Abstract
Background
Maspin is known to be a tumor suppressor protein and its prognostic significance in patients with several types of cancer has been reported. To date, however, no study has focused on the association between maspin expression and the prognosis of patients with adenocarcinoma of the uterine cervix. We explored the prognostic value of maspin expression with particular reference to its subcellular localization in patients with adenocarcinoma of the uterine cervix.
Methods
Paraffin-embedded tissue samples from 46 patients diagnosed as adenocarcinoma of the uterine cervix were immunohistochemically analyzed using an antibody for maspin. The patients were followed up for 3 to 165 months (median: 64.2 months) and the prognostic value was evaluated by the log-rank test and the Cox regression hazard model.
Results
A sample was considered maspin-positive if maspin was expressed in only the cytoplasm; 69.6% (32 cases) of the specimens were maspin-positive, and there was significant correlation between positivity and recurrence (P = 0.022). Maspin-positive patients had both shorter disease free survival and shorter overall survival by the log-rank test (P = 0.023, P = 0.043, respectively). By Cox’s multivariate analysis, the International Federation of Obstetrics and Gynecology (FIGO) status was the only independent prognostic factor for disease free survival and overall survival in patients with adenocarcinoma of the uterine cervix.
Conclusion
This is the first report to reveal an association between cytoplasmic maspin expression and the prognosis of patients with adenocarcinoma of the uterine cervix. Although further studies with a larger series of patients and a longer follow up period are necessary, the present results suggest that cytoplasmic maspin expression could be an indicator of unfavorable prognosis in patients with adenocarcinoma of the uterine cervix.
Keywords: adenocarcinoma, immunohistochemistry, maspin, uterine cervix
Adenocarcinoma of the uterine cervix is a relatively uncommon histological subtype of uterine cancer comprising 10–20% of all cervical cancer cases.1 Although squamous cell carcinoma (SCC) of the uterine cervix has declined markedly in developed countries, both the relative and absolute incidences of adenocarcinoma of the uterine cervix have increased over the past four decades.1, 2 The majority of reports have shown that adenocarcinoma of the uterine cervix carries a worse prognosis than SCC in 5-year overall survival rates, and that its survival rates are significantly lower than SCC’s in each tumor stage.3–5 Differences have also been reported between SCC and adenocarcinoma of the uterine cervix in the pattern of dissemination, recurrences and response to chemotherapy.3 The important prognostic factors have been considered to be the International Federation of Obstetrics and Gynecology (FIGO) stage, tumor size and the occurrence of lymph nodes metastasis. However, the search for another predictive factor for adenocarcinoma of the uterine cervix may be important for deciding on a therapeutic strategy. Maspin, a member of the serine protease inhibitor (serpin) family, was originally detected in normal breast epithelial cells.6 Maspin expression was previously shown to be down-regulated as breast epithelial cells progressed to a higher grade of breast tumor and to be lost in metastasis and it has been shown to have tumor suppressor activity attributable to the inhibition of breast cancer cell motility, invasion and metastasis.7 Although the precise biochemical pathways leading to these biological endpoints remains to be elucidated, many reports describe the association between maspin expression and clinicopathological factors in many types of cancer.8 We have also reported on associations between maspin expression and clinicopathological factors in several types of cancer such as breast cancer,9–11 colorectal cancer,12 endometrioid endometrial carcinoma,13 ovarian mucinous borderline tumor,14 lung adenocarcinoma and soft tissue sarcoma;15, 16 and we have suggested that cytoplasmic maspin expression may be an indicator of poor prognosis. To our knowledge, this is the first report to investigate the association between maspin expression and the prognosis of patients with adenocarcinoma of the uterine cervix. The aim of this study was to clarify whether cytoplasmic expression of maspin could be a predictive factor for patients with adenocarcinoma of the uterine cervix as well as other types of cancers.
