Abstract
In primates, despite the fact that GnRH neurons are mature at birth, a gonadal steroid independent central inhibition restrains the initiation of puberty. The neural substrates responsible for this central inhibition, however, are unclear. In this study, we tested the hypothesis that neuroestradiol release in the hypothalamus decreases prior to the pubertal increase in GnRH release. We found that in female monkeys at the prepubertal stage, when GnRH release was low, estradiol (E2) levels in the stalk-median eminence of the hypothalamus were higher than those in older, early pubertal females in which nocturnal GnRH release begins to increase. Furthermore, estrone (E1) levels were higher in the stalk-median eminence of prepubertal and early pubertal monkeys compared with midpubertal monkeys, which have the highest GnRH release. The elevated E2 and E1 levels at the prepubertal stage are likely hypothalamic in origin because circulating E2 and E1 levels in prepubertal and early pubertal monkeys were much lower than those in midpubertal monkeys. Heightened synthesis and release of neuroestradiol during the prepubertal period and subsequent reduction at puberty onset indicate possible roles for neuroestradiol in central inhibition of GnRH release. The mechanism governing the reduction in neuroestradiol synthesis at puberty onset remains to be determined.
An increase in pulsatile release of GnRH heralds puberty onset (1). In primates GnRH neurons are mature at birth and active during the neonatal period (2, 3). However, shortly after the neonatal period, GnRH neurons enter a dormant state, and this low activity state remains throughout the prepubertal period until the time of puberty onset (1, 4). Importantly, the low GnRH neuronal activity during the prepubertal period in humans and nonhuman primates is due to a central suppression within the hypothalamus, rather than a negative feedback influence of gonadal steroids (1, 4). To date, neural substrates responsible for hypothalamic central suppression remain unclear.
Despite the abundant presence of aromatase enzyme necessary for de novo estradiol (E2) synthesis in the hypothalamus (5), little is presently known about its role in neuroendocrine function. Previously we reported that neuroestradiol, synthesized and released in the hypothalamus, participates in the regulation of pulsatile GnRH release in adult ovariectomized female monkeys because estradiol benzoate (EB)-induced GnRH and E2 release is blocked by the aromatase inhibitor, letrozole (6).
It is possible that neuroestradiol plays a role in central inhibition of GnRH release during the prepubertal period. In the present study, we tested the hypothesis that neuroestradiol release in the stalk-median eminence (S-ME) decreases prior to the pubertal increase in GnRH release. Here we report that elevated levels of E2 in the S-ME at the prepubertal stage dramatically decrease at puberty onset in female rhesus monkeys.
Materials and Methods
Animals
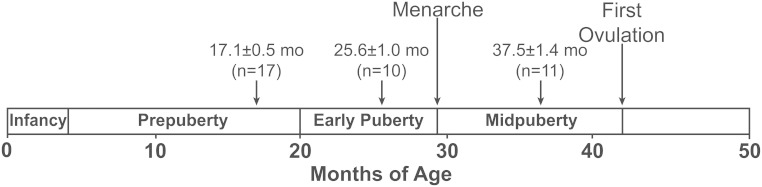
Thirty-two female rhesus monkeys (Macaca mulatta) at 13.7–46.1 month of age, born and raised at the Wisconsin National Primate Research Center, were used in this study. They were housed in pairs under controlled lighting conditions (lights on at 6:00 am through 6:00 pm and lights off 6:00 pm through 6:00 am) at a maintained temperature and were fed as previously described (7). The pubertal stages are defined as follows: prepubertal stage, no signs of puberty; early pubertal stage, some signs of puberty but before menarche, and midpubertal stage, after menarche but before first ovulation (8). The age of menarche and first ovulation in this study was 29.4 ± 1.1 and 41.8 ± 1.2 months of age, respectively (Figure 1). The protocol was approved by the University of Wisconsin-Madison Animal Care and Use Committee in accordance with the guidelines of the National Institutes of Health and the US Department of Agriculture.
Figure 1.
Developmental stages from birth through puberty in the female rhesus monkey. Average ages and the number of females used for developmental stages in this study are shown (short arrows). The definition of the developmental stages in females are described in Materials and Methods.
Samples were obtained from 53 archived push-pull perfusion experiments. Specifically, they consisted of 21 experiments in 17 prepubertal animals (17.1 ± 0.5 mo of age, 2.4 ± 0.1 kg), 13 experiments in 10 early pubertal animals (25.6 ± 1.0 mo, 2.9 ± 0.1 kg), and 19 experiments in 11 midpubertal animals (37.5 ± 1.4 mo, 4.5 ± 0.2 kg). Although most of the experiments in each stage consisted of different animals, three to four animals contributed two or three times to the data within or between stages; in these animals, multiple experiments were conducted at different ages. As previously described (7), push-pull perfusates (200 μL) were collected from the S-ME of female monkeys at 10-minute intervals for 6 hours (6:00 am through 12:00 pm) in the morning and 6 hours (6:00 pm through 12:00 am) in the evening and stored at −80°C. Because 150 μL of the 200-μL samples were previously used in earlier studies (7, 9), in this study we pooled all remaining perfusates from each morning and evening experiment. For multisteroid measurement by liquid chromatography-tandem mass spectrometry (LC-MS/MS), 1 mL of each pooled sample was used. Archived serum samples collected at 8:00–9:30 am from the same monkeys (5.1 ± 0.2 d after the push-pull experiments) were used for measurements of LH (100 μL, RIA) and multisteroid analysis (500 μl, LC-MS/MS).
