Abstract
IGF-I/insulin-like growth factor binding protein 2 (IGFBP-2) coordinately stimulate osteoblast differentiation but the mechanisms by which they function have not been determined. AMP-activated protein kinase (AMPK) is induced during differentiation and AMPK knockout mice have reduced bone mass. IGF-I modulates AMPK in other cell types; therefore, these studies determined whether IGF-I/IGFBP-2 stimulate AMPK activation and the mechanism by which AMPK modulates differentiation. Calvarial osteoblasts and MC-3T3 cells expressed activated AMPK early in differentiation and AMPK inhibitors attenuated differentiation. However, expression of constitutively activated AMPK inhibited differentiation. To resolve this discrepancy we analyzed the time course of AMPK induction. AMPK activation was required early in differentiation (day 3–6) but down-regulation of AMPK after day 9 was also necessary. IGF-I/IGFBP-2 induced AMPK through their respective receptors and blocking-receptor activation blocked AMPK induction. To determine the mechanism by which AMPK functioned we analyzed components of the autophagosome. Activated AMPK stimulated ULK-1 S555 phosphorylation as well as beclin-1 and microtubule-associated protein 1A/1B light-chain phosphatidylethanolamine conjugate (LC3II) induction. Inhibition of AMPK attenuated these changes and direct inhibition of autophagy inhibited differentiation. Conversely, expression of activated AMPK was associated with persistence of these changes beyond day 9 and inhibited differentiation. Blocking AMPK activation after day 9 down-regulated these autophagosome components and rescued differentiation. This allowed induction of mechanistic target of rapamycin and AKT, which suppressed autophagy. The results show that early induction of AMPK in response to IGF-I/IGFBP-2 followed by suppression is required for osteoblast differentiation. AMPK functions through stimulation of autophagy. The findings suggest that these early catabolic changes are important for determining the energy source for osteoblast respiration and down-regulation of these components may be required for induction of glycolysis, which is required during the final anabolic stages of differentiation.
Insulin-like growth factor I (IGF-I) is a potent stimulant of osteoblast proliferation and gene-knockout studies have shown that it plays an important role in determining bone size, mass, and mineralization (1, 2). Recent studies have shown that a member of the insulin-like growth factor binding protein (IGFBP) family, IGFBP-2, is also required for optimal IGF-I-stimulated osteoblast proliferation and differentiation (3, 4). Deletion of IGFBP-2 resulted in decreased femoral bone volume/total volume (BV/TV) and reduced femoral length, and it diminished osteoblastic proliferation and differentiation (5). Rescue of cells in which IGFBP-2 expression had been deleted with exogenous addition of IGFBP-2 or a peptide that contains the active domain of IGFBP-2 restored normal growth and differentiation (3, 5). The effect of IGFBP-2 is mediated through a distinct cell surface receptor termed receptor tyrosine phosphatase β (RPTPβ), which functions as a tyrosine phosphatase and dephosphorylates phosphatase and tensin homolog (PTEN) constitutively (6). IGFBP-2 binding to RPTPβ inhibits its phosphatase activity resulting in increased PTEN tyrosine phosphorylation that reduces PTEN-mediated inhibition of AKT (4, 6). However, IGF-I stimulation of AKT activation is required at a time point that is relatively late in the differentiation cycle; therefore, it is not clear whether there are signaling events that are stimulated by IGF-I/IGFBP-2 early in the differentiation cycle, and whether these changes are required for differentiation.
AMP-activated protein kinase (AMPK), a cellular modulator of energy availability, is expressed in low levels in proliferating preosteoblasts and is activated during osteoblast differentiation (7, 8). Activation of AMPK has been shown to both stimulate (9–11) and inhibit osteoblast differentiation (12). Some studies have reported that AMPK activation is induced early during osteoblast differentiation and that its induction is required for normal bone formation in vitro and in vivo (9–11). AMPK-knockout mice have low bone mass and increased bone turnover with enhanced resorption (13, 14). Furthermore, following ovariectomy the rate of bone loss in AMPK−/− mice is retarded compared with controls and both cortical and trabecular bone thickness is reduced (15). Additional studies using knockdown of AMPK in cultured osteoblasts showed that this resulted in attenuated osteogenesis (16). These studies also showed that AMPK was induced early in differentiation and that addition of compound C, an AMPK inhibitor, attenuated differentiation.
In contrast several studies have shown that AMPK inhibits AKT, a known stimulant of osteoblast differentiation (17). AMPK phosphorylates TSC-2 S1345, which enhances its ability to inhibit mechanistic target of rapamycin (mTOR) activation (18). The TORC2 complex that contains activated mTOR mediates AKT S473 activation (19). Additional studies have shown that AMPK inhibits IGF-I-stimulated AKT activation (20). Therefore, it was not clear whether IGFBP-2 and IGF-I could stimulate AMPK activation in osteoblasts or why AMPK, a known inhibitor of AKT activation (which is required for osteogenic differentiation), would enhance differentiation. Hence, these studies were undertaken to determine whether IGF-I/IGFBP-2 could regulate AMPK in osteoblasts, to determine the downstream signaling events that occurred in response to AMPK induction and if AMPK induction was required for IGF-I/IGFBP-2 stimulation of osteoblast differentiation. Furthermore, we analyzed the time course of AMPK activation to determine whether changes in the timing of AMPK activation or its inhibition could explain conflicting results reported previously, thereby clarifying the role of AMPK in osteoblast differentiation.
Materials and Methods
Human IGF-I was a gift from Genentech. Immobilon-P membrane, an anti-pAMPK(T172) antibody and compound C were purchased from EMDmillipore Corp. α-MEM, streptomycin, and penicillin were purchased from Life Technologies. Antibodies against phospho-AKT (S473), pAMPK(S485), pmTOR (S2448), phospho-ULK-1 (S555 and S757), LC3I/II, and Beclin-1 were purchased from Cell Signaling Technology, Inc. Antiosteocalcin and antifibronectin (Fn-3) antibodies were purchased from Santa Cruz Biotechnology, Inc. Bafilomycin A1 and PQ401 were purchased from R&D systems. IGFBP-2 antiserum was prepared as previously described (21). The horseradish peroxidase–conjugated mouse antirabbit, goat antimouse, and mouse antirabbit light chain–specific antibodies were purchased from Jackson ImmunoResearch Laboratories. All other reagents were obtained from Sigma unless otherwise stated.
Mice
Generation of the original mixed background strain B6;129-Igfbp2<tm1Jep>, which we refer to as Igfbp2−/− mice, has been described previously (5). The mice were backcrossed onto C57BL/6J background for 10 generations. Igfbp2+/+ mice were C57BL/6J controls. All of the experimental studies were performed with male mice. All of the animal studies were reviewed and approved by the Institutional Animal Care and Use Committee of University of North Carolina at Chapel Hill.
Cell culture
MC-3T3 E1 clone 4 (CL4) cells were obtained from ATCC. Cells were cultured in α-MEM (glucose 1000 mg/L) containing 10% fetal bovine serum (ThermoFisher Scientific). After confluency, culture medium was changed to differentiation medium (DM), which contained 10% fetal bovine serum plus 50 ug/mL ascorbic acid and 4mM β-glycerol phosphate. Fresh DM was applied every 72 hours. In some experiments IGFBP-2 (1 ug/mL) and/or IGF-I (100 ng/mL) was added to the DM and replaced every 72 hours unless otherwise stated. Exposure to IGFBP-2 did not affect cell number, apoptosis, or MAPK activation during differentiation (4).
Neonatal calvarial osteoblasts were isolated from 3–5-day-old mice. Cells were obtained from IGFBP-2 (−/−) mice and control (+/+) littermates (3). Briefly, calvaria were digested five times with collagenase type 2 (250 U/mL) and trypsin (0.05%) plus EDTA (0.02%) in the PBS. The cells released from digests 2–5 were collected as primary calvarial osteoblasts and maintained in α-MEM (glucose 1000 mg/L) supplemented with 10% fetal bovine serum and nonessential amino acids.