MATERIALS AND METHODS
Tissue specimens
This study enrolled a total of 46 consecutive patients with adenocarcinoma of the uterine cervix diagnosed at the Department of Pathology Tottori University Hospital between 1999 and 2010. The median follow up period was 64.2 months (range: 3–165 months). Since a considerable number of patients had received neoadjuvant chemotherapy, radiotherapy or both before tumor resection, we selected tissues mainly from biopsy specimens to prevent therapy-related changes from disturbing histological or immunohistochemical assessment. The patients’ clinicopathological data were retrieved from the hospital’s computerized medical records system. Each histological diagnosis was re-evaluated and established according to the World Health Organization classification17 by experienced pathologists (K.N. and Y.U.). Most patients underwent surgical treatments including total hysterectomy with lymph node dissection (35 cases), radical trachelectomy (3 cases), and simple hysterectomy (1 case); the inoperable patients received either chemoradiotherapy (5 cases) or chemotherapy only (2 cases). Written informed consent was obtained from all patients, and the study was performed with the approval of the Ethics Committee of the Faculty of Medicine, Tottori University (No. 1558).
Immunohistochemical procedures
All specimens were fixed in 10% neutrally buffered formalin and embedded in paraffin. A representative block from each case containing an adequate amount of tumor tissue was selected. After the sections (4 μm thick) were deparaffinized and endogenous peroxidase activity was blocked, they were pretreated in citrate buffer (0.01 M, pH 6.0) using a microwave oven (RE-DD6-S; Sharp, Osaka, Japan) for 20 minutes. After cooling to room temperature, the sections were incubated at 4 ˚C overnight with monoclonal anti-human maspin antibody (clone EAW24; diluted 1:50; Leica Biosystems, Newcastle, UK). The sections were incubated with biotinylated anti-mouse IgG antibody (BA-2000; diluted 1:150; Vector Laboratories, Burlingame, VT) for 20 minutes, followed by streptavidin biotinylated-HRP conjugate (diluted 1:150; Invitrogen, Camarillo, CA) for 20 minutes. The sections were then incubated with 3,3’-Diaminobenzidine (DAB) solution (Liquid DAB+ Substrate, Imidazole-HCl buffer, pH 7.5, containing hydrogen peroxide and an anti-microbial agent; Dako, Glostrup, Denmark) for 4 minutes, and finally were counterstained with hematoxylin.
Immunohistochemical evaluation
To evaluate maspin expression status, the sections were divided into two groups: maspin-positive and -negative. Specimens were considered maspin-positive if staining occurred only in the cytoplasm with a staining area of > 10% of the whole tumor for each section irrespective of staining intensity. The remaining specimens were considered maspin-negative. These evaluations were performed independently by two authors (K.N. and Y.U.), who were blinded to the patients’ outcome data. Normal squamous epithelium served as an internal positive control if it was present in the same specimen. Myoepithelial cells in normal breast tissue specimens were also used as positive controls.
Statistical analysis
All statistical analyses were performed using the Statistical Package for Social Sciences version 21 (IBM SPSS Statistics; IBM, Armonk, NY). The association between maspin status and clinicopathological parameters was evaluated by non-parametric tests. The chi-square test was used when there were two categorical variables of interest and the Kruskal-Wallis test when there were three or more variables. Survival curves were calculated using the Kaplan-Meier method and compared by log-rank test. The Cox proportional hazard regression model was used to analyze the independence of each parameter of disease free survival (DFS) or overall survival (OS).
RESULTS
Clinicopathological features
The clinicopathological profiles of the 46 patients are summarized in Table 1. The age distribution was 22–74 years (median: 50.0 years). Thirty-three cases were at FIGO Stage I/II and 13 cases were at Stage III/IV. In 23 cases (50%) the tumor was larger than 40mm, and in 10 cases (21.7%) lymph node metastasis was recognized either at operation or at first radiographic examination. Nineteen cases (41.3%) had recurrent diseases: 6 distal metastasis (lung: 4, brain: 2, liver: 1, bone: 1), 11 local recurrences and 2 peritoneal disseminations.
Table 1.
