Abstract
A potentially novel approach for treating obesity includes attenuating myostatin as this increases muscle mass and decreases fat mass. Notwithstanding, conflicting studies report that myostatin stimulates or inhibits adipogenesis and it is unknown whether reduced adiposity with myostatin attenuation results from changes in fat deposition or adipogenesis. We therefore quantified changes in the stem, transit amplifying and progenitor cell pool in white adipose tissue (WAT) and brown adipose tissue (BAT) using label-retaining wild-type and mstn−/− (Jekyll) mice. Muscle mass was larger in Jekyll mice, WAT and BAT mass was smaller and label induction was equal in all tissues from both wild-type and Jekyll mice. The number of label-retaining cells, however, dissipated quicker in WAT and BAT of Jekyll mice and was only 25% and 17%, respectively, of wild-type cell counts 1 month after induction. Adipose cell density was significantly higher in Jekyll mice and increased over time concomitant with label-retaining cell disappearance, which is consistent with enhanced expansion and differentiation of the stem, transit amplifying and progenitor pool. Stromal vascular cells from Jekyll WAT and BAT differentiated into mature adipocytes at a faster rate than wild-type cells and although Jekyll WAT cells also proliferated quicker in vitro, those from BAT did not. Differentiation marker expression in vitro, however, suggests that mstn−/− BAT preadipocytes are far more sensitive to the suppressive effects of myostatin. These results suggest that myostatin attenuation stimulates adipogenesis in vivo and that the reduced adiposity in mstn−/− animals results from nutrient partitioning away from fat and in support of muscle.
Myostatin is a highly conserved member of the TGF-β superfamily and a potent negative regulator of skeletal muscle mass (1). In adult tissues, myostatin is expressed predominantly in skeletal muscle, although it is also minimally expressed in adipose tissue and the heart, all of which are phenotypically affected with myostatin attenuation (2–4). The hypermuscularity appears to result from a combination of muscle cell hyperplasia and hypertrophy as myostatin regulates myogenesis developmentally and its attenuation promotes stem cell (also known as satellite cell) based mechanisms of muscle growth and repair postnatally (5, 6). Moreover, recent studies suggest that the pool of stem, transit amplifying and progenitor (STP) cells, and thus tissue potency, in cardiac muscle and fat, both brown and white, may also be influenced by myostatin (7).
Reduced adiposity is common to myostatin null (mstn−/−) animals and with the pharmacological attenuation of myostatin (2, 3, 8, 9). Some studies suggest that the reduced adiposity in mstn−/− mice is likely due to muscle depletion of metabolic reserves and the repartitioning of glucose away from fat rather than to direct effects on adipogenesis or to fat turnover (2, 10). In fact, the reduced expression of the adipogenic markers CCAAT/enhancer binding protein (C/EBP)α, peroxisome proliferator-activated receptor (PPAR)γ and leptin in mstn−/− fat suggests that adipogenesis is impaired in mstn−/− mice and conversely, that myostatin promotes adipogenesis (8). In vitro support for this mechanism is lacking, however, as myostatin control of adipogenesis is hotly debated due to in vitro studies with various mesenchymal adipocyte progenitor cells that demonstrate myostatin to either inhibit or stimulate their differentiation (11–15). For example, myostatin inhibits differentiation of bone marrow-derived mesenchymal stem cells and of primary preadipocytes (12), but stimulates C3H10T(1/2) commitment towards an adipogenic rather than myogenic fate (13, 16). More confusing is the demonstration that myostatin blocks BMP7-stimulated adipogenesis in C3H10T(1/2) and 3T3-L1 cells (17). Differentiation in each of these studies was induced before myostatin treatment and the myokine's effects on adipogenesis in vivo are currently unknown. Better in vivo models are therefore needed to resolve the controversy.
Adipocytes and myocytes are both derived from the same mesodermal precursor cells during development and as both muscle and fat are altered with myostatin attenuation, it is highly possible that myostatin directly regulates adipogenesis despite the controversial results generated with the different in vitro studies. We therefore assessed differences in the heterologous STP pools in white adipose tissue (WAT) and brown adipose tissue (BAT) of label-retaining wild-type and mstn−/− (Jekyll) mice. Our studies indicate that these pools rapidly deplete in Jekyll mice in vivo and that the mstn−/− stromal vascular fraction of adipose cells proliferates and differentiates faster. These studies strongly suggest that myostatin inhibits adipogenesis as this process is actually enhanced in mstn−/− mice. They also suggest that expanding the adipogenic pool is a legitimate long-term consequence of using myostatin-attenuating therapeutics, many of which are currently being developed.
Materials and Methods
Animals
All mice used in this research were female and were housed and bred in an environmentally controlled room. Animals were fed ad libitum and kept under a 12-hour light, 12-hour dark cycle with lights on at 5 am. Mice were used according to a protocol preapproved by the Institutional Animal Care and Use Committee of Washington State University.
Label-retaining wild-type (mstn+/+) and Jekyll (mstn−/−) mice were previously described (7) and were used herein to identify STP cells as quantifying label-retaining cells (LRCs) in any particular tissue is an accurate estimate of its heterologous STP pool. These mice expressed a fusion protein of histone 2B and green fluorescent protein (H2B-GFP) under control of a doxycycline-inducible promoter (18, 19). Treating mice with doxycycline labeled all cells with H2B-GFP while label was subsequently diluted during a chase period without doxycycline. This resulted in the retention of label in only quiescent STP cells, which helped maintain tissue potency. Doxycycline was administered to mice at 6 weeks of age and at a concentration of 400-μg/mL 5% sucrose drinking water that was kept in red-frosted bottles to prevent degradation from ultraviolent light. Mice were dosed with doxycycline for 2 weeks, changing solution every 3 days, followed by chase periods of 0 days or 1, 2, 3, and 4 months.