Construction of cDNAs and establishment of MC3T3 cells expressing wild-type IGFBP-2, AMPK (1–312) T172D, and β-galactosidase (LacZ)
The preparation of the constructs that contained IGFBP-2 (4), AMPK (1–312) T172D, and LacZ (20) have been described previously. That these constructs contained the correct sequences was verified by DNA sequencing. 293FT cells (Life Technologies) were prepared for generation of virus stocks and CL4 expressing IGFBP-2, AMPK T172D, and LacZ were established using procedures that have been described previously (22).
Construction of cDNAs and establishment of IGFBP-2 Si and LacZ Si cells
Based on Life Technologies' website design tools, a sequence containing 21 oligonucleotides (GGAAAGAGACCAACACTGAGC) was used to construct the shRNA template plasmid to inhibit the translation of mouse IGFBP-2 mRNA. The oligonucleotides were synthesized by Nucleic Acids Core Facility at the University of North Carolina annealed and ligated into BLOCK-iT U6 RNAi Entry Vector (Cat No. K4945-00, Life Technologies) following the manufacturer's instructions. The complete sequence was verified by DNA sequencing. The expression vector was generated using the Gateway LR recombination reaction between the Entry Vector and BLOCK-iT Lentiviral RNAi Gateway Vector (Cat No. K4943-00, Life Technologies). A sequence targeting LacZ was used as a control. After confirmation of the sequence, plasmid DNA was prepared using a Plasmid Midi Kit (Promega). 293FT cells (Life Technologies) were transfected and used to prepare for generation of virus stocks. CL4 cells expressing small hairpin RNA sequence targeting IGFBP-2 (IGFBP-2 Si) and corresponding control CL4 expressing small hairpin RNA sequence targeting LacZ (Ctrl Si) were established using procedures described previously (22)
Immunoblotting
The cell monolayers were lysed in a modified radioimmunoprecipitation assay buffer as previously described (22). Immunoblotting was performed as previously described (22) using a dilution 1:1000 for anti- pAKT (Ser473), pAMPK (T172, S485), pmTOR (S2448), pULK (S555 and S757), LC3I/II, Beclin-1 and β-actin antibodies. A dilution of 1:150 was used for antiosteocalcin and a dilution 1:10000 for anti-IGFBP-2. The proteins were visualized using enhanced chemiluminescence (ThermoFisher Scientific). Total cellular protein in the lysates was determined using bicinchoninic acid assay (ThermoFisher Scientific).
Alizarin Red staining
Cells were washed with PBS twice before they were fixed with 10% formalin. After 10 minutes' fixation, 1% Alizarin Red (pH 4.2) was applied and incubated for another 10 minutes before it was removed. Cells were washed with ddH2O twice and dried. Images were captured using Leica M420 Microscope. The time course of the change in Alizarin red staining as well as osteocalcin expression during the course of differentiation in MC3T3 cells is shown in Supplemental Table 1.
Statistical analysis
Densitometry results are expressed as the mean ± SD. All experiments were replicated at least three times to assure reproducibility. The results were analyzed for statistically significant differences using Student t test or ANOVA followed by the Bonferroni multiple comparison post-hoc test. Statistical significance was set at P < .05.
Results
Biphasic regulation of AMPK is required for optimal osteoblast differentiation
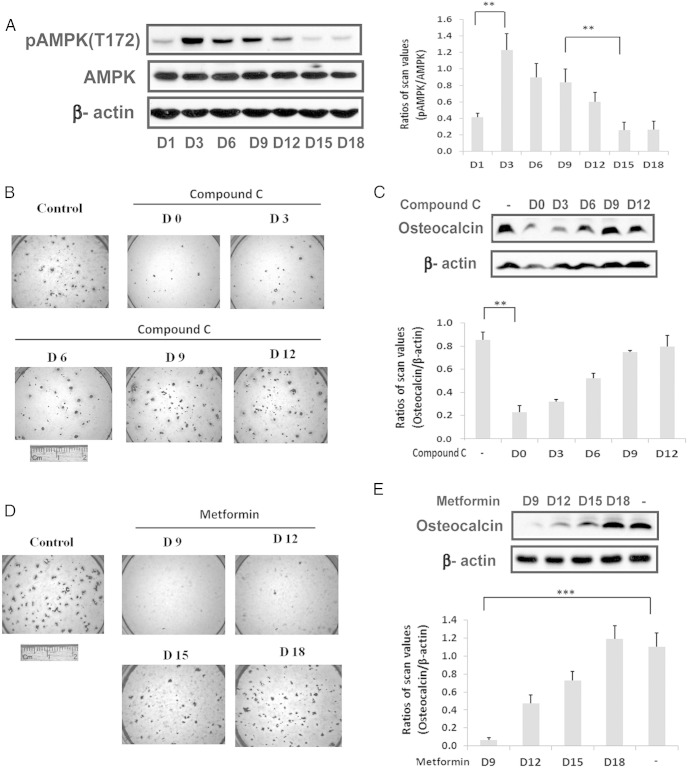
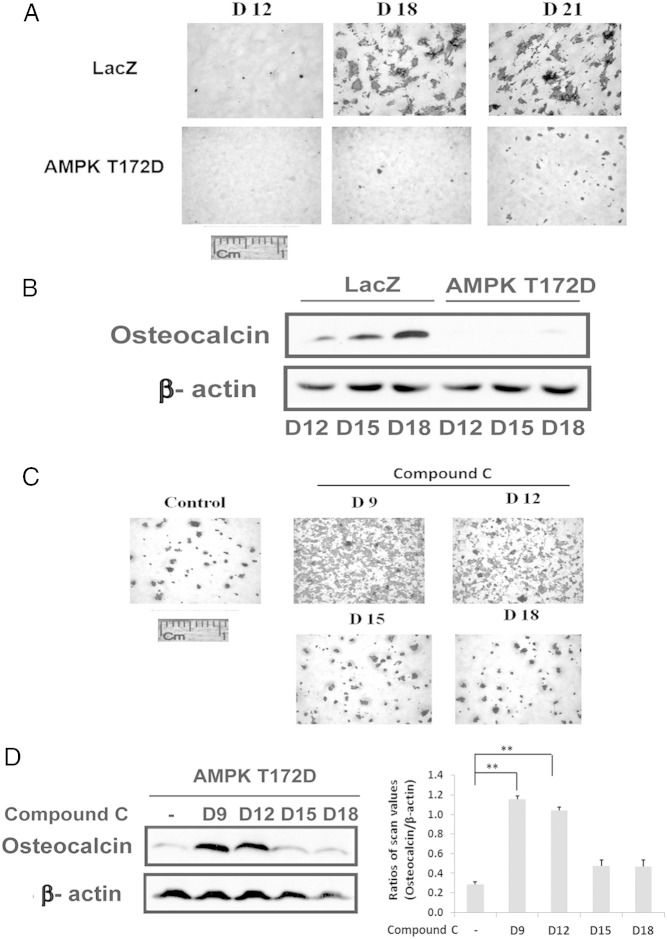
To determine whether AMPK was induced early in differentiation MC-3T3 cells were exposed to beta glycerol phosphate, and ascorbic acid in 10% fetal bovine serum and the abundance of AMPK phospho (T172) (located in the activation loop) assessed by direct immunoblotting. Phospho AMPK (T172) increased 2.9 ± 0.4-fold (P < .01) between days 1 and 3 of differentiation (Figure 1A). This was followed by a significant decrease (70.6 ± 0.6%; P < .01) between days 9 and 15 whereas there was no significant change in AMPK protein. To assess the significance of this change for osteoblast differentiation, compound C, an AMPK inhibitor, was added early in the differentiation cycle and at later time points. The addition of this AMPK inhibitor on day 0 or 3 resulted in attenuation of differentiation (inhibition of alizarin red staining) whereas addition on day 9 or later had no effect (Figure 1B). Similarly, osteocalcin expression was reduced 73.1 ± 6.4% (P < .01) in the day-0-treated cultures compared with control (Figure 1C). The results suggest that induction of AMPK early in the differentiation cycle is required and that prior studies that reported AMPK inhibited differentiation might be explained by persistent activation of AMPK at later time points (12). To test this hypothesis we repeated the experiment but metformin was added to stimulate AMPK between days 9 and 18. As shown in Figure 1D, addition of metformin on days 9 and 12 attenuated differentiation whereas addition on day 18 had no effect. Measurement of osteocalcin expression confirmed this result (Figure 1E). To confirm that this change was due to AMPK activation we used cells that expressed an AMPK mutant that was constitutively activated (T172D). Expression of this mutant resulted in near-complete suppression of osteoblast differentiation as assessed both by Alizarin Red staining or osteocalcin expression (Figure 2, A and B). Given that activated AMPK was expressed at relatively high levels through day 9 in nontransfected cells but then decreased (Figure 1A), we determined the effect of adding compound C on day 9 to cells that expressed the constitutively active mutant. Addition of compound C on days 9 and 12 rescued differentiation but addition on day 15 or 18 had no effect (Figure 2C). Analysis of osteocalcin expression showed that it was increased 4.1 ± 0.7-fold (P < .01) in the cultures exposed to compound C on day 9 compared with control (Figure 2D). These results demonstrate that during the midphase of differentiation suppression of AMPK activity is required to complete the differentiation cycle.
