epab−/− mice after 5 and 10 minutes EGF stimulation (10 ng/mL). As expected, EGFR phosphorylation significantly increased in the GCs of WT mice after EGF stimulation (Figure 6). However, this increase in p-EGFR in response to EGF treatment was not observed in the GCs of Epab−/− mice (Figure 6). Therefore, impaired EGFR phosphorylation observed in Epab−/− GCs may be a contributing factor leading to failure of MEK/ERK/p90RSK activation in response to LH, EGF, and AREG.
Figure 6.
The activation of EGFR is impaired in GCs of Epab−/− mice. A–C, Western blot analysis was performed to compare EGF-induced phosphorylation of EGFR in GCs from WT and Epab−/− mice. GCs were collected from WT and Epab−/− mice at 12 weeks of age after 44–48 hours of 5-IU PMSG injection, cultured, serum starved, and treated with EGF (10 ng/mL) for 5 and 10 minutes. Representative Western blottings are shown in A. The intensity of Western blot bands corresponding to total (T) EGFR (B) and phosphorylated (p) EGFR (C) were analyzed using densitometry. The ratio of p-EGFR to total EGFR was normalized to untreated WT. Data are presented as mean ± SEM from 3 separate experiments. *, significant difference between groups (*, P < .05; ***, P < .001; ***, P < .0001). Significance was determined by two-way ANOVA followed by Tukey's multiple comparisons test.
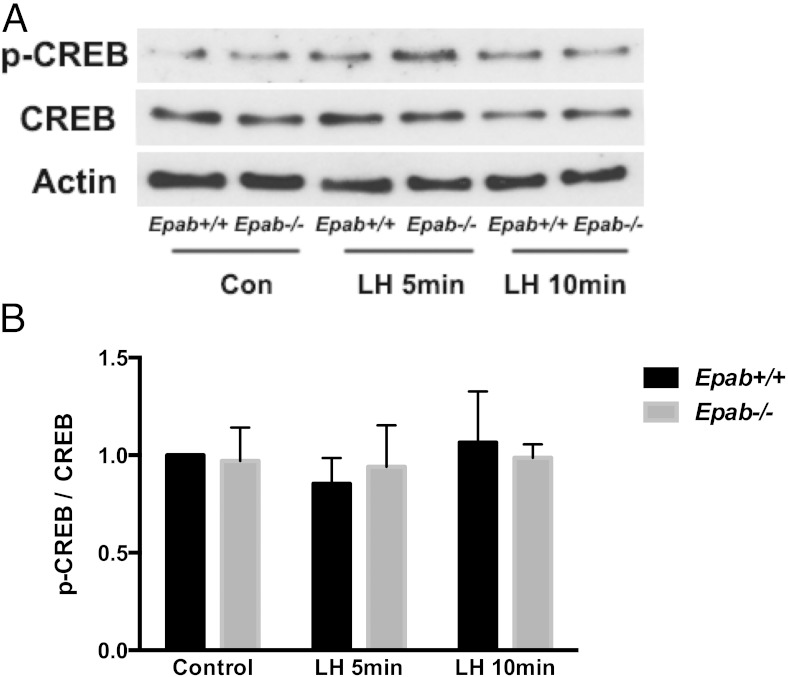
Although EGF mediates many of the responses of LH, LH-induced activation of ERK signaling can also occur in the absence of EGFR activation (25, 62). Thus, LH acts through additional pathways downstream of cAMP to activate ERK. To address whether Epab deficiency affects ERK activation independently of EGF signaling, we examined p-CREB levels in WT and Epab−/− GCs after LH treatment. We did not observe CREB activation in WT or Epab−/− GCs after 5 or 10 minutes of LH treatment (Figure 7). Because p-CREB activation appears to require longer LH treatment, it is likely that impaired ERK activation observed in Epab−/− GCs in response to 5 and 10 minutes of LH treatment is due to impaired EGFR phosphorylation.
Figure 7.
CREB activation does not increase in response to short-term LH treatment in GCs from WT or Epab−/− mice. Western bot analysis was performed to compare LH-induced phosphorylation of CREB in GCs from WT and Epab−/− mice. GCs were collected from WT and Epab−/− mice at 12 weeks of age 44–48 hours after 5-IU PMSG injection, cultured, serum starved, and treated with LH (1 μg/mL) for 5 and 10 minutes. Representative Western blottings of total (T) and phosphorylated (p) CREB are shown in A. The intensity of Western blot bands was analyzed using densitometry (B). The ratio of p-CREB to total CREB was normalized to untreated WT control. Data are presented as mean ± SEM from 3 separate experiments. Phosphorylation of CREB was not significantly different in WT or Epab−/− GCs after 5 or 10 minutes of LH treatment.
