Abstract
Numerous musculoskeletal pain disorders are based in dysfunction of peripheral perfusion and are often comorbid with altered cardiovascular responses to muscle contraction/exercise. We have recently found in mice that 24 h peripheral ischemia induced by a surgical occlusion of the brachial artery (BAO) induces increased paw-guarding behaviors, mechanical hypersensitivity, and decreased grip strength. These behavioral changes corresponded to increased heat sensitivity as well as an increase in the numbers of chemosensitive group III/IV muscle afferents as assessed by an ex vivo forepaw muscles/median and ulnar nerves/dorsal root ganglion (DRG)/spinal cord (SC) recording preparation. Behaviors also corresponded to specific upregulation of the ADP-responsive P2Y1 receptor in the DRGs. Since group III/IV muscle afferents have separately been associated with regulating muscle nociception and exercise pressor reflexes (EPRs), and P2Y1 has been linked to heat responsiveness and phenotypic switching in cutaneous afferents, we sought to determine whether upregulation of P2Y1 was responsible for the observed alterations in muscle afferent function, leading to modulation of muscle pain-related behaviors and EPRs after BAO. Using an afferent-specific siRNA knockdown strategy, we found that inhibition of P2Y1 during BAO not only prevented the increased mean blood pressure after forced exercise, but also significantly reduced alterations in pain-related behaviors. Selective P2Y1 knockdown also prevented the increased firing to heat stimuli and the BAO-induced phenotypic switch in chemosensitive muscle afferents, potentially through regulating membrane expression of acid sensing ion channel 3. These results suggest that enhanced P2Y1 in muscle afferents during ischemic-like conditions may dually regulate muscle nociception and cardiovascular reflexes.
SIGNIFICANCE STATEMENT Our current results suggest that P2Y1 modulates heat responsiveness and chemosensation in muscle afferents to play a key role in the development of pain-related behaviors during ischemia. At the same time, under these pathological conditions, the changes in muscle sensory neurons appear to modulate an increase in mean systemic blood pressure after exercise. This is the first report of the potential peripheral mechanisms by which group III/IV muscle afferents can dually regulate muscle nociception and the exercise pressor reflex. These data provide evidence related to the potential underlying reasons for the comorbidity of muscle pain and altered sympathetic reflexes in disease states that are based in problems with peripheral perfusion and may indicate a potential target for therapeutic intervention.
Keywords: cardiovascular assays, electrophysiology, muscle pain, protein analysis, siRNAs
Introduction
Musculoskeletal pain resulting from reduced blood flow and oxygenation to the periphery is a common clinical complaint (McDermott et al., 2004; Norgren et al., 2006; Davies, 2012). This occurs in prevalent disorders, such as peripheral vascular disease (PVD) or complex regional pain syndrome (CRPS; Coderre and Bennett, 2010). Ischemia is first associated with intermittent fatigue but can progress to pain at rest in these patients (Ouriel, 2001; Meru et al., 2006).
Thinly myelinated (group III) and unmyelinated (group IV) primary afferents that innervate skeletal muscles can function as nociceptors and are the initiators of pain states resulting from peripheral injuries, such as ischemia (Mense, 2003; Birdsong et al., 2010; Ross et al., 2014). However, there is also an abundance of evidence that these same group III/IV muscle afferents comprise the sensory component of the exercise pressor reflex (EPR), which is a well studied cardiovascular response that causes an increase in cardiac output and blood pressure during exercise or muscle contraction (McCloskey and Mitchell, 1972; Kaufman et al., 1983; Mitchell et al., 1983; McMahon and McWilliam, 1992; Amann et al., 2010). Muscle tissue generates heat (González-Alonso et al., 2000) and releases metabolites, such as protons, ATP, and lactic acid, during contraction (Bockman, 1983; Bockman and McKenzie, 1983; MacLean et al., 1998). The group III/IV muscle sensory neurons are known to respond to thermal stimuli (Taguchi et al., 2005; Jankowski et al., 2013; Queme et al., 2013; Ross et al., 2014) and different combinations of these same metabolites (Light et al., 2008). Specifically, there is one population of muscle afferents that responds to a combination of ATP, lactic acid, and protons that resembles the environment in the muscle during moderate exercise, and a second population that responds exclusively to the same metabolites but in a higher concentration, similar to what is produced in muscle tissue during ischemic contractions (Alam and Smirk, 1937; Kniffki et al., 1978; Kaufman and Rybicki, 1987; Light et al., 2008; Jankowski et al., 2013; Pollak et al., 2014; Ross et al., 2014). Human subjects that received intramuscular injections of these metabolite mixtures reported that the solution associated with moderate exercise produced a sensation of fatigue while injection of the more concentrated mixture caused a feeling of muscle pain (Pollak et al., 2014).
Numerous channels/receptors that respond to acidic conditions or ATP have been linked to the production of muscle pain, but also to the regulation of the EPR. These channels/receptors include acid sensing ion channels (ASICs) and various P2 receptors (Hanna and Kaufman, 2003; North, 2004; Hayes et al., 2007, 2008a,b; Tsuchimochi et al., 2011). Evidence shows that, in addition to afferents that respond to these aforementioned metabolites, there are thermally sensitive afferents that also play a role in EPR alterations after injury (Gao et al., 2007; Sluka et al., 2007). One type of purinergic receptor, P2Y1, has been shown to modulate heat sensitivity and phenotypic changes in the expression of transient receptor potential vanilloid 1 in cutaneous C-polymodal fibers during inflammation (Molliver et al., 2011; Jankowski et al., 2012a). We have further shown that P2Y1 is also upregulated in the dorsal root ganglia (DRGs) after muscle ischemia (Ross et al., 2014), and mice with 24 h ischemic injury experience spontaneous pain-like behaviors, decreased mechanical thresholds to von Frey hair stimulation of the plantar surface of the affected forepaw, and decreased muscle strength (Ross et al., 2014). These behaviors have been associated with an increase in the response intensity to heat stimulation of the muscles and in the prevalence of chemosensitive group III/IV muscle afferents (Ross et al., 2014). Based on these facts, we sought to test the hypothesis that upregulation of P2Y1 in the DRGs during BAO-induced ischemia would not only alter observed changes in the specific responses to different thermal and chemical stimuli in muscle afferents, but would also block alterations in pain-related behaviors and EPRs.
Materials and Methods
Animals.
Experiments were conducted with young adult (3–8 weeks) male Swiss Webster mice (Charles River). All animals were housed in a barrier facility in group cages of ≤4 mice, maintained on a 12 h light/dark cycle with a temperature-controlled environment, and given food and water ad libitum. All procedures were approved by the Cincinnati Children's Hospital Research Foundation Institutional Animal Care and Use Committee and adhered to National Institutes of Health Standards of Animal Care and Use under practices approved by the Association for Assessment and Accreditation of Laboratory Animal Care.
Induction of ischemia.
A complete brachial artery occlusion (BAO) was used to induce an ischemic injury that at the same time allowed for precise control of the of blood flow received by the forepaw muscles. The procedure was performed as previously described in Ross et al. (2014). Briefly, mice were anesthetized with 3% isoflurane, and then, under a dissection microscope, a small incision was made in the right forelimb above the elbow. The right brachial artery was exposed proximal to the ulnar artery/radial artery split. The vessels were gently loosened from adjacent connective tissue and then the brachial artery was tied using a 7-0 silk suture. Incisions were closed with 6-0 silk sutures. Except for the baseline behavioral time points, all assessments were performed 1 d after the BAO.
Nerve-specific siRNA injections.
Specific targeting siRNAs were used to selectively knock down the expression of the ADP receptor P2Y1 (Thermo Fisher Scientific) and conjugated to penetratin-1 (MP Biomedicals) as described by Davidson et al. (2004) and Jankowski et al. (2006, 2010, 2012a,b). This particular duplex was previously determined to have the highest targeting efficiency to P2Y1 based on the knockdown efficacy of four different P2Y1-targeting siRNAs in vitro (Jankowski et al., 2012a, data not shown). The one duplex used in the current study and in the experiments of the previous report is as follows: sense: 5′-S-S-GAAGUUAUUUCAUCUACAGUU; antisense: 5′-P-CUGUAGAUGAAAUAACUUCUU (Jankowski et al., 2012a). Our nontargeting control siRNA used in this report and in others (Jankowski et al., 2006, 2009, 2010, 2012a,b) has been determined to not target any gene in the mouse genome (Thermo Fisher Scientific, catalog #D-001206-14-05). The sequence for the nontargeting siRNA duplex is as follows: sense: 5′-S-S-UAGCGACUAAACACAUCAAUU; antisense: 5′-DY547-UUGAUGUGUUUAGUCGCUAUU.
Two days before BAO, mice were anesthetized as described. A small incision was made in the inner midforelimb region, proximal to the elbow, and exposed the ulnar and median nerves to be injected. siRNAs were heated to 65°C for 5 min before injection. Using a quartz microelectrode connected to a picospritzer, we pressure injected 0.1–0.2 μl of 90 μm penetratin-1 linked nontargeting, control (PenCON) or P2Y1-targeting siRNAs (PenY1) directly into the median and ulnar nerves. We have previously shown that this injection protocol does not significantly injure the affected sensory afferents, nor does it provoke antiviral-related responses (Jankowski et al., 2009, 2010, 2012a), but it does enable us to specifically determine whether dynamic changes in gene expression in specific affected sensory afferents play a role in observed alterations in sensory fiber responses or animal behaviors.
Nociceptive behaviors and measurement of EPRs.