LC-MS/MS measurement
Steroid extractions were performed as previously described (6). For internal standard, 50 pg deuterated 5-E2 for estrone (E1) and E2, 100 pg of deuterated 5-T for androstenedione (A) and T, and 100 pg of deuterated 9-P4 for progesterone (P4) were used. Dansyl chloride-derivitized samples were analyzed on a QTRAP 5500 quadruple linear ion trap mass spectrometer (AB Sciex) as previously described (6). Standard curves for E2 and E1 were 625, 313, 156, 78, 39, 20, 9.76, 4.88, 2.44, and 1.22 pg/tube and for T, A, and P4 3125, 1563, 781, 391, 195, 98, 48.8, 24.4, 12.2, and 6.1 pg/tube. No steroids were detected in blank or double-blank samples. Interassay and intraassay coefficients of variation from a pooled sample of 20 μL of artificial cerebrospinal fluid with 15 pg of E2 and E1, and 100 pg of T, A, and P4 were 15.8%, 17.8%, 22.7%, 20.4%, and 17.2% (interassay) and 5.8%, 6.0%, 10.1%, 10.6%, and 10.2% (intraassay), respectively. The detection limit of E2, E1, T, A, and P4 were 0.6, 0.6, 1.5, 1.5, 1.5 pg/tube, respectively.
Peptide hormone RIAs
GnRH in push-pull perfusates was previously measured by RIA (7). LH in serum samples was measured as previously described (10) (see Table 1). Interassay and intraassay coefficients of variation for GnRH were 8.1% and 11.3%, and for LH, 3.2% and 5.0%, respectively.
Table 1.
Antibody Table
| Peptide/Protein Target | Antigen Sequence | Name of Antibody | Manufacturer, Catalog Number, and/or Name of Individual Providing the Antibody | Species Raised (Monoclonal or Polyclonal) | Dilution Used |
|---|---|---|---|---|---|
| GnRH | Mammalian GnRH | R1245 | Dr Terry M. Nett (Colorado State University, Fort Collins, CO) | Rabbit (polyclonal) | 1:420 000 |
| LH | Recombinant cynomolgus LH-1-1 (AFP-6936A) | AFP-342994 | National Hormone and Peptide Program (Torrance, CA) | Rabbit (polyclonal) | 1:3 000 000 |
Statistical analysis
GnRH levels presented in this study for each age group were obtained from the control monkeys previously reported (7). That is, GnRH levels were recalculated for three age groups (prepubertal, early pubertal, and midpubertal stages) from the original data set, which were presented at 3- to 5-month intervals (7). GnRH and steroid levels were assessed from respective morning and evening perfusate samples. Additionally, morning and evening samples were averaged to illustrate overall developmental trajectory as total values. A one-way ANOVA with a Neuman-Keuls post hoc test was used for statistical analysis of total hypothalamic changes and serum changes across the pubertal stage. A two-way ANOVA with Bonferroni post hoc test was used for comparison of morning to evening changes across pubertal stages in perfusates. The limit of significance was set at P < .05.
