Abstract
Background:
Blockade of human epidermal growth factor receptor type 2 (HER2) has dramatically improved outcome for patients with HER2-positive breast cancer. Trastuzumab, an anti-HER2 monoclonal antibody, has previously demonstrated improvement in overall survival (OS) in patients with metastatic and early stage HER2-positive breast cancer. However, trastuzumab can cause congestive heart failure (CHF) with an increased frequency for patients who have also received an anthracycline. The current trial was designed to evaluate the impact of the duration of trastuzumab on CHF.
Methods:
E2198 included 227 eligible women with histologically confirmed stage II or IIIA HER2-positive breast cancer. The patients were randomised to receive 12 weeks of paclitaxel and trastuzumab followed by four cycles of doxorubicin and cyclophosphamide (abbreviated Arm) or the aforementioned treatment with additional 1 year of trastuzumab (conventional Arm). The primary end point was to evaluate the safety of this variable duration of trastuzumab therapy, particularly cardiac toxicity defined as CHF or left ventricular ejection fraction decrease >10%. Secondary end points included disease-free survival (DFS) and OS.
Results:
Compared with 12-week treatment with trastuzumab, 1 year of trastuzumab-based therapy did not increase the frequency or severity of cardiac toxicity: three patients on the abbreviated Arm and four on the conventional Arm experienced CHF. The 5-year DFS was 76% and 73% for the abbreviated and conventional Arms, respectively, with a hazard ratio (HR) of 1.3 (95% CI: 0.8–2.1; P=0.3). There was also no statistically significance difference in OS (HR, 1.4; P=0.3).
Conclusions:
Compared with 12 weeks of treatment, 1 year of treatment with trastuzumab did not significantly increase the risk of cardiac toxicity. Although not powered for efficacy comparisons, the longer duration of trastuzumab therapy did not demonstrate a signal for marked superiority.
Keywords: HER2+, trastuzumab, congestive heart failure, breast cancer, adjuvant trial, duration of therapy
Blockade of human epidermal growth factor receptor type 2 (HER2) has dramatically improved outcome in both the metastatic and adjuvant settings for tumours with HER2 overexpression or gene amplification (Slamon et al, 2001; Piccart-Gebhart et al, 2005; Romond et al, 2005; Slamon et al, 2011). Multiple anti-HER2 agents have demonstrated improvement in progression-free survival (PFS) and/or overall survival (OS) for patients with metastatic disease (Geyer et al, 2006; Verma et al, 2012; Swain et al, 2015). The use of trastuzumab in the adjuvant setting has also demonstrated improvement in OS when added to a backbone of chemotherapy compared with chemotherapy alone (Slamon et al, 2001). Many other agents used in the metastatic setting are actively being tested in the adjuvant and neoadjuvant settings. Although the therapeutic indices for these agents are excellent, trastuzumab is not void of toxicity. Specifically, congestive heart failure (CHF) is the most serious toxicity (Telli et al, 2007) and its frequency is increased when combined with an anthracycline (Romond et al, 2012).
Currently, the optimal duration of adjuvant trastuzumab therapy for patients with HER2-positive disease is 1 year (Pivot et al, 2013). This duration was based on the results of two major trials. The HERA trial demonstrated that 2 years of trastuzumab therapy after standard chemotherapy did not improve disease-free survival (DFS) nor OS compared with 1 year of trastuzumab therapy (Piccart-Gebhart et al, 2005). The PHARE trial compared the outcome of 1 year of trastuzumab therapy with 6 months trastuzumab therapy. This was a non-inferiority trial and demonstrated that outcome of trastuzumab administration for 6 months was not non-inferior to the same for 12 months (Pivot et al, 2013). Even shorter durations of trastuzumab, however, have been compared with no therapy and have demonstrated activity. Specifically, the FINHER trial showed that 3 months of adjuvant trastuzumab was superior to none (Joensuu et al, 2009). E2198 was primarily designed to evaluate the impact of duration of trastuzumab on CHF. Here we also report the results for a comparison of the conventional 1-year duration trastuzumab therapy with a shorter exposure in the adjuvant setting.