Figure 1.
Biphasic regulation of AMPK activation is required for osteoblast differentiation. A, Cell lysates from MC-3T3 cells following exposure to DM for the indicated number of days were prepared and immunoblotted with an anti-pAMPK (T172) antibody as described in Materials and Methods. β-actin was immunoblotted as a loading control. Each bar is the ratio of the scan value of the pT172AMPK band divided by the AMPK band. B and D, MC-3T3 cells were stained by Alizarin Red on day 21 after DM alone (control) or DM plus compound C (1uM) (B) or metformin (0.5mM) (D), which had been added on the indicated day. C and E, Cell lysates were obtained from MC-3T3 cells on day 21 following DM exposure in the absence or presence of compound C (1uM) (C) or metformin (0.5mM) (E) that had been added on the indicated day. They were immunoblotted with antiosteocalcin or anti-β-actin. Each bar is the ratio of the scan value of the osteocalcin band divided by the β-actin band. **, P < .01 and ***, P < .001 indicate significant differences between two treatments.
Figure 2.
Constitutively activated AMPK impaires osteoblast differentiation. A, LacZ- or AMPK T172D-overexpressing cells were stained by Alizarin Red on the indicated day after DM exposure. B, Lysates obtained from the cells expressing LacZ or AMPK T172D on the indicated day following DM exposure were immunoblotted with antiosteocalcin or β-actin. C, AMPK T172D-overexpressing cells were stained with Alizarin Red on 21 days after DM exposure in the absence (control) or the presence of compound C (1uM) that was added on the indicated day. D, Lysates obtained from cells overexpressing AMPK T172D after 21 days of DM exposure in the absence or in the presence of compound C (1uM) added on the indicated day were immunoblotted with antiosteocalcin or β-actin. Each bar is the ratio of the scan value of the osteocalcin band divided by the β-actin band. **, P < .01 indicates significant differences between two treatments.
IGF-I and IGFBP-2 stimulate AMPK activation at the early stage of differentiation
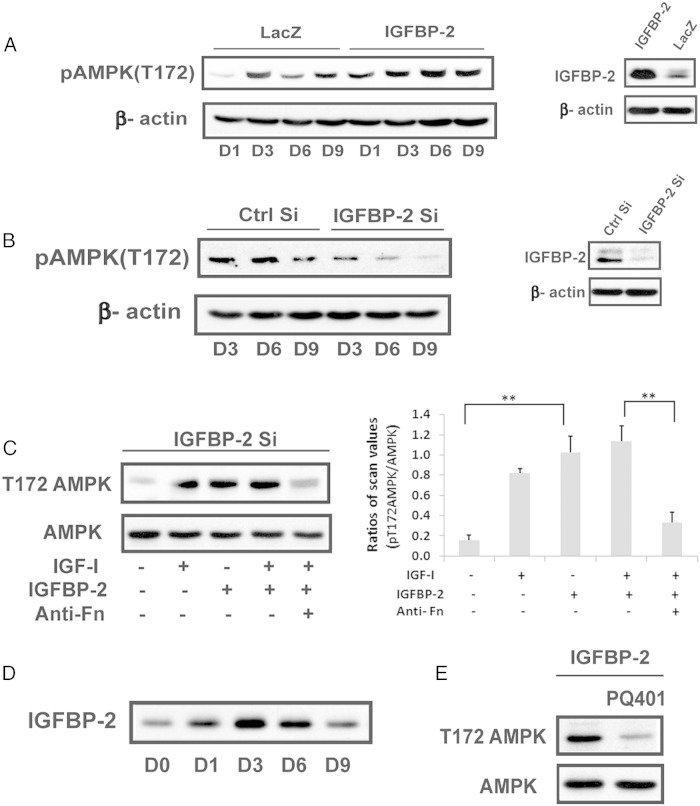
To determine whether IGF-I and IGFBP-2 played a regulatory role in AMPK induction we used cells overexpressing IGFBP-2. IGFBP-2 overexpression stimulated AMPK (phosphoT172) on days 3–9 and the levels on day 6 and 9 were 2.2 ± 0.1 (P < .01) and 1.4 ± 0.1 (P < .05) -fold greater than control cultures (Figure 3A). To confirm the role of IGFBP-2, SiRNA was used. Cultures in which IGFBP-2 was significantly decreased showed a significant reduction in AMPK T172 phosphorylation between days 3–9 compared with control cultures (56 ± 3%, P < .01; 70 ± 5%, P < .01; and 73 ± 17%, P < .05 decreases on days 3,6, and 9, respectively) (Figure 3B). Activation of AMPK could be restored by adding IGF-I or IGFBP-2 to the Si RNA treated cultures (Figure 3C). Given that these results showed that IGFBP-2 stimulated AMPK activation we measured IGFBP-2 secretion in nontransfected MC-3T3 cells early in differentiation. IGFBP-2 was increased 3.8 ± 0.6 (P < .01) -fold on day 3. This was sustained on day 6 but decreased significantly on day 9 (62 ± 7% reductionn; P < .01) (Figure 3D). To determine whether IGF-I and IGFBP-2 were stimulating AMPK though binding to their respective receptors, PQ401, an IGF-I receptor tyrosine kinase inhibitor, and an antifibronectin-3 antibody that inhibits IGFBP-2 binding to RPTPβ (4, 6) were used. As shown in Figure 3, C and E, these inhibitors attenuated the ability of IGF-I and IGFBP-2 to stimulate AMPK (eg, 74 ± 7%, P < .01; 67 ± 7%, P < .05, reductions, respectively) thereby confirming that activation of both receptors was required.
Figure 3.
Activation of AMPK at the early stage of differentiation is positively regulated by IGFBP-2 and requires IGF-I receptor activation. A, Cell lysates obtained from LacZ- or IGFBP-2-overexpressing cells on indicated day after exposure to DM were immunoblotted with anti-pAMPK (T172), IGFBP-2 or β-actin. B, Lysates from MC-3T3 cells expressing shRNA sequence targeting LacZ (Ctrl Si) or IGFBP-2 (IGFBP-2 Si) obtained on the indicated day after DM exposure were immunoblotted with anti-pAMPK (T172), IGFBP-2, or β-actin. C, Lysates from MC-3T3 cells expressing shRNA sequence targeting IGFBP-2 (IGFBP-2 Si) following a 3-day DM exposure in the absence or the presence of IGF-I (100 ng/mL) alone or IGFBP-2 (1 ug/mL) alone or both or IGF-I and IGFBP-2 plus antifibronectin antibody-3 (anti-Fn) (500 ng/mL) were immunoblotted with anti-pAMPK (T172) or anti-AMPK. Each bar is the ratio of the scan value of the pT172AMPK band divided by the AMPK band. **, P < .01 indicates significant differences between two treatments. D, Lysates from MC-3T3 cells obtained on the indicated day after DM exposure were immunoblotted with anti-IGFBP-2. E, Lysates from IGFBP-2 overexpressing cells obtained on day 3 after DM exposure in the absence or the presence of PQ401 (25uM) were immunoblotted with anti-pAMPK (T172) or anti-AMPK.
