Abstract
CONTEXT:
Kangaroo mother care (KMC) is an intervention aimed at improving outcomes among preterm and low birth weight newborns.
OBJECTIVE:
Conduct a systematic review and meta-analysis estimating the association between KMC and neonatal outcomes.
DATA SOURCES:
PubMed, Embase, Web of Science, Scopus, African Index Medicus (AIM), Latin American and Caribbean Health Sciences Information System (LILACS), Index Medicus for the Eastern Mediterranean Region (IMEMR), Index Medicus for the South-East Asian Region (IMSEAR), and Western Pacific Region Index Medicus (WPRIM).
STUDY SELECTION:
We included randomized trials and observational studies through April 2014 examining the relationship between KMC and neonatal outcomes among infants of any birth weight or gestational age. Studies with <10 participants, lack of a comparison group without KMC, and those not reporting a quantitative association were excluded.
DATA EXTRACTION:
Two reviewers extracted data on study design, risk of bias, KMC intervention, neonatal outcomes, relative risk (RR) or mean difference measures.
RESULTS:
1035 studies were screened; 124 met inclusion criteria. Among LBW newborns, KMC compared to conventional care was associated with 36% lower mortality(RR 0.64; 95% [CI] 0.46, 0.89). KMC decreased risk of neonatal sepsis (RR 0.53, 95% CI 0.34, 0.83), hypothermia (RR 0.22; 95% CI 0.12, 0.41), hypoglycemia (RR 0.12; 95% CI 0.05, 0.32), and hospital readmission (RR 0.42; 95% CI 0.23, 0.76) and increased exclusive breastfeeding (RR 1.50; 95% CI 1.26, 1.78). Newborns receiving KMC had lower mean respiratory rate and pain measures, and higher oxygen saturation, temperature, and head circumference growth.
LIMITATIONS:
Lack of data on KMC limited the ability to assess dose-response.
CONCLUSIONS:
Interventions to scale up KMC implementation are warranted.
An estimated 4 million infants die each year during their first 4 weeks of life.1 Although important progress has been made toward Millennium Development Goal 4 to reduce mortality in children <5 years old, less improvement has been achieved in the neonatal period.2 Infants born before term or at low birth weight (LBW) are at elevated risk of neonatal mortality and morbidity, inhibited growth and development, and chronic disease.1,3,4 Health technologies such as incubators can help improve outcomes in high-risk infants; however, such equipment is not widely available in low- and middle-income countries, where 99% of all neonatal deaths occur.1 Effective and low-cost alternative methods of neonatal care are needed.
In 1978, Dr Edgar Rey Sanabria introduced kangaroo mother care (KMC) in Bogotá, Colombia as an alternative to incubators for LBW infants.5 The World Health Organization defines KMC with 4 components: early, continuous, and prolonged skin-to-skin contact (SSC) between the newborn and mother, exclusive breastfeeding, early discharge from the health facility, and close follow-up at home.6 KMC is postulated to improve neonatal outcomes by maintaining the infant’s temperature and other vital sign parameters through SSC and by providing the benefits of breastfeeding.5 These effects are thought to be beneficial for all newborns but may be especially advantageous for preterm infants.
In previous meta-analyses, KMC was found to reduce the risk of morbidity and mortality among LBW infants.7,8 In randomized controlled trials (RCTs), SSC alone has also been associated with improved breastfeeding, cardiorespiratory stability, and improved responses to procedural pain.9,10 Although these reviews have provided important evidence on the effectiveness of KMC, they are limited to specific outcome measures and newborn populations, and they have included only RCTs, with the exception of Lawn et al 2010.7 To give a more complete understanding of the potential benefits and drawbacks of KMC, this systematic review and meta-analysis aims to provide a comprehensive summary of observational studies and RCTs on KMC and neonatal outcomes.
Methods
Search Strategy and Selection Criteria
The literature search for this review included original reports, direct queries of authors of published articles, and program reports. We identified studies through electronic database searches of PubMed, Embase, Web of Science, Scopus, African Index Medicus (AIM), Latin American and Caribbean Health Sciences Information System (LILACS), Index Medicus for the Eastern Mediterranean Region (IMEMR), Index Medicus for the South-East Asian Region (IMSEAR), and Western Pacific Region Index Medicus (WPRIM) by using the terms “kangaroo mother care,” “kangaroo care,” and “skin to skin care” through April 24, 2014. We also conducted hand searches of reference lists of published systematic reviews. To search the gray literature for unpublished studies, we explored programmatic reports and requested data from programs implementing KMC.
We included studies using any definition of KMC with at least the SSC component and any neonatal outcome. We excluded studies with nonhuman subjects, <10 participants, nonprimary data collection or analysis, lack of a comparison group without KMC, and those that did not report a quantitative effect measure. We did not limit our analysis to studies of newborns with a specific gestational age or birth weight.
Data Abstraction
All article abstracts were screened by 2 independent reviewers. When eligibility for inclusion was unclear from the abstract, the full text was screened. Two reviewers then abstracted data from all articles meeting the inclusion criteria. At each stage, reviewers compared results to ensure agreement, and in cases of disagreement, a third party acted as tiebreaker. Data from articles in English, Spanish, and Portuguese were abstracted by 2 fluent speakers. For articles in less common languages, a single native speaker abstracted data. If an article was missing key information, we contacted authors by e-mail to request data.
We collected information on study design, setting, participant characteristics, description of KMC and comparison groups, follow-up time, outcomes, assessment of bias, and measures of association. Relative risks (RRs) or mean difference (MD) effect estimates with 95% confidence intervals (CIs) were extracted. We collected exposure data on KMC components, clinical stabilization criteria for starting KMC, and duration of SSC promoted and practiced.
Study Quality
Two independent reviewers assessed