Results
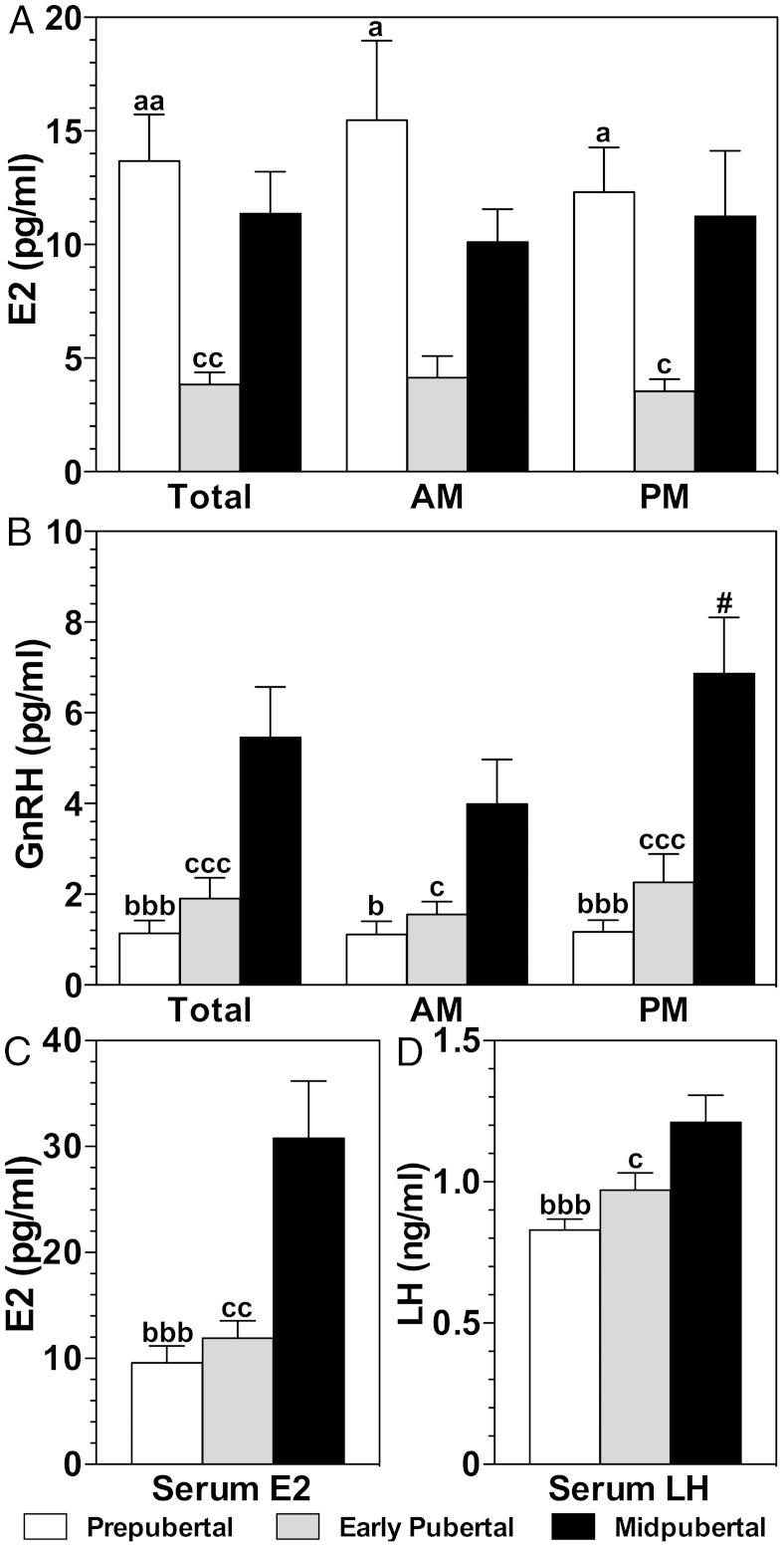
Consistent with previous observations (8, 11, 12), GnRH levels in push-pull perfusates were lowest in the prepubertal monkeys, elevated in the early pubertal monkeys, and highest in the midpubertal monkeys (Figure 2B). Moreover, nocturnal GnRH increases were seen in the midpubertal monkeys (Figure 2B). Serum LH levels followed a similar developmental trajectory as GnRH release, ie, LH levels in midpuberty were higher than prepuberty and early puberty (Figure 2D). Likewise, serum E2 levels were higher in the midpubertal animals than the prepubertal or early pubertal animals. (Figure 2C and Table 2). These data suggest that the females in this study underwent a typical progression toward maturity.
Figure 2.
Developmental changes in the release of E2 (A) and GnRH (B) in push-pull perfusates during the 6-hour morning (AM) and 6-hour evening (PM) periods. Developmental changes in serum E2 (C) and LH (D) in the same monkeys are also shown. GnRH, serum E2, and serum LH in prepubertal (white bars) and early pubertal (gray bars) monkeys were significantly lower than those in midpubertal (black bars) monkeys. In contrast, E2 in push-pull perfusates of prepubertal and midpubertal animals were significantly higher than those of early pubertal animals. a, P < .05, aa, P < .01 prepubertal vs early pubertal; b, P < .05, bbb, P < .001 prepubertal vs midpubertal; c, P < .05, cc, P < .01, ccc, P < .001 early pubertal vs midpubertal; #, P < .05 AM vs PM.
Table 2.