Activation of AMPK stimulates autophagy, which is required for osteoblast differentiation
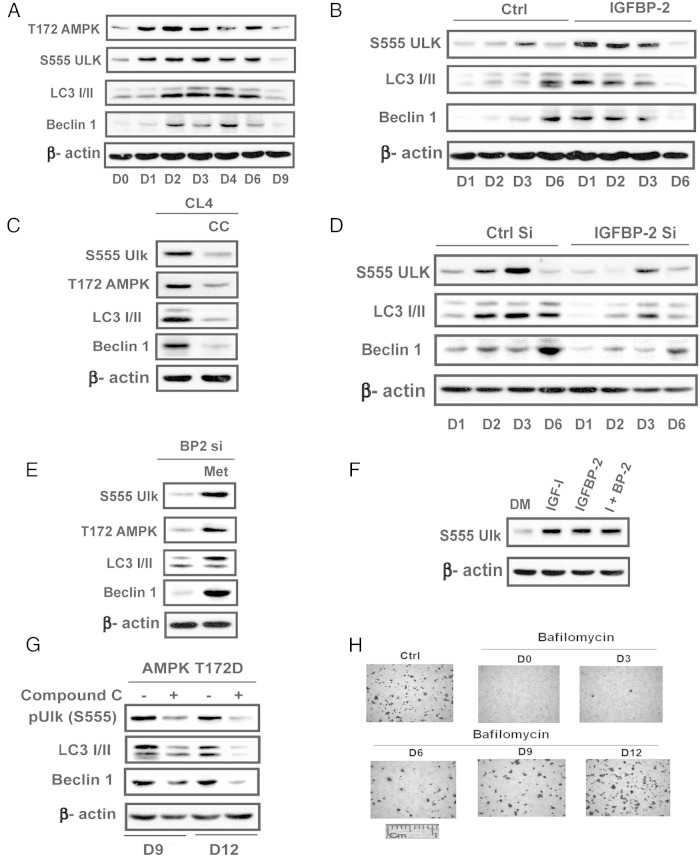
Recent reports have suggested that stimulation of autophagy is necessary for early osteoblast differentiation. Given that AMPK directly phosphorylates unc-51 like autophagy activating kinase-1(ULK-1), thereby stimulating ULK-1 activation and this is required to assemble essential components of the autophagosome, we investigated the possibility that the transient induction of AMPK early in differentiation was leading to stimulation of autophagy. Characterization of the time course of these changes in nontransfected MC-3T3 cells showed that phosphorylation of ULK-1 S555 (a known AMPK phosphorylation site) was increased 2.7 ± 0.5-fold (P < .05) on day 2 and 2.4 ± 0.2-fold (P < .01) on day 3 in parallel with AMPK activation (Figure 4A). Consistent with the changes in AMPK activation ULK-1 S555 phosphorylation on day 9 was markedly attenuated (72 ± 9%; P < .01, decrease compared with day 3). Similarly, microtubule-associated protein 1A/1B light-chain phosphatidylethanolamine conjugate (LC3I/II) and beclin-1, important components of the autophagosome, showed increased expression on days 2–6 (eg, 3.0 ± 0.5-fold, P < .01, and 4.8 ± 0.9-fold, P < .001 on day 2) that was attenuated on day 9. Enhanced expression of IGFBP-2 accelerated the time course of the increase in ULK-1 serine 555 phosphorylation and beclin-1 and LC3I/II expression (3.1 ± 0.5, P < .05; 7.8 ± 0.5, P < .001; 4.9 ± 0.9 P < .01 -fold increases on day 1 compared with control) (Figure 4B). The addition of compound C markedly attenuated the changes in these components that occurred on day 3 (Figure 4C). IGFBP-2 knockdown attenuated ULK-1 phosphorylation (54 ± 6% reduction; P < .05 on day 3) and beclin-1 and LC3I/II induction (53 ± 2 and 59 ± 3% reductions; P < .01, on day 6) (Figure 4D). In contrast, metformin stimulated ULK-1 phosphorylation, LC3I/II, and beclin-1 on day 3 even in the absence of IGFBP-2 (Figure 4E). As for AMPK induction, both IGF-I and IGFBP-2 stimulated ULK-1 S555 on days 1–3 (3.4 ± 0.4, P < .01; and 4.4 ± 0.8, P < .01, -fold increases, respectively) (Figure 4F). The addition of compound C on day 9 or 12, which rescued differentiation in the AMPK-T172D-overexpressing cells, significantly suppressed ULK-1 S555 phosphorylation, beclin-1, and LC3I/II expression (48 ± 2%, P < .01; 58 ± 8%, P < .01, and 60 ± 2%, P < .05, decreases, respectively) (Figure 4G). To determine whether these changes were relevant to autophagy we measured differentiation in response to the addition of bafilomycin, an autophagy inhibitor. The addition of bafilomycin between days 0 and 6 inhibited osteoblast differentiation whereas addition on day 12 had no effect (Figure 4H). These results clearly demonstrate that autophagy is required but only early in the differentiation cycle concomitantly with AMPK activation. Taken together, the findings strongly suggest that IGF-I/IGFBP-2 induce AMPK early in differentiation and that this is required to activate autophagy but that AMPK activation must also be attenuated after day 9 for differentiation to proceed.
Figure 4.
Activation of autophagy at the early stage of differentiation is required for optimal osteoblast differentiation. A, Lysates from MC-3T3 cells obtained following exposure to DM for the indicated days were immunoblotted with anti-pAMPK (T172), pULK (S555), LC3 I/II, Beclin-1, or β-actin. B and D, Lysates from LacZ- (Ctrl) or IGFBP-2-overexpressing cells (B) or from MC-3T3 cells expressing shRNA sequence targeting LacZ (Ctrl Si) or IGFBP-2 (IGFBP-2 Si) (D) obtained on indicated day after DM exposure were immunoblotted with an anti-pULK (S555), LC3 I/II, Beclin-1, or β-actin antibody. C, Lysates from MC-3T3 cells after 3 days of DM exposure in the absence or the presence of compound C (CC) (1uM) were immunoblotted with an anti-pAMPK (T172), pULK (S555), LC3 I/II, Beclin-1, or β-actin. E, Lysates from MT-3T3 cells expressing the shRNA-targeting IGFBP-2 (IGFBP-2 Si) after 3 days of DM exposure in the absence or the presence of metformin (Met) (0.5mM) were immunoblotted with an anti-pAMPK (T172), pULK (S555), LC3 I/II, Beclin-1, or β-actin. F, Lysates from MC-3T3 cells after 3 days of DM exposure in the absence or the presence of IGF-I (100 ng/mL) or IGFBP-2 (1 ug/mL) or both were immunoblotted with an anti-pULK (S555) or β-actin. G, Lysates obtained from cells overexpressing AMPK T172D after 9 or 12 days of DM exposure in the absence or in the presence of compound C (1uM) were immunoblotted with an anti-pULK (S555), LC3 I/II, Beclin-1, or β-actin. H, MC-3T3 cells were stained by Alizarin Red on day 21 after DM alone (Ctrl) or DM plus bafilomycin-1A (2nM) added on the indicated day.