Surface body temperatures were obtained of day 0 neonates and in 1- and 2-month-old wild-type and mstn−/− mice using a thermography imager and infrared camera. Neonates were placed in 6-well cell culture plates and imaged simultaneously, whereas adult mice were imaged individually, all on a flat surface. With each image, mice were removed from their cage for no more than 10 seconds in order to prevent and normalize surface temperature adjustments to the environmental change.
Tissue processing and imaging
Mice were euthanized using CO2 after different chase periods. Inguinal WAT, intrascapular BAT, and small intestine (SI) were collected and fixed with 4% paraformaldehyde in PBS for 4 hours. Tissues were rinsed in PBS and stored in 70% ethanol overnight and then lysed by a second overnight incubation in 20mM Tris, 1mM EDTA, 150mM NaCl, 0.5% Triton X-100, and 1mM EGTA in PBS. After again rinsing in PBS, tissues were then incubated in 10-μg/mL RNAse A for 1 hour at 37°C and WAT and BAT were stained with Dapi for 4 hours and overnight, respectively. Whole mount tissues were then imaged on a Leica TCS SP5 LASAF confocal microscope.
Cell count analysis
All color images were converted to color-free 8-bit images using ImageJ 1.47v. For all tissues, an upper GFP threshold limit of 255 and a lower limit vary from 10 to 15 was used. The background was subtracted with a 15-pixel radius and images were despeckled to remove additional fluorescent noise. All GFP-positive cells were then normalized to nuclei counts.
Cell culture
The stromal vascular cellular fraction of adipose tissue was analogous to the quiescent STP pool and is fully capable of differentiating into mature adipocytes in culture (20). Fresh inguinal WAT and intrascapular BAT were collected and minced into smaller pieces with 1 ml digestion buffer containing 0.75-U/mL collagenase D, 0.5-μL/mL CaCl, 0.5-μL/mL MgCl, and 1.0-U/mL dispersate type II. These tissues were incubated in a 37°C shaker for 45 minutes. The dispersate was then filtered through 40- and 100-μm cell strainers and centrifuged at 0.4g for 5 minutes. The pelleted stromal vascular cellular fractions were then resuspended in F12 culture media supplemented with 15% fetal bovine serum and 1% penicillin-streptomycin. Cells from WAT and BAT were initially plated in 3.5- and 6-mm Petri dishes for 4 days, respectively, and cultured at 37°C in a humidified atmosphere of 5% CO2 and allowed to expand, changing media every 2 days.
Proliferation assay
Cultured cells were passed to 96-well plates with 8000 and 16 000 cells per well from WAT and BAT, respectively. In the presence of serum, cells were treated with and without 100-ng/mL (13nM) recombinant LR3-IGF1 (an analog that does not binding IGF binding proteins) for 24, 48, 72, and 92 hours and incubated in humidified atmosphere of 5% CO2 under 37°C. In separate experiments, cells were serum starved for 16 hours before treating with 13nM LR3-IGF1, 20nM myostatin, or both. Recombinant human myostatin was expressed and purified from CHO cells as we previously described (21). Cell number was assessed in both experiments using the CellTiter 96 AQ One cell proliferation assay (Promega) according to the manufacturer's protocol. Plates were incubated at 37°C in a humidified, 5% CO2 atmosphere for 1 hour before recording the absorbance at 490 nm.
Differentiation assay
Cultured cells were passed to 12-well plates, grown to confluency and treated with or without 20nM myostatin. Adipogenic differentiation was induced in WAT cells with 0.5mM 3-isobutyl-1-methylxanthine, 1μM dexamethasone, and 10-μg/mL insulin (all from Sigma-Aldrich) for 8 days (20). BAT was induced with 5-μg/mL insulin, 50μM indomethacin, 1μM dexamethasone, 0.5μM 3-isobutyl-1-methylxanthine, and 1nM T3 (all from Sigma-Aldrich) for 8 days. These cells were incubated in a humidified atmosphere of 5% CO2 at 37°C, and media were changed every 2 days. Adipocytes were then fixed by incubating in 10% formaldehyde for 40 minutes and then stained with 200-μL Oil Red O solution for 10 minutes, all at room temperature. Unincorporated stain was then washed away with water and differentiation was quantified as a consequence of fat accumulation by extracting the Oil Red O solution (isopropanol) and recording the absorbance at 510 nm in an ELISA plate reader. The expression of adipogenic markers was also quantified using real-time RT-PCR assays as previously described (20, 22).
Statistical analysis
Differences between means (±SEM) were determined by a two-way ANOVA coupled to a Tukey post hoc test for multiple mean comparisons. In each comparison, P ≤ .05 was used to determine significance differences.
Results
Enhanced muscle and diminished fat mass in mstn−/− mice
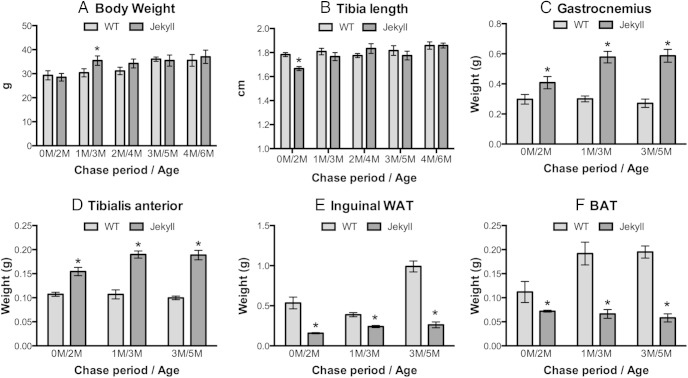
We measured body weight and tibia length representing aggregate growth rate between wild-type and Jekyll mice, and only minor differences were noted in 3- and 2-month-old mice, respectively (Figure 1, A and B). By contrast, the weights of gastrocnemius and tibialis anterior skeletal muscles were higher in Jekyll mice as compared with wild-type at all time points (Figure 1, C and D). Weights of inguinal WAT and BAT, however, were smaller in Jekyll mice (Figure 1, E and F). This suggests that the similar body weight between wild-type and Jekyll mice despite larger muscle mass in Jekyll mice is likely due to the compensatory loss of fat.