Developmental Changes in Steroids in the S-ME and Serum
| Steroid | Developmental Stage | S-ME |
Serum | Total-ME to Serum Ratiob | ||
|---|---|---|---|---|---|---|
| AM | PM | Totala | ||||
| E2 | Prepubertal | 15.5 ± 3.5c | 12.3 ± 2.0c | 13.7 ± 2.1c | 8.7 ± 1.4d | 3.3 ± 1.1c,e |
| Early pubertal | 4.1 ± 1.0 | 3.5 ± 0.5f | 3.8 ± 0.5g | 11.9 ± 1.6g | 0.4 ± 0.1 | |
| Midpubertal | 10.1 ± 1.5 | 11.2 ± 2.9 | 11.4 ± 1.8 | 29.2 ± 5.36 | 0.6 ± 0.2 | |
| E1 | Prepubertal | 11.2 ± 4.4c | 6.3 ± 2.3c | 9.7 ± 2.7c | 5.3 ± 1.6 | 6.4 ± 2.3e |
| Early pubertal | 11.1 ± 0.4f | 5.4 ± 3.4f | 8.3 ± 3.5f | 3.6 ± 0.7 | 3.1 ± 1.7f | |
| Midpubertal | 0.4 ± 0.2 | 0.6 ± 0.2 | 0.5 ± 0.2 | 4.9 ± 0.9 | 0.1 ± 0.06 | |
| A | Prepubertal | 20.6 ± 9.4 | 19.3 ± 8.4 | 19.9 ± 6.2 | 1673 ± 383 | 0.01 ± 0.003 |
| Early pubertal | 30.5 ± 15.5 | 31.8 ± 18.6 | 31.1 ± 11.9 | 1370 ± 388 | 0.02 ± 0.008 | |
| Midpubertal | 22.0 ± 9.4 | 19.4 ± 8.0 | 21.6 ± 6.1 | 1914 ± 527 | 0.01 ± 0.002 | |
| T | Prepubertal | 2.2 ± 0.9 | 3.0 ± 1.3 | 2.5 ± 0.8 | 204 ± 21 | 0.02 ± 0.007 |
| Early pubertal | 4.1 ± 1.5 | 2.3 ± 1.0 | 3.2 ± 0.9 | 200 ± 34 | 0.03 ± 0.01 | |
| Midpubertal | 5.7 ± 2.7 | 4.9 ± 1.5 | 5.2 ± 1.5 | 230 ± 40 | 0.04 ± 0.02 | |
| P4 | Prepubertal | 4.4 ± 1.6 | 3.6 ± 1.6 | 3.9 ± 1.1 | 60 ± 18e | 0.17 ± 0.07 |
| Early pubertal | 3.3 ± 1.2 | 3.3 ± 1.2 | 3.3 ± 0.8 | 76 ± 36f | 0.20 ± 0.10 | |
| Midpubertal | 4.1 ± 2.5 | 3.9 ± 1.9 | 3.9 ± 1.5 | 265 ± 84 | 0.07 ± 0.03 | |
Abbreviations: AM, morning; PM, evening. Values presented (mean ± SEM) are picograms per milliliter, except for ratio.
Total is the mean of AM and PM values.
Ratio is the total S-ME level divided by serum level.
P < .05, prepubertal vs early pubertal.
P < .001, prepubertal vs midpubertal.
P < .05, prepubertal vs midpubertal.
P < .05, early pubertal vs midpubertal.
P < .01, early pubertal vs midpubertal.
In contrast to serum E2, total brain E2 in S-ME perfusates in the prepubertal female monkeys were elevated compared with the older, early pubertal females (Figure 2A and Table 2). Additionally, brain E2 levels in both morning and evening perfusates in prepubertal monkeys were higher than those in early pubertal monkeys. Transitioning from the early to midpubertal stage, total and evening levels of brain E2 in midpubertal monkeys were higher than in the early pubertal monkeys (Figure 2A).
Steroid precursors of E2 exhibited interesting developmental profiles. Notably, total brain E1 levels in perfusates obtained from prepubertal and early pubertal animals were higher than those in midpubertal animals (Table 2). Although brain and serum A were higher than those of T, values remained unchanged throughout puberty. Serum P4 was elevated (although not ovulatory levels) in the midpubertal monkeys as compared with those in the prepubertal and early pubertal monkeys; however, brain P4 stayed stable throughout puberty.
Because steroids measured in the S-ME may be from the general circulation, we calculated total S-ME steroid levels over serum steroid levels as a ratio. The S-ME to serum E2 ratio in prepubertal and E1 ratios in prepubertal and early pubertal monkeys were larger than 1 (Table 2), indicating that E2 and E1 levels were higher in the brain than circulation. In contrast, E2 ratios in the early and midpubertal animals and E1 ratio in the midpubertal animals were less than 1, indicating that E2 and E1 levels were higher in circulation than in the brain. The S-ME to serum ratios for A, T, and P4 did not significantly vary throughout puberty.
Discussion
In the present study, we found that E2 levels in the S-ME of prepubertal female monkeys were higher than those found in early pubertal monkeys. Furthermore, E1 levels in the S-ME at both prepubertal and early pubertal stages were higher than those at the midpubertal stage. Because circulating E2 levels in the prepubertal and early pubertal monkeys were lower than those in midpubertal monkeys, elevated levels of E2 in the S-ME at the prepubertal stage are likely hypothalamic in origin.