Patients and methods
Study design and treatment
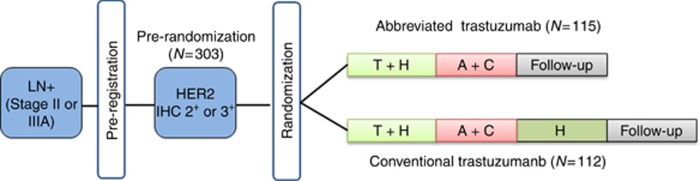
E2198 was a randomised phase II study (Figure 1). All patients were pre-registered to the pre-randomisation Arm and tumour blocks were sent to the ECOG-ACRIN Pathology Coordinating Office for central review of HER2 status. Patients with a score of 2–3+ determined by immunohistochemistry (IHC) were classified as eligible for randomisation to an abbreviated trastuzumab Arm or a conventional trastuzumab Arm. Treatments were assigned using permuted blocks within strata with dynamic balancing within main institutions and their affiliate networks. Patients were stratified based on whether radiation therapy was planned vs not planned. All patients received paclitaxel 175 mg m−2 on day 1 every 3 weeks for four cycles and trastuzumab with a 4 mg kg−1 loading dose in week 1 followed by 2 mg kg−1 weekly through week 10 (TH). Three weeks after the last doses of TH, patients received doxorubicin 60 mg m−2 and cyclophosphamide 600 mg m−2 on day 1 every 3 weeks for four cycles (AC). Within 3 weeks after the completion of AC and completion of radiation therapy (if administered), patients randomised to the conventional Arm received additional trastuzumab with a 4 mg kg−1 loading dose in week 1 followed by 2 mg kg−1 weekly for 1 year (Figure 1). Radiation therapy, if clinically indicated, was administered according to institutional guidelines after the completion of AC. Patients with tumours classified as oestrogen receptor (ER) and/or progesterone receptor (PgR) positive must have received tamoxifen at the completion of AC for 5 years. For patients randomised to the conventional Arm, tamoxifen could have been administered concomitantly with the maintenance trastuzumab.
Figure 1.
Trial schema.
Patients
Female patients ⩾18 years old with histologically confirmed stage II or IIIA HER2-positive breast cancer were eligible if they had not received previous chemotherapy or hormonal therapy. Patients who had received previous tamoxifen for chemoprevention were required to have discontinued tamoxifen for at least 1 year before enrolment. Patients who had their last definitive surgical procedure within the previous 12 weeks before receiving radiation therapy were considered eligible. Additional inclusion criterial included adequate cardiac function with a left ventricular ejection fraction (LVEF) ⩾50%, and adequate renal, hepatic and haematological function.
Patients were excluded if they had a history of congestive cardiomyopathy, CHF, uncontrolled hypertension, myocardial infraction or uncontrolled arrhythmia within the prior 6 months. Patients were also excluded if they had another cancer within the previous 5 years except basal or squamous cell carcinoma of the skin or in situ cervical cancer.
Methods
Assessments of the patient's overall health, history, physical exam and performance status were performed at baseline, day 1 of each cycle, and then post AC every 3 months for the first year, every 6 months for the second year and then annually. Haematological evaluations were obtained with the same frequency with additional tests between TH and at the start of AC and during each cycle. A chest X-ray and EKG were required at baseline. LVEF was to be performed via MUGA or echocardiogram (as long as the method was consistent) at baseline, post TH, post AC, 1 year post AC (abbreviated arm only) and at the following three time points for the conventional Arm: 6 months after beginning maintenance trastuzumab; within 1 month of completing; and 1 year post maintenance trastuzumab. Annual mammograms were required with the long-term follow-up form submitted each time that a patient was seen.
Since the standard definition of HER2 positivity was either 2+ or 3+ with the HercepTest (DAKO, Carpinteria, CA, USA) when this trial was conducted, we repeated the HER2 scoring centrally (for available cases) using IHC and reflex FISH in November 2013 according to the College of American Pathologist (CAP) guidelines (Wolff et al, 2013). Those patients with an IHC of 0 or, 1+ were considered HER2-negative, whereas those with 3+ were considered positive. Those patients who were 2+ were tested by FISH. Patients were considered HER2-negative with a HER2/CEP17 ratio <1.8; positive with a ratio ⩾2.2; and equivocal with a ratio ⩾1.8 and <2.2.
Statistical considerations
The primary objective was to evaluate the safety of these treatment regimens, particularly with respect to CHF and LVEF. Toxicities were graded according to the Common Toxicity Criteria (CTC) version 2.0. CHF (defined as grade 3-4 LVEF dysfunction), Grade 3-4myocarditis, and decreases in LVEF of >10% were of interest. The study was originally designed to enrol a total of 100 eligible patients. The accrual goal was later increased to allow entry of 200 eligible patients in order to better assess the cardiac toxicity. CHF rate was summarised as a proportion with corresponding exact 95% confidence interval (CI). Fisher's exact test was used to compare Arms with respect to proportion with >10% decrease in LVEF post TH and post AC. The proportion of patients with >10% decrease in LVEF 1 year post AC treatment and 1 year post maintenance trastuzumab was also evaluated. Although the trial was not powered for time-to-event comparisons, secondary end points included DFS and OS. DFS was defined as time from randomisation to disease recurrence, development of invasive second primary or death. Patients without documented DFS event were censored at date of last disease evaluation. Survival was defined as the time from randomisation to death from any cause, otherwise censored at date last known alive. Distributions of DFS and OS were estimated using the Kaplan–Meier method. Toxicity summaries included all patients who received protocol treatment. Summaries of baseline demographics, disease characteristics, and analysis of outcome data included eligible patients. Two-sided P-values <0.05 were considered statistically significant.