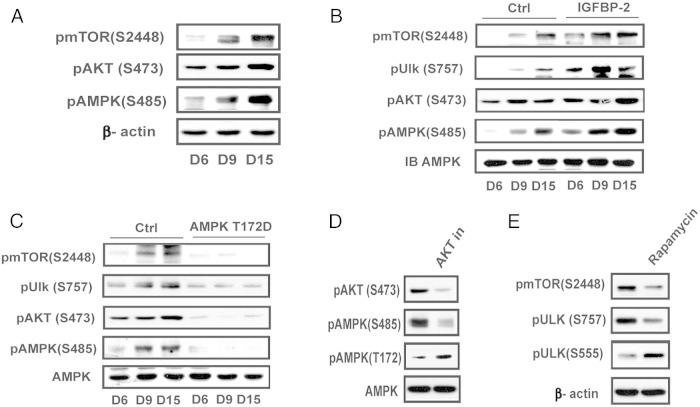
Activation of AKT and mTOR are responsible for suppression of AMPK at the late stage of differentiation
To understand the underlying mechanism for suppression of AMPK activation and autophagy in the middle of the differentiation cycle, we focused on the changes that occurred between days 6 and 15. Late activation of mTOR/AKT has been observed in osteogenic differentiation of human meschymal stem cells (16); therefore, the changes in phospho mTOR (S2448) and phospho AKT (S473) were determined. The results show that activation of mTOR and AKT increased substantially between days 6 and 15 (Figure 5A). Phosphorylation of AMPK S485, which is mediated by AKT and negatively regulates AMPK activity (20), was also increased. Enhanced IGFBP-2 expression was accompanied by increased AKT S473 (2.0 ± 0.2-fold increase; P < .01 on day 15) and AMPK S485 phosphorylation (2.1 ± 0.2-fold increase; P < .01 on day 9) (Figure 5B). Similarly, IGFBP-2 overexpression stimulated mTOR activation (3.5 ± 0.4-fold; P < .001 on day 9) compared with control cultures. This led to enhanced phosphorylation of ULK-1 S757, a known mTOR phosphorylation site (3.2 ± 0.8-fold increase on day 9; P < .001) (Figure 5B). This change down-regulates ULK-1 activity and inhibits autophagy (23). In contrast, overexpression of constitutively active AMPK (T172D) prevented the increases in mTOR Ser2448 and AKT S473 phosphorylation on days 9 and 15; consequently, there was no increase in ULK-1 S757 or AMPK S485 phosphorylation (Figure 5C). To confirm that the change in AMPK S485 led to a decrease in AMPK activation we used an AKT inhibitor. This reduced S485 phsophorylation by 69 ± 5% (P < .01) and increased T172 phsophorylation 2.3 ± 0.4-fold, (P < .05) (Figure 5D). Similarly, the addition of an mTOR inhibitor reduced ULK-1 phsophorylation by 65 ± 6% (P < .01), which was associated with a 2.4 ± 0.3-fold (P < .01) increase in ULK-1 S555 (Figure 5E). Therefore, our results show that activation of AKT signaling is responsible for down-regulation of AMPK activation and mTOR down-regulates ULK-1 during the middle and late phases of differentiation.
Figure 5.
Activation AMPK is suppressed at the middle and late stage of differentiation via activation of mTOR and AKT. A, Cell lysates from MC-3T3 cells following exposure to DM for the indicated days were immunoblotted with an anti-pmTOR (S2448), pAKT (S473), pAMPK (S485), or β-actin. B and C, Lysates from LacZ- (Ctrl) or IGFBP-2-overexpressing cells (B) or from MC-3T3 cells expressing AMPK T172D (C) obtained on the indicated day after DM exposure were immunoblotted with anti-pmTOR (S2448), pULK (S757), pAKT (S473), pAMPK (S485), or AMPK. D, Lysates from MC-3T3 cells following exposure to DM for 9 days in the presence of or absence of AKT inhibitor (AKT in) (500nM) were immunoblotted with an anti-pAKT (S473), pAMPK (S485, T172), or AMPK. E, Lysates from MC-3T3 cells following exposure to DM for 9 days in the presence of or absence of rapamycin (500nM) were immunoblotted with an anti-pmTOR (S2448), pULK (S757, S555), or β-actin.
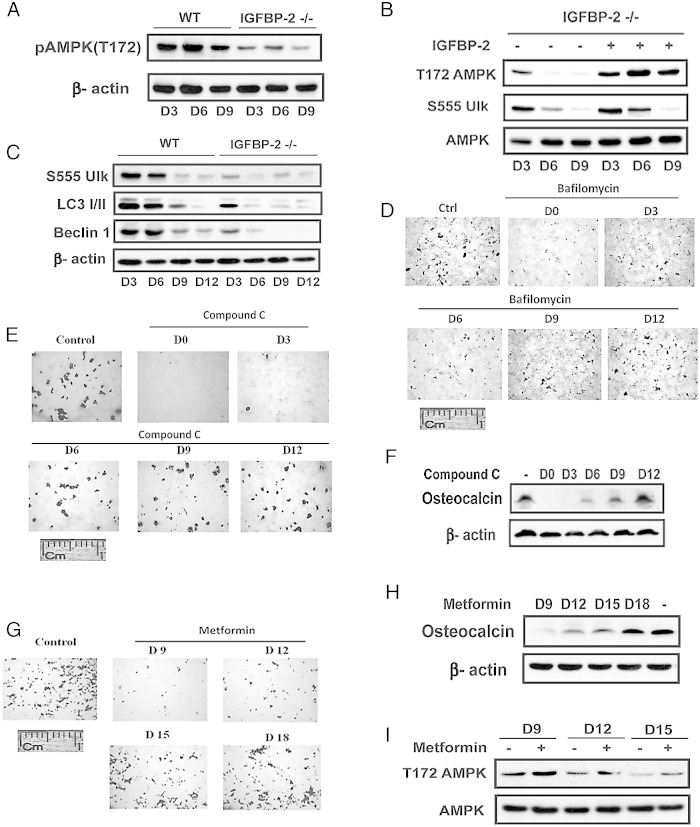
Biphasic regulation of AMPK and autophagy is required for calvarial osteoblast differentiation
To determine the physiologic relevance of these findings for normal osteoblast differentiation primary mouse calvarial osteoblasts, isolated from control and IGFBP-2−/− mice were subjected to similar treatments. Analysis of AMPK T172 expression in the absence of IGFBP-2 showed that it was significantly suppressed on days 3–9 (Figure 6A). The addition of IGFBP-2 to the −/− cultures resulted in significant stimulation of AMPK T172 phosphorylation (eg, 1.5 ± 0.2, P < .05; 7.6 ± 0.8, P < .001; and 8.3 ± 1.2, P < .01, fold increases on days 3, 6, and 9 compared with control). This was accompanied by corresponding changes in ULK-1 phosphorylation (eg, 1.3 ± 0.1-fold increase on day 3, P < .05; and 2.8 ± 0.2-fold on day 6, P < .05) (Figure 6B). As for the MC-3T3 cells, the loss of expression of IGFBP-2 resulted in attenuated ULK-1 S555 phosphorylation on days 3 and 6 and there was a decrease in beclin-1and LC3I/II abundance (Figure 6C). Consistently, addition of bafilomycin at the early stage, such as on day 0 or day 3, significantly impaired wild-type (WT) osteoblast differentiation (Figure 6D). To determine whether AMPK was required for differentiation, compound C was added to osteoblast cultures derived from WT mice between days 0 and 12 and Alizarin Red staining determined. The addition of compound C on day 0 or 3 attenuated differentiation whereas addition on days 9 and 12 had no effect (Figure 6E). These findings were confirmed when osteocalcin expression was determined (Figure 6F). To assess the importance of attenuation AMPK stimulation later in the differentiation cycle metformin was added on days 9–18. As shown in Figure 6G the addition of metformin on day 9 or 12 resulted in significant inhibition of differentiation whereas addition at later times had no effect. When osteocalcin expression was determined this finding was confirmed (Figure 6H). The addition of metformin on day 9, 12, and 15 stimulated AMPK T172 phosphorylation (Figure 6I). The results clearly demonstrate that induction of AMPK early in the differentiation cycle in primary osteoblasts is required for differentiation. IGFBP-2 stimulates these changes and attenuation of AMPK activation between days 9 and 15 is also required.
Figure 6.