Discussion
In our previous study, we demonstrated that EPAB-deficient mice are infertile due to impaired oocyte maturation, cumulus expansion and ovulation. In this study, we investigated the cause of defective cumulus expansion and ovulation in EPAB-deficient mice. We first demonstrated that Epab−/− oocytes fail to promote cumulus expansion in WT CCs. However, this is not due to decreased expression of oocyte-derived factors GDF9 and BMP15. Additionally, we found that the CCs from Epab−/− mice fail to undergo expansion even in the presence of WT oocytes. Finally, we demonstrated that MEK/ERK/p90RSK activation in response to LH and EGF signaling is impaired in GCs as a result of Epab deficiency.
Because EPAB is oocyte specific, we hypothesized that the dysfunction in GCs from Epab−/− mice is due to factors inherent to the oocyte. We utilized a coculture system of OOXs with DOs under EGF stimulation. Our results show that WT-OOXs expand in WT-oocyte-conditioned media in response to EGF treatment, consistent with previous reports (53), and is similar to that observed with intact WT COC complexes. However, when WT-OOX/KO-oocyte group were treated with EGF, cumulus expansion did not occur and the expression of mediators of cumulus expansion (Ptgs2, Btc, and Tnfaip6) did not change. Because EPAB is required for translational activation upon stimulation of oocyte maturation (34), it is likely that the failure of promoting cumulus expansion of Epab−/− oocytes is due to impaired translation of maternally derived mRNAs in the oocyte. Consequently, Epab−/− oocytes fail to express optimal levels of specific proteins that are required by the somatic cells in order to achieve cumulus expansion. Additionally, the oocyte's production of cumulus enabling factors is developmentally regulated such that oocytes incapable of spontaneous germinal vesicle breakdown are unable to promote cumulus expansion (55). Epab−/− oocytes fail to undergo germinal vesicle breakdown and mature to metaphase II and, therefore, may not be at the right stage to produce the appropriate factors required for cumulus expansion.
Evidence emerging from earlier studies demonstrates that oocyte-specific factors, including GDF9 and BMP15, are indispensable for regulating somatic cell functions. Gdf9-null mice are infertile due to an arrest of follicle development at the primary follicle stage (63). GDF9 promotes GC proliferation and differentiation leading to the formation of the 2-layer preantral follicle and subsequent recruitment of the thecal layer (58). GDF9 also promotes cumulus expansion by stimulating Ptgs2 and Has2 mRNA expression and suppressing urokinase plasminogen activator (17, 64). Thus, in addition to playing a key role during early folliculogenesis, GDF9 functions as an oocyte-secreted paracrine factor that regulates key enzymes involved in cumulus expansion. Unlike Gdf9-null mice, Bmp15-null mice are fertile but display impaired terminal cumulus-oocyte maturation and decreased ovulation rates. BMP15 has been reported to regulate cumulus expansion via a mechanism requiring EGFR signaling (16, 65). In addition, oocyte-derived BMP15 and/or GDF9 suppress LH signaling in CCs by preventing Lhcgr mRNA expression (17, 64, 66, 67), thereby leading to heterogeneity in LH receptor (LHR) expression between cumulus and mural GCs in preovulatory follicles. In our study, WT and Epab−/− oocytes did not show a difference in GDF9 or BMP15 expression, suggesting that the failure of cumulus expansion of EPAB-deficient mice is not due to the absence of these oocyte factors. Therefore, EPAB is either downstream of these factors, or EPAB could be required for the activation of an independent signaling pathway that regulates folliculogenesis. Another possibility is that EPAB-deficient oocytes produce the necessary factors, but are unable to transport them to the somatic compartment. Perhaps EPAB is important for maintaining communication between the oocyte and somatic cells, either by regulating the expression of components required for junction formation or the assembly of transzonal processes.
Interestingly, Epab−/− OOXs fail to expand in response to EGF even when cocultured with WT oocytes. This suggests that impaired cumulus expansion is also due to a defect in Epab−/− CCs. If the CCs from Epab−/− mice were not properly differentiated, they would be incapable of supporting cumulus expansion. This is an important consideration, because the oocyte drives the CC lineage (30, 57, 68) and also because EPAB is required in the oocyte at the preantral stage of folliculogenesis before CC differentiation (69). The expression of cumulus markers (Amh, Slc38a3, Ar) (Supplemental Figure 1) suggests that Epab−/− CCs are phenotypically normal; however, other transcripts may be abnormally expressed. Another possibility is that in addition to GCs, CCs from Epab−/− mice fail to activate MEK/ERK signaling, thereby leading to impaired cumulus expansion.