Clinicopathologic characteristics of patients with adenocarcinoma of the uterine cervix
| n | % | ||||
| Age (years old) | |||||
| <30 | 1 | 2.2 | |||
| 30–39 | 8 | 17.4 | |||
| 40–49 | 13 | 28.3 | |||
| 50–59 | 14 | 30.4 | |||
| 60–69 | 8 | 17.4 | |||
| ≥ 70 | 2 | 4.3 | |||
| Recurrence | |||||
| None | 27 | 58.7 | |||
| Local recurrence | 11 | 17.4 | |||
| Distant metastasis | 6 | 19.6 | |||
| Peritoneal dissemination | 2 | 4.3 | |||
| FIGO stage | |||||
| IB | 22 | 47.8 | |||
| IIA | 2 | 4.3 | |||
| IIB | 9 | 19.6 | |||
| IIIA | 1 | 2.2 | |||
| IIIB | 7 | 15.2 | |||
| IV | 5 | 10.9 | |||
| Tumor size | |||||
| < 40mm | 21 | 45.7 | |||
| ≥ 40mm | 23 | 50.0 | |||
| Lymph node metastasis | |||||
| Negative | 30 | 65.2 | |||
| Positive | 10 | 21.7 | |||
| Unknown | 6 | 13.0 | |||
| Histological subtype | |||||
| Mucinous adenocarcinoma | 20 | 43.4 | |||
| Endocervical type | 17 | 37.0 | |||
| Intestinal type | 3 | 6.5 | |||
| Endometrioid adenocarcinoma | 16 | 34.8 | |||
| Others | 5 | 10.8 | |||
| Adenosquamous carcinoma | 5 | 10.8 | |||
| Treatment procedure | |||||
| Resection only | 13 | 28.3 | |||
| Resection with neoadjuvant chemotherapy | 13 | 28.3 | |||
| Resection with adjuvant therapy only | 13 | 28.3 | |||
| Chemo-radiation therapy only | 5 | 10.9 | |||
| Chemotherapy only | 2 | 4.3 | |||
FIGO, The International Federation of Gynecology and Obstetrics.
Immunohistochemical findings and correlation with clinicopathological factors
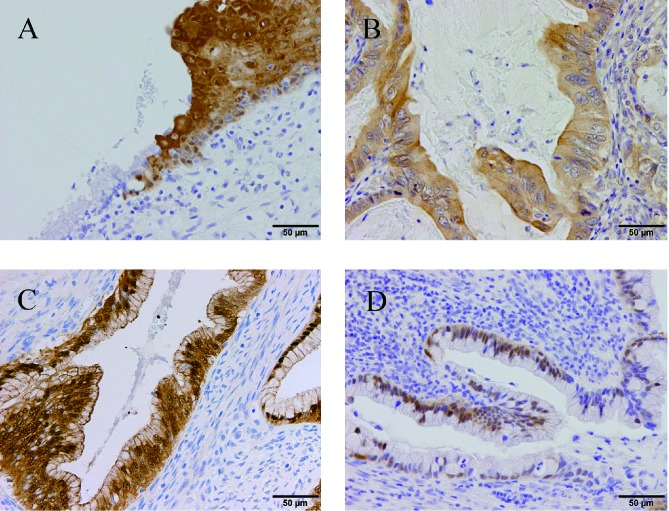
Representative staining patterns of maspin expression are shown in Fig. 1. Subcellular localization was classified into three categories: cytoplasmic staining only (32 cases, 69.6%), pancellular staining (both nuclear and cytoplasm; 4 cases, 8.7%), nuclear staining only (4 cases, 8.7%) and no staining (6 cases, 13%). The latter three patterns were judged as maspin-negative. Maspin-positive status was significantly correlated with recurrence (P = 0.022) and slightly correlated with the non-mucinous subtype (P = 0.042) (Table 2).
Fig. 1.
Representative immunoreactivity for maspin in formalin-fixed paraffin-embedded adenocarcinoma tissues of the uterine cervix.
A: Normal squamous epithelium showed both cytoplasmic and nuclear staining, while normal columnar epithelium was not stained at the squamo-columnar junction.
B: Mucinous adenocarcinoma showing only cytoplasmic staining for maspin.
C: Mucinous adenocarcinoma showing both cytoplasmic and nuclear staining for maspin.
D: Mucinous adenocarcinoma showing only nuclear staining for maspin.
Table 2.
Associations between maspin expression and clinicopathological factors in adenocarcinoma of the uterine cervix
| Maspin expression | ||||
| Positive | Negative | P-value | ||
| FIGO stage | ||||
| I and II | 20 | 13 | 0.072 | |
| III and IV | 12 | 1 | ||
| Tumor size | ||||
| < 40mm | 13 | 8 | 0.179 | |
| ≥ 40mm | 19 | 4 | ||
| Lymph node metastasis | ||||
| Present | 19 | 11 | 0.279 | |
| Absent | 11 | 2 | ||
| Histological subtype | ||||
| Mucinous | 10 | 10 | 0.042* | |
| Endometrioid | 13 | 3 | ||
| Others | 9 | 1 | ||
| Recurrence | ||||
| Present | 17 | 2 | 0.022* | |
| Absent | 15 | 12 | ||
*P < 0.05.