Biphasic regulation of AMPK is required for calvarial osteoblast differentiation. A and B, Calvarial osteoblasts isolated from IGFBP-2+/+ WT or IGFBP-2−/− mice were exposed to differentiation medium (DM) for indicated number of days. A, Lysates were immunoblotted with anti-pAMPK(T172) or β-actin. B, Calvarial osteoblasts isolated from IGFBP-2−/− mice were exposed to DM for indicated days in the absence or the presence of IGFBP-2 (1 ug/mL). Lysates were immunoblotted with anti-pAMPK (T172), pULK (S555), or AMPK. C, Lysates were immunoblotted with an anti-pULK (S555), LC3 I/II, Beclin-1 or β-actin. D, E, and G, Cells were stained by Alizarin Red on day 21 after DM alone (control) or DM plus bafilomycin A1 (2nM) (D), compound C (1uM) (E), or metformin (0.5 mM) (G) that had been added on the indicated day. F and H, Cell lysates from the same treatments as panel E (F) or G (H) were immunoblotted with an antiosteocalcin or β-actin antibody. I, Lysates from cells that had been exposed to DM for indicated number of days in the absence or presence of metformin (0.5mM) for 2 hours were immunoblotted with anti-pAMPK (T172) or AMPK.
Discussion
Our results demonstrate that the requirement for AMPK activation and its role in stimulating osteoblast differentiation is biphasic. Specifically, induction of AMPK activation early in the differentiation cycle is required for differentiation to proceed but failure to down-regulate AMPK after day 9 results in attenuation of differentiation. That AMPK induction is required is supported by the fact that expression of osteocalcin and Alizarin Red staining were inhibited when AMPK activation was suppressed between days 0 and 6. This requirement was clearly context specific and time limited given that addition of an AMPK inhibitor at later time points had no effect. The necessity for down-regulating AMPK activation after day 9 was confirmed using a different strategy. Specifically, overexpression of a constitutively activated form of AMPK inhibited differentiation but the addition of an AMPK inhibitor between days 9 and 12 resulted in complete restoration of the osteoblast differentiation. This strongly suggests that AMPK activity must be down-regulated at later stages in order for differentiation to proceed.
Prior studies reported that activation of AMPK is required for osteoblast differentiation in vitro (9–11). Both MC-3T3 and primary murine osteoblasts showed enhanced differentiation in response to metformin or 5-aminoimidazole-4-carboxamide ribonucleotide (AICAR), agents that induce AMPK (9–11, 24–26), but the increases in AMPK were transient (eg, 1–3 h) making it difficult to conclude that AMPK induced a change in differentiation that occurred over 14 days. Other studies showed that an 8–14-day exposure to AICAR increased osteocalcin and Runx2 expression but did not document that this enhanced AMPK activity throughout this interval (11, 26). Shah et al (9) reported metformin stimulated mineralized nodule formation in primary rat osteoblasts after 17 days and that this was inhibited with compound C but AMPK activation was not measured. Therefore, none of these reports demonstrated sustained activation of AMPK during the course of differentiation.
Three studies analyzed the effect of altering AMPK activity in vivo. Shah et al (9) reported that AMPK-knockout mice had reduced trabecular number and BV/TV as well as trabecular and cortical thickness. Quinn et al (25) knocked out the AMPK beta 2 subunit which led to reduced BV/TV, trabecular number, and bone mineral density (BMD). However, treatment of normal mice with AICAR also resulted in decreased BV/TV, trabecular thickness and BMD. This was attributed to increased osteoclast formation in response to AMPK activation but whether prolonged exposure AICAR also inhibited osteoblast differentiation was not analyzed. In contrast, Kasai et al (12) showed that a transient increase in AMPK expression inhibited primary mouse osteoblast differentiation. Unlike the other reports these investigators showed that AMPK was induced at several points during the 18-day treatment period. In an attempt to resolve these differences Pantovik et al (16) showed that dexamethasone stimulated an increase in AMPK and suppressed mTOR on days 1 and 3 in dental pulp mesenchymal stem cells. Knockdown of AMPK at day 1 inhibited differentiation but knockdown of mTOR reduced alkaline phosphatase induction at day 7. They concluded that AMPK and autophagy were activated early in differentiation but they did not address the significance of changes in either AMPK or autophagy that occurred after day 5.
Our study significantly extends these observations by confirming that AMPK induction is required early during differentiation and that IGF-I and IGFBP-2 stimulate AMPK. More importantly, we delineated the downstream events that occur following AMPK activation that lead to osteoblast differentiation (Figure 7). Although prior studies have suggested that direct phosphorylation of AKT or induction of DLX5-dependent Runx2 expression (26) are mechanisms by which AMPK stimulates differentiation, our finding that AMPK stimulates ULK-1 phosphorylation at the same time that autophagy is being initiated provides a mechanism for coordinating changes in energy metabolism and autophagy. Prior reports have suggested that induction of autophagy is required for osteoblast differentiation. Pantovik et al (16) showed that activation of AMPK on days 1–5 was accompanied by an increase in LC3I/II and beclin-1 and that the autophagy inhibitor, bafilomycin, inhibited alkaline phosphatase induction. Chang et al (27) demonstrated in bone marrow osteoblasts deletion of p62, a protein that transports cargo to the autophagosome, resulted in inhibition of differentiation, whereas induction of p62 resulted in full differentiation. Two studies in mice have confirmed the importance of autophagy for normal bone formation. Liu et al (14) demonstrated that deletion of an essential component of the autophagosome complex (FIP 200) led to multiple autophagy defects, deficient mineralization, decreased BV/TV, trabecular number, and cortical thickness. Using osteoblasts isolated from these mice they confirmed that there was a defect in differentiation. Nollet et al (13) showed that knockdown of ATG 5, a component of the autophagosome resulted in decreased bone volume and mineralization as well as an increase in osteoclast number. BV/TV, trabecular width, and number were also reduced significantly. Taken together, these findings strongly support the conclusion that autophagy is required for normal bone cell differentiation.
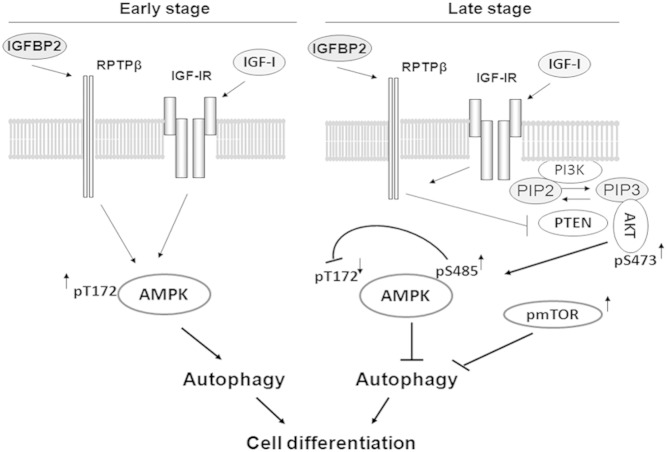
Figure 7.
Biphasic regulation of AMPK and autophagy is required for osteoblast differentiation. At the early stage of differentiation, IGF-I and IGFBP-2 stimulate AMPK activation via IGF-I receptor and RPTPβ dependent pathways, respectively, resulting in activation of autophagy. This early activation of AMPK and autophagy is required for optimal osteoblast differentiation. At the late stage of differentiation, IGF-I and IGFBP-2 stimulate RPTPβ oligomerization and inactivation, leading to inactivation of PTEN and activation of AKT (S473 phosphorylation). Activation of AKT stimulates AMPK S485 phosphorylation, leading to the suppression of AMPK activation (reduction of T172 phosphorylation) and the termination of autophagy. Activation of mTOR at this stage further suppresses autophagy via activation of ULK S757. These signaling events at the late stage are also essential for optimal osteoblast differentiation.