Separate groups of naive (Naive), BAO, PenCON plus BAO (PenCON+BAO), or PenY1 plus BAO (PenY1+BAO) mice were used for behavioral analysis (n = 10 per group). Testing of pain-related behaviors was performed as previously described by Ross et al. (2014). Briefly, mice were first tested at baseline, ∼2 h before injury (BAO), and then again 1 d after injury. All behavioral testing was performed in the morning. While it was not possible to blind the experimenter to the presence of injury due to the necessary incision in the forelimb, the experimenter was blinded to the pretreatment status of the animal (PenY1 siRNA injected, PenCON siRNA injected, or BAO alone). Nociceptive testing included three behavioral assessments: forepaw guarding, von Frey filament stimulation of the plantar surface of the forepaws, and forelimb grip strength, in this order. Mice were placed in a raised acrylic glass chamber with a steel mesh bottom and allowed to habituate for ≥30 min. To evaluate guarding behavior, mice were assigned a score of 0–2 (0, mouse places foot firmly on mesh; 1, mouse does not bear full weight on foot; 2, mouse holds foot completely above mesh) every 5 min for 12 total observations. The average score for the 12 trials was determined for each mouse per behavioral day. Mechanical withdrawal thresholds on the forepaws were then determined by stimulating the plantar surface with an increasing series of von Frey filaments (0.07–4 g). Threshold to withdrawal was recorded for ≥3 trials with 5 min intervals between stimulations, and the average of the three trials was used for analysis. Last, mice were assayed for forepaw muscle strength using a grip-strength meter (BioSeb). Animals were held by the tail over a metal grid until they firmly held it with both forepaws, but were not allowed to grip the grid with their hindpaws. Then they were quickly pulled back horizontally (along the axis of the force sensor) until they could not retain their grip. Grip strength was measured (in grams) in three rounds of three trials each, with 5 min between each round. The average of the nine trials was used for analysis.
After these assessments were made, we designed a low-intensity, forced-run protocol to evaluate cardiovascular function. This protocol was based on previous work by Kemi et al. (2002) and Billat et al. (2005). Before and immediately after the exercise session, each mouse was placed in a small acrylic restrainer adequate for the mouse's size and weight, and allowed a short period of acclimation (∼5 min). Then its blood pressure and heart rate were measured 60 times (maximum number obtained per mouse) with a tail-cuff blood pressure system (Kent Scientific). Data were collected using Coda software (Kent Scientific) and analyzed off-line. The first 20 measurements were used to acclimate the mice to tail-cuff inflation and were not used for analysis. Unreliable measurements were automatically discarded by the software, and manual discards were made when the animal showed excessive movement that generated signal artifacts. As a control to verify that the exercise activity was not influencing the pain-related hypersensitivity due to BAO, we performed all the behavioral assessments before and immediately after exercise but before BAO and again 1 d after occlusion surgery. For this analysis, a blinded experimenter evaluated two different groups of mice, one exposed to BAO (n = 8) and another to sham surgeries (a thread was placed around the brachial artery but was not tied to block the blood flow, n = 4).
For the exercise protocol, the mice were run at baseline (immediately before BAO) and 1 d after surgery on a modular treadmill with an Oxymax sensor (Columbus Instruments), calibrated with standardized gas mixtures before every testing session, to monitor oxygen consumption (VO2). VO2 monitoring enables us to assess the transition from aerobic to anaerobic exercise and ensured that oxygen consumption remained below exhaustion levels in our mice (Billat et al., 2005; Rocco et al., 2014). The running protocol consisted of a slowly increasing speed from 9 m/min up to a maximal speed of 13 m/min at 0° of inclination for a total distance of 500 m. Speed was increased 1 m/min per minute. Thus the entire running protocol lasted ∼40 min. This speed is ∼75% the mean critical speed for mice, which correlates well with the lactate threshold (Billat et al., 2005), and is well below the speed and distance previously reported to induce muscle damage in mice (Nakamura et al., 2005; Thabet et al., 2005).
Ex vivo recording preparation.
Ex vivo recording was performed as previously described by Jankowski et al. (2013) and Ross et al. (2014). Briefly, mice were anesthetized with an intramuscular hindlimb injection of ketamine and xylazine (90 and 10 mg/kg, respectively) and perfused transcardially with oxygenated (95% O2–5% CO2) artificial CSF (aCSF; in mm: 127.0 NaCl, 1.9 KCl, 1.2 KH2PO4, 1.3 MgSO4, 2.4 CaCl2, 26.0 NaHCO3, and 10.0 d-glucose) at 12–15°C. The right forelimb and the SC were then excised and placed in a bath of the same aCSF. The forelimb skin was removed along with the cutaneous branches of the median and ulnar nerves. The SC was hemisected and the median and ulnar nerves along with the forelimb muscles they innervate (with bone left intact) were dissected in continuity with their respective DRGs (C7, C8, and T1). After dissection, the preparation was transferred to a separate recording chamber containing cold oxygenated aCSF. The forepaw was pinned on an elevated platform, keeping the entire paw perfused in a chamber isolated from the DRGs and the SC. Finally, the bath was slowly warmed to 32°C before recording from the DRGs.
All single-unit recordings were made from the C7, C8, and T1 DRGs as these are the primary source of muscle afferent fibers in the median and ulnar nerves. Sensory neuron somata were impaled with quartz microelectrodes (impedance, >150 MΩ) containing 5% neurobiotin (Vector Laboratories) in 1 m potassium acetate. Electrical search stimuli were delivered through a suction electrode on the nerve to locate sensory neuron somata with axons in the median and ulnar nerves. The latency from the onset of this stimulus and the conduction distance between the DRG and the stimulation site (measured directly along the nerve) were used to calculate the conduction velocity (CV) of the fibers. Group IV afferents were classified as those with a CV ≤1.2 m/s, and group III afferents were those with CVs between 1.2 and 15 m/s (Jankowski et al., 2013). Peripheral receptive fields (RFs) in the muscles were localized by electrically stimulating the muscles with a concentric bipolar electrode. Only driven cells with RFs in the muscles then underwent mechanical, thermal, and chemical testing. Mechanical response characteristics were assessed with an increasing series of von Frey hairs ranging from 0.4 to 10 g (with diameters of 0.23–0.36 mm). Mechanical stimulation of the RF was held for ∼1–2 s. Thermal responses were determined by applying hot (≥52°C) or cold (≤3°C) saline directly to the paw muscles at the electrically determined RF. Each application lasted ∼1–2 s. After that, the muscles were exposed to an oxygenated “low” metabolite mixture (15 mm lactate, 1 μm ATP, pH 7.0) and then to a “high” metabolite mixture (50 mm lactate, 5 μm ATP, pH 6.6) delivered by a valve controller with an in-line heater to maintain solutions at bath temperature. ATP was added to the mixture immediately before delivery of metabolites. Adequate recovery times (∼20–30 s) were used between stimulations. All elicited responses were recorded digitally for off-line analysis (Spike2 software, Cambridge Electronic Design). After physiological characterization, select cells were labeled by iontophoretic injection of 5% neurobiotin (1 or 2 cells/DRG).
Immunohistochemistry.
Once a sensory neuron was characterized and intracellularly filled with neurobiotin, the DRG containing the injected cell was removed and immersion fixed with 3% paraformaldehyde in 0.1 m phosphate buffer (PB). DRGs were fixed for 30 min at room temperature and then changed to PB for storage before embedding in 10% gelatin. Embedded DRGs were then postfixed in 3% paraformaldehyde in 0.1 m PB, and cryoprotected in 20% sucrose. 50 μm DRG, sections were collected in PB and then processed overnight for ASIC3 (guinea pig anti-ASIC3, 1:2000; Millipore) and P2X3 (rabbit anti-P2X3, 1:2000; Thermo Fisher Scientific). After incubation in primary antiserum, tissue was washed and sections were fluorescently labeled with secondary antibodies (anti-guinea pig AlexaFluor-647, 1:400, or anti-rabbit AlexaFluor-594, 1:400; Jackson ImmunoResearch Laboratories) as well as FITC–avidin (1:750; Vector Laboratories) to label neurobiotin-filled cells. Sections were mounted with Fluoromount G (Electron Microscopy Sciences) on gelatin-coated slides and stored in the dark at room temperature. To test the specificity of our antibody labeling, DRGs from a naive mouse were processed under the same protocol without adding a primary antibody for ASIC3 to one set of DRG samples as a negative control since a peptide is not commercially available to perform a peptide block control (Millipore). Exposure time during microscopic analysis for each positive and negative image was performed at the same intensity level to confirm staining above background. Distribution of fluorescent staining was determined with a Leica confocal microscope with sequential scanning to avoid bleed-through of the fluorophores. Images for publication were prepared using Photoshop Elements 6 software (Adobe).
Quantification of DRG neurons.
Total cells containing P2X3 and ASIC3 were quantified in DRGs (either C7, C8, or T1) from Naive, BAO, PenCON+BAO, or PenY1+BAO mice (n = 3–5). The DRGs were taken after electrophysiological experiments and were processed for immunohistochemical analysis as described above. The numbers of positive cells were determined using a slightly modified methodology as previously reported by Christianson et al. (2006) and Jankowski et al. (2009). In brief, three nonconsecutive sections were randomly chosen and Z-stacks were generated at 3 μm intervals to create 15-μm-thick optical sections using a Leica confocal microscope with sequential scanning. The number of P2X3-positive and ASIC3-positive cells was counted using Neurolucida software, ensuring that the same cell was not counted twice, averaged in the top, middle, and bottom optical section of each stack, and reported as mean ± SEM.
Western blotting.
C7-T1 DRGs were collected from the right side of Naive, BAO, PenCON+BAO, and PenY1+BAO mice (n = 3 each). PenCON+BAO and PenY1+BAO groups were injected with siRNAs 2 d before performing the BAO similar to other experiments and tissue was collected 1 d after BAO in all groups. Tissue was pooled (DRGs from two mice per sample) and homogenized in lysis buffer containing 1% SDS, 10 mm Tris-HCl, pH 7.4, and protease inhibitors (1 μg/ml pepstatin, 1 μg/ml leupeptin, 1 μg/ml aprotinin, 1 mm sodium orthovanadate, and 100 μg/ml phenylmethylsulfonyl fluoride; Sigma-Aldrich Biochemicals). For isolation of membrane protein fractions, samples were processed using a Mem-PER Plus Membrane protein extraction kit (Thermo Fisher Scientific) following the provided instructions. Briefly, samples (n = 3 per condition) were pooled and washed in provided wash buffer, then homogenized in permeabilization buffer and incubated for 10 min on ice with constant mixing, and then centrifuged at 16,000 × g for 15 min at 4°C to pellet permeabilized cells. The supernatant containing cytosolic proteins was removed and stored at −80°C until use. The pellet containing the membrane-associated proteins was resuspended in solubilization buffer and incubated for 30 min at 4°C with constant mixing, then centrifuged at 16,000 × g for 15 min at 4°C, and the supernatant containing the membrane proteins was used for subsequent analysis. All samples (20 μg) were then centrifuged, boiled 10 min in a denaturing buffer containing β-mercaptoethanol and SDS, separated on a 12.5% precast polyacrylamide gel (Bio-Rad Laboratories) and transferred to an polyvinylidene difluoride membrane (PVDF; Merck Millipore) that was blocked in LiCor Blocking Buffer diluted 1:1 in 0.1 m PB. Membranes were then incubated in this same blocking solution containing 0.2% Tween 20 and primary antibodies overnight at 4°C. Antibodies used for Western blot were as follows: rabbit anti-P2Y1 (1:400; Alomone Labs); chicken anti-GAPDH (1:2000; Pro-Sci) and, for membrane fractions, guinea pig anti-ASIC3 (1:600; Merck Millipore). After incubation in primary antibodies, PVDF membranes were then washed and incubated with secondary antibody (donkey anti-chicken Dy680RD, 1:20,000; donkey anti-rabbit Dy800CW, 1:20,000; donkey anti-guinea pig IRDy800CW or Dy680RD, 1:15,000; Li-Cor Biosciences) diluted in blocking buffer containing 0.2% Tween 20 and 0.01% SDS. Membranes were washed and imaging was performed using a Li-Cor laser scanner for protein analysis. Immunoreactive bands were analyzed by measuring and plotting the band intensity and subsequently calculating the area under the curve using National Institutes of Health ImageJ software. Band intensity was normalized to GAPDH and reported as a ratio of P2Y1/GAPDH expression. For membrane fraction analysis, equal parts of each isolated fraction were loaded for direct quantification.