Figure 1.
Body morphology of wild-type (WT) (mstn+/+) and Jekyll (mstn−/−) mice. A and B, Body weights and tibia lengths from mice of the indicated chase period/age; M, month. C–F, Mass of gastrocnemius and tibialis anterior (TA) muscles and of inguinal WAT and BAT. In all panels, significant differences between WT and Jekyll mice of given chase/age groups are indicated by asterisks (mean ± SEM, P ≤ .05, n = 6/group).
Adipose tissue LRCs rapidly deplete in mstn−/− mice
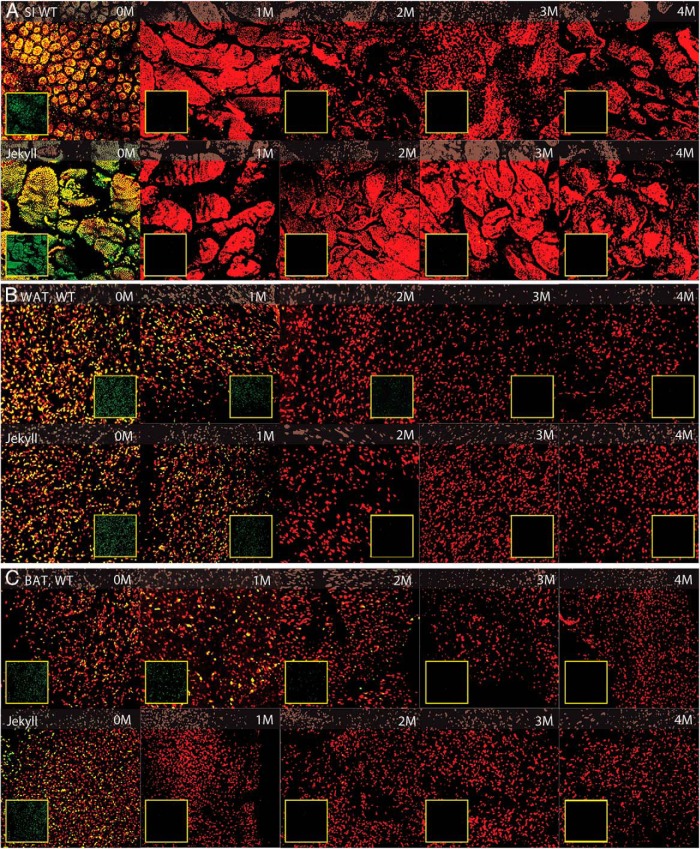
The LRC pools were quantified in SI, WAT, and BAT using confocal microscopy (Figure 2). In each panel, all nuclei are stained red with propidium iodide, LRC nuclei are green (inset images), and overlapping nuclei are orange. The confocal images shown for each chase period are composites constructed from 25 4-μm scans of each tissue and represent the top 100 μm. SI was used as a control for dosing efficacy because this tissue does not express a mstn−/− phenotype. Indeed, SI of wild-type and Jekyll mice were labeled equally, as indicated by similar GFP staining on day 0, and label disappeared similarly in both groups (Figure 2A). The LRCs were evenly dispersed in WAT and BAT of both wild-type and Jekyll mice on day 0 with an approximately equal number of GFP+ cells (Figure 2, B and C). The LRCs quickly dissipated as mice from both groups aged, although it was apparent that the rate of disappearance was greater in Jekyll mice. For example, LRCs were still readily detected in wild-type fat after a 2-month chase but not in Jekyll fat. This was particularly evident in BAT, because large differences were clearly detected even at 1 month. By 4 months, however, there were almost no GFP+ cells detected in either group regardless of tissue.
Figure 2.
Histological assessment of adipose tissue and small intestine (SI). Wild-type (WT) and Jekyll H2B-GFPxM2 mice were pulsed with doxycycline for 2 weeks followed by 0-, 1-, 2-, 3-, and 4-month chase periods. Whole-mount SI and WAT and BAT were imaged by confocal microscopy at ×250 (red, propidium iodide-stained nuclei; green, LRC nuclei [inset boxes]; yellow, overlay). Representative composite image stacks representing 25–4-μm digital sections are shown for each chase period.
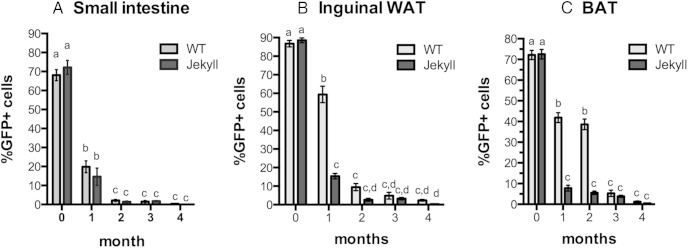
All of these differences were more apparent after quantifying LRCs in each tissue. SI LRCs were in fact equally labeled on day 0 and changes in LRC detection were also equally rapid as approximately 80% of label was lost in both groups after just 1 month, and LRCs were almost undetectable by 2 months (Figure 3A). This verified that exposure to doxycycline and induction of label was identical in both wild-type and Jekyll mice and that any differences in the adipose tissue LRC counts, or those in any other myostatin-sensitive tissue, would not be due to differential tissue labeling. In both WAT and BAT, wild-type and Jekyll mice started with an equal number of LRCs at 0 day. By 1 month, however, substantial differences were detected as wild-type LRC counts in WAT and BAT were 4- and 6-fold higher, respectively, than those in Jekyll tissues (Figure 3, B and C). LRC counts were similar at 2 months in WAT, despite slightly higher wild-type values (not significant) and continued to decline over time in both groups. By contrast, wild-type LRC counts remained elevated in BAT and were 39% of total cell counts compared with 5.5% for Jekyll BAT. There was no significant difference in LRC counts between the wild-type and Jekyll mice, regardless of tissue, after 3 or 4 months of chase. These results nevertheless suggest that the LRC pools in Jekyll fat are more labile than those in wild-type fat. Such changes in STP pools are more likely to be associated with cells rapidly proliferating, as when pools renew, and with increased differentiation into mature adipocytes.