Results
Patients and treatment
Pre-registration of E2198 began in August 1999, but suspended on 23 February 2000 while an amendment to increase accrual was under review. The pre-registration was re-activated in May 2000 and closed in October 2000. A total of 303 patients were originally pre-registered to pre-randomisation Arm and 234 were randomised to the abbreviated or conventional trastuzumab Arm. However, only 227 patients met the eligibility criteria, of whom 115 were assigned to abbreviated and 112 conventional trastuzumab Arm (Figure 1). The two groups of patients were well balanced at baseline with respect to prognostic characteristics (Table 1). The median age was 49 years (range 22–78). Approximately 54% of the patients had 1–3 positive lymph nodes, 29% had 4–9 positive nodes and 17% had 10 or more positive nodes. Sixty two percent of the patients had ER- and/or PgR-positive tumours. Patients received a median of eight cycles of the treatment for both Arms (range, 2–8); 93% and 87% of patients on abbreviated and conventional Arms completed protocol treatment through AC, respectively. Out of the 112, 90 patients on conventional Arm (80%), including nine patients who did not complete eight cycles of TH→AC, began the maintenance treatment of trastuzumab. Out of these 90, 67 patients (74%) completed the entire duration of maintenance trastuzumab. Therefore 60% of the patients assigned to conventional Arm completed treatment per protocol.
Table 1. Patient demographics and disease characteristics.
| Abbreviated trastuzumab (N=115) | Conventional trastuzumab (N=112) | |
|---|---|---|
|
Race – no. (%) | ||
| White | 106 (92) | 97 (87) |
| Hispanic | 2 (2) | 3 (3) |
| Black | 4 (3.5) | 9 (8) |
| Other | 3 (2.5) | 3 (2) |
|
Age (years) – no. (%) | ||
| <40 | 21 (18) | 21 (19) |
| ⩾40 | 94 (82) | 91 (81) |
| Age – median (range) | 49 (26–78) | 48 (22–76) |
|
Nodal status – no. (%) | ||
| 1–3 positive nodes | 58 (51) | 64 (57) |
| 4–9 positive nodes | 35 (30) | 32 (29) |
| ⩾10 positive nodes | 22 (19) | 16 (14) |
| Number of positive nodes – median (range) | 3 (1–29) | 3 (1–24) |
|
ER/PgR status – no. (%) | ||
| ER−/PgR− | 45 (39) | 41 (37) |
| ER−/PgR+ | 6 (5) | 5 (14) |
| ER+/PgR− | 9 (9) | 16 (4) |
| ER+/PgR+ | 54 (47) | 50 (45) |
| Missing | 1 (1) | 0 (0) |
|
ECOG performance status – no. (%) | ||
| 0 | 100 (87) | 99 (88) |
| 1 | 14 (12) | 12 (11) |
| 2 | 1 (1) | 1 (1) |
|
Most extensive surgery – no. (%) | ||
| Less than mastectomy | 32 (28) | 41 (36) |
| Modified radical mastectomy | 70 (61) | 59 (53) |
| Total (simple) mastectomy | 13 (11) | 12 (11) |
In November 2013, HER2 status was reassessed based on more contemporary definitions. Samples from 176 patients were available for repeat testing (Supplementary Figure 1). Tumours from 31 out of the 176 patients (17.6%) that were previously classified as HER2-positive were found to be 0 or 1+ by IHC, and thus reclassified as HER2-negative; 105 patients (59.7%) were 3+ and thus confirmed as HER2-positive; 40 patients (22.7%) were HER2 2+ and subsequent tested by FISH: 9 out of 40 patients did not have sufficient additional material for FISH and were removed from further analyses; 15 had HER2 amplification and 16 did not. In total, 120 patients were confirmed with HER2-positive tumours by more contemporary definitions of positivity. Except for a minor difference in median age (median 47 for those confirmed HER2+ and 51 for those not), there were no differences with respect to baseline demographics and disease characteristics between those patients confirmed HER2+ vs those not.
Safety
Seven out of the 234 patients (3%) had CHF (95% CI, 1.2–6%) including one patient with grade 3 myocarditis; three of the patients were on the abbreviated Arm and four on the conventional Arm. There were no differences between Arms in the proportion with LVEF decrease >10% post TH treatment (P=0.6) or post AC therapy (P=0.6) (Table 2). Patients had no unexpected frequency or severity of toxicity related to the similar treatment combination (Supplementary Table 1). No lethal toxicities were observed.