Statistical analysis.
All values are presented as mean ± SEM unless stated differently. Behavioral assays were tested with two-way repeated-measures ANOVA with Bonferroni's post hoc test. Differences in phenotype frequency between groups were compared using a χ2 test. Mean peak instantaneous frequencies and thresholds to different peripheral stimuli were compared via Kruskal–Wallis one-way ANOVA on ranks with Dunn's post hoc test. Analysis of P2Y1 protein samples were assessed using a one-way ANOVA with Tukey's post hoc and for ASIC3 membrane protein using a one-way ANOVA with Holm–Sidak post hoc. Critical significance level was set at p < 0.05.
Results
P2Y1 regulates the development of pain-related behaviors and alterations in the cardiovascular response to exercise during BAO
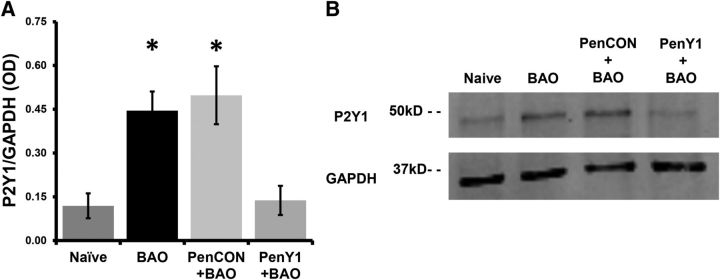
To assess the role of upregulated P2Y1 in primary afferents during BAO, we used our novel nerve-specific siRNA-mediated knockdown strategy (Jankowski et al., 2006, 2009, 2010, 2012a,b) to specifically inhibit the upregulation of P2Y1 in median and ulnar afferents during BAO. We first verified the specificity of our siRNA injections in blocking the reported upregulation of P2Y1 in the DRGs during BAO at the protein level using Western blotting analysis of DRG lysates from Naive, BAO, PenCON+BAO, and PenY1+BAO mice. We confirmed a significant increase in the expression of P2Y1 protein in the DRGs during this ischemic insult (Naive, 0.12 ± 0.04; BAO, 0.44 ± 0.07; p < 0.05 vs Naive), and the same increase was observed in the PenCON+BAO group (0.50 ± 0.10, p < 0.05 vs Naive). Injecting P2Y1-targeting siRNAs into the median and ulnar nerves, however, effectively blocked the increase in DRG P2Y1 protein expression in mice with BAO (PenY1+BAO, 0.14 ± 0.05; p < 0.05 vs BAO and PenCON+BAO), confirming the efficacy of our technique to prevent P2Y1 upregulation (Fig. 1).
Figure 1.
P2Y1-targeted siRNA injections into the median and ulnar nerves selectively knock down ischemia-induced protein overexpression in the DRGs. A, Ischemia (BAO or PenCON+BAO) induces an increase in the expression levels of P2Y1 compared with Naive mice and this is prevented by injections of P2Y1-targeted siRNAs (PenY1+BAO) into the ulnar and median nerves 2 d before the ischemic injury. B, Representative Western blot for all conditions assessed showing inhibition of P2Y1 upregulation during BAO or PenCON+BAO by P2Y1-targeting siRNA injections. One-way ANOVA/Tukey's post hoc, *p < 0.01 versus Naive and PenY1+BAO.
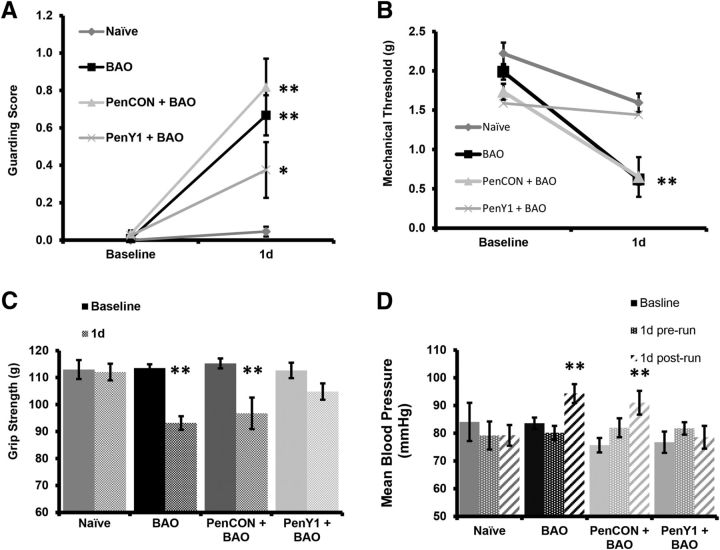
To investigate the effects of BAO on muscle pain-related behaviors in addition to EPRs, we performed three different assessments that evaluate spontaneous pain-like behaviors (paw guarding), mechanical hypersensitivity (withdrawal thresholds to mechanical stimulation of the forepaws using von Frey filaments), and muscle function (forepaw grip strength) followed by evaluation of changes in the cardiovascular function (systemic blood pressure and heart rate) after forced exercise. We have previously shown that a sham surgery (insertion of a suture under the artery without tying) does not alter any other these measurements compared with naive (Ross et al., 2014). While naive animals did not experience any change in guarding scores during 2 consecutive days of observation (baseline Naive, 0.00 ± 0.00; vs 1 d Naive, 0.04 ± 0.03, n = 11), the mice exposed to BAO showed increased guarding scores of the injured forepaw when compared with their baseline, as did the group injected with nontargeting control siRNAs (baseline BAO, 0.02 ± 0.06; vs 1 d BAO, 0.74 ± 0.06; baseline PenCON+BAO, 0.03 ± 0.02; vs 1 d PenCON+BAO, 0.82 ± 0.15, n = 10, p < 0.001). A different group of mice was injected with P2Y1-targeted siRNAs in the ulnar and median nerve of the right forepaw 2 d prior to performing a BAO. The PenY1+BAO group also showed a small but significant increase in guarding score when compared with its baseline and the Naive group (baseline PenY1+BAO, 0.02 ± 0.08; vs 1 d PenY1+BAO, 0.38 ± 0.08; n = 10, p < 0.01); however, this increase was significantly lower than both BAO control groups (Fig. 2A; p < 0.01).
Figure 2.
Ischemic insult increases spontaneous and evoked pain-related behaviors, reduces grip strength, and increases mean arterial blood pressure after exercise. A, One day after BAO or PenCON injection with BAO, paw-guarding scores are significantly increased compared with Naive mice; however, selective knockdown of P2Y1 (PenY1+BAO) partially prevents the observed injury-induced changes in guarding. B, Mechanical withdrawal thresholds are also found to be reduced after ischemic injury, but P2Y1 knockdown also partially prevents the development of mechanical hypersensitivity. C, Grip strength was significantly reduced compared with baseline in the BAO and PenCON+BAO groups, but not in the Naive or P2Y1-knockdown mice. D, The BAO and PenCON+BAO groups also showed a significant increase in systemic MBP specifically when low-intensity exercise was combined with the ischemic injury, but this was also prevented by afferent-specific inhibition of P2Y1. Two-way repeated-measures ANOVA with Bonferroni's post hoc, *p < 0.05 vs 1 d BAO, **p < 0.01 vs baseline and 1 d Naive.
The BAO and the PenCON+BAO groups also experienced significant decreases in the mechanical withdrawal thresholds versus their baseline measurements (baseline BAO, 1.99 ± 0.15 g; vs 1 d BAO, 0.65 ± 0.10 g; baseline PenCON+BAO, 1.73 ± 0.12 g vs 1 d PenCON+BAO, 0.65 ± 0.10 g, p < 0.001, n = 10). Mechanical withdrawal thresholds in the Naive group were surprisingly also found to be slightly decreased after 24 h when compared with their own baseline (baseline Naive, 2.22 ± 0.14 g; vs 1 d Naive, 1.59 ± 0.14 g; n = 11 p < 0.01), but this was not detected in the PenY1+BAO group (baseline PenY1+BAO, 1.59 ± 0.15 g; vs 1 d PenY1+BAO, 1.44 ± 0.15 g, n = 10). Regardless, the mechanical withdrawal thresholds in the BAO control groups were still significantly lower versus the time-matched Naive (p < 0.001) and PenY1+BAO (p < 0.003) groups at 1 d (Fig. 2B). We did not observe any changes in guarding scores or mechanical withdrawal thresholds in the contralateral limb in any of the conditions (data not shown).