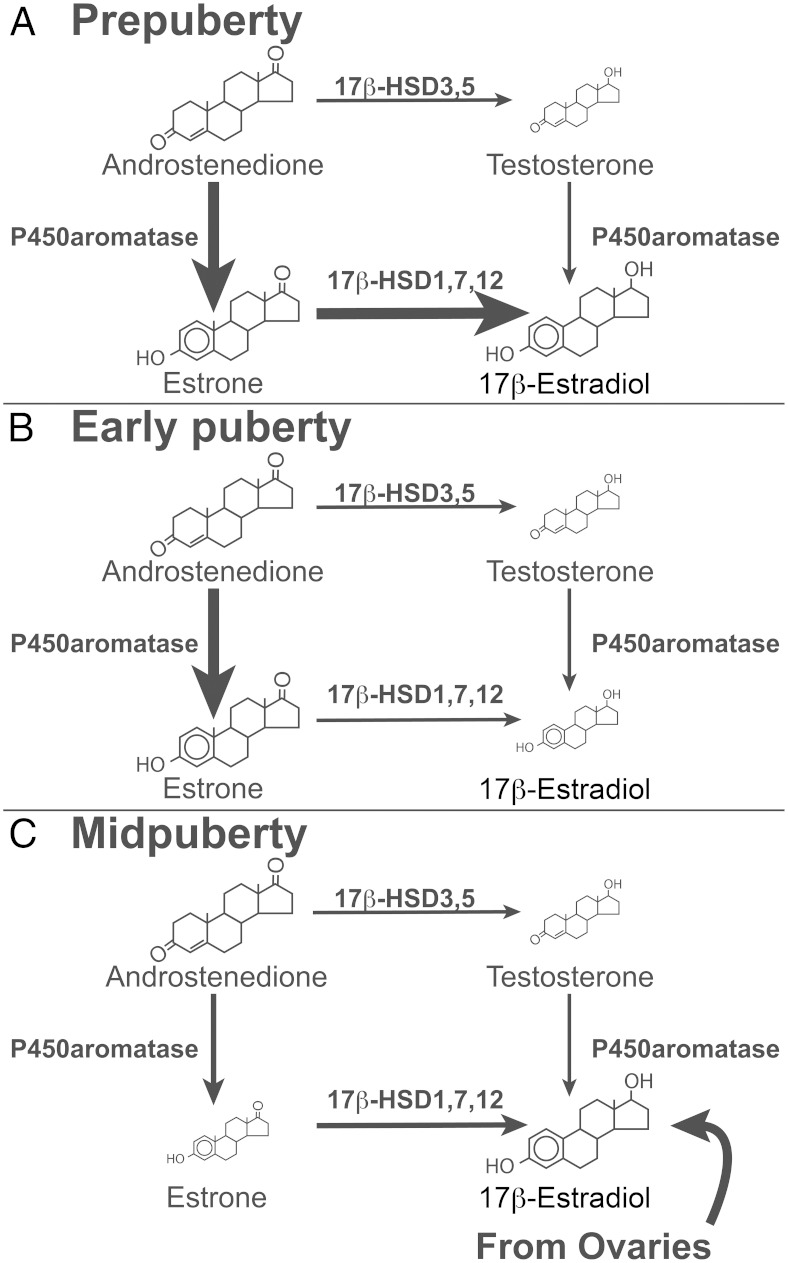
Synthesis of steroid hormones requires the activity of cytochrome P450 superfamily members and hydroxysteroid dehydrogenases (HSDs). Specifically, conversion of A to E2 requires the activity of aromatase and a 17β-HSD (Figure 3). The 17β-HSD subtypes 1, 7, and 12 are estrogen specific, whereas 17β-HSD subtypes 3 and 5 are androgen specific (13). The monkey hypothalamus contains aromatase (5, 14) and expresses 17β-HSD activity (15). The present data suggest that active synthesis and release of steroids occurs in the hypothalamus of developing female monkeys. Furthermore, multisteroid analysis suggests that aromatase and 17β-HSD activity appear to be relatively high in the hypothalamus in the prepubertal period because both E1 and E2 in the S-ME are elevated, whereas E2 falls in the early pubertal period (Figure 3). Elevated E2 in the S-ME during the midpubertal period, however, is likely due to elevated E2 in circulation because the ratio of E2 levels (S-ME vs serum) between early puberty and midpuberty are similar, despite the fact that during early puberty both serum and S-ME E2 levels are low, whereas during midpuberty serum and S-ME E2 levels are elevated. Additionally, in a collateral study, we found that E2 levels (∼90 pg/mL) in the S-ME of adult ovariectomized females bearing E2 capsule implants were similar to the E2 levels in circulation (∼80–100 pg/mL) and higher than the S-ME levels (∼2 pg/mL) in adult ovariectomized females (Terasawa, E., unpublished observation). Measurements from ovariectomized females and direct assessments of enzyme activity in the hypothalamus throughout pubertal development are necessary to confirm the view that in midpuberty E2 found in the S-ME is predominantly of ovarian origin. Nevertheless, the data suggest that at puberty onset E2 biosynthesis shifts from the brain to the ovary.