Figure 3.
Quantification of adipose tissue and SI LRCs. Tissue images were analyzed using ImageJ, and the total number of GFP+ LRCs was normalized to total nuclei in each image (A, SI; B, inguinal WAT; C, BAT). Chase periods are indicated on the x-axes, and mean ± SEM values are plotted (n = 6/group). In each histogram, different letters indicate statistical significance (P ≤ .05) between any particular group (WT, wild type), whereas the same letters indicate no difference.
Adipocyte density and body temperature are elevated in mstn−/− mice
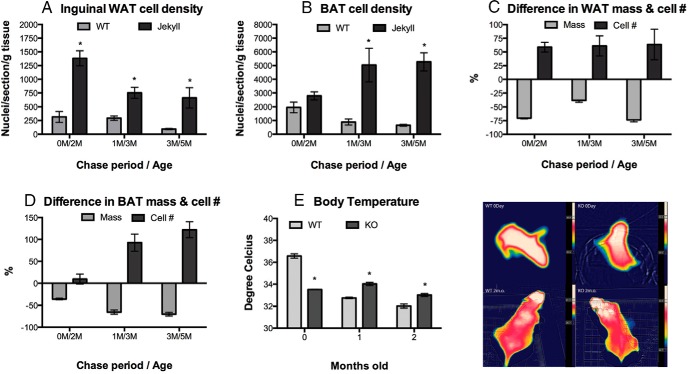
Increased adipogenesis and renewal of adipose STP pools could increase cellular densities, which were quantified in adipose tissues of both groups. Previous reports demonstrated reduced adiposity and greater distribution of smaller adipocytes in inguinal fat pads from adult mstn−/− mice (2, 3). Adipocyte density was indeed greater in Jekyll mice (Figure 4A). This was true for WAT at all chase periods and for BAT after 1 and 3 months (Figure 4B). Jekyll values were approximately 4-, 2.5-, and 7-fold higher, respectively, than wild-type values at day 0 and after 1 and 3 months of chase. Although BAT cell density was not different at 0 day, it was 5.5- and 8-fold higher than wild-type values at 1 and 3 months, respectively.
Figure 4.
Adipose cell density and surface body temperature. A and B, Total number of nuclei per sectioned tissue (inguinal WAT and BAT) was normalized to tissue weight. Significant differences between wild-type and Jekyll mice are indicated by asterisks (P ≤ .05, n = 6/group). C and D, Relative changes in tissue mass and cell number for Jekyll mice are expressed as a % of wild-type values (0 line; n = 6/group). E and F, Surface body temperature of wild-type and mstn−/− (KO) mice quantified and imaged. The temperature variations of each individual mouse are indicated by the thermal bars adjacent to the images. Significant differences between wild-type and Jekyll mice are indicated by asterisks (P ≤ .05, n = 6–8/group).
Jekyll fat pad weights were similar among mice of different ages, suggesting that the higher adipocyte density and smaller size was not necessarily due to increased lipolysis. Indeed, plotting changes in adipose mass vs total cell number indicates that these parameters are disproportionate in WAT (Figure 4C) and particularly in BAT (Figure 4D). Compared with wild type, for example, cell number was approximately 50% and 125% higher in Jekyll WAT and BAT, respectively, although the mass of each was 70%–75% smaller. Thus, the difference in cell densities is not simply due to the loss of stored triglycerides (lipolysis) but likely to the formation of more mature adipocytes, consistent with LRC depletion.
Jekyll neonates had significantly lower body temperatures than wild-type neonates (Figure 4, E and F), and although wild-type temperatures fell during the first month, they were then maintained below those of Jekyll mice. Mice are born with BAT throughout much of the body, and as they age, these fat stores deplete and only the BAT beneath the subscapular WAT pad remains in adults. In fact, differences in adult BAT between wild-type and Jekyll/mstn−/− mice are reflected in surface body temperature (Figures 1F and 4F). It is well known that newborns respond to cold exposure by increasing heat production from BAT stores (23, 24). Thus, our data suggest that the lower BAT reserves in mstn−/− neonates could compromise heat production.