Muscle function, as assessed by grip strength, was not altered in the Naive group over consecutive days (baseline Naive, 112.97 ± 3.54 g; vs 1 d Naive, 112.05 ± 3.06 g). The BAO and the PenCON+BAO controls, however, showed significantly decreased grip strength during ischemia compared with their baselines (baseline BAO, 113.54 ± 1.40 g; vs 1 d BAO, 93.14 ± 2.51 g, p < 0.001; baseline PenCON+BAO, 115.26 ± 1.84 g; vs 1 d PenCON+BAO, 96.73 ± 5.83 g, p < 0.001) and compared with the Naive group at 1 d (p < 0.01). The PenY1+BAO group showed a significant decrease compared with its baseline (p < 0.05) but the decrease was not to the same extent as the BAO or PenCON+BAO controls at the 1 d time point (Fig. 2C; p < 0.05). Results from BAO and PenCON+BAO confirm our previous work (Ross et al., 2014).
We did not find any differences between the baseline measurements for systemic blood pressure or heart rate before or after 40 min of forced running in uninjured mice. Thus data were combined for baseline comparisons (Naive, 84.08 ± 6.88 mmHg; BAO, 83.58 ± 2.07 mmHg; PenCON+BAO, 75.70 ± 2.60 mmHg; PenY1+BAO, 76.76 ± 2.56 mmHg). On the day after the BAO but before the exercise, no changes in the mean blood pressure (MBP) were observed compared with the baseline in any of the conditions (Naive 1 d prerun, 79.16 ± 5.05 mmHg; BAO 1 d prerun, 80.14 ± 2.52 mmHg; PenCON+BAO 1 d prerun, 81.96 ± 3.43 mmHg; PenY1+BAO 1 d prerun, 81.74 ± 2.24 mmHg). Immediately after the running protocol, however, the BAO and PenCON+BAO mice showed a significant increase in their MBP (BAO 1 d postrun, 94.71 ± 5.33 mmHg; PenCON+BAO 1 d postrun, 91.00 ± 4.28 mmHg) compared with their baseline (p < 0.05) and their prerun (p < 0.05) measurements. This increase was not observed in the Naive or PenY1+BAO groups (Naive 1 d postrun, 79.25 ± 3.74 mmHg; PenY1+BAO 1 d postrun, 78.54 ± 4.11 mmHg; Fig. 2D). We did not find any significant changes in the heart rate of the animals, under any condition, or during ischemia [Naive (n = 11): baseline, 689 ± 23.58 bpm; d 1 prerun, 703.82 ± 21.39 bpm; d 1 postrun, 680.81 ± 22.40 bpm; BAO (n = 10): baseline, 722.12 ± 12.73 bpm; d 1 prerun, 709.47 ± 17.15 bpm; d 1 postrun, 700.13 ± 19.02 bpm; PenCON+BAO (n = 10): baseline, 740.30 ± 15.92 bpm; d 1 prerun, 694.99 ± 8.08 bpm; d 1 postrun, 691.82 ± 10.27 bpm; PenY1+BAO (n = 10): baseline, 705.46 ± 15.92 bpm; d 1 prerun, 684.11 ± 18.84 bpm; d 1 postrun, 709.50 ± 14.90 bpm].
To determine whether the animals may have experienced increased pain-related behaviors or mechanical hypersensitivity during BAO due to exercise, we evaluated a separate cohort of mice before and immediately after exercise before performing either a BAO or a sham injury and then evaluated pain-related behaviors again 1 d after the surgery. In the Sham group, we did not detect any significant change before or after exercise in the guarding score (Sham: baseline prerun, 0.00 ± 0.00; baseline postrun, 0.10 ± 0.06; 1 d prerun, 0.21 ± 0.13; 1 d postrun, 0.19 ± 0.07), or mechanical thresholds (Sham: baseline prerun, 3.45 ± 0.55 g; baseline postrun, 3.33 ± 0.27 g; 1 d prerun, 2.83 ± 0.16 g; 1 d postrun, 2.53 ± 0.54 g) in the affected limb, or in grip strength (Sham: baseline prerun, 132.53 ± 5.14 g; baseline postrun, 126.17 ± 4.02 g; 1 d prerun 124.00 ± 4.77 g; 1 d postrun, 123.22 ± 6.65 g). As expected, the guarding scores were increased 1 d after surgery compared with the baseline, both before and after exercise (BAO: baseline prerun, 0.00 ± 0.00; baseline postrun, 0.00 ± 0.00; 1 d prerun, 1.61 ± 0.11; 1 d postrun, 1.82 ± 0.10; p < 0.001 vs baseline prerun and postrun) while the mechanical thresholds (BAO: baseline prerun, 3.17 ± 0.57 g; baseline postrun, 3.48 ± 0.59 g; 1 d prerun, 0.92 ± 0.11 g; 1 d postrun, 0.64 ± 0.08 g, p < 0.001 vs baseline prerun and postrun) and grip strength (BAO: baseline prerun, 132.96 ± 4.56 g; baseline postrun, 131.90 ± 2.16 g; 1 d prerun, 106.96 ± 3.41 g; 1 d postrun, 100.79 ± 2.66 g; p < 0.001 vs baseline prerun and postrun) were decreased at 1 d after the BAO. Thus the minor exercise had no effect on the evaluated pain-related behaviors in either the Sham or the BAO group before or after the surgeries but was sufficient to induce the EPR.
Increased responses to heat and phenotypic changes in group III/IV afferents require the upregulation of P2Y1 in the DRGs
To determine whether upregulation of P2Y1 directly played a role in altering the muscle afferents during ischemia, we performed intracellular recordings from single DRG neurons using our ex vivo forepaw muscle preparation on all four conditions described above. We recorded 79 cells from Naive mice, 70 cells from BAO mice, 65 cells from the PenCON+BAO group, and 69 cells from the PenY1+BAO group. CVs ranged from 0.22 m/s to 14.92 m/s with a mean of 0.57 ± 0.17 m/s for the group IV afferents and a mean of 8.44 ± 0.28 m/s for the group III fibers. Since we did not observe any significant differences between group III and group IV afferents for any response property analyzed (data not shown), we combined these data for ease of presentation.
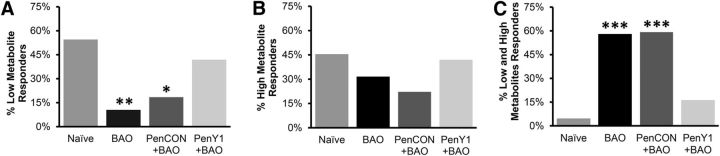
There were no significant differences in the responsiveness to the low or high mixture of metabolites among the Naive, BAO, PenCON+BAO, and PenY1+BAO groups (data not shown). However, we did find a significant change in the proportions of cells that responded to each specific combination of metabolites (p < 0.01, χ2). In the Naive mice, there was a similar proportion of cells that responded to low (55%, 12 of 22 cells) and high (45%, 10 of 22 cells) metabolite mixtures, while very few cells were found to respond to both of the metabolite mixtures (5%, 1 of 22 cells). The BAO mice showed a completely different distribution of the metabolite-sensitive cells with very few fibers responding to the low mixture of metabolites (11%, 2 of 19 cells), a larger percentage responding to the high mixture (32%, 6 of 19 cells) and an even larger number responding to both metabolites (58%, 11 of 19), confirming previous reports by Ross et al. (2014). This was very similar to what we observed in the PenCON+BAO mice, in which few cells responded to the low metabolite mixture (19%, 5 of 27), a similar number responded to the high metabolite mixture (22%, 6 of 27), and a very high number responded to both metabolites (59%, 16 of 27). The PenY1+BAO group, however, did not experience this observed phenotypic switch and displayed a distribution similar to that of the Naive animals with 42% (13 of 31 cells) of cells responding to the low metabolite mixture, an equal number of cells responding to the high solution (42%, 13 of 31), and a very low percentage of cells that responded to both low and high mixtures (16%, 5 of 31 cells; Fig. 3A–C).
Figure 3.
Inhibition of P2Y1 during BAO prevents the injury-induced alterations in chemosensitive muscle afferent prevalence. A, A decrease in the percentage of cells responsive to low metabolite concentrations is observed after ischemic injury (BAO or PenCON+BAO) compared with Naive mice, but this is not observed in the selective P2Y1-knockdown group. B, There are no significant alterations in the numbers of group III/IV muscle afferents that respond to high concentrations of metabolites in any condition. C, The observed increase in afferents that respond to both high and low concentrations of metabolites in the BAO and PenCON+BAO groups is also prevented by selective knockdown of P2Y1. χ-Squared analysis, *p < 0.05, **p < 0.01, ***p < 0.001 versus Naive and PenY1+BAO.
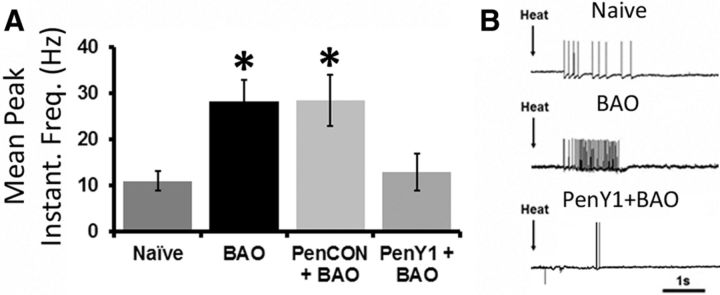
Similar to findings in our previous report (Ross et al., 2014), we also observed a significant increase in responsiveness to heat in the BAO (28.20 ± 4.72 Hz; n = 12) and PenCON+BAO (28.51 ± 5.58 Hz; n = 14) groups, when compared with the Naive (10.94 ± 2.06 Hz; n = 12, p < 0.04) and the PenY1+BAO groups (12.83 ± 4.00 Hz; n = 13, p < 0.05). The PenY1+BAO mice did not show any changes when compared with Naives (Fig. 4). We did not observe any significant difference in mechanical threshold (Naive, 6.21 ± 0.69 g, n = 30; BAO, 4.36 ± 0.53 g, n = 33; PenCON+BAO, 4.35 ± 0.76 g, n = 23; PenY1+BAO, 4.67 ± 0.79 g, n = 23) or mechanical responsiveness (Naive, 75.48 ± 13.90 Hz, n = 28; BAO, 77.55 ± 17.20 Hz, n = 24; PenCON+BAO, 65.36 ± 15.18 Hz, n = 22; PenY1+BAO, 47.93 ± 10.44 Hz, n = 20). Response to cold saline was also not different between any group (Naive, 18.71 ± 7.18 Hz, n = 13; BAO, 11.90 ± 2.96 Hz, n = 11; PenCON+BAO, 25.99 ± 10.45 Hz, n = 14; PenY1+BAO, 15.25 ± 5.38 Hz, n = 11).