Table 2. LVEF assessmenta.
| Abbreviated trastuzumab | Conventional trastuzumab | |
|---|---|---|
| Baseline and post TH LVEF available – no. | 112 | 111 |
| Median post TH LVEF – no. | 61 | 61 |
| Post TH LVEF decline >10% – no. (%) | 12 (11) | 9 (8) |
| Post TH LVEF decline >10% and LVEF <LLN – no. (%) | 3 (3) | 1 (1) |
| Baseline and post AC LVEF available – no. | 95 | 104 |
| Median post AC LVEF – no. | 59 | 60 |
| Post AC LVEF decline >10% – no. (%) | 15 (16) | 13 (13) |
| Post AC LVEF decline >10% and LVEF <LLN – no. (%) | 3 (3) | 5 (5) |
| Baseline and post 1 year of treatmenta LVEF available – no. | 78 | 40 |
| Median post 1 year of treatment LVEF – no. | 62 | 63 |
| Post 1 year of treatment LVEF decline >10% – no. (%) | 13 (17) | 9 (23) |
| Post one year of treatment LVEF decline >10% and LVEF <LLN – no. (%) | 1 (1) | 2 (5) |
Abbreviation: LVEF=left ventricular ejection fraction.
Timepoint for Abbreviated trastuzumab Arm is 1 year post AC. Timepoint for conventional trastuzumab Arm is one year after completion of maintenance trastuzumab.
Efficacy
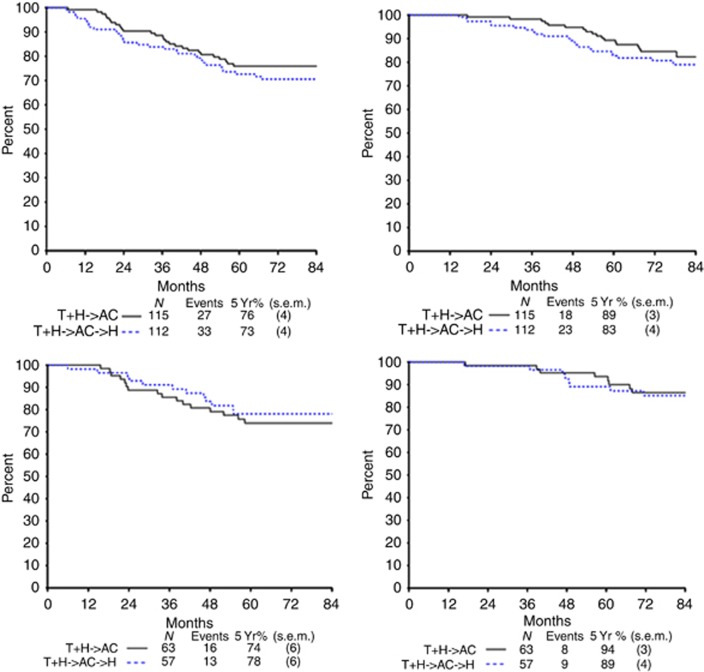
Data reported here are as of November 2013 at a median follow-up of 77 months. Efficacy was evaluated separately in the 227 patients enrolled and randomised in the trial, and in the 120 patients re-tested centrally and HER2-positive. Figure 2 shows the Kaplan–Meier curves for DFS and OS of the aforementioned two groups of patients randomised into the two Arms. For the 227 patients, there was no statistically significant difference in DFS (HR for conventional (29 recurrences) vs abbreviated (25 recurrences), 1.3; 95% CI, 0.8–2.1; P=0.3; 5-year DFS, 73 and 76%, respectively) or OS (HR, 1.4; P=0.3; 5-year OS, 83 and 89%, respectively) (Table 3a). For the 120 patients, there was no significant difference in either DFS (HR, 0.9; P=0.7; 5-year, DFS 78% and 74%, respectively) or OS (HR, 1.2; P=0.7; 5-year OS, 89% and 94%, respectively) (Table 3b).
Figure 2.
(Top) Comparison of DFS (left) and OS (right) for the 227 patients enrolled and randomised. (Bottom) Comparison of DFS (left) and OS (right) for the 120 patients centrally re-tested and HER2-positive.
Table 3a. DFS and OS outcomes for all patients.
|
5-Year rate |
||||
|---|---|---|---|---|
| Hazard ratio (95% CI)a | P-valueb | Abbreviated trastuzumab N=115 | Conventional trastuzumab N=112 | |
| DFS | 1.31 (0.79–2.12) | 0.31 | 76% | 73% |
| OS | 1.37 (0.74–2.54) | 0.32 | 89% | 83% |
Abbreviations: CI=confidence interval; DFS=disease-free survival; OS=overall survival.