Failure of cumulus expansion in Epab-deficient mice in vivo could also be due to impaired signaling in GCs in response to LH. The LHR is a G protein-coupled receptor expressed on theca and mural GCs. Because CCs and oocytes are insensitive to direct LH stimulation due to lack of LHRs (70, 71), they rely on signals initiated by the GCs. Specifically, LH stimulates meiotic maturation, cumulus expansion, and ovulation by inducing expression of EGF-like factors in GCs (26). EGF-like factors, in turn, activate the EGFR signaling pathway in CCs to induce COC expansion and oocyte maturation (60). In vitro, soluble EGF (an AREG and EREG analog) stimulates mRNA expression of Ptgs2, Tnfaip6, and Has2 in primary GC cultures (72). These genes are necessary for synthesis and stabilization of the extracellular matrix of CCs and are required for cumulus expansion (73–75). EGF also acts as a potent stimulator of cumulus expansion in intact cumulus-oocyte complexes in mice (24, 76). However, EGF cannot induce cumulus expansion in the absence of the oocyte, which suggests that oocyte secreted factors in vitro are necessary for the CCs to undergo expansion in response to EGF (53).
Disruption of the EGFR signaling in mice compromises cumulus expansion and ovulation, indicating that activation of this pathway is essential for LH-induced ovulation (28, 62). Mice in which ERK1 and ERK2 have been disrupted in GCs exhibit normal follicle growth, but in response to LH, the COCs fail to expand, oocytes fail to reenter meiosis, and follicles fail to either ovulate or luteinize (77). The phenotypes of mice with genetic disruption of EGFR or ERK1/ERK2 in GCs show significant similarities to the phenotype of Epab−/− mice, which exhibit female infertility due to impaired cumulus expansion and ovulation, in addition to an inability to achieve oocyte maturation (52). Importantly, phosphorylation of ERK1/2 in Epab−/− GCs was completely abolished in response to EGF, whereas the effect was less prominent (although significant) in response to LH (Figures 4 and 5). This finding, combined with the observation that EGFR phosphorylation is suppressed in Epab−/− GCs (Figure 6), whereas LHR expression is unchanged (Supplemental Figure 1), suggests that the main defect in Epab−/− somatic cells involves impaired EGF signaling and ERK activation.
LH and EGF-like factors work together in a coordinated fashion to induce oocyte maturation and cumulus expansion and both result in MAPK (MEK/ERK) activation. LHR is coupled primarily to Gs protein and activation of adenylyl cyclase. Thus, the primary signal emanating from LHR is the accumulation of cAMP. In GCs, ERK1/2 phosphorylation in response to LH, forskolin, and 8-Bromoadenosine 3′,5′-cyclic monophosphate is blocked by H89, a potent and selective inhibitor of protein kinase A (PKA) (78), suggesting that stimulation of ERK1/2 phosphorylation in GCs by LH is mediated by cAMP-dependent PKA activation. The cAMP/PKA pathway leads to production of EGF-like factors that will subsequently bind and activate the EGFR (79) and result in ERK1/2 phosphorylation (25). Importantly, LH-induced ERK1/2 phosphorylation is only partially dependent on EGFR, suggesting that MEK/ERK pathway is activated upstream (or in parallel) as well as downstream of the EGFR (25, 62). Thus, additional pathways downstream of cAMP contribute to LH-mediated activation of MAPK. In our study, we did not observe activation of CREB in response to 5 and 10 minutes LH treatment in WT or Epab−/− GCs. Thus, rapid ERK activation in response to 5–10 minutes of LH treatment appears to occur independently of the cAMP pathway and is the result of EGFR signaling. This finding supports the conclusion that impaired phosphorylation of MEK1/2, ERK1/2, and p90RSK observed in Epab−/− GCs is most likely due to insufficient EGFR activation.
The inability of Epab−/− GCs to properly respond to LH and EGF could be due to abnormal GC maturation during folliculogenesis. Although the expressions of LHR, Cyp11a1, and Cd34 were similar to WT, the expression of Cyp19a1 was significantly lower in Epab−/− GCs. This finding suggests that some aspect of GC maturation or differentiation is affected by EPAB deficiency. Cyp19a1 expression is regulated by FSH (80), which is necessary to induce growth and maturation of ovarian follicles to the preovulatory stage. FSH induces a complex pattern of gene expression in GCs that is mediated by several different signaling cascades including ERK, MAPK, and phosphatidylinositol-3 kinase (81). FSH also leads to the production of EGF-like growth factors in CCs and results in meiotic resumption (82). Therefore, it is possible that FSH signaling is also impaired in GCs from Epab−/− mice and will be examined in future studies.