FIGO, The International Federation of Gynecology and Obstetrics.
Survival analysis
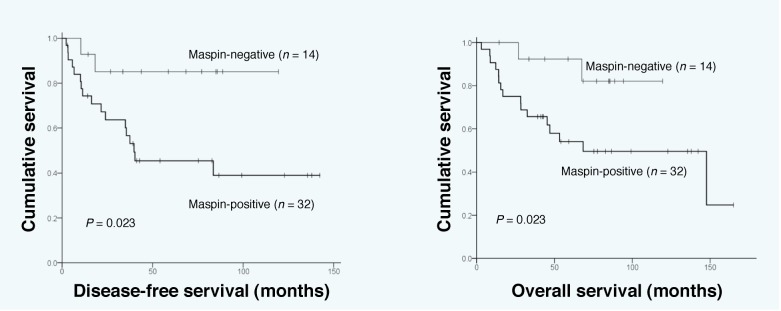
During the follow up period, 19 patients experienced recurrent disease and 18 patients died of the disease. In the log-rank test, maspin-positive patients showed significantly shorter DFS (P = 0.023) and OS (P = 0.043) (Fig. 2). The 5 years DFS rates of the maspin-positive and -negative groups were 45.5% (95% CI 0.27–0.64) and 85.1% (95% CI 0.66–1.04), respectively. The 5 years OS rates of the maspin-positive and -negative groups were 54.0% (95% CI 0.36–0.72) and 82.1% (95% CI 0.59–1.07), respectively. There were significant differences in DFS and OS in the Kaplan-Meier curves according to FIGO stage (P < 0.001, P < 0.001, respectively) and lymph node metastasis (P = 0.002, P < 0.001, respectively). There was significant difference of OS in Kaplan-Meier curves according to tumor size (P = 0.005). According to Cox multivariate analysis, advanced FIGO stage was the only independent prognostic factor for both DFS (P = 0.001) and OS (P = 0.002) (Table 3).
Fig. 2.
Disease-free survival (left) and overall survival (right) curves of 46 patients with uterine cervical adenocarcinoma according to maspin positivity status.
Table 3.
Multivariate analysis of clinicopathological factors for disease-free survival and overall survival
| Disease-free survival | Overall survival | ||||||
| HR | 95% Cl | P-value | HR | 95% Cl | P-value | ||
| Maspin | |||||||
| Positive vs. negative | 2.733 | 0.609–12.262 | 0.189 | 3.505 | 0.684–17.953 | 0.132 | |
| FIGO stage | |||||||
| I,II vs. III, IV | 0.070 | 0.015–0.324 | 0.001* | 0.101 | 0.024–0.419 | 0.002* | |
| Tumor size | |||||||
| < 40mm vs. ≥ 40mm | 0.958 | 0.345–2.665 | 0.935 | 2.350 | 0.693–7.968 | 0.170 | |
| Lymph node metastasis | |||||||
| Positive vs. negative | 0.823 | 0.215–3.142 | 0.775 | 2.117 | 0.573–7.823 | 0.261 | |
*P < 0.05.
CI, confidence interval; FIGO, The International Federation of Gynecology and Obstetrics; HR, hazard ratio.