Hazard ratio for conventional trastuzumab vs abbreviated trastuzumab.
Based on log-rank test.
Table 3b. DFS and OS for patients centrally re-tested and HER2-positive.
|
5-year rate |
||||
|---|---|---|---|---|
| Hazard ratio (95% CI)a | P-valueb | Abbreviated trastuzumab N=63 | Conventional trastuzumab N=57 | |
| DFS | 0.85 (0.41–1.77) | 0.66 | 74% | 78% |
| OS | 1.21 (0.46–3.13) | 0.70 | 94% | 89% |
Abbreviations: CI=confidence interval; DFS=disease-free survival; HER2=human epidermal growth factor receptor type 2; OS=overall survival.
Hazard ratio for conventional trastuzumab vs abbreviated trastuzumab.
Based on log-rank test.
Discussion
Targeting HER2 has drastically improved the outcome for patients with HER2-positive breast cancer. In the metastatic setting, the addition of trastuzumab to chemotherapy significantly improved both PFS and OS. The addiction to this pathway has been evidenced by continued benefit to anti-HER2 blockade even after progression on trastuzumab (Geyer et al, 2006; Schaller et al, 2007). More recently, dual blockade with trastuzumab and either lapatinib or pertuzumab has also demonstrated further improved PFS and OS in the metastatic setting (Swain et al, 2015). Trastuzumab has improved DFS and OS in the adjuvant setting compared with chemotherapy alone (Slamon et al, 2011). Although dual therapy with trastuzumab plus pertuzumab and trastuzumab plus lapatinib demonstrated an improvement in pathological complete response over trastuzumab alone, the addition of lapatinib to trastuzumab did not improve either DFS or OS in the large adjuvant ALTTO trial (Piccart-Gebhart et al, 2014). We now await the results of the APHINITY trial, testing the combination of pertuzumab with trastuzumab, to determine whether we have truly reached a plateau in terms of benefit from single agent HER2 blockade. From a duration standpoint, recently the ExteNET trial (Chan et al, 2015) reported improved invasive DFS in early stage HER2-positive breast cancer patients who received neratinib after completion of 1 year of trastuzumab. It is not clear if this improvement was due to a longer duration of therapy or the addition of second effective anti-HER2 therapy. The HERA trial included a comparator arm that focused entirely on duration and demonstrated that 2 years of trastuzumab administration does not confer benefit over 1 year, suggesting we may have reached a duration plateau for single agent therapy (Piccart-Gebhart et al, 2005). Although we may have reached a ceiling in terms of incremental benefit from the amount and the duration of HER2 blockade, many questions remain as to whether we can minimise the amount of chemotherapy and trastuzumab in specific populations and whether less than 12 months of therapy may be beneficial. Recent data have demonstrated that women with small tumours (T1c) may indeed benefit from HER2 blockade (O'Sullivan et al, 2014); although the outcomes for T1a/T1b tumours appear to have exceptionally good outcome in the absence of additional therapy. In addition, Tolaney et al. (2015) demonstrated that the use of single agent paclitaxel and trastuzumab alone might provide outstanding efficacy for small, node-negative tumours. Thus, there are many evidence-based options for patients diagnosed with early stage HER2-positive breast cancer; ranging from traditional full doses of chemotherapy with dual antibody blockade to single agent paclitaxel and trastuzumab in the adjuvant setting. Shorter durations of trastuzumab have also recently been reported. The PHARE trial (Pivot et al, 2013), which was a non-inferiority trial, could not exclude the inferiority of 6 months of trastuzumab compared with 1 year. Despite this, there is some evidence of benefit to the use of trastuzumab for shorter durations compared with none (Joensuu et al, 2009). Thus, it is not clear where on the risk spectrum that the incremental gain in outcome from longer therapy can be optimally captured.
Opened to accrual in 1999, E2198 was designed to capture the toxicity for various durations of trastuzumab therapy; most importantly cardiac toxicity. The previously reported rates of CHF from the large adjuvant trastuzumab trials are consistently low (<4% incidence) (Ewer et al, 2005; Suter et al, 2007; Perez et al, 2008; Procter et al, 2010; Russell et al, 2010; Pivot et al, 2013; de Azambuja et al, 2014). Exposure to anthracyclines, low baseline ejection fraction, older age, hypertension and high BMI appear to predict a higher likelihood (Perez et al, 2008; Russell et al, 2010; Bowles et al, 2012). The majority of patients who experience severe cardiac toxicity, however, do appear to have either partial or complete recovery of function with cessation of therapy (Ewer et al, 2005; Procter et al, 2010; Russell et al, 2010; de Azambuja et al, 2014). Two years of trastuzumab did not increase the risk of severe cardiac damage compared to one year in the HERA trial (de Azambuja et al, 2014). There was a significantly higher rate of CHF for those receiving 1 year of therapy compared with 6 months in the PHARE study, but the rates were very low in both arms with recovery in the vast majority of patients (Pivot et al, 2013). As expected from these prior reports, there were no unexpected toxicities for the combination of chemotherapy with trastuzumab. Specifically, the rates of CHF and drops in LVEF >10% were modest and not substantially different based on duration of therapy. Unfortunately this trial used the older CTCv. 2.0 criteria for scoring and there was neither central review nor long-term follow-up, which is a limitation of this study.