Figure 3.
Schematic illustration of the developmental changes in E2 biosynthesis in the S-ME of female monkeys. The two main enzymatic pathways from androgens to estrogens are shown. A, During the prepubertal stage P450 aromatase and 17β-HSD1, HSD7, and HSD12 activities in the S-ME appear to be elevated because A, E1, and E2 levels are high, whereas T levels are low. B, During the early pubertal stage, P450 aromatase activity, but not 17β-HSD1, HSD7, and HSD12 activity, remains elevated because A and E1 are still elevated, whereas E2 levels are low. Finally, in panel C, in the midpubertal stage, moderate activities of P450 aromatase and 17β-HSD1, HSD7, and HSD12 activities in the S-ME are hypothesized because E2 levels in the brain and serum are both high, but brain and serum E1 levels are both low. It is speculated that elevated E2 levels in the S-ME are largely of ovarian origin. Note that the size of each arrow represents an estimated enzyme activity based on the measured steroids. The sizes of the steroid icons in the figure reflect an approximate amount of steroids.
Previously we showed that infusion of EB into the S-ME of ovariectomized adult female monkeys stimulated GnRH release and neuroestradiol release in a pulsatile manner (6). Although there are several differences between the two studies, ie, methods (push-pull vs microdialysis), sampling periods (6 h pooled perfusates vs 20 min microdialysates), age of monkeys (adolescent vs adult), and gonadal status (ovarian intact vs ovariectomized), neuroestradiol levels in this study were comparable with the control period averaged over 6 hours of the previous study (6).
In monkeys and humans, GnRH neurons are mature and active at birth. An increase in pulsatile GnRH release, however, does not occur until the age of puberty. The low activity of GnRH neurons during the prepubertal period has been attributed as a consequence of a central inhibition mechanism independent of gonadal steroid feedback. At puberty onset, GnRH release increases, especially during the evening, and, subsequently, GnRH release reaches a maximum during the midpubertal period (1). In this study we found that E2 released in the S-ME was high in prepuberty, decreased in early puberty, and increased again in midpuberty. Interestingly, in prepubertal animals both morning and evening neuroestradiol levels were higher than those in early pubertal counterparts; upon animals reaching the midpubertal stage, only evening neuroestradiol levels were higher than those in the early pubertal stage. This developmental E2 pattern is quite different from the progressive rise in GnRH and LH release and circulating E2 levels during pubertal development.
To date, γ-aminobutyric acid (GABA) and neuropeptide Y (NPY) neurons, and the makorin RING-finger protein 3 (MKRN3) gene have been postulated as substrates associated with central inhibition. Mutations of MKRN3 in human patients exhibiting central precocious puberty were identified, indicating a possible role of the putative E3 ubiquitin ligase, MKRN3, in central inhibition (16). Moreover, MKRN3 mRNA levels in the arcuate nucleus of mice are high during the neonatal period and start to decrease at postnatal day 15, reaching mature levels by postnatal day 18 (16). Developmental changes in MKRN3 expression or activity in primates are unknown. Nevertheless, it is possible that MKRN3 interacts with neuroestradiol resulting in inhibitory inputs to GnRH neurons.
The role of GABA neurons is based on observations in female monkeys that GABA release in the S-ME is elevated at the prepubertal stage, when GnRH release is low; GABA release decreases when the pubertal increase in GnRH release occurs; infusion of the GABAA antagonist bicuculline stimulates release of GnRH and kisspeptin at the prepubertal stage but not the pubertal stage; and chronic infusion of bicuculline into the S-ME induces precocious puberty (17–19). The role of NPY neurons is based on data that NPY mRNA expression in neonatal male monkeys is low when GnRH mRNA expression is high, whereas NPY mRNA expression is elevated when GnRH mRNA expression is low in prepubertal males, regardless of the presence or absence of testes (20).
Presently the questions of whether there are interactions between neuroestradiol and the potential inhibitory substrates, GABA and NPY neurons, or whether and how neuroestradiol regulates upstream input to GnRH neurons remain unanswered. Whereas the following discussion is speculative, there is evidence to suggest neuroestradiol may play a role in the prepubertal restraint of GnRH release. During the prepubertal period, neuroestradiol binds to estrogen receptors in upstream inhibitory and/or stimulatory neurons known to express estrogen receptors (see reference 1) and facilitate inhibitory neurons and/or suppress stimulatory neurons. For example, inhibitory neurons, such as GABA and NPY neurons, may be stimulated by neuroestradiol to a higher activity state during the prepubertal period, thereby inhibiting GnRH release because chronic administration of bicuculline induces precocious puberty (18) and NPY mRNA expression decreases during the transition between prepuberty and puberty (20). Alternatively, during the prepubertal period, activation of GnRH neurons directly or indirectly through upstream stimulatory neurons (ie, glutamate, kisspeptin, and neurokinin B neurons) may be suppressed by neuroestradiol, and maturational changes in the hypothalamus may remove neuroestradiol inhibition prior to puberty onset. In fact, electrical stimulation of the mediobasal hypothalamus or kisspeptin agonist administration stimulates GnRH release in prepubertal monkeys (21, 22). Moreover, chronic infusion of N-methyl-D-aspartate induces precocious puberty in prepubertal monkeys (23).