Figure 4.
Knockdown of P2Y1 during BAO blocks the increased firing in response heat stimuli in muscle afferents. A, Increased mean peak instantaneous frequencies in response to heat stimulation of the muscles is observed in mice with BAO and mice with PenCON+BAO compared with Naives, but this is not observed in the PenY1+BAO group. B, Examples of responses to heat stimulation of the muscles during ex vivo recording in Naive, BAO, and PenY1+BAO mice. Kruskall–Wallis one-way ANOVA on ranks with Dunn's post hoc test, *p < 0.05 vs Naive and PenY1.
ASIC3 as a potential downstream effector of P2Y1 upregulation after ischemia
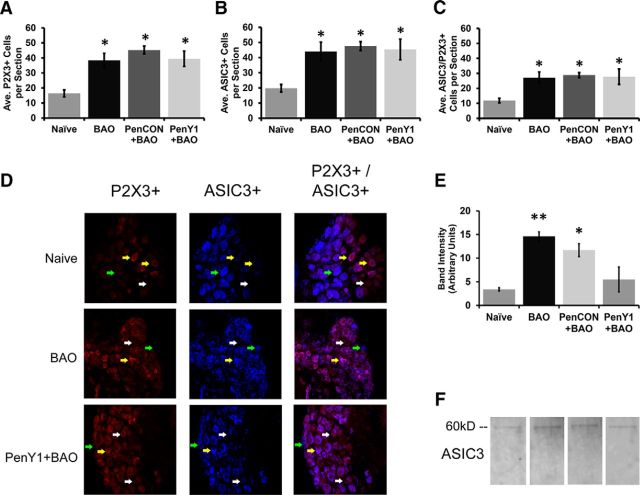
We then assessed the expression of ASIC3 and the ATP-sensing P2X3 channel with immunohistochemical staining of electrophysiologically characterized group III/IV afferents. A total of 51 cells were intracellularly labeled, processed, and recovered from all four conditions. Of these cells, 35 responded to ≥1 variety of chemical stimulation. Two of four cells that responded to high metabolite solutions (metabonociceptors) in the Naive group were positive for ASIC3. One of four stained cells that responded to low metabolite mixtures (metaboreceptors) in Naive animals was positive for ASIC3 and this cell was also found to be P2X3 immunoreactive (Fig. 5; Table 1). This latter result was the first time that we have observed a low-responding cell in the naive condition that was positive for this ion channel (Jankowski et al., 2013; Ross et al., 2014). However, it is important to note that this cell was also responsive to mechanical deformation of the muscles (in addition to heat and cold stimuli), and ASIC3 could thus have a more direct effect on this response property for this particular cell. In the BAO group, three of six metaboreceptors were ASIC3 positive while in the PenCON+BAO group, two of seven metaboreceptors were ASIC3 immunoreactive (combined Control BAO groups: 5 of 13), confirming previous studies (Ross et al., 2014). Surprisingly, inhibition of P2Y1 expression did not prevent metaboreceptors from positively labeling for ASIC3 after BAO (two of five). The metabonociceptors showed the same number of ASIC3-positive cells during ischemia as the Naive animals, and this expression was again not changed by the PenY1 injections during BAO. P2X3 was found in both metabolite-responsive subpopulations in all conditions (Fig. 5; Table 1).
Figure 5.
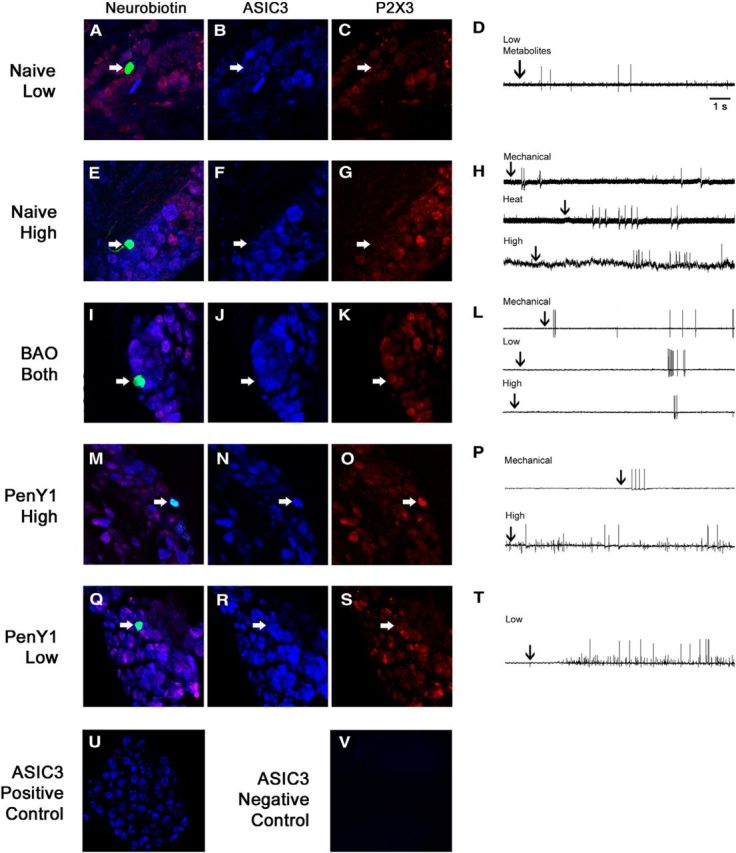
Immunostaining of different intracellularly filled and functionally characterized muscle afferents from ex vivo recording in Naive, BAO Control, and PenY1+BAO conditions. A–D, Example of a characterized muscle afferent from a Naive mouse that responded only to low metabolites. This cell was negative for ASIC3 and P2X3. E–H, Another cell from a Naive mouse that responded to mechanical and heat stimuli in addition to high metabolites was positive for ASIC3 but not P2X3. I–L, A cell from a mouse with BAO that responded not only to mechanical stimulation but also to low and high mixtures of metabolites was positive for both ASIC3 and P2X3. M–T, A cell from a P2Y1-knockdown animal after ischemic injury that responded to mechanical stimulation and also to the high mixture of metabolites was found to be positive for ASIC3 and P2X3 (M–P), while another cell from this condition that only responded to the low-metabolite mixture was also immunopositive for ASIC3 but not P2X3 (Q–T). U, V, Positive (U) and negative (V) immunohistochemistry controls show specific binding of ASIC3 antibody.
Table 1.
Immunostaining of ASIC3 and P2X3 in functionally characterized muscle afferents recovered from the ex vivo recording preparations
| Receptor type | Condition | ASIC3+ | P2X3+ | ASIC3+/P2X3+ |
|---|---|---|---|---|
| Metaboreceptors | Naive | 1/4 | 2/4 | 1/4 |
| Control BAO | 5/13 | 7/13 | 3/13 | |
| BAO | 3/6 | 4/6 | 2/6 | |
| PenCON + BAO | 2/7 | 3/7 | 1/7 | |
| PenY1 + BAO | 2/5 | 2/5 | 1/5 | |
| Metabonociceptors | Naive | 2/4 | 1/4 | 0/4 |
| Control BAO | 2/4 | 2/4 | 2/4 | |
| BAO | 1/3 | 1/3 | 1/3 | |
| PenCON + BAO | 1/1 | 1/1 | 1/1 | |
| PenY1 + BAO | 3/5 | 2/5 | 1/5 |
Quantification of the average number of positive cells per DRG section also revealed that there was a significant increase in the number of P2X3-positive cells in the DRGs of the BAO group (38.33 ± 5.38; n = 5, p < 0.05) and the PenCON+BAO mice (45.25 ± 2.59; n = 5, p < 0.001) compared with the Naive animals (16.33 ± 3.50; n = 3). The selective knockdown of P2Y1 did not prevent this increase in the number of P2X3-positive cells (PenY1+BAO, 39.44 ± 6.55; n = 3, p < 0.001). The total number of ASIC3-positive cells was also higher in the BAO group (44.00 ± 6.19; n = 5 p < 0.01) and the PenCON+BAO group (47.58 ± 2.90, n = 5, p < 0.001), compared with Naive mice (19.78 ± 2.48, n = 3). Contrary to what we expected, however, PenY1 siRNA-injected mice with BAO again showed a significant increase of ASIC3-positive cells compared with the Naive mice (PenY1+BAO, 45.33 ± 9.23; n = 3, p < 0.01; Fig. 6A–D) and were not found to be different from the BAO mice (p > 0.999) or PenCON+BAO mice (p > 0.999).
Figure 6.
Selective knockdown of P2Y1 in muscle afferents does not alter the de novo expression of P2X3 or ASIC3 in DRGs after BAO but does reduce the membrane expression of ASIC3. A–C, The average number of cells per section that were positive for P2X3 (A) and ASIC3 (B) and that coexpressed ASIC3 and P2X3 (C) was significantly increased during ischemia (BAO and PenCON+BAO) compared with Naive, but this was not reversed by the P2Y1 knockdown (PenY1+BAO). Kruskal–Wallis with Dunn's post hoc; *p < 0.02 vs Naive. D, Examples of immunostaining in DRGs from Naive, BAO, and PenY1+BAO conditions. E, The membrane protein fraction isolated from DRGs of ischemic mice (BAO and PenCON+BAO) shows increased expression of ASIC3 versus Naive mice; inhibition of P2Y1 partially blocked this increase in membrane-bound ASIC3 in DRGs during BAO (one-way ANOVA with Holm–Sidak post hoc; **p < 0.02 vs Naive and PenY1+BAO; *p < 0.03 vs Naive and p = 0.069 vs PenY1+BAO). F, Examples of DRG membrane fraction Western blots for ASIC3 in Naive, BAO, PenCON+BAO, or PenY1+BAO mice.
As our current and previous (Ross et al., 2014) data suggested that ASIC3 was likely an important player in mediating afferent chemosensitivity, we proceeded to evaluate whether the effect of P2Y1 was not due to changes in total protein expression but rather to membrane expression of specific channels. We thus isolated the membrane protein fraction of DRGs from the various experimental conditions. Western blotting analysis of membrane-bound proteins revealed that afferent-specific knockdown of P2Y1 was sufficient to reduce the increased membrane expression of ASIC3 (Fig. 6E,F).