Enhanced proliferation and differentiation rate of adipose stromal vascular cells from mstn−/− mice
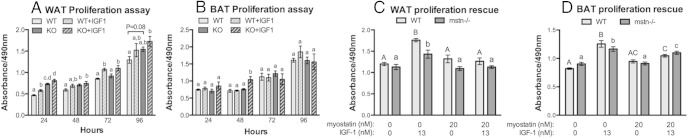
Overall, Jekyll WAT stromal vascular cells proliferated quicker than cells from wild-type WAT (Figure 5A). Differences were not substantial, however, and were only statistically significant after 24 and 48 hours, although nonsignificant trends were noted after 72 and 96 hours. Responsiveness to low dose IGF-1 was nevertheless similar in cells from both animal groups, suggesting that the enhanced basal proliferation rate of Jekyll WAT adipoprogenitor cells in vitro is due to the absence of myostatin rather than to changes in IGF-1 sensitivity. These results were similar to those with mstn−/− muscle satellite cells (5, 6) as their self-renewal is enhanced. By contrast, the proliferation rate of BAT stromal vascular cells isolated from wild-type and Jekyll mice did not differ and were equally unresponsive to IGF-1 in the presence of serum (Figure 5B). Cells were also assayed after a prolonged serum starvation to suppress basal proliferation and increase IGF-1 sensitivity (Figure 5, C and D). Under these conditions, IGF-1 increased the proliferation of both WAT and BAT cells, whereas 20nM myostatin fully suppressed these effects in WAT and partially suppressed them in BAT. These results together suggest that myostatin suppresses both basal and IGF-1-stimulated proliferation of both WAT and BAT preadipocytes, actions that are again similar to those in muscle satellite cells (5, 6, 25, 26).
Figure 5.
Proliferation of adipose stromal vascular cells. A and B, Proliferation rate of stromal vascular cells from both adipose tissues (inguinal WAT and BAT) was estimated by quantifying cell number using the CellTiter 96 AQ 1 solution assay. Cells were treated ± LR3-IGF1 (n = 12, P ≤ .05), which does not interact with IGF binding proteins, and in the presence of serum. Significant differences within each time period are indicated by different letters whereas the same letters indicate no difference. C and D, Cells were also treated with LR3-IGF1, recombinant myostatin or both for 24 hours after a prolonged (16 h) serum starvation to increase IGF-1 sensitivity (n = 8/group, P ≤ .05). Significant differences among wild-type (WT) or mstn−/− (KO) cells are indicated separately by different letters (eg, capital for WT).
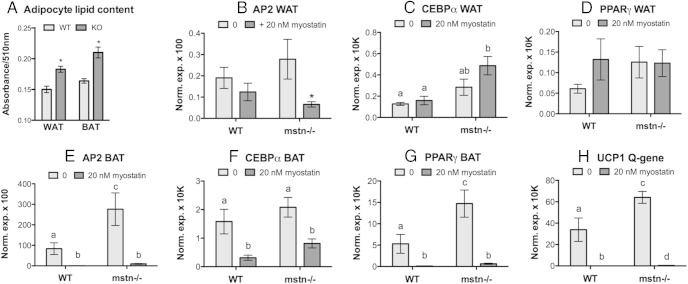
Differentiation of these cells in vitro was assessed by quantifying total lipids after induced differentiation and by quantifying known markers of the process. In fact, terminal differentiation of both WAT and BAT cells from Jekyll mice was enhanced as lipid staining was approximately 45% higher in Jekyll adipocytes 8 days after induction (Figure 6A). Expression of adipogenic markers was especially elevated in BAT and, conversely, suppressed by myostatin. These effects were far more pronounced in BAT than in WAT (Figure 6, B–H) as only adipocyte lipid-binding protein 2 (AP2) expression was suppressed by myostatin in WAT. This suggests that the mechanisms underlying enhanced adipogenesis in mstn−/− mice may actually differ in WAT and BAT and could be explained by altered rates in proliferation and differentiation, respectively.
Figure 6.
Differentiation of adipose stromal vascular cells. A, WAT and BAT cells from wild-type and mstn−/− (KO) mice were differentiated and adipocyte lipid content, as a marker of terminal differentiation, was quantified with Oil Red O staining (n = 6/group; *, P ≤ .05 compared with wild type [WT]). Cells were also treated with or without recombinant myostatin (A–H), and gene expression of adipogenic markers was quantified. Markers include adipocyte lipid-binding protein (AP2), C/EBPα, and PPARγ, which are common to both WAT and BAT, as well as uncoupling protein (UCP)1, which is unique to BAT. In C–H, significant differences among all groups are indicated by different letters (n = 6/group, P ≤ .05), and in B by an asterisks (compared with 0 control).
Discussion
To our knowledge, this is the first study to quantify STP cells in fat using the doxycycline-inducible H2B-GFP system and adult pulsing as our previous research incorporated embryonic pulsing (7). This previous study was the first to quantify muscle, heart, fat, and tibia STP cells in neonates and similarly assessed the influence of myostatin using Jekyll mice. It determined that Jekyll mice were born with fewer cardiac and skeletal muscle LRCs as the muscle STP pool was depleted, consistent with enhanced myogenesis. By contrast, Jekyll mice were born with more WAT and BAT LRCs, which quickly disappeared as adipose cell density increased beyond wild-type levels. This is consistent with data presented herein indicating that the adult STP adipose pools are quickly depleted in Jekyll mice as once again adipose cell density increases. Thus, myostatin inhibits adipogenesis independent of changes in adiposity and this conclusion is supported by data demonstrating the enhanced capacity of mstn−/− stromal vascular cells to proliferate and/or differentiate in vitro and by the myokine's direct inhibitory actions in these cells.
Several studies suggested that attenuating myostatin can affect adipose tissue mass and growth in vivo (2, 27, 28) and can induce adipose browning (29) either due to the loss of myostatin or to the compensatory up-regulation of irisin, an adipogenic myokine (30). Indeed, a growing consensus suggests that myostatin indirectly influences adiposity by altering metabolic rate through changes in muscle mass; more specifically, that the enhanced musculature of mstn−/− animals consumes metabolites and prevents fat accumulation (2, 27). However, several in vitro studies have also demonstrated direct effects of myostatin or other ActRIIb ligands on adipocyte growth and differentiation (13, 16, 17, 31, 32), suggesting that the effects noted herein result from the loss of myostatin's direct and indirect actions on adipogenesis and lipogenesis. Several of these in vitro studies, however, report conflicting results as myostatin can either stimulate or inhibit adipocyte differentiation depending on the cell line.