Overall, EPAB-deficient mice display impaired cumulus expansion, ovulation, and follicle development (52). In the current study, we demonstrate the basic molecular mechanisms of follicular somatic cell dysfunction in Epab−/− mice. Because EPAB is expressed exclusively in the oocyte, the underlying cause is likely to be failed communication of oocyte-specific factors that ultimately affects the differentiation and function of the somatic cells. EPAB promotes CC responsiveness to EGF as well as the activation of the ERK cascade in GCs in response to LH and EGF. The findings reported here open a new perspective on oocyte-somatic cell communication and cooperation. Studies are in progress in order to further understand how oocyte-specific EPAB regulates CC/GC differentiation and function.
Acknowledgments
We thank the Lalor Foundation for providing Katie Lowther with a postdoctoral fellowship.
This work was supported by the National Institutes of Health Award R01HD059909 (to E.S.). K.M.L. was supported by a fellowship from Lalor Foundation. M.D.L. was partly supported by the National Center for Research Resources and the National Center for Advancing Translational Science Grant KL2-RR024138.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AREG
- amphiregulin
- BMP15
- bone morphogenic protein 15
- BTC
- betacellulin
- CC
- cumulus cell
- COC
- cumulus oophorus complex
- DO
- denuded oocyte
- EGF
- epidermal growth factor
- EGFR
- EGF receptor
- EPAB
- embryonic poly(A)-binding protein
- EREG
- epiregulin
- GAPDH
- Glyceraldehyde-3-Phosphate Dehydrogenase
- GC
- granulosa cell
- GDF9
- growth differentiation factor 9
- KO
- knockout
- OOX
- oocytectomized COC
- p
- phosphorylated
- p90RSK
- p90 ribosomal S6 kinase
- PKA
- protein kinase A
- PMSG
- pregnant mare serum gonadotropin
- qRT-PCR
- quantitative reverse transcription PCR
- TBS-T
- Tris-buffered saline with Tween-20
- WT
- wild-type.
References
- 1. Eppig JJ, Schultz RM, O'Brien M, Chesnel F. Relationship between the developmental programs controlling nuclear and cytoplasmic maturation of mouse oocytes. Dev Biol. 1994;164:1–9. [DOI] [PubMed] [Google Scholar]
- 2. Adashi EY. Endocrinology of the ovary. Hum Reprod. 1994;9:815–827. [DOI] [PubMed] [Google Scholar]
- 3. Wigglesworth K, Lee KB, O'Brien MJ, Peng J, Matzuk MM, Eppig JJ. Bidirectional communication between oocytes and ovarian follicular somatic cells is required for meiotic arrest of mammalian oocytes. Proc Natl Acad Sci USA. 2013;110:E3723–E3729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Eppig JJ. Intercommunication between mammalian oocytes and companion somatic cells. BioEssays. 1991;13:569–574. [DOI] [PubMed] [Google Scholar]
- 5. Richards JS, Russell DL, Ochsner S, Espey LL. Ovulation: new dimensions and new regulators of the inflammatory-like response. Annu Rev Physiol. 2002;64:69–92. [DOI] [PubMed] [Google Scholar]
- 6. Mehlmann LM. Stops and starts in mammalian oocytes: recent advances in understanding the regulation of meiotic arrest and oocyte maturation. Reproduction. 2005;130:791–799. [DOI] [PubMed] [Google Scholar]
- 7. Hussein TS, Thompson JG, Gilchrist RB. Oocyte-secreted factors enhance oocyte developmental competence. Dev Biol. 2006;296:514–521. [DOI] [PubMed] [Google Scholar]
- 8. Blondin P, Sirard MA. Oocyte and follicular morphology as determining characteristics for developmental competence in bovine oocytes. Mol Reprod Dev. 1995;41:54–62. [DOI] [PubMed] [Google Scholar]
- 9. Huang Z, Wells D. The human oocyte and cumulus cells relationship: new insights from the cumulus cell transcriptome. Mol Hum Reprod. 2010;16:715–725. [DOI] [PubMed] [Google Scholar]
- 10. Eppig JJ. Oocyte control of ovarian follicular development and function in mammals. Reproduction. 2001;122:829–838. [DOI] [PubMed] [Google Scholar]
- 11. McPherron AC, Lee SJ. GDF-3 and GDF-9: two new members of the transforming growth factor-β superfamily containing a novel pattern of cysteines. J Biol Chem. 1993;268:3444–3449. [PubMed] [Google Scholar]