Discussion
This report is the first to show that upregulation of a purinergic receptor (P2Y1) in muscle sensory neurons during peripheral ischemic insults drives the alterations in afferent heat sensitivity in addition to changes in phenotypic identity (Figs. 3, 4) to dually modulate pain-related behaviors and the cardiovascular response to exercise (Fig. 2). This study is also the first account of unilateral gene upregulation only in specified muscle afferents playing a role in systemic alterations in cardiovascular function while still retaining limb-specific effects on muscle nociception after injury. Mechanistically, the effect of P2Y1 upregulation in muscle afferents may be mediating these effects on sensory function and behavior by regulating membrane expression of ASIC3 (Figs. 5, 6).
BAO sensitizes muscle nociceptors through a P2Y1-mediated mechanism to dually regulate nociception and cardiovascular reflexes
We have shown that BAO increases spontaneous pain-related behaviors, induces mechanical hypersensitivity, and reduces grip strength. At the same time, group III/IV muscle afferents show an increased response to heat stimulation and a phenotypic switch from single-modality chemosensitivity to polymodal chemosensitivity, which appears to play an important role in the behavioral changes observed during ischemic injury. Data from these new cohorts are in agreement with our previous reports (Ross et al., 2014). Although these tests by themselves are not specific for muscle hypersensitivity and, under our conditions, ischemia could affect cutaneous tissue, collectively the behavioral tests being used, in conjunction with the recording analyses of muscle afferents specifically, provide insight into the ongoing deep-tissue sensitivity during ischemia in the affected limb.
ASICs (Benson et al., 1999; Immke and McCleskey, 2001; Naves and McCleskey, 2005; Yagi et al., 2006; Birdsong et al., 2010), P2X receptors (Dessem et al., 2010; Noma et al., 2013), and inflammatory cytokine receptors (Dina et al., 2008; Noma et al., 2013; Alvarez et al., 2014) have all been implicated in modulating nociceptive responses in muscle afferents. P2Y1 has been specifically linked to reduced heat responses (Molliver et al., 2011) and phenotypic switching in cutaneous afferents during inflammation of the skin (Jankowski et al., 2012a). In the current study, we observed a change in the firing of afferents to heat stimulation of the muscles during ischemia in addition to an altered prevalence of specific chemosensitive fibers (Figs. 3, 4). In a rat model of thrombus-induced ischemic pain, P2Y1 has been shown to play an important role in behavioral heat responsiveness (Kwon et al., 2014), and we showed here that heat hypersensitivity in afferents was prevented by selective knockdown of P2Y1 (Figs. 1–4).
P2Y1 knockdown also prevented or reduced all pain-related behavioral changes observed during ischemia (Fig. 2), yet we did not observe any changes in the mechanical sensitivity in muscle afferents. It is possible that the in vivo mechanical hypersensitivity (Fig. 2) observed during BAO may be due to a combination of de novo expression of ASIC3 and subsequent direct influence of P2Y1 on membrane expression of this channel in metaboreceptors (Figs. 5, 6) as this ion channel has been specifically implicated in mechanical hypersensitivity in other models of muscle pain (Sluka et al., 2003; Hori et al., 2010; Walder et al., 2010, 2011; Taguchi et al., 2015). Furthermore, as shown previously (Light et al., 2008; Jankowski et al., 2013), there are two populations of chemosensitive afferents innervating muscles. While low responders probably fulfill a metaboreceptor function, high responders seem to work as metabonociceptors. Here we observed a population of muscle afferents that normally responds to a relatively innocuous chemical stimulus produced by contracting muscles responding to a more noxious environment (Figs. 4, 5). This switch in subtype prevalence was prevented by selective knockdown of P2Y1, suggesting that P2Y1 plays an important roles in not only sensing the environment around the nerve endings but also in driving the sensitization and phenotypic identity of muscle afferents, likely leading to the pain-related behaviors observed (Kumazawa and Mizumura, 1977; Kaufman et al., 1984, 1988; Rybicki et al., 1985; Thimm and Baum, 1987; Sinoway et al., 1993; Mense, 2009).
Group III/IV fibers can thus function as nociceptors or metaboreceptors, but the latter function has been classically associated with the afferent arm of the EPR (Kaufman, 2012). There are multiple studies in which arterial occlusion with a 10–20% blood flow reduction increased EPRs (Tsuchimochi et al., 2010; Stone et al., 2015). In patients with peripheral artery disease, for example, the cardiovascular response to mild exercise was increased when compared with healthy human subjects (Baccelli et al., 1999). An interesting result from our study and others (Tsuchimochi et al., 2010) was the finding that a full occlusion of a major artery does not induce any detectable cardiovascular changes by itself. However, combination of both an ischemic injury and moderate exercise produced a significant change in systemic MBP, which was ablated by selective unilateral inhibition of P2Y1 upregulation in median and ulnar muscle afferents (Figs. 1, 2). Together with results from pain-related behavioral assessments, this not only implies that P2Y1 plays an important role in the generation of the EPR during ischemia, but also that the afferents involved in the dysregulation of the EPR during ischemia are the same population of muscle-sensory neurons that function as nociceptors to regulate pain-like behaviors in the same ischemic setting. Although we did not detect a significant change in the heart rate in any of our experimental conditions as was shown in other preclinical (Hayes et al., 2007; Tsuchimochi et al., 2010) and clinical (Baccelli et al., 1999; Muller et al., 2015) reports, this is likely due to faster recuperation of this parameter between the end of forced exercise and the initiation of cardiovascular monitoring in addition to our low-intensity exercise protocol.
Role of ASIC3 after ischemic injury
ASIC3 has been linked to mechanosensation as well as responsiveness to lactate and protons (Immke and McCleskey, 2001; Naves and McCleskey, 2005; Light et al., 2008; Birdsong et al., 2010). Although we recognize the limitation of not being able to verify antibody specificity in a knock-out mouse, controls presented here (Fig. 5) and in previous reports by our group (Ross et al., 2014) and others (Liu et al., 2010; Xing et al., 2012) have shown that after ischemia, ASIC3 is highly upregulated in the DRGs over naive levels (Molliver et al., 2005; Jankowski et al., 2013). It is interesting that most cells that function as metaboreceptors tend to be ASIC3 negative (Jankowski et al., 2013; Ross et al., 2014; this report). However, in this study, we found for the first time that one cell that responded to the innocuous mixture of metabolites was ASIC3 positive. In any case, this is a very rare occurrence and it is possible that this cell may serve a purpose different from metaboreception as it was also mechanically sensitive. Nevertheless, we have confirmed in this report that cells responding to both metabolite mixtures appear to increase (Fig. 4) and contain ASIC3 (Fig. 5) after ischemia. Since P2Y1 inhibition was effective in reverting both the phenotypic switch and the heat hypersensitivity observed in muscle afferents during ischemia, we hypothesized that this could be due to a P2Y1-mediated increase in the expression of ASIC3. However, we found that selective knockdown of P2Y1 was unable to prevent the observed increase in the number of cells positive for ASIC3 in the DRGs. Since P2Y1 is a G-protein-coupled receptor, there are a variety of different mechanisms that could be involved in the modulation of ASIC3. We thus analyzed the membrane expression of ASIC3 in the DRGs from our groups to determine whether P2Y1 upregulation may be modulating insertion of this channel into the cell membrane during ischemia. We indeed found that the membrane fraction of ASIC3 is increased during ischemic injury in DRGs and this increase was altered by P2Y1 inhibition during BAO (Fig. 6). Thus ASIC3 likely plays an important part in the observed phenotypic switching in muscle afferents during BAO and this process is at least in part regulated by P2Y1. However, the mechanism of how this purinergic receptor interacts with ASIC3 is not clear and warrants further investigation.
Significance
We have described for the first time a mechanism by which muscle afferents regulate two distinct biological processes simultaneously (nociception and EPRs) during conditions of reduced blood flow and oxygenation of the periphery. The upregulation of the ADP-sensitive P2Y1 receptor in the affected muscle afferents controls heat responsiveness and chemosensitivity to regulate these behavioral responses by possibly regulating the membrane expression of ASIC3. Although it is difficult to determine whether nociception precedes alterations in EPRs or vice versa, the dual regulation of these phenomena through the group III/IV muscle afferents may explain the concurrent expression of altered cardiovascular function and peripheral perfusion anomalies, in addition to muscle pain, in patients with ischemic conditions like PVD or CRPS (Veldman et al., 1993; Jänig and Baron, 2003; Hodges et al., 2006; Bartur et al., 2014). Results may thus provide a unique direction of therapeutic development for patients with ischemic myalgia and altered cardiovascular function.
Footnotes
This work was supported by grants from the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01AR064551-01A1; M.P.J.), the Rita Allen Foundation/American Pain Society (M.P.J.), and the International Association for the Study of Pain Early Career Program (M.P.J.). We thank Dr. Zaza Khuchua for use of the treadmill system and Dr. Robert Hinton for use of the tail-cuff system.
The authors declare no competing financial interests.