Our study adds to these findings by demonstrating induction of autophagy is required early in differentiation and that IGF-I and IGFBP-2 stimulate increases in important components of the autophagosome (Figure 7). To determine the significance of AMPK induction for autophagy, we analyzed the downstream signaling target ULK-1 given that this kinase is a principal regulator of the initial phase of autophagosome formation and AMPK has been shown to activate ULK-1 through S555 phosphorylation. Our results also show that phosphorylation of ULK-1 S555 is increased at the time when beclin-1 and LC3I/II, essential components of the autophagosome, are also increased and that inhibition of AMPK decreased ULK-1 S555 phosphorylation and inhibited LC3I/II and beclin-1 induction. Studies in other cell types have demonstrated that AMPK stimulates autophagy through direct phosphorylation of ULK-1 S555 and mutagenesis of that serine inhibits autophagy (28). Models of nutrient deprivation or other stresses that are known to activate AMPK result in direct phosphorylation of ULK-1 S555 and this enhances ULK-1 mediated stimulation of autophagosome formation (23, 29, 30). Given that addition of compound C between days 0 and 3 completely attenuated osteoblast differentiation as well as the induction of autophagosome components and direct inhibition of autophagy using bafilomycin added at the same time points inhibited differentiation, we conclude that AMPK phosphorylation of ULK-1 S555 is inducing autophagy and this is required for differentiation. Importantly, we demonstrated that IGF-I/IGFBP-2 regulated beclin-1 and LC3I/II expression, changes that led to autophagosome formation (29).
Our results also emphasize the importance of AMPK down-regulation for termination of autophagy and that this change is required for differentiation. The decrease in AMPK activation after day 9 in nontransfected cells was associated with an increase in AKT activation, which stimulated AMPK S 485 phosphorylation a change that down-regulates AMPK activity (20, 31). Concomitantly, mTOR phosphorylated ULK-1 on S757 and this inhibits ULK-1 activity. This has been shown to be the mechanism by which mTOR down-regulates autophagy (27, 32). Our finding that expression of constitutively activated AMPK resulted in persistent ULK-1 S555 phosphorylation, beclin-1 induction, and suppression of ULK-1 757 phosphorylation beyond day 9, whereas compound C addition inhibited these changes and rescued differentiation, provides further evidence of the importance of AMPK down-regulation in the later stages of differentiation. AMPK phosphorylates TSC-2 S1345, which enables it to inhibit mTOR activation (18). Our results show that AMPK down-regulation after day 9 permits induction of mTOR and AKT activation and that AKT supresses AMPK activity through S485 phosphorylation. However, the event that catalyzes the change from IGF-I/IGFBP-2 stimulation of AMPK activity early in differentiation to their subsequent stimulation of AKT and mTOR leading to AMPK and ULK-1inhibition was not defined. Other investigators have shown that induction of the TORC2 complex, which contains activated mTOR at day 9 is critical for osteoblast differentiation and that this is involved in the switch in energy metabolism from oxidative phosphorylation to glycolysis (33). In that report the changes were mediated by WNT-5 an important osteoblast trophic factor. Given that AMPK is a major stimulant of oxidative phosphorylation and it inhibits glycolysis, our observation that there is a decline in AMPK activity and increased mTOR and AKT activation at the time at which TORC2 has been reported to be increased suggests that down-regulation of AMPK activation is part of a coordinated series of events that is necessary to the switch from oxidative phosphorylation to glycolysis a metabolic reprograming response that has been shown to be important for osteoblast differentiation (33, 34).
Our findings also highlight the importance of coordinate stimulation by both IGF-I and IGFBP-2 for osteoblast differentiation. Several investigators have shown that IGF-I is an important anabolic factor for bone and that deletion of the IGF-I receptor results in major attenuation of late-stage differentiation events such as calcium incorporation and extracellular matrix synthesis (1, 2, 35, 36). IGF-I knockout mice exhibit decreased BV/TV and decreased BMD (1, 2, 37). Similarly, IGFBP-2 deletion results in a phenotype in which males and estrogen deficient females have attenuated bone formation, decreased trabecular number, and decreased BV/TV (5) (38). Exposure to both IGF-I and IGFBP-2 is required for stimulation of osteoblast differentiation and the two distinct signaling pathways that are stimulated by each protein through its distinct receptor interact to enhance AKT activation, which is required for progression through the differentiation cycle (4). These studies extend those observations to show that inhibition of IGF-I receptor activation or inhibition of IGFBP-2 induced RPTPβ inactivation results in attenuation of AMPK induction and ULK-1 S555 phosphorylation. Therefore, it is likely that IGF-I and IGFBP-2 are inducing autophagy through this mechanism.
The basis for understanding why anabolic factors such as IGF-I/IGFBP-2 induce AMPK and autophagy and the linkage between these events and the progression of osteoblast differentiation to the anabolic phase was not addressed in the studies. However, the finding that a temporal order of events described herein whereby AMPK and autophagy are induced early and then have to be suppressed later in differentiation provides a new perspective for analyzing how anabolic growth factors may stimulate more than one physiologically significant event to induce osteoblasts to progress to the final stages of differentiation. Other investigators have proposed that autophagy may be required as a checkpoint prior to entry into differentiation because rapidly proliferating cell types require selective protein editing to eliminate errors that occur during the rapid proliferative phrase and there are examples of anabolic growth factors stimulating autophagy under these conditions (39). Additional reports have emphasized the role of metabolic reprogramming plays in differentiation and both AMP kinase and autophagy induction have been directly linked to this process during myofibroblast differentiation (40). Further study should be directed toward defining the role of IGF-I/IGFBP-2 in stimulating these catabolic events and why they are necessary prior to entry into the final anabolic stage. Although trophic factors such as IGF-I have important role in the final stages of osteoblasts differentiation including calcification and matrix protein synthesis, the necessity for undergoing catabolism prior to initiation these anabolic events and the roles played by IGF-I/IGFBP-2 in regulating these processes requires further investigation.
Acknowledgments
The authors thank Ms Laura Lindsay for her help in preparing the manuscript. We also thank Ms Christine Wai for her technical assistance.
This work was supported by Grant 501AR061164-05 from the National Institutes of Health to D.R.C. and C.J.R.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AICAR
- 5-aminoimidazole-4-carboxamide ribonucleotide
- BV/TV
- bone volume/total volume
- CL4
- MC-3T3 E1 clone 4
- DM
- differentiation medium
- LacZ
- β-galactosidase
- LC3I/II
- microtubule-associated protein 1A/1B light-chain phosphatidylethanolamine conjugate
- mTOR
- mechanistic target of rapamycin
- PTEN
- phosphatase and tensin homolog
- RPTPβ
- receptor tyrosine phosphatase β
- WT
- wild type.