- 12. Chang H, Brown CW, Matzuk MM. Genetic analysis of the mammalian transforming growth factor-β superfamily. Endocr Rev. 2002;23:787–823. [DOI] [PubMed] [Google Scholar]
- 13. McGrath SA, Esquela AF, Lee SJ. Oocyte-specific expression of growth/differentiation factor-9. Mol Endocrinol. 1995;9:131–136. [DOI] [PubMed] [Google Scholar]
- 14. Dube JL, Wang P, Elvin J, Lyons KM, Celeste AJ, Matzuk MM. The bone morphogenetic protein 15 gene is X-linked and expressed in oocytes. Mol Endocrinol. 1998;12:1809–1817. [DOI] [PubMed] [Google Scholar]
- 15. Otsuka F, Yao Z, Lee T, Yamamoto S, Erickson GF, Shimasaki S. Bone morphogenetic protein-15. Identification of target cells and biological functions. J Biol Chem. 2000;275:39523–39528. [DOI] [PubMed] [Google Scholar]
- 16. Yoshino O, McMahon HE, Sharma S, Shimasaki S. A unique preovulatory expression pattern plays a key role in the physiological functions of BMP-15 in the mouse. Proc Natl Acad Sci USA. 2006;103:10678–10683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Elvin JA, Clark AT, Wang P, Wolfman NM, Matzuk MM. Paracrine actions of growth differentiation factor-9 in the mammalian ovary. Mol Endocrinol. 1999;13:1035–1048. [DOI] [PubMed] [Google Scholar]
- 18. Gilchrist RB, Lane M, Thompson JG. Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum Reprod Update. 2008;14:159–177. [DOI] [PubMed] [Google Scholar]
- 19. Chen L, Russell PT, Larsen WJ. Functional significance of cumulus expansion in the mouse: roles for the preovulatory synthesis of hyaluronic acid within the cumulus mass. Mol Reprod Dev. 1993;34:87–93. [DOI] [PubMed] [Google Scholar]
- 20. Hess KA, Chen L, Larsen WJ. Inter-α-inhibitor binding to hyaluronan in the cumulus extracellular matrix is required for optimal ovulation and development of mouse oocytes. Biol Reprod. 1999;61:436–443. [DOI] [PubMed] [Google Scholar]
- 21. Freimann S, Ben-Ami I, Dantes A, Ron-El R, Amsterdam A. EGF-like factor epiregulin and amphiregulin expression is regulated by gonadotropins/cAMP in human ovarian follicular cells. Biochem Biophys Res Commun. 2004;324:829–834. [DOI] [PubMed] [Google Scholar]
- 22. Ashkenazi H, Cao X, Motola S, Popliker M, Conti M, Tsafriri A. Epidermal growth factor family members: endogenous mediators of the ovulatory response. Endocrinology. 2005;146:77–84. [DOI] [PubMed] [Google Scholar]
- 23. Conti M, Hsieh M, Park JY, Su YQ. Role of the epidermal growth factor network in ovarian follicles. Mol Endocrinol. 2006;20:715–723. [DOI] [PubMed] [Google Scholar]
- 24. Hsieh M, Zamah AM, Conti M. Epidermal growth factor-like growth factors in the follicular fluid: role in oocyte development and maturation. Semin Reprod Med. 2009;27:52–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Panigone S, Hsieh M, Fu M, Persani L, Conti M. Luteinizing hormone signaling in preovulatory follicles involves early activation of the epidermal growth factor receptor pathway. Mol Endocrinol. 2008;22:924–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Park JY, Su YQ, Ariga M, Law E, Jin SL, Conti M. EGF-like growth factors as mediators of LH action in the ovulatory follicle. Science. 2004;303:682–684. [DOI] [PubMed] [Google Scholar]
- 27. Sekiguchi T, Mizutani T, Yamada K, et al. Expression of epiregulin and amphiregulin in the rat ovary. J Mol Endocrinol. 2004;33:281–291. [DOI] [PubMed] [Google Scholar]
- 28. Hsieh M, Lee D, Panigone S, et al. Luteinizing hormone-dependent activation of the epidermal growth factor network is essential for ovulation. Mol Cell Biol. 2007;27:1914–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Luetteke NC, Qiu TH, Fenton SE, et al. Targeted inactivation of the EGF and amphiregulin genes reveals distinct roles for EGF receptor ligands in mouse mammary gland development. Development. 1999;126:2739–2750. [DOI] [PubMed] [Google Scholar]