References
- Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol. 1937;89:372–383. doi: 10.1113/jphysiol.1937.sp003485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez P, Green PG, Levine JD. Role for monocyte chemoattractant protein-1 in the induction of chronic muscle pain in the rat. Pain. 2014;155:1161–1167. doi: 10.1016/j.pain.2014.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol. 2010;109:966–976. doi: 10.1152/japplphysiol.00462.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baccelli G, Reggiani P, Mattioli A, Corbellini E, Garducci S, Catalano M. The exercise pressor reflex and changes in radial arterial pressure and heart rate during walking in patients with arteriosclerosis obliterans. Angiology. 1999;50:361–374. doi: 10.1177/000331979905000502. [DOI] [PubMed] [Google Scholar]
- Bartur G, Vatine JJ, Raphaely-Beer N, Peleg S, Katz-Leurer M. Heart rate autonomic regulation system at rest and during paced breathing among patients with CRPS as compared to age-matched healthy controls. Pain Med. 2014;15:1569–1574. doi: 10.1111/pme.12449. [DOI] [PubMed] [Google Scholar]
- Benson CJ, Eckert SP, McCleskey EW. Acid-evoked currents in cardiac sensory neurons: a possible mediator of myocardial ischemic sensation. Circ Res. 1999;84:921–928. doi: 10.1161/01.res.84.8.921. [DOI] [PubMed] [Google Scholar]
- Billat VL, Mouisel E, Roblot N, Melki J. Inter- and intrastrain variation in mouse critical running speed. J Appl Physiol. 2005;98:1258–1263. doi: 10.1152/japplphysiol.00991.2004. [DOI] [PubMed] [Google Scholar]
- Birdsong WT, Fierro L, Williams FG, Spelta V, Naves LA, Knowles M, Marsh-Haffner J, Adelman JP, Almers W, Elde RP, McCleskey EW. Sensing muscle ischemia: coincident detection of acid and ATP via interplay of two ion channels. Neuron. 2010;68:739–749. doi: 10.1016/j.neuron.2010.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bockman EL. Blood flow and oxygen consumption in active soleus and gracilis muscles in cats. Am J Physiol Heart Circ Physiol. 1983;244:H546–H551. doi: 10.1152/ajpheart.1983.244.4.H546. [DOI] [PubMed] [Google Scholar]
- Bockman EL, McKenzie JE. Tissue adenosine content in active soleus and gracilis muscles of cats. Am J Physiol Heart Circ Physiol. 1983;244:H552–H559. doi: 10.1152/ajpheart.1983.244.4.H552. [DOI] [PubMed] [Google Scholar]
- Christianson JA, Mcllwrath SL, Koerber HR, Davis BM. Transient receptor potential vanilloid 1-immunopositive neurons in the mouse are more prevalent within colon affeents compared to skin and muscle afferents. Neuroscience. 2006;140:247–257. doi: 10.1016/j.neuroscience.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Coderre TJ, Bennett GJ. A hypothesis for the cause of complex regional pain syndrome-type I (reflex sympathetic dystrophy): pain due to deep-tissue microvascular pathology. Pain Med. 2010;11:1224–1238. doi: 10.1111/j.1526-4637.2010.00911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson TJ, Harel S, Arboleda VA, Prunell GF, Shelanski ML, Greene LA, Troy CM. Highly efficient small interfering RNA delivery to primary mammalian neurons induces microRNA-like effects before mRNA degradation. J Neurosci. 2004;24:10040–10046. doi: 10.1523/JNEUROSCI.3643-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies MG. Critical limb ischemia: epidemiology. Methodist Debakey Cardiovasc J. 2012;8:10–14. doi: 10.14797/mdcj-8-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dessem D, Ambalavanar R, Evancho M, Moutanni A, Yallampalli C, Bai G. Eccentric muscle contraction and stretching evoke mechanical hyperalgesia and modulate CGRP and P2X3 expression in a functionally relevant manner. Pain. 2010;149:284–295. doi: 10.1016/j.pain.2010.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dina OA, Green PG, Levine JD. Role of interleukin-6 in chronic muscle hyperalgesic priming. Neuroscience. 2008;152:521–525. doi: 10.1016/j.neuroscience.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Li JD, Sinoway LI, Li J. Effect of muscle interstitial pH on P2X and TRPV1 receptor-mediated pressor response. J Appl Physiol. 2007;102:2288–2293. doi: 10.1152/japplphysiol.00161.2007. [DOI] [PubMed] [Google Scholar]
- González-Alonso J, Quistorff B, Krustrup P, Bangsbo J, Saltin B. Heat production in human skeletal muscle at the onset of intense dynamic exercise. J Physiol. 2000;524:603–615. doi: 10.1111/j.1469-7793.2000.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna RL, Kaufman MP. Role played by purinergic receptors on muscle afferents in evoking the exercise pressor reflex. J Appl Physiol. 2003;94:1437–1445. doi: 10.1152/japplphysiol.01011.2002. [DOI] [PubMed] [Google Scholar]
- Hayes SG, Kindig AE, Kaufman MP. Blockade of acid sensing ion channels attenuates the exercise pressor reflex in cats. J Physiol. 2007;581:1271–1282. doi: 10.1113/jphysiol.2007.129197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes SG, McCord JL, Kaufman MP. Role played by P2X and P2Y receptors in evoking the muscle chemoreflex. J Appl Physiol. 2008a;104:538–541. doi: 10.1152/japplphysiol.00929.2007. [DOI] [PubMed] [Google Scholar]
- Hayes SG, McCord JL, Rainier J, Liu Z, Kaufman MP. Role played by acid-sensitive ion channels in evoking the exercise pressor reflex. Am J Physiol Heart Circ Physiol. 2008b;295:H1720–H1725. doi: 10.1152/ajpheart.00623.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges LD, Sandercock GR, Das SK, Brodie DA. Cardiac pumping capability in patients with peripheral vascular disease. Clin Physiol Funct Imaging. 2006;26:185–190. doi: 10.1111/j.1475-097X.2006.00673.x. [DOI] [PubMed] [Google Scholar]
- Hori K, Ozaki N, Suzuki S, Sugiura Y. Upregulations of P2X(3) and ASIC3 involve in hyperalgesia induced by cisplatin administration in rats. Pain. 2010;149:393–405. doi: 10.1016/j.pain.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Immke DC, McCleskey EW. ASIC3: a lactic acid sensor for cardiac pain. ScientificWorldJournal. 2001;1:510–512. doi: 10.1100/tsw.2001.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jänig W, Baron R. Complex regional pain syndrome: mystery explained? Lancet Neurol. 2003;2:687–697. doi: 10.1016/S1474-4422(03)00557-X. [DOI] [PubMed] [Google Scholar]
- Jankowski MP, Cornuet PK, McIlwrath S, Koerber HR, Albers KM. SRY-box containing gene 11 (Sox11) transcription factor is required for neuron survival and neurite growth. Neuroscience. 2006;143:501–514. doi: 10.1016/j.neuroscience.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski MP, McIlwrath SL, Jing X, Cornuet PK, Salerno KM, Koerber HR, Albers KM. Sox11 transcription factor modulates peripheral nerve regeneration in adult mice. Brain Res. 2009;1256:43–54. doi: 10.1016/j.brainres.2008.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski MP, Rau KK, Soneji DJ, Anderson CE, Koerber HR. Enhanced artemin/GFRα3 levels regulate mechanically insensitive, heat-sensitive C-fiber recruitment after axotomy and regeneration. J Neurosci. 2010;30:16272–16283. doi: 10.1523/JNEUROSCI.2195-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski MP, Rau KK, Soneji DJ, Ekmann KM, Anderson CE, Molliver DC, Koerber HR. Purinergic receptor P2Y1 regulates polymodal C-fiber thermal thresholds and sensory neuron phenotypic switching during peripheral inflammation. Pain. 2012a;153:410–419. doi: 10.1016/j.pain.2011.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski MP, Soneji DJ, Ekmann KM, Anderson CE, Koerber HR. Dynamic changes in heat transducing channel TRPV1 expression regulate mechanically insensitive, heat sensitive C-fiber recruitment after axotomy and regeneration. J Neurosci. 2012b;32:17869–17873. doi: 10.1523/JNEUROSCI.3148-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowski MP, Rau KK, Ekmann KM, Anderson CE, Koerber HR. Comprehensive phenotyping of group III and IV muscle afferents in mouse. J Neurophysiol. 2013;109:2374–2381. doi: 10.1152/jn.01067.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MP. The exercise pressor reflex in animals. Exp Physiol. 2012;97:51–58. doi: 10.1113/expphysiol.2011.057539. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Rybicki KJ. Discharge properties of group III and IV muscle afferents: their responses to mechanical and metabolic stimuli. Circ Res. 1987;61:I60–I65. [PubMed] [Google Scholar]
- Kaufman MP, Longhurst JC, Rybicki KJ, Wallach JH, Mitchell JH. Effects of static muscular contraction on impulse activity of groups III and IV afferents in cats. J Appl Physiol Respir Environ Exerc Physiol. 1983;55:105–112. doi: 10.1152/jappl.1983.55.1.105. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Waldrop TG, Rybicki KJ, Ordway GA, Mitchell JH. Effects of static and rhythmic twitch contractions on the discharge of group III and IV muscle afferents. Cardiovasc Res. 1984;18:663–668. doi: 10.1093/cvr/18.11.663. [DOI] [PubMed] [Google Scholar]
- Kaufman MP, Rotto DM, Rybicki KJ. Pressor reflex response to static muscular contraction: its afferent arm and possible neurotransmitters. Am J Cardiol. 1988;62:58E–62E. doi: 10.1016/S0002-9149(88)80013-4. [DOI] [PubMed] [Google Scholar]
- Kemi OJ, Loennechen JP, Wisløff U, Ellingsen Ø. Intensity-controlled treadmill running in mice: cardiac and skeletal muscle hypertrophy. J Appl Physiol. 2002;93:1301–1309. doi: 10.1152/japplphysiol.00231.2002. [DOI] [PubMed] [Google Scholar]
- Kniffki KD, Mense S, Schmidt RF. Responses of group IV afferent units from skeletal muscle to stretch, contraction and chemical stimulation. Exp Brain Res. 1978;31:511–522. doi: 10.1007/BF00239809. [DOI] [PubMed] [Google Scholar]