Using the C3H10T(1/2) mesenchymal cell line that is capable of differentiating into muscle or fat cells, recombinant myostatin reduces expression of myogenic markers, increases that of adipogenic markers, stimulates terminal differentiation into lipid-containing adipocytes and can even substitute for glucocorticoids during induction (13, 16). Stem and progenitor cells from muscle or fat also possess myogenic and adipogenic potential, yet we saw no indication of fatty infiltration of muscle (or vice versa) in Jekyll mice. This suggests that transdifferentiation of such precursors does not appear to occur with myostatin attenuation. In contrast to the C3H10T(1/2) studies, others report that myostatin inhibits 3T3-L1 preadipocyte differentiation as indicated by changes in cellular morphology, reduced expression of PPARγ and C/EBPα (known markers of adipocyte differentiation) and reduced lipid accumulation (31–35). These latter studies were consistent with our results and were supported by studies with primary brown preadipocytes where myostatin reduces expression of BAT markers (PPARγ, uncoupling protein-1, PGCα, and PRDM16) and terminal differentiation through novel signaling pathways that include β-catenin (36). Furthermore, previous studies have also reported elevated protein expression of mature adipocyte-specific markers (eg, adiponectin, AMP-activated protein kinase, PPARγ, and leptin) in adipogenic cells from mstn−/− mice (29, 37) or with myostatin knockdown (38). These studies and our results together suggest that myostatin action in the C3H10T(1/2) cell line may have little physiological relevance as myostatin clearly inhibits differentiation of 3T3-L1 and primary WAT and BAT preadipocytes in vitro, whereas adipogenic mstn−/− cells more readily self-renew and differentiate.
Browning of WAT also occurs in mstn−/− fat pads and is mediated by the fibronectin type III domain-containing protein 5 or irisin-induction of BAT-specific markers (30). Although difficult to determine, it is unlikely that mature WAT cells revert to BAT as the WAT adipogenic pool is fully capable of differentiating into both cell types. Thus, the enhanced WAT adipogenesis noted herein may also contribute to the browning of WAT. This is very important because the expansion of WAT could have potentially negative consequences long term, whereas WAT browning is highly beneficial and is associated with improved insulin sensitivity.
To determine whether mstn−/− mice had more adipocytes, despite smaller adipose tissue weights, we normalized adipocyte cell counts to tissue weights and cell number per unit. Our data revealed that the decrease in adipose tissue weight and increase in cell density was only partly due to reduced adipocyte size, as previously reported (2, 3, 39). This is because the relative differences between wild-type and Jekyll mice in adipose tissue mass and cell number did not change similarly over time. This was particularly evident in BAT, where differences in cell density increased at a rate faster than that for mass. These findings perfectly corresponded with a more rapid disappearance rate of Jekyll WAT and BAT LRCs and are even supported by the proliferation and differentiation studies with stromal vascular cells.
To determine the functional relevance of differences in BAT, we measured the surface body temperature of wild-type and mstn−/− neonates and 1- and 2-month-old mice. Unlike in WAT, BAT oxidation of fat is unique to thermoregulation and is particularly important to neonates and infants (40, 41). The lower body temperature and BAT mass in mstn−/− neonates was suggestive of a causative relationship and although an inverse relationship exists in older mice (1 and 2 mo old), it was consistent with the fact that basic metabolic rate (energy expenditure) is elevated in mstn−/− mice and that circulating metabolites are diverted away from fat stores (ie, reduced lipogenesis) to support the enhanced musculature (10, 42). Thus, higher body temperatures with less BAT in mstn−/− mice is not necessarily due to increased brown fat oxidation, but to enhanced resting energy expenditure and the generation of metabolic heat.
It is important to emphasize that the differences noted in adipose LRCs between wild-type and Jekyll mice were not due to differential dosing of doxycycline as LRC counts were identical at time 0 and in the intestines, a tissue unaffected by myostatin or in mstn−/− animals. In fact, the H2B-GFP doxycycline inducible system is superior to the more common method of bromodeoxyridine labeling, another nonradioactive technique that requires tissue fixation and labels only the S phase cells (43, 44). Bromodeoxyridine can also integrate into apoptotic cells as a result of DNA damage and repair (45) and precludes fate mapping/lineage tracing as labeled cells can no longer proliferate or differentiate. By contrast, the H2B-GFP system labeled all cells independent of cell cycle and the label remained for long chase periods. Labeled cells were still viable and this enables LRCs to be isolated and further characterized in vitro or in vivo. Future studies with Jekyll mice will therefore be useful in determining myostatin's niche-specific effects on the progenitor cell pools of different tissues as LRCs from wild-type and Jekyll mice can easily be transplanted and monitored.
This study clearly suggests that myostatin inhibits adipogenesis in vivo and in vitro and resolves an important controversy, although BAT appears more sensitive to myostatin than does WAT. The diversity of stem or progenitor cell types within adipose tissues is limited compared with many if not most other tissues. Nevertheless, future studies are needed to determine how myostatin influences the cellular identities and relative compositions of these pools, which include adipose-derived stem cells, preadipocytes, mesenchymal stem cells, endothelial progenitors and like most tissues, a small percentage of immune cells (46). Browning of WAT can significantly improve insulin sensitivity and can be accomplished by attenuating myostatin (30). Thus, a better understanding of how myostatin regulates adipogenic pools in different adipose stores could be important to understanding and possibly treating obesity and type 2 diabetes.
Acknowledgments
We like to particularly thank Melissa F. Jackson, PhD for the assistance provided.