Although this trial was not powered for efficacy outcome comparisons, there appeared to be no substantial signal to demonstrate marked benefit for longer duration. Thus, while these data should not challenge the current standard of care for one year of anti-HER2 therapy, it does add to the existing body of data to support some benefit for even short durations of trastuzumab. For patients with low-risk disease, where the value of therapy was uncertain at the outset and where cardiac (or other toxicity) issues become apparent, it may provide support to truncate therapy early with some benefit, rather than extending therapy to complete an entire year of therapy. These efficacy results must be interpreted with caution because of the different definition of HER2 positivity at the time of the trial initiation. To address this concern, we centrally repeated HER2 testing using more contemporary definitions of HER2 positivity. It must be noted that several patients were removed simply because of lack of sufficient tumour material for testing or were found to be HER2 equivocal. In addition, even for those patients clearly HER2-negative on re-testing, these tumours may have had molecular heterogeneity and thus have gained benefit despite the negative test. Further, there are some data to support benefit from those with even low levels of HER2 protein expression (Paik et al, 2008). Regardless, in both the parent group and retested subset, there was no substantial improvement in outcomes for the longer duration of therapy.
In summary, this trial supports the safety of combining chemotherapy with trastuzumab. Further, although underpowered and exploratory, it identified no substantial signal for marked inferiority for a shorter duration of trastuzumab. Although 1 year of anti-HER2 blockade should remain the standard of care, this adds additional data to support efficacy for shorter durations and this may be beneficial for decision making in patients having difficulty in tolerating the therapy. In addition, from a global perspective, 1 year of trastuzumab administration for every patient with HER2-positive breast cancer may simply not be feasible from a financial standpoint (Benjamin et al, 2013). Thus, additional trials addressing shorter durations remain critical.
Acknowledgments
This study was conducted by the ECOG-ACRIN Cancer Research Group (Robert L Comis, MD and Mitchell D Schnall, MD, PhD, Group Co-Chairs) and supported in part by Public Health Service Grants CA23318, CA66636, CA21115, CA180820, CA180794, CA49883, CA180795, CA180816, CA180798, CA35103, CA35267, CA17145, CA39229, CA180844, CA16116, CA180802 and from the National Cancer Institute, National Institutes of Health and the Department of Health and Human Services. Its content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies this paper on British Journal of Cancer website (http://www.nature.com/bjc)
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
Supplementary Material
References
- Benjamin L, Buthion V, Iskedjian M, Farah B, Rioufol C, Vidal-Trecan G (2013) Budget impact analysis of the use of oral and intravenous anti-cancer drugs for the treatment of HER2-positive metastatic breast cancer. J Med Econ 16(1): 96–107. [DOI] [PubMed] [Google Scholar]
- Bowles EJ, Wellman R, Feigelson HS, Onitilo AA, Freedman AN, Delate T, Allen LA, Nekhlyudov L, Goddard KA, Davis RL, Habel LA, Yood MU, McCarty C, Magid DJ, Wagner EH Pharmacovigilance Study Team (2012) Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J Natl Cancer Inst 104(17): 1293–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan A, Delaloge S, Holmes FA, Moy B, Iwata H, Harvey VJ, Robert NJ, Silovski T, Gokmen E, Von Minckwitz G, Ejlertsen B, Chia SKL, Mansi J, Barrios CH, Gnant M, Wong A, Bryce R, Yao B, Martin M (2015) Neratinib after adjuvant chemotherapy and trastuzumab in HER2-positive early breast cancer: primary analysis at 2 years of a phase 3, randomized, placebo-controlled trial (ExteNET). ASCO Meet Abstr 33(15-Suppl): 508. [Google Scholar]