Changes in neuroestradiol synthesis may also indirectly affect inhibitory or stimulatory neurons by the availability of upstream neurosteroids and their metabolites. It has been shown that pregnenolone, dehydroepiandrosterone, and their sulfates are important allosteric modulators of neurotransmitter receptors such as GABAA receptor and N-methyl-D-aspartate receptors (24). Finally, although the mechanism is unclear, we previously observed that unlike pubertal and adult monkeys, GnRH release in prepubertal female monkeys was insensitive to ovariectomy and systemic EB injection (12, 25). Taken together, during the prepubertal period when the ovaries are minimally active, elevated levels of brain estrogens are attributable to heightened neuroestradiol synthesis, but this elevated prepubertal neuroestradiol synthesis appears to be subsequently impacted by circulating gonadal steroids from the ovaries after puberty onset. The report showing that similar hypothalamic E2 synthesis is insensitive to gonadectomy and adrenalectomy in neonatal rats (26) is consistent with this view.
Our work that neuroestradiol release in adult ovariectomized monkeys is stimulated by exogenous EB infusion into the S-ME (6) suggests that there are interactions between circulating E2 and neuroestradiol under certain physiological conditions. However, it is unknown how neuroestradiol and circulating E2 interact with each other after the ovaries begin releasing E2 resulting in negative/positive feedback effects.
In conclusion, we found that neuroestradiol in the S-ME of female monkeys decreases in association with puberty onset. This finding has important clinical and evolutional implications. An elevated gonadotropin response to GnRH challenge reported in aromatase-deficient girls (27) might be due to E2 deficiency in the hypothalamus in addition to the ovaries. Additionally, in primates, a prolonged period of neocortical development may require suppression of GnRH neural activity by neuroestradiol. Although the precise mechanism for the role of neuroestradiol in puberty is unknown, the present finding opens up the exciting possibility that neuroestradiol synthesized and released locally may be involved in the prepubertal restraint of GnRH release in primates.
Acknowledgments
We thank Dr Hemanta Shrestha for his technical assistance.
This work was supported by National Institutes of Health Grant R21HD077447 (to E.T.). The work was also possible by support for the Wisconsin National Primate Research Center by National Institutes of Health Grant 5P51OD011106.
Disclosure Summary: The authors have nothing to disclose.
For News & Views see page 42
- A
- androstenedione
- E1
- estrone
- E2
- estradiol
- EB
- estradiol benzoate
- GABA
- γ-aminobutyric acid
- HSD
- hydroxysteroid dehydrogenase
- LC-MS/MS
- liquid chromatography-tandem mass spectrometry
- MKRN3
- makorin RING-finger protein 3
- NPY
- neuropeptide Y
- P4
- progesterone
- S-ME
- stalk-median eminence.
References
- 1. Terasawa E, Fernandez DL. Neurobiological mechanisms of the onset of puberty in primates. Endocr Rev. 2001;22:111–151. [DOI] [PubMed] [Google Scholar]
- 2. Plant TM. A study of the role of the postnatal testes in determining the ontogeny of gonadotropin secretion in the male rhesus monkey (Macaca mulatta). Endocrinology. 1985;116:1341–1350. [DOI] [PubMed] [Google Scholar]
- 3. Grumbach MM. The neuroendocrinology of human puberty revisited. Horm Res. 2002;57(suppl 2):2–14. [DOI] [PubMed] [Google Scholar]
- 4. Plant TM. Neuroendocrine control of the onset of puberty. Front Neuroendocrinol. 2015;38:73–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Naftolin F, Horvath TL, Jakab RL, Leranth C, Harada N, Balthazart J. Aromatase immunoreactivity in axon terminals of the vertebrate brain. An immunocytochemical study of quail, rat, monkey, and human tissues. Neuroendocrinology. 1996;63:149–155. [DOI] [PubMed] [Google Scholar]
- 6. Kenealy BP, Kapoor A, Guerriero KA, et al. Neuroestradiol in the hypothalamus contributes to the regulation of gonadotropin releasing hormone release. J Neurosci. 2013;33:19051–19059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Windsor-Engnell BM, Kasuya E, Mizuno M, Keen KL, Terasawa E. An increase in in vivo release of LHRH and precocious puberty by posterior hypothalamic lesions in female rhesus monkeys (Macaca mulatta). Am J Physiol Endocrinol Metab. 2007;292:E1000–E1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Terasawa E, Bridson WE, Nass TE, Noonan JJ, Dieschke DJ. Developmental changes in the luteinizing hormone secretory pattern in peripubertal female rhesus monkeys: comparisons between gonadally intact and ovariectomized animals. Endocrinology. 1984;115:2233–2240. [DOI] [PubMed] [Google Scholar]