- Kumazawa T, Mizumura K. Thin-fibre receptors responding to mechanical, chemical, and thermal stimulation in the skeletal muscle of the dog. J Physiol. 1977;273:179–194. doi: 10.1113/jphysiol.1977.sp012088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon SG, Roh DH, Yoon SY, Moon JY, Choi SR, Choi HS, Kang SY, Han HJ, Beitz AJ, Oh SB, Lee JH. Acid evoked thermal hyperalgesia involves peripheral P2Y1 receptor mediated TRPV1 phosphorylation in a rodent model of thrombus induced ischemic pain. Mol Pain. 2014;10:2. doi: 10.1186/1744-8069-10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Light AR, Hughen RW, Zhang J, Rainier J, Liu Z, Lee J. Dorsal root ganglion neurons innervating skeletal muscle respond to physiological combinations of protons, ATP, and lactate mediated by ASIC, P2X, and TRPV1. J Neurophysiol. 2008;100:1184–1201. doi: 10.1152/jn.01344.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Gao Z, Li J. Femoral artery occlusion increases expression of ASIC3 in dorsal root ganglion neurons. Am J Physiol Heart Circ Physiol. 2010;299:H1357–H1364. doi: 10.1152/ajpheart.00612.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean DA, LaNoue KF, Gray KS, Sinoway LI. Effects of hindlimb contraction on pressor and muscle interstitial metabolite responses in the cat. J Appl Physiol. 1998;85:1583–1592. doi: 10.1152/jappl.1998.85.4.1583. [DOI] [PubMed] [Google Scholar]
- McCloskey DI, Mitchell JH. Reflex cardiovascular and respiratory responses originating in exercising muscle. J Physiol. 1972;224:173–186. doi: 10.1113/jphysiol.1972.sp009887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott MM, Liu K, Greenland P, Guralnik JM, Criqui MH, Chan C, Pearce WH, Schneider JR, Ferrucci L, Celic L, Taylor LM, Vonesh E, Martin GJ, Clark E. Functional decline in peripheral arterial disease: associations with the ankle brachial index and leg symptoms. JAMA. 2004;292:453–461. doi: 10.1001/jama.292.4.453. [DOI] [PubMed] [Google Scholar]
- McMahon SE, McWilliam PN. Changes in R–R interval at the start of muscle contraction in the decerebrate cat. J Physiol. 1992;447:549–562. doi: 10.1113/jphysiol.1992.sp019017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mense S. The pathogenesis of muscle pain. Curr Pain Headache Rep. 2003;7:419–425. doi: 10.1007/s11916-003-0057-6. [DOI] [PubMed] [Google Scholar]
- Mense S. Algesic agents exciting muscle nociceptors. Exp Brain Res. 2009;196:89–100. doi: 10.1007/s00221-008-1674-4. [DOI] [PubMed] [Google Scholar]
- Meru AV, Mittra S, Thyagarajan B, Chugh A. Intermittent claudication: an overview. Atherosclerosis. 2006;187:221–237. doi: 10.1016/j.atherosclerosis.2005.11.027. [DOI] [PubMed] [Google Scholar]
- Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol. 1983;45:229–242. doi: 10.1146/annurev.ph.45.030183.001305. [DOI] [PubMed] [Google Scholar]
- Molliver DC, Immke DC, Fierro L, Paré M, Rice FL, McCleskey EW. ASIC3, an acid-sensing ion channel, is expressed in metaboreceptive sensory neurons. Mol Pain. 2005;1:35. doi: 10.1186/1744-8069-1-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molliver DC, Rau KK, McIlwrath SL, Jankowski MP, Koerber HR. The ADP receptor P2Y1 is necessary for normal thermal sensitivity in cutaneous polymodal nociceptors. Mol Pain. 2011;7:13. doi: 10.1186/1744-8069-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller MD, Drew RC, Ross AJ, Blaha CA, Cauffman AE, Kaufman MP, Sinoway LI. Inhibition of cyclooxygenase attenuates the blood pressure response to plantar flexion exercise in peripheral arterial disease. Am J Physiol Heart Circ Physiol. 2015;309:H523–H528. doi: 10.1152/ajpheart.00267.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura A, Yoshida K, Ueda H, Takeda S, Ikeda S. Up-regulation of mitogen activated protein kinases in mdx skeletal muscle following chronic treadmill exercise. Biochim Biophys Acta. 2005;1740:326–331. doi: 10.1016/j.bbadis.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Naves LA, McCleskey EW. An acid-sensing ion channel that detects ischemic pain. Braz J Med Biol Res. 2005;38:1561–1569. doi: 10.1590/s0100-879x2005001100001. [DOI] [PubMed] [Google Scholar]
- Noma N, Shinoda M, Honda K, Kiyomoto M, Dezawa K, Nakaya Y, Komiyama O, Imamura Y, Iwata K. Interaction of IL-1β and P2X(3) receptor in pathologic masseter muscle pain. J Dent Res. 2013;92:456–460. doi: 10.1177/0022034513483770. [DOI] [PubMed] [Google Scholar]
- Norgren L, Hiatt WR, Dormandy JA, Nehler MR, Harris KA, Fowkes FG, Rutherford RB. Inter-society consensus for the management of peripheral arterial disease (TASC II) Eur J Vasc Endovasc Surg. 2006;33(Suppl 1):S1–S75. doi: 10.1016/j.ejvs.2006.09.024. [DOI] [PubMed] [Google Scholar]
- North RA. P2X3 receptors and peripheral pain mechanisms. J Physiol. 2004;554:301–308. doi: 10.1113/jphysiol.2003.048587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouriel K. Peripheral arterial disease. Lancet. 2001;358:1257–1264. doi: 10.1016/S0140-6736(01)06351-6. [DOI] [PubMed] [Google Scholar]
- Pollak KA, Swenson JD, Vanhaitsma TA, Hughen RW, Jo D, White AT, Light KC, Schweinhardt P, Amann M, Light AR. Exogenously applied muscle metabolites synergistically evoke sensations of muscle fatigue and pain in human subjects. Exp Physiol. 2014;99:368–380. doi: 10.1113/expphysiol.2013.075812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queme F, Taguchi T, Mizumura K, Graven-Nielsen T. Muscular heat and mechanical pain sensitivity after lengthening contractions in humans and animals. J Pain. 2013;14:1426–1436. doi: 10.1111/pme.12166. [DOI] [PubMed] [Google Scholar]
- Rocco AB, Levalley JC, Eldridge JA, Marsh SA, Rodgers BD. A novel protocol for assessing exercise performance and dystropathophysiology in the mdx mouse. Muscle Nerve. 2014;50:541–548. doi: 10.1002/mus.24184. [DOI] [PubMed] [Google Scholar]
- Ross JL, Queme LF, Shank AT, Hudgins RC, Jankowski MP. Sensitization of group III and IV muscle afferents in the mouse after ischemia and reperfusion injury. J Pain. 2014;15:1257–1270. doi: 10.1016/j.jpain.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybicki KJ, Waldrop TG, Kaufman MP. Increasing gracilis muscle interstitial potassium concentrations stimulate group III and IV afferents. J Appl Physiol. 1985;58:936–941. doi: 10.1152/jappl.1985.58.3.936. [DOI] [PubMed] [Google Scholar]
- Sinoway LI, Hill JM, Pickar JG, Kaufman MP. Effects of contraction and lactic acid on the discharge of group III muscle afferents in cats. J Neurophysiol. 1993;69:1053–1059. doi: 10.1152/jn.1993.69.4.1053. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Price MP, Breese NM, Stucky CL, Wemmie JA, Welsh MJ. Chronic hyperalgesia induced by repeated acid injections in muscle is abolished by the loss of ASIC3, but not ASIC1. Pain. 2003;106:229–239. doi: 10.1016/S0304-3959(03)00269-0. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Radhakrishnan R, Benson CJ, Eshcol JO, Price MP, Babinski K, Audette KM, Yeomans DC, Wilson SP. ASIC3 in muscle mediates mechanical, but not heat, hyperalgesia associated with muscle inflammation. Pain. 2007;129:102–112. doi: 10.1016/j.pain.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone AJ, Copp SW, McCord JL, Kaufman MP. Femoral artery ligation increases the responses of thin fiber muscle afferents to contraction. J Neurophysiol. 2015;113:3961–3966. doi: 10.1152/jn.00288.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi T, Sato J, Mizumura K. Augmented mechanical response of muscle thin-fiber sensory receptors recorded from rat muscle-nerve preparations in vitro after eccentric contraction. J Neurophysiol. 2005;94:2822–2831. doi: 10.1152/jn.00470.2005. [DOI] [PubMed] [Google Scholar]
- Taguchi T, Katanosaka K, Yasui M, Hayashi K, Yamashita M, Wakatsuki K, Kiyama H, Yamanaka A, Mizumura K. Peripheral and spinal mechanisms of nociception in a rat reserpine-induced pain model. Pain. 2015;156:415–427. doi: 10.1097/01.j.pain.0000460334.49525.5e. [DOI] [PubMed] [Google Scholar]
- Thabet M, Miki T, Seino S, Renaud JM. Treadmill running causes significant fiber damage in skeletal muscle of KATP channel-deficient mice. Physiol Genomics. 2005;22:204–212. doi: 10.1152/physiolgenomics.00064.2005. [DOI] [PubMed] [Google Scholar]
- Thimm F, Baum K. Response of chemosensitive nerve fibers of group III and IV to metabolic changes in rat muscles. Pflugers Arch. 1987;410:143–152. doi: 10.1007/BF00581907. [DOI] [PubMed] [Google Scholar]
- Tsuchimochi H, McCord JL, Hayes SG, Koba S, Kaufman MP. Chronic femoral artery occlusion augments exercise pressor reflex in decerebrated rats. Am J Physiol Heart Circ Physiol. 2010;299:H106–H113. doi: 10.1152/ajpheart.00141.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchimochi H, Yamauchi K, McCord JL, Kaufman MP. Blockade of acid sensing ion channels attenuates the augmented exercise pressor reflex in rats with chronic femoral artery occlusion. J Physiol. 2011;589:6173–6189. doi: 10.1113/jphysiol.2011.217851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldman PH, Reynen HM, Arntz IE, Goris RJ. Signs and symptoms of reflex sympathetic dystrophy: prospective study of 829 patients. Lancet. 1993;342:1012–1016. doi: 10.1016/0140-6736(93)92877-V. [DOI] [PubMed] [Google Scholar]
- Walder RY, Rasmussen LA, Rainier JD, Light AR, Wemmie JA, Sluka KA. ASIC1 and ASIC3 play different roles in the development of hyperalgesia after inflammatory muscle injury. J Pain. 2010;11:210–218. doi: 10.1016/j.jpain.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walder RY, Gautam M, Wilson SP, Benson CJ, Sluka KA. Selective targeting of ASIC3 using artificial miRNAs inhibits primary and secondary hyperalgesia after muscle inflammation. Pain. 2011;152:2348–2356. doi: 10.1016/j.pain.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xing J, Lu J, Li J. Acid-sensing ion channel subtype 3 function and immunolabelling increases in skeletal muscle sensory neurons following femoral artery occlusion. J Physiol. 2012;590:1261–1272. doi: 10.1113/jphysiol.2011.221788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagi J, Wenk HN, Naves LA, McCleskey EW. Sustained currents through ASIC3 ion channels at the modest pH changes that occur during myocardial ischemia. Circ Res. 2006;99:501–509. doi: 10.1161/01.RES.0000238388.79295.4c. [DOI] [PubMed] [Google Scholar]