- 30. Diaz FJ, O'Brien MJ, Wigglesworth K, Eppig JJ. The preantral granulosa cell to cumulus cell transition in the mouse ovary: development of competence to undergo expansion. Dev Biol. 2006;299:91–104. [DOI] [PubMed] [Google Scholar]
- 31. Mendez R, Hake LE, Andresson T, Littlepage LE, Ruderman JV, Richter JD. Phosphorylation of CPE binding factor by Eg2 regulates translation of c-mos mRNA. Nature. 2000;404:302–307. [DOI] [PubMed] [Google Scholar]
- 32. Oh B, Hwang S, McLaughlin J, Solter D, Knowles BB. Timely translation during the mouse oocyte-to-embryo transition. Development. 2000;127:3795–3803. [DOI] [PubMed] [Google Scholar]
- 33. Matova N, Cooley L. Comparative aspects of animal oogenesis. Dev Biol. 2001;231:291–320. [DOI] [PubMed] [Google Scholar]
- 34. Seli E, Lalioti MD, Flaherty SM, Sakkas D, Terzi N, Steitz JA. An embryonic poly(A)-binding protein (ePAB) is expressed in mouse oocytes and early preimplantation embryos. Proc Natl Acad Sci USA. 2005;102:367–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Groisman I, Huang YS, Mendez R, Cao Q, Theurkauf W, Richter JD. CPEB, maskin, and cyclin B1 mRNA at the mitotic apparatus: implications for local translational control of cell division. Cell. 2000;103:435–447. [DOI] [PubMed] [Google Scholar]
- 36. Stutz A, Conne B, Huarte J, et al. Masking, unmasking, and regulated polyadenylation cooperate in the translational control of a dormant mRNA in mouse oocytes. Genes Dev. 1998;12:2535–2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Radford HE, Meijer HA, de Moor CH. Translational control by cytoplasmic polyadenylation in Xenopus oocytes. Biochim Biophys Acta. 2008;1779:217–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Barnard DC, Ryan K, Manley JL, Richter JD. Symplekin and xGLD-2 are required for CPEB-mediated cytoplasmic polyadenylation. Cell. 2004;119:641–651. [DOI] [PubMed] [Google Scholar]
- 39. Gebauer F, Richter JD. Synthesis and function of Mos: the control switch of vertebrate oocyte meiosis. BioEssays. 1997;19:23–28. [DOI] [PubMed] [Google Scholar]
- 40. Hake LE, Richter JD. Translational regulation of maternal mRNA. Biochim Biophys Acta. 1997;1332:M31–38. [DOI] [PubMed] [Google Scholar]
- 41. Piqué M, López JM, Foissac S, Guigó R, Méndez R. A combinatorial code for CPE-mediated translational control. Cell. 2008;132:434–448. [DOI] [PubMed] [Google Scholar]
- 42. Vasudevan S, Seli E, Steitz JA. Metazoan oocyte and early embryo development program: a progression through translation regulatory cascades. Genes Dev. 2006;20:138–146. [DOI] [PubMed] [Google Scholar]
- 43. Chen J, Melton C, Suh N, et al. Genome-wide analysis of translation reveals a critical role for deleted in azoospermia-like (Dazl) at the oocyte-to-zygote transition. Genes Dev. 2011;25:755–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Padmanabhan K, Richter JD. Regulated Pumilio-2 binding controls RINGO/Spy mRNA translation and CPEB activation. Genes Dev. 2006;20:199–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Collier B, Gorgoni B, Loveridge C, Cooke HJ, Gray NK. The DAZL family proteins are PABP-binding proteins that regulate translation in germ cells. EMBO J. 2005;24:2656–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Guzeloglu-Kayisli O, Pauli S, Demir H, Lalioti MD, Sakkas D, Seli E. Identification and characterization of human embryonic poly(A) binding protein (EPAB). Mol Hum Reprod. 2008;14:581–588. [DOI] [PubMed] [Google Scholar]
- 47. Guzeloglu-Kayisli O, Lalioti MD, Babayev E, Torrealday S, Karakaya C, Seli E. Human embryonic poly(A)-binding protein (EPAB) alternative splicing is differentially regulated in human oocytes and embryos. Mol Hum Reprod. 2014;20:59–65. [DOI] [PubMed] [Google Scholar]