- de Azambuja E, Procter MJ, van Veldhuisen DJ, Agbor-Tarh D, Metzger-Filho O, Steinseifer J, Untch M, Smith IE, Gianni L, Baselga J, Jackisch C, Cameron DA, Bell R, Leyland-Jones B, Dowsett M, Gelber RD, Piccart-Gebhart MJ, Suter TM (2014) Trastuzumab-associated cardiac events at 8 years of median follow-up in the Herceptin Adjuvant trial (BIG 1-01). J Clin Oncol 32(20): 2159–2165. [DOI] [PubMed] [Google Scholar]
- Ewer MS, Vooletich MT, Durand JB, Woods ML, Davis JR, Valero V, Lenihan DJ (2005) Reversibility of trastuzumab-related cardiotoxicity: new insights based on clinical course and response to medical treatment. J Clin Oncol 23(31): 7820–7826. [DOI] [PubMed] [Google Scholar]
- Geyer CE, Forster J, Lindquist D, Chan S, Romieu CG, Pienkowski T, Jagiello-Gruszfeld A, Crown J, Chan A, Kaufman B, Skarlos D, Campone M, Davidson N, Berger M, Oliva C, Rubin SD, Stein S, Cameron D (2006) Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 355(26): 2733–2743. [DOI] [PubMed] [Google Scholar]
- Joensuu H, Bono P, Kataja V, Alanko T, Kokko R, Asola R, Utriainen T, Turpeenniemi-Hujanen T, Jyrkkio S, Moykkynen K, Helle L, Ingalsuo S, Pajunen M, Huusko M, Salminen T, Auvinen P, Leinonen H, Leinonen M, Isola J, Kellokumpu-Lehtinen PL (2009) Fluorouracil, epirubicin, and cyclophosphamide with either docetaxel or vinorelbine, with or without trastuzumab, as adjuvant treatments of breast cancer: final results of the FinHer Trial. J Clin Oncol 27(34): 5685–5692. [DOI] [PubMed] [Google Scholar]
- O'Sullivan CCM, Bradbury I, Azambuja ED, Perez EA, Rastogi P, Spielmann M, Joensuu H, Ballman KV, Costantino JP, Delaloge S, Zardavas D, Piccart-Gebhart MJ, Zujewski J, Holmes EM, D GR (2014) Efficacy of adjuvant trastuzumab (T) compared with no T for patients (pts) with HER2-positive breast cancer and tumors ≤2 cm: A meta-analysis of the randomized trastuzumab trials. J Clin Oncol 32(5 Suppl): abstr 508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paik S, Kim C, Wolmark N (2008) HER2 status and benefit from adjuvant trastuzumab in breast cancer. N Engl J Med 358(13): 1409–1411. [DOI] [PubMed] [Google Scholar]
- Perez EA, Suman VJ, Davidson NE, Sledge GW, Kaufman PA, Hudis CA, Martino S, Gralow JR, Dakhil SR, Ingle JN, Winer EP, Gelmon KA, Gersh BJ, Jaffe AS, Rodeheffer RJ (2008) Cardiac safety analysis of doxorubicin and cyclophosphamide followed by paclitaxel with or without trastuzumab in the North Central Cancer Treatment Group N9831 adjuvant breast cancer trial. J Clin Oncol 26(8): 1231–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccart-Gebhart MJ, Holmes AP, Baselga J, De Azambuja E, Dueck AC, Viale G, Zujewski JA, Goldhirsch A, Santillana S, Pritchard KI, Wolff AC, Jackisch C, Lang I, Untch M, Smith IE, Boyle F, Xu B, Gomez HL, Gelber RD, Perez EA (2014) First results from the phase III ALTTO trial (BIG 2-06; NCCTG [Alliance] N063D) comparing one year of anti-HER2 therapy with lapatinib alone (L), trastuzumab alone (T), their sequence (T→L), or their combination (T+L) in the adjuvant treatment of HER2-positive early breast cancer (EBC). J Clin Oncol 32(5s): abstr LBA4. [Google Scholar]
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, Cameron D, Dowsett M, Barrios CH, Steger G, Huang CS, Andersson M, Inbar M, Lichinitser M, Lang I, Nitz U, Iwata H, Thomssen C, Lohrisch C, Suter TM, Ruschoff J, Suto T, Greatorex V, Ward C, Straehle C, McFadden E, Dolci MS, Gelber RD Herceptin Adjuvant (HERA) Trial Study Team (2005) Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med 353(16): 1659–1672. [DOI] [PubMed] [Google Scholar]
- Pivot X, Romieu G, Debled M, Pierga JY, Kerbrat P, Bachelot T, Lortholary A, Espie M, Fumoleau P, Serin D, Jacquin JP, Jouannaud C, Rios M, Abadie-Lacourtoisie S, Tubiana-Mathieu N, Cany L, Catala S, Khayat D, Pauporte I, Kramar A PHARE trial investigators (2013) 6 months versus 12 months of adjuvant trastuzumab for patients with HER2-positive early breast cancer (PHARE): a randomised phase 3 trial. Lancet Oncol 14(8): 741–748. [DOI] [PubMed] [Google Scholar]