References
- 1. Mohan S, Kesavan C. Role of insulin-like growth factor-1 in the regulation of skeletal growth. Curr Osteoporos Rep. 2012;10:178–186. [DOI] [PubMed] [Google Scholar]
- 2. Giustina A, Mazziotti G, Canalis E. Growth hormone, insulin-like growth factors, and the skeleton. Endocr Rev. 2008;29:535–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kawai M, Breggia AC, DeMambro VE, et al. The heparin-binding domain of IGFBP-2 has insulin-like growth factor binding-independent biologic activity in the growing skeleton. J Biol Chem. 2011;286:14670–14680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xi G, Wai C, DeMambro V, Rosen CJ, Clemmons DR. IGFBP-2 directly stimulates osteoblast differentiation. J Bone Miner Res. 2014;29:2427–2438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. DeMambro VE, Clemmons DR, Horton LG, Bouxsein, et al. Gender-specific changes in bone turnover and skeletal architecture in igfbp-2-null mice. Endocrinol. 2008;149:2051–2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Shen X, Xi G, Maile LA, Wai C, Rosen CJ, Clemmons DR. Insulin-like growth factor (IGF) binding protein 2 functions coordinately with receptor protein tyrosine phosphatase β and the IGF-I receptor to regulate IGF-I-stimulated signaling. Mol Cell Biol. 2012;32:4116–4130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wang C, Lin K, Chang J, Sun J. Osteogenesis and angiogenesis induced by porous β-CaSiO(3)/PDLGA composite scaffold via activation of AMPK/ERK1/2 and PI3K/Akt pathways. Biomaterials. 2013;34:64–77. [DOI] [PubMed] [Google Scholar]
- 8. Kim EK, Lim S, Park JM, et al. Human mesenchymal stem cell differentiation to the osteogenic or adipogenic lineage is regulated by AMP-activated protein kinase. J Cell Physiol. 2012;227:1680–1687. [DOI] [PubMed] [Google Scholar]
- 9. Shah M, Kola B, Bataveljic A, et al. AMP-activated protein kinase (AMPK) activation regulates in vitro bone formation and bone mass. Bone. 2010;47:309–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kanazawa I, Yamaguchi T, Yano S, Yamauchi M, Sugimoto T. Metformin enhances the differentiation and mineralization of osteoblastic MC3T3–E1 cells via AMP kinase activation as well as eNOS and BMP-2 expression. Biochem Biophys Res Commun. 2008;375:414–419. [DOI] [PubMed] [Google Scholar]
- 11. Kanazawa I, Yamaguchi T, Yano S, Yamauchi M, Sugimoto T. Activation of AMP kinase and inhibition of Rho kinase induce the mineralization of osteoblastic MC3T3–E1 cells through endothelial NOS and BMP-2 expression. Am J Physiol Endocrinol Metab. 2009;296:E139–E146. [DOI] [PubMed] [Google Scholar]
- 12. Kasai T, Bandow K, Suzuki H, et al. Osteoblast differentiation is functionally associated with decreased AMP kinase activity. J Cell Physiol. 2009;221:740–749. [DOI] [PubMed] [Google Scholar]
- 13. Nollet M, Santucci-Darmanin S, Breuil V, et al. Autophagy in osteoblasts is involved in mineralization and bone homeostasis. Autophagy. 2014;10:1966–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Liu F, Fang F, Yuan H, et al. Suppression of autophagy by FIP200 deletion leads to osteopenia in mice through the inhibition of osteoblast terminal differentiation. J Bone Miner Res. 2013;28:2414–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jeyabalan J, Shah M, Viollet B, et al. Mice lacking AMP-activated protein kinase α1 catalytic subunit have increased bone remodelling and modified skeletal responses to hormonal challenges induced by ovariectomy and intermittent PTH treatment. J Endocrinol. 2012;214:349–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Pantovic A, Krstic A, Janjetovic K, et al. Coordinated time-dependent modulation of AMPK/Akt/mTOR signaling and autophagy controls osteogenic differentiation of human mesenchymal stem cells. Bone. 2013;52:524–531. [DOI] [PubMed] [Google Scholar]
- 17. Fujita T, Azuma Y, Fukuyama R, et al. Runx2 induces osteoblast and chondrocyte differentiation and enhances their migration by coupling with PI3K-Akt signaling. J Cell Biol. 2004;166:85–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Inoki K, Zhu T, Guan KL. TSC2 mediates cellular energy response to control cell growth and survival. Cell. 2003;115:577–590. [DOI] [PubMed] [Google Scholar]
- 19. Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1089–1101. [DOI] [PubMed] [Google Scholar]
- 20. Ning J, Clemmons DR. AMP-activated protein kinase inhibits IGF-I signaling and protein synthesis in vascular smooth muscle cells via stimulation of insulin receptor substrate 1 S794 and tuberous sclerosis 2 S1345 phosphorylation. Mol Endocrinol. 2010;24:1218–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cohick WS, Clemmons DR. Regulation of insulin-like growth factor binding protein synthesis and secretion in a bovine epithelial cell line. Endocrinology. 1991;129:1347–1354. [DOI] [PubMed] [Google Scholar]
- 22. Xi G, Shen X, Clemmons DR. p66shc negatively regulates insulin-like growth factor I signal transduction via inhibition of p52shc binding to Src homology 2 domain-containing protein tyrosine phosphatase substrate-1 leading to impaired growth factor receptor-bound protein-2 membrane recruitment. Mol Endocrinol. 2008;22:2162–2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dunlop EA, Tee AR. The kinase triad, AMPK, mTORC1 and ULK1, maintains energy and nutrient homoeostasis. Biochem Soc Trans. 2013;41:939–943. [DOI] [PubMed] [Google Scholar]
- 24. Jang WG, Kim EJ, Bae IH, et al. Metformin induces osteoblast differentiation via orphan nuclear receptor SHP-mediated transactivation of Runx2. Bone. 2011;48:885–893. [DOI] [PubMed] [Google Scholar]
- 25. Quinn JM, Tam S, Sims NA, et al. Germline deletion of AMP-activated protein kinase beta subunits reduces bone mass without altering osteoclast differentiation or function. FASEB J. 2010;24:275–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Jang WG, Kim EJ, Lee KN, Son HJ, Koh JT. AMP-activated protein kinase (AMPK) positively regulates osteoblast differentiation via induction of Dlx5-dependent Runx2 expression in MC3T3E1 cells. Biochem Biophs Res Comm. 2011;404:1004–1009. [DOI] [PubMed] [Google Scholar]
- 27. Chang KH, Sengupta A, Nayak RC, et al. p62 is required for stem cell/progenitor retention through inhibition of IKK/NF-κB/Ccl4 signaling at the bone marrow macrophage-osteoblast niche. Cell Rep. 2014;9:2084–2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kim J, Kundu M, Viollet B, Guan KL. AMPK and mTOR regulate autophagy through direct phosphorylatiohn of ULk1. Nature Cell Biol. 2010;13:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wirth M, Joachim J, Tooze SA. Autophagosome formation- The role of ULK1 and Beclin1-PI3KC3 complexes in setting the stage. Semin Cancer Biol. 2013;23:301–309. [DOI] [PubMed] [Google Scholar]
- 30. Egan DF, Shackelford DB, Mihaylova MM, et al. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science. 2011;331:456–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Valentine RJ, Coughlan KA, Ruderman NB, Saha AK. Insulin inhibits AMPK activity and phosphorylates AMPK Ser485/491 through Akt in hepatocytes, myotubes and incubated rat skeletal muscle. Arch Biochem Biophys. 2014;562:62–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wong PM, Puente C, Ganley IG, Jiang X. The ULK1 complex. Sensing nutrient signals for autophagy activation. Autophagy. 2013;2:124–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Esen E, Chen J, Karner CM, Okunade AL, Patterson BW, Long F. WNT-LRP5 signaling induces Warburg effect through mTORC2 activation during osteoblast differentiation. Cell Metab. 2013;17:745–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wu SB, Wu YT, Wu TP, Wei YH. Role of AMPK-mediated adaptive responses in human cells with mitochondrial dysfunction to oxidative stress. Biochimica et Biophysica Acta. 2014;1840:1331–1344. [DOI] [PubMed] [Google Scholar]
- 35. Xian L, Wu X, Pang L, et al. Matrix IGF-1 maintains bone mass by activation of mTOR in mesenchymal stem cells. Nat Med. 2012;18:1095–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Zhang M, Xuan S, Bouxsein ML, et al. Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signaling in bone matrix mineralization. J Biol Chem. 2002;277:44005–44012. [DOI] [PubMed] [Google Scholar]
- 37. Bikle DD, Sakata T, Leary C, et al. Insulin-like growth factor I is required for the anabolic actions of parathyroid hormone on mouse bone. J Bone Miner Res. 2002;17:1570–1578. [DOI] [PubMed] [Google Scholar]
- 38. DeMambro VE, Le PT, Guntur AR, et al. IGFBP2 deletion in ovariectomized mice enhances energy expenditure but accelerates bone loss. Endocrinology. 2015;156(11):4129–4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Belleudi F, Purpura V, Caputo S, Torrisi MR. FGF7/KGF regulates autophagy in keratinocytes: A novel dual role in the induction of both assembly and turnover of autophagosomes. Autophagy. 2014;10(5):803–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bernard K, Logsdon NJ, Ravi S, et al. Metabolic reprogramming is required for myofibroblast contractility and differentiation. J Biol Chem. 2015;290(42):25427–25438. [DOI] [PMC free article] [PubMed] [Google Scholar]