- 48. Kim JH, Richter JD. RINGO/cdk1 and CPEB mediate poly(A) tail stabilization and translational regulation by ePAB. Genes Dev. 2007;21:2571–2579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Voeltz GK, Ongkasuwan J, Standart N, Steitz JA. A novel embryonic poly(A) binding protein, ePAB, regulates mRNA deadenylation in Xenopus egg extracts. Genes Dev. 2001;15:774–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wilkie GS, Gautier P, Lawson D, Gray NK. Embryonic poly(A)-binding protein stimulates translation in germ cells. Mol Cell Biol. 2005;25:2060–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Friend K, Brook M, Bezirci FB, Sheets MD, Gray NK, Seli E. Embryonic poly(A)-binding protein (ePAB) phosphorylation is required for Xenopus oocyte maturation. Biochem J. 2012;445:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Guzeloglu-Kayisli O, Lalioti MD, Aydiner F, et al. Embryonic poly(A)-binding protein (EPAB) is required for oocyte maturation and female fertility in mice. Biochem J. 2012;446:47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Buccione R, Vanderhyden BC, Caron PJ, Eppig JJ. FSH-induced expansion of the mouse cumulus oophorus in vitro is dependent upon a specific factor (s) secreted by the oocyte. Dev Biol. 1990;138:16–25. [DOI] [PubMed] [Google Scholar]
- 54. Sugiura K, Su YQ, Li Q, Wigglesworth K, Matzuk MM, Eppig JJ. Estrogen promotes the development of mouse cumulus cells in coordination with oocyte-derived GDF9 and BMP15. Mol Endocrinol. 2010;24:2303–2314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Vanderhyden BC, Caron PJ, Buccione R, Eppig JJ. Developmental pattern of the secretion of cumulus expansion-enabling factor by mouse oocytes and the role of oocytes in promoting granulosa cell differentiation. Dev Biol. 1990;140:307–317. [DOI] [PubMed] [Google Scholar]
- 56. Schneider CA, Rasband WS, Eliceiri KW. NIH Image to ImageJ: 25 years of image analysis. Nat Methods. 2012;9:671–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Diaz FJ, Wigglesworth K, Eppig JJ. Oocytes determine cumulus cell lineage in mouse ovarian follicles. J Cell Sci. 2007;120:1330–1340. [DOI] [PubMed] [Google Scholar]
- 58. Su YQ, Wu X, O'Brien MJ, et al. Synergistic roles of BMP15 and GDF9 in the development and function of the oocyte-cumulus cell complex in mice: genetic evidence for an oocyte-granulosa cell regulatory loop. Dev Biol. 2004;276:64–73. [DOI] [PubMed] [Google Scholar]
- 59. Yan C, Wang P, DeMayo J, et al. Synergistic roles of bone morphogenetic protein 15 and growth differentiation factor 9 in ovarian function. Mol Endocrinol. 2001;15:854–866. [DOI] [PubMed] [Google Scholar]
- 60. Kawashima I, Liu Z, Mullany LK, Mihara T, Richards JS, Shimada M. EGF-like factors induce expansion of the cumulus cell-oocyte complexes by activating calpain-mediated cell movement. Endocrinology. 2012;153:3949–3959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Reizel Y, Elbaz J, Dekel N. Sustained activity of the EGF receptor is an absolute requisite for LH-induced oocyte maturation and cumulus expansion. Mol Endocrinol. 2010;24:402–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hsieh M, Thao K, Conti M. Genetic dissection of epidermal growth factor receptor signaling during luteinizing hormone-induced oocyte maturation. PLoS One. 2011;6:e21574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Dong J, Albertini DF, Nishimori K, Kumar TR, Lu N, Matzuk MM. Growth differentiation factor-9 is required during early ovarian folliculogenesis. Nature. 1996;383:531–535. [DOI] [PubMed] [Google Scholar]
- 64. Vitt UA, Hayashi M, Klein C, Hsueh AJ. Growth differentiation factor-9 stimulates proliferation but suppresses the follicle-stimulating hormone-induced differentiation of cultured granulosa cells from small antral and preovulatory rat follicles. Biol Reprod. 2000;62:370–377. [DOI] [PubMed] [Google Scholar]
- 65. Guéripel X, Brun V, Gougeon A. Oocyte bone morphogenetic protein 15, but not growth differentiation factor 9, is increased during gonadotropin-induced follicular development in the immature mouse and is associated with cumulus oophorus expansion. Biol Reprod. 2006;75:836–843. [DOI] [PubMed] [Google Scholar]