- 9. Fernandez DL, Terasawa E. Changes in growth hormone-releasing hormone (GHRH) release in vivo in prepubertal and pubertal female rhesus monkeys. Presented at: Abstracts of the 29th Annual Meeting of the Society for Neuroscience; 1999; Miami Beach, FL; 25:798 (320.11). [Google Scholar]
- 10. Kenealy BP, Keen KL, Garcia JP, Richter DJ, Terasawa E. Prolonged infusion of estradiol benzoate into the stalk median eminence stimulates release of GnRH and kisspeptin in ovariectomized female rhesus macaques. Endocrinology. 2015;156:1804–1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Watanabe G, Terasawa E. In vivo release of luteinizing hormone releasing hormone increases with puberty in the female rhesus monkey. Endocrinology. 1989;125:92–99. [DOI] [PubMed] [Google Scholar]
- 12. Chongthammakun S, Claypool LE, Terasawa E. Ovariectomy increases in vivo luteinizing hormone-releasing hormone release in pubertal, but not prepubertal, female rhesus monkeys. J Neuroendocrinol. 1993;5:41–50. [DOI] [PubMed] [Google Scholar]
- 13. Luu-The V. Assessment of steroidogenesis and steroidogenic enzyme functions. J Steroid Biochem Mol Biol. 2013;137:176–182. [DOI] [PubMed] [Google Scholar]
- 14. MacLusky NJ, Naftolin F, Goldman-Rakic PS. Estrogen formation and binding in the cerebral cortex of the developing rhesus monkey. Proc Natl Acad Sci USA. 1986;83:513–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Resko JA, Stadelman HL, Norman RL. 17β-Hydroxysteroid dehydrogenase activity in the pituitary gland and neural tissue of rhesus monkeys. J steroid Biochem. 1979;11:1429–1434. [DOI] [PubMed] [Google Scholar]
- 16. Abreu AP, Dauber A, Macedo DB, et al. Central precocious puberty caused by mutations in the imprinted gene MKRN3. N Engl J Med. 2013;368:2467–2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mitsushima D, Marzban F, Luchansky LL, et al. Role of glutamic acid decarboxylase in the prepubertal inhibition of the luteinizing hormone-releasing hormone release in prepubertal female monkeys. J Neurosci. 1993;16:2563–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Keen KL, Burich AJ, Mitsushima D, Kasuya E, Terasawa E. Effects of pulsatile infusion of the GABA(A) receptor blocker bicuculline on the onset of puberty in female rhesus monkeys. Endocrinology. 1999;140:5257–5266. [DOI] [PubMed] [Google Scholar]
- 19. Kurian JR, Keen KL, Guerriero KA, Terasawa E. Tonic control of kisspeptin release in prepubertal monkeys: implications to the mechanism of puberty onset. Endocrinology. 2012;153:3331–3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. El Majdoubi M, Sahu A, Ramaswamy S, Plant TM. Neuropeptide Y: a hypothalamic brake restraining the onset of puberty in primates. Proc Natl Acad Sci USA. 2000;97:6179–6184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Claypool LE, Watanabe G, Terasawa E. Effects of electrical stimulation of the medial basal hypothalamus on the in vivo release of luteinizing hormone-releasing hormone in the prepubertal and peripubertal female monkey. Endocrinology. 1990;127:3014–3022. [DOI] [PubMed] [Google Scholar]
- 22. Guerriero KA, Keen KL, Millar RP, Terasawa E. Developmental changes in GnRH release in response to kisspeptin agonist and antagonist in female rhesus monkeys (Macaca mulatta): implication for the mechanism of puberty. Endocrinology. 2012;153:825–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Plant TM, Gay VL, Marshall GR, Arslan M. Puberty in monkeys is triggered by chemical stimulation of the hypothalamus. Proc Natl Acad Sci USA. 1989;86:2506–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baulieu EE. Neurosteroids: a novel function of the brain. Psychoneuroendocrinology. 1998;23:963–987. [DOI] [PubMed] [Google Scholar]
- 25. Chongthammakun S, Terasawa E. Negative feedback effects of estrogen on luteinizing hormone-releasing hormone release occur in pubertal, but not prepubertal, ovariectomized female rhesus monkeys. Endocrinology. 1993;132:735–743. [DOI] [PubMed] [Google Scholar]
- 26. Konkle AT, McCarthy MM. Developmental time course of estradiol, testosterone, and dihydrotestosterone levels in discrete regions of male and female rat brain. Endocrinology. 2011;152:223–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Jones ME, Boon WC, McInnes K, Maffei L, Carani C, Simpson ER. Recognizing rare disorders: aromatase deficiency. Nat Clin Pract Endocrinol Metab. 2007;3:414–421. [DOI] [PubMed] [Google Scholar]