This work was supported by the National Science Foundation Grant 1147275 (to B.D.R.), National Institutes of Health Grants R01HD067449 (to M.D.) and R01GM114640 (to T.B.T.), and the Muscular Dystrophy Association Grant 240087 (to T.B.T.).
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BAT
- brown adipose tissue
- C/EBP
- CCAAT/enhancer binding protein
- H2B-GFP
- histone 2B and green fluorescent protein
- PPAR
- peroxisome proliferator-activated receptor
- SI
- small intestine
- STP
- stem, transit amplifying and progenitor
- WAT
- white adipose tissue.
References
- 1. Rodgers BD, Garikipati DK. Clinical, agricultural, and evolutionary biology of myostatin: a comparative review. Endocr Rev. 2008;29:513–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. McPherron AC, Lee SJ. Suppression of body fat accumulation in myostatin-deficient mice. J Clin Invest. 2002;109:595–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jackson MF, Luong D, Vang D, et al. Cardiac hypertrophy, enhanced stress response, reduced adiposity and sexual dimorphism in aging myostatin null mice. J Endocrinol. 2012;213:263–275. [DOI] [PubMed] [Google Scholar]
- 4. Rodgers BD, Interlichia JP, Garikipati DK, et al. Myostatin represses physiological hypertrophy of the heart and excitation-contraction coupling. J Physiol. 2009;587:4873–4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McCroskery S, Thomas M, Maxwell L, Sharma M, Kambadur R. Myostatin negatively regulates satellite cell activation and self-renewal. J Cell Biol. 2003;162:1135–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wagner KR, Liu X, Chang X, Allen RE. Muscle regeneration in the prolonged absence of myostatin. Proc Natl Acad Sci USA. 2005;102:2519–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jackson MF, Li N, Rodgers BD. Myostatin regulates tissue potency and cardiac calcium-handling proteins. Endocrinology. 2014;155:1771–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lin J, Arnold HB, Della-Fera MA, Azain MJ, Hartzell DL, Baile CA. Myostatin knockout in mice increases myogenesis and decreases adipogenesis. Biochem Biophys Res Commun. 2002;291:701–706. [DOI] [PubMed] [Google Scholar]
- 9. McPherron AC, Lawler AM, Lee SJ. Regulation of skeletal muscle mass in mice by a new TGF-β superfamily member. Nature. 1997;387:83–90. [DOI] [PubMed] [Google Scholar]
- 10. Guo T, Jou W, Chanturiya T, Portas J, Gavrilova O, McPherron AC. Myostatin inhibition in muscle, but not adipose tissue, decreases fat mass and improves insulin sensitivity. PLoS One. 2009;4:e4937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Artaza JN, Singh R, Ferrini MG, Braga M, Tsao J, Gonzalez-Cadavid NF. Myostatin promotes a fibrotic phenotypic switch in multipotent C3H 10T1/2 cells without affecting their differentiation into myofibroblasts. J Endocrinol. 2008;196:235–249. [DOI] [PubMed] [Google Scholar]
- 12. Guo W, Flanagan J, Jasuja R, Kirkland J, Jiang L, Bhasin S. The effects of myostatin on adipogenic differentiation of human bone marrow-derived mesenchymal stem cells are mediated through cross-communication between Smad3 and Wnt/β-catenin signaling pathways. J Biol Chem. 2008;283:9136–9145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Artaza JN, Bhasin S, Magee TR, et al. Myostatin inhibits myogenesis and promotes adipogenesis in C3H 10T(1/2) mesenchymal multipotent cells. Endocrinology. 2005;146:3547–3557. [DOI] [PubMed] [Google Scholar]
- 14. Lei H, Yu B, Yang X, et al. Inhibition of adipogenic differentiation by myostatin is alleviated by arginine supplementation in porcine-muscle-derived mesenchymal stem cells. Sci China Life Sci. 2011;54:908–916. [DOI] [PubMed] [Google Scholar]
- 15. Geng J, Peng F, Xiong F, et al. Inhibition of myostatin promotes myogenic differentiation of rat bone marrow-derived mesenchymal stromal cells. Cytotherapy. 2009;11:849–863. [DOI] [PubMed] [Google Scholar]
- 16. Feldman BJ, Streeper RS, Farese RV, Jr, Yamamoto KR. Myostatin modulates adipogenesis to generate adipocytes with favorable metabolic effects. Proc Natl Acad Sci USA. 2006;103:15675–15680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rebbapragada A, Benchabane H, Wrana JL, Celeste AJ, Attisano L. Myostatin signals through a transforming growth factor β-like signaling pathway to block adipogenesis. Mol Cell Biol. 2003;23:7230–7242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hochedlinger K, Yamada Y, Beard C, Jaenisch R. Ectopic expression of Oct-4 blocks progenitor-cell differentiation and causes dysplasia in epithelial tissues. Cell. 2005;121:465–477. [DOI] [PubMed] [Google Scholar]
- 19. Tumbar T, Guasch G, Greco V, et al. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Yang QY, Liang JF, Rogers CJ, Zhao JX, Zhu MJ, Du M. Maternal obesity induces epigenetic modifications to facilitate Zfp423 expression and enhance adipogenic differentiation in fetal mice. Diabetes. 2013;62:3727–3735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cash JN, Rejon CA, McPherron AC, Bernard DJ, Thompson TB. The structure of myostatin:follistatin 288: insights into receptor utilization and heparin binding. EMBO J. 2009;28:2662–2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang S, Liang X, Yang Q, et al. Resveratrol induces brown-like adipocyte formation in white fat through activation of AMP-activated protein kinase (AMPK) α1. Int J Obes (Lond). 2015;39:967–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aherne W, Hull D. Brown adipose tissue and heat production in the newborn infant. J Pathol Bacteriol. 1966;91:223–234. [DOI] [PubMed] [Google Scholar]