- 66. Eppig JJ, Wigglesworth K, Pendola F, Hirao Y. Murine oocytes suppress expression of luteinizing hormone receptor messenger ribonucleic acid by granulosa cells. Biol Reprod. 1997;56:976–984. [DOI] [PubMed] [Google Scholar]
- 67. Otsuka F, Yamamoto S, Erickson GF, Shimasaki S. Bone morphogenetic protein-15 inhibits follicle-stimulating hormone (FSH) action by suppressing FSH receptor expression. J Biol Chem. 2001;276:11387–11392. [DOI] [PubMed] [Google Scholar]
- 68. Su YQ, Sugiura K, Eppig JJ. Mouse oocyte control of granulosa cell development and function: paracrine regulation of cumulus cell metabolism. Semin Reprod Med. 2009;27:32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lowther KM, Mehlmann LM. Embryonic poly(A) binding protein (EPAB) is required during early stages of mouse oocyte development for chromatin organization, transcriptional silencing, and meiotic competence. Biol Reprod. 2015;93:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Amsterdam A, Koch Y, Lieberman ME, Lindner HR. Distribution of binding sites for human chorionic gonadotropin in the preovulatory follicle of the rat. J Cell Biol. 1975;67:894–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Peng XR, Hsueh AJ, LaPolt PS, Bjersing L, Ny T. Localization of luteinizing hormone receptor messenger ribonucleic acid expression in ovarian cell types during follicle development and ovulation. Endocrinology. 1991;129:3200–3207. [DOI] [PubMed] [Google Scholar]
- 72. Sayasith K, Lussier J, Doré M, Sirois J. Human chorionic gonadotropin-dependent up-regulation of epiregulin and amphiregulin in equine and bovine follicles during the ovulatory process. Gen Comp Endocrinol. 2013;180:39–47. [DOI] [PubMed] [Google Scholar]
- 73. Davis BJ, Lennard DE, Lee CA, et al. Anovulation in cyclooxygenase-2-deficient mice is restored by prostaglandin E2 and interleukin-1β. Endocrinology. 1999;140:2685–2695. [DOI] [PubMed] [Google Scholar]
- 74. Ochsner SA, Day AJ, Rugg MS, Breyer RM, Gomer RH, Richards JS. Disrupted function of tumor necrosis factor-α-stimulated gene 6 blocks cumulus cell-oocyte complex expansion. Endocrinology. 2003;144:4376–4384. [DOI] [PubMed] [Google Scholar]
- 75. Fülöp C, Szántó S, Mukhopadhyay D, et al. Impaired cumulus mucification and female sterility in tumor necrosis factor-induced protein-6 deficient mice. Development. 2003;130:2253–2261. [DOI] [PubMed] [Google Scholar]
- 76. Downs SM. Specificity of epidermal growth factor action on maturation of the murine oocyte and cumulus oophorus in vitro. Biol Reprod. 1989;41:371–379. [DOI] [PubMed] [Google Scholar]
- 77. Fan HY, Liu Z, Shimada M, et al. MAPK3/1 (ERK1/2) in ovarian granulosa cells are essential for female fertility. Science. 2009;324:938–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Seger R, Hanoch T, Rosenberg R, et al. The ERK signaling cascade inhibits gonadotropin-stimulated steroidogenesis. J Biol Chem. 2001;276:13957–13964. [DOI] [PubMed] [Google Scholar]
- 79. Wayne CM, Fan HY, Cheng X, Richards JS. Follicle-stimulating hormone induces multiple signaling cascades: evidence that activation of Rous sarcoma oncogene, RAS, and the epidermal growth factor receptor are critical for granulosa cell differentiation. Mol Endocrinol. 2007;21:1940–1957. [DOI] [PubMed] [Google Scholar]
- 80. Carlone DL, Richards JS. Functional interactions, phosphorylation, and levels of 3′,5′-cyclic adenosine monophosphate-regulatory element binding protein and steroidogenic factor-1 mediate hormone-regulated and constitutive expression of aromatase in gonadal cells. Mol Endocrinol. 1997;11:292–304. [DOI] [PubMed] [Google Scholar]
- 81. Hunzicker-Dunn M, Maizels ET. FSH signaling pathways in immature granulosa cells that regulate target gene expression: branching out from protein kinase A. Cell Signal. 2006;18:1351–1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Downs SM, Chen J. EGF-like peptides mediate FSH-induced maturation of cumulus cell-enclosed mouse oocytes. Mol Reprod Dev. 2008;75:105–114. [DOI] [PubMed] [Google Scholar]