DISCUSSION
It has been reported that adenocarcinoma of the uterine cervix is clearly different from SCC based on its epidemiology, molecular pathogenesis and clinical behavior, including the response to chemotherapy and the patterns of recurrence.3 It has also been reported that adenocarcinoma is more likely to involve both lymph node and distant metastasis compared to SCC resulting in a worse prognosis.18, 19 The main prognostic factors are considered to be the presence of lymph nodes metastasis, tumor size and FIGO stage. However, few molecular alterations of adenocarcinoma of the uterine cervix have been reported, in contrast to the case with SCC. Therefore, it will be important to explore new molecular markers. The p53 gene is a candidate molecular marker for adenocarcinoma of the uterine cervix. It has been reported that nucleotide changes in the p53 gene were significantly more frequent in adenocarcinoma of the uterine cervix than in SCC, and the highest incidence of p53 gene mutations was observed in adenocarcinoma of the uterine cervix from Asia and Europe.20 On the other hand, maspin is a member of the serpin family of protease inhibitors and was originally thought to be a tumor suppressor due to its ability to inhibit invasion, motility, lymphangiogenesis and metastasis of mammary tumors.6, 21 However, the loss of maspin expression in several cancers such as pancreatic, colorectal and ovarian cancers cannot typically be observed because of the lack of maspin expression in the corresponding normal tissue. The most compelling data regarding the clinical significance of maspin in cancer progression and metastasis emerged from survival studies of cancer patients. Although the original studies revealed the association of reduced maspin expression with cancer progression and worse prognosis, it has been demonstrated that this correlation was far more complex than originally suspected.8 Factors contributing to this complexity include differences in cancer type (for example, adenocarcinoma vs. SCC), cut-off values of positive criteria, antibodies used, methods of detection used, and subcellular maspin distribution. Recent evidence indicates that nuclear localization of maspin in cancer cells is necessary for its tumor suppressor activity and that, when excluded from the nucleus, maspin exhibited no tumor suppressor activity.22, 23 These results suggest that maspin localized in the nucleus may result in better prognosis, while cytoplasmic maspin accumulation may result in an unfavorable prognosis, which is consistent with our previous reports suggesting that cytoplasmic maspin could be a predictor of worse prognosis in several types of cancers.9–16 In turn, it has been reported that p53 activated the maspin promoter by binding directly to the p53 consensus-binding site present in the maspin promoter, suggesting the close association between maspin and the p53 gene.24 As nucleotide changes in the p53 gene were significantly more frequent in adenocarcinoma of the uterine cervix than in SCC,20 further investigation concerning the gene or protein alteration of both maspin and p53 may be intriguing. Although the present study has the limitations of a small number of patients and a retrospective nature, we for the first time suggested that cytoplasmic maspin expression might be a candidate molecular marker of progression in adenocarcinoma of the uterine cervix.
In conclusion, to our knowledge, this is the first investigation of the association between maspin expression and the prognosis of patients with adenocarcinoma of the uterine cervix. Although further studies with a larger series of patients and a longer follow-up period should be undertaken, the present study suggests the potential usefulness of cytoplasmic maspin expression as a prognostic factor for patients with adenocarcinoma of the uterine cervix. The immunohistochemical detection of maspin could be a new marker of aggressive behavior in patients with adenocarcinoma of the uterine cervix.
Acknowledgments
Ackonwlegments: We are grateful to N. Itaki for his excellent technical assistance.
The authors declare no conflict of interest.
REFERENCES
- 1. Schorge JO, Knowles LM, Lea JS. Adenocarcinoma of the cervix. Curr Treat Options Oncol. 2004; 5: 119-27. . [DOI] [PubMed] [Google Scholar]
- 2. Bray F, Carstensen B, Moller H, Zappa M, Zakelj MP, Lawrence G, et al. Incidence trends of adenocarcinoma of the cervix in 13 European countries. Cancer Epidemiol Biomark Prev. 2005; 14: 2191-9. . [DOI] [PubMed] [Google Scholar]
- 3. Gien LT, Beauchemin MC, Thomas G. Adenocarcinoma: A unique cervical cancer. Gynecol Oncol. 2010; 116: 140-6. . [DOI] [PubMed] [Google Scholar]