- Procter M, Suter TM, de Azambuja E, Dafni U, van Dooren V, Muehlbauer S, Climent MA, Rechberger E, Liu WT, Toi M, Coombes RC, Dodwell D, Pagani O, Madrid J, Hall M, Chen SC, Focan C, Muschol M, van Veldhuisen DJ, Piccart-Gebhart MJ (2010) Longer-term assessment of trastuzumab-related cardiac adverse events in the Herceptin Adjuvant (HERA) trial. J Clin Oncol 28(21): 3422–3428. [DOI] [PubMed] [Google Scholar]
- Romond EH, Jeong JH, Rastogi P, Swain SM, Geyer CE Jr., Ewer MS, Rathi V, Fehrenbacher L, Brufsky A, Azar CA, Flynn PJ, Zapas JL, Polikoff J, Gross HM, Biggs DD, Atkins JN, Tan-Chiu E, Zheng P, Yothers G, Mamounas EP, Wolmark N (2012) Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer. J Clin Oncol 30(31): 3792–3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE Jr., Davidson NE, Tan-Chiu E, Martino S, Paik S, Kaufman PA, Swain SM, Pisansky TM, Fehrenbacher L, Kutteh LA, Vogel VG, Visscher DW, Yothers G, Jenkins RB, Brown AM, Dakhil SR, Mamounas EP, Lingle WL, Klein PM, Ingle JN, Wolmark N (2005) Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med 353(16): 1673–1684. [DOI] [PubMed] [Google Scholar]
- Russell SD, Blackwell KL, Lawrence J, Pippen JE Jr., Roe MT, Wood F, Paton V, Holmgren E, Mahaffey KW (2010) Independent adjudication of symptomatic heart failure with the use of doxorubicin and cyclophosphamide followed by trastuzumab adjuvant therapy: a combined review of cardiac data from the National Surgical Adjuvant breast and Bowel Project B-31 and the North Central Cancer Treatment Group N9831 clinical trials. J Clin Oncol 28(21): 3416–3421. [DOI] [PubMed] [Google Scholar]
- Schaller G, Fuchs I, Gonsch T, Weber J, Kleine-Tebbe A, Klare P, Hindenburg HJ, Lakner V, Hinke A, Bangemann N (2007) Phase II study of capecitabine plus trastuzumab in human epidermal growth factor receptor 2 overexpressing metastatic breast cancer pretreated with anthracyclines or taxanes. J Clin Oncol 25(22): 3246–3250. [DOI] [PubMed] [Google Scholar]
- Slamon D, Eiermann W, Robert N, Pienkowski T, Martin M, Press M, Mackey J, Glaspy J, Chan A, Pawlicki M, Pinter T, Valero V, Liu MC, Sauter G, von Minckwitz G, Visco F, Bee V, Buyse M, Bendahmane B, Tabah-Fisch I, Lindsay MA, Riva A, Crown J Breast Cancer International Research Group (2011) Adjuvant trastuzumab in HER2-positive breast cancer. N Engl J Med 365(14): 1273–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L (2001) Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344(11): 783–792. [DOI] [PubMed] [Google Scholar]
- Suter TM, Procter M, van Veldhuisen DJ, Muscholl M, Bergh J, Carlomagno C, Perren T, Passalacqua R, Bighin C, Klijn JG, Ageev FT, Hitre E, Groetz J, Iwata H, Knap M, Gnant M, Muehlbauer S, Spence A, Gelber RD, Piccart-Gebhart MJ (2007) Trastuzumab-associated cardiac adverse effects in the herceptin adjuvant trial. J Clin Oncol 25(25): 3859–3865. [DOI] [PubMed] [Google Scholar]
- Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, Ciruelos E, Ferrero JM, Schneeweiss A, Heeson S, Clark E, Ross G, Benyunes MC, Cortes J, Group CS (2015) Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med 372(8): 724–734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telli ML, Hunt SA, Carlson RW, Guardino AE (2007) Trastuzumab-related cardiotoxicity: calling into question the concept of reversibility. J Clin Oncol 25(23): 3525–3533. [DOI] [PubMed] [Google Scholar]
- Tolaney SM, Barry WT, Dang CT, Yardley DA, Moy B, Marcom PK, Albain KS, Rugo HS, Ellis M, Shapira I, Wolff AC, Carey LA, Overmoyer BA, Partridge AH, Guo H, Hudis CA, Krop IE, Burstein HJ, Winer EP (2015) Adjuvant paclitaxel and trastuzumab for node-negative, HER2-positive breast cancer. N Engl J Med 372(2): 134–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, Pegram M, Oh DY, Dieras V, Guardino E, Fang L, Lu MW, Olsen S, Blackwell K, Group ES (2012) Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med 367(19): 1783–1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF American Society of Clinical OncologyCollege of American Pathologists (2013) Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol 31(31): 3997–4013. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.