- 24. Harms M, Seale P. Brown and beige fat: development, function and therapeutic potential. Nat Med. 2013;19:1252–1263. [DOI] [PubMed] [Google Scholar]
- 25. Garikipati DK, Rodgers BD. Myostatin stimulates myosatellite cell differentiation in a novel model system: evidence for gene subfunctionalization. Am J Physiol Regul Integr Comp Physiol. 2012;302:R1059–R1066. [DOI] [PubMed] [Google Scholar]
- 26. Garikipati DK, Rodgers BD. Myostatin inhibits myosatellite cell proliferation and consequently activates differentiation: evidence for endocrine-regulated transcript processing. J Endocrinol. 2012;215:177–187. [DOI] [PubMed] [Google Scholar]
- 27. Yang J, Zhao B. Postnatal expression of myostatin propeptide cDNA maintained high muscle growth and normal adipose tissue mass in transgenic mice fed a high-fat diet. Mol Reprod Dev. 2006;73:462–469. [DOI] [PubMed] [Google Scholar]
- 28. Zhao B, Wall RJ, Yang J. Transgenic expression of myostatin propeptide prevents diet-induced obesity and insulin resistance. Biochem Biophys Res Commun. 2005;337:248–255. [DOI] [PubMed] [Google Scholar]
- 29. Braga M, Reddy ST, Vergnes L, et al. Follistatin promotes adipocyte differentiation, browning, and energy metabolism. J Lipid Res. 2014;55:375–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Shan T, Liang X, Bi P, Kuang S. Myostatin knockout drives browning of white adipose tissue through activating the AMPK-PGC1α-Fndc5 pathway in muscle. FASEB J. 2013;27:1981–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim HS, Liang L, Dean RG, Hausman DB, Hartzell DL, Baile CA. Inhibition of preadipocyte differentiation by myostatin treatment in 3T3-L1 cultures. Biochem Biophys Res Commun. 2001;281:902–906. [DOI] [PubMed] [Google Scholar]
- 32. Hirai S, Matsumoto H, Moriya NH, Kawachi H, Yano H. Follistatin rescues the inhibitory effect of activin A on the differentiation of bovine preadipocyte. Domest Anim Endocrinol. 2007;33:269–280. [DOI] [PubMed] [Google Scholar]
- 33. Hirai S, Matsumoto H, Hino N, Kawachi H, Matsui T, Yano H. Myostatin inhibits differentiation of bovine preadipocyte. Domest Anim Endocrinol. 2007;32:1–14. [DOI] [PubMed] [Google Scholar]
- 34. Allen DL, Cleary AS, Speaker KJ, et al. Myostatin, activin receptor IIb, and follistatin-like-3 gene expression are altered in adipose tissue and skeletal muscle of obese mice. Am J Physiol Endocrinol Metab. 2008;294:E918–E927. [DOI] [PubMed] [Google Scholar]
- 35. Li F, Yang H, Duan Y, Yin Y. Myostatin regulates preadipocyte differentiation and lipid metabolism of adipocyte via ERK1/2. Cell Biol Int. 2011;35:1141–1146. [DOI] [PubMed] [Google Scholar]
- 36. Kim WK, Choi HR, Park SG, Ko Y, Bae KH, Lee SC. Myostatin inhibits brown adipocyte differentiation via regulation of Smad3-mediated β-catenin stabilization. Int J Biochem Cell Biol. 2012;44:327–334. [DOI] [PubMed] [Google Scholar]
- 37. Braga M, Pervin S, Norris K, Bhasin S, Singh R. Inhibition of in vitro and in vivo brown fat differentiation program by myostatin. Obesity (Silver Spring). 2013;21:1180–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lu J, Wei C, Zhang X, et al. The effect of myostatin silencing by lentiviral-mediated RNA interference on goat fetal fibroblasts. Mol Biol Rep. 2013;40:4101–4108. [DOI] [PubMed] [Google Scholar]
- 39. Rodgers BD, Wiedeback BD, Hoversten KE, Jackson MF, Walker RG, Thompson TB. Myostatin stimulates, not inihibits, C2C12 myoblast proliferation. Endocrinology. 2014;155:670–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Schulz TJ, Huang TL, Tran TT, et al. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc Natl Acad Sci USA. 2011;108:143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Seale P, Conroe HM, Estall J, et al. Prdm16 determines the thermogenic program of subcutaneous white adipose tissue in mice. J Clin Invest. 2011;121:96–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Allen DL, Hittel DS, McPherron AC. Expression and function of myostatin in obesity, diabetes, and exercise adaptation. Med Sci Sports Exerc. 2011;43:1828–1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Rothaeusler K, Baumgarth N. Assessment of cell proliferation by 5-bromodeoxyuridine (BrdU) labeling for multicolor flow cytometry. Curr Protoc Cytom. 2007; Chapter 7:Unit7 31. [DOI] [PubMed] [Google Scholar]
- 44. Terry NH, White RA. Flow cytometry after bromodeoxyuridine labeling to measure S and G2+M phase durations plus doubling times in vitro and in vivo. Nat Protoc. 2006;1:859–869. [DOI] [PubMed] [Google Scholar]
- 45. Magavi SS, Macklis JD. Identification of newborn cells by BrdU labeling and immunocytochemistry in vivo. Methods Mol Biol. 2008;438:335–343. [DOI] [PubMed] [Google Scholar]
- 46. Harasymiak-Krzyanowska I, Niedojadło A, Karwat J, et al. Adipose tissue-derived stem cells show considerable promise for regenerative medicine applications. Cell Mol Biol Lett. 2013;18:479–493. [DOI] [PMC free article] [PubMed] [Google Scholar]