- 4. Nakanishi T, Ishikawa H, Suzuki Y, Inoue T, Nakamura S, Kuzuya K. A comparison of prognoses of pathologic stage IIB adenocarcinoma and squamous cell carcinoma of the uterine cervix. Gynecol Oncol. 2000; 79: 289-93. . [DOI] [PubMed] [Google Scholar]
- 5. Park JY, Kim DY, Kim JH, Kim YM, Kim YT, Nam JH. Outcomes after radical hysterectomy in patients with early-stage adenocarcinoma of uterine cervix. Br J Cancer. 2010; 102: 1692-8. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zou Z, Anisowicz Z, Hendrix MJ, Thor A, Neveu M, Sheng S, et al. Maspin, a serpin with tumor-suppressing activity in human mammary epithelial cells. Science. 1994; 263: 526-9. . [DOI] [PubMed] [Google Scholar]
- 7. Bodenstine TM, Seftor RE, Khalkhali-Ellis Z, Seftor EA, Pemberton PA, Sager R. Maspin: molecular mechanisms and therapeutic implications. Cancer metastasis Rev. 2012; 31: 529-51. . [DOI] [PubMed] [Google Scholar]
- 8. Berardi R, Morgese F, Onofri A, Mazzanti P, Pistelli M, Ballatore Z, et al. Role of maspin in cancer. Clin Transl Med. 2013; 2: 8. DOI: 10.1186/2001-1326-2-8. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Umekita Y, Ohi Y, Sagara Y, Yoshida H. Expression of maspin predicts poor prognosis in breast cancer patients. Int J Cancer. 2002; 100: 452-5. . [DOI] [PubMed] [Google Scholar]
- 10. Umekita Y, Yoshida H. Expression of maspin is up-regulated during the progression of mammary ductal carcinoma. Histopathol. 2003; 42: 541-5. . [DOI] [PubMed] [Google Scholar]
- 11. Umekita Y, Ohi Y, Souda M, Rai Y, Sagara Y, Sagara Y, et al. Maspin expression is frequent and correlates with basal markers in triple-negative breast cancer. Diagn Pathol. 2011; 6: 36. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Umekita Y, Souda M, Yoshida H. Expression of maspin in colorectal cancer. in vivo. 2006; 13: 317-21. . [PubMed] [Google Scholar]
- 13. Tsuji T, Umekita Y, Ohi Y, Kamino M, Douchi T, Yoshida H, et al. Maspin expression is up-regulated during the progression of endometrioid endometrial carcinoma. Histopathol. 2007; 51: 871-4. . [DOI] [PubMed] [Google Scholar]
- 14. Tsuji T, Togami S, Douchi T, Umekita Y. Difference in subcellular localization of maspin expression in ovarian mucinous borderline tumour. Histopathol. 2009; 55: 130-2. . [DOI] [PubMed] [Google Scholar]
- 15. Takagi Y, Matsuoka Y, Shiomi T, Nosaka K, Takeda C, Haruki T, et al. Cytoplasmic maspin expression is a predictor of poor prognosis in patients with lung adenocarcinoma measuring less than 3 cm. Histopathol. 2015; 66: 732-9. . [DOI] [PubMed] [Google Scholar]
- 16. Takeda C, Takagi Y, Shiomi T, Nosaka K, Yamashita H, Osaki M, et al. Cytoplasmic maspin expression predicts poor prognosis of patients with soft tissue sarcomas. Diagn Pathol. 2014; 30: 205. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tavassoli FA. Devilee P. WHO Classification of Tumours of Breast and Female Genital Organs, 3rd edition Lyon: IARC Press; 2003. p.272-4. [Google Scholar]
- 18. Eifel PJ, Morris M, Oswald MJ, Wharton JT, Delclos L. Adenocarcinoma of the uterine cervix. Prognosis and pattern of failure in 367 cases. Cancer. 1990; 65: 2507-14. . [DOI] [PubMed] [Google Scholar]
- 19. Irie T, Kigawa J, Minagawa Y, Itamochi H, Sato S, Akeshima R, et al. Prognosis and clinicopathological characteristics of Ib-IIb adenocarcinoma of the uterine cervix in patients who have had radical hysterectomy. European J Surg Oncol. 2000; 26: 464-7. . [DOI] [PubMed] [Google Scholar]
- 20. Tornesello ML, Buonaguro L, Buonaguro FM. Mutations of the TP53 gene in adenocarcinoma and squamous cell carcinoma of the cervix: A systematic review. Gynecol Oncol. 2013; 128: 442-8. . [DOI] [PubMed] [Google Scholar]
- 21. Liu Z, Shi Y, Meng W, Liu Y, Yang K, Wu S, et al. Expression and localization of maspin in cervical cancer and its role in tumor progression and lymphangiogenesis. J Gynecol Oncol. 2014; 289: 373-82. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Goulet B, Kennette W, Ablack A, Postenka CO, Hague MN, Mymryk JS, et al. Nuclear localization of maspin is essential for its inhibition of tumor growth and metastasis. Lab Invest. 2011; 91: 1181-7. . [DOI] [PubMed] [Google Scholar]
- 23. Machowska M, Wachowicz K, Sopel M, Rzepecki R1. Nuclear location of tumor suppressor protein maspin inhibits proliferation of breast cancer cells without affecting proliferation of normal epithelial cells. BMC Cancer. 2014; 28: 142. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zou Z, Gao C, Nagaich AK, Connell T, Saito S, Moul JW, et al. p53 regulates the expression of the tumor suppressor gene maspin. J Biol Chem. 2000; 275: 6051-4. . [DOI] [PubMed] [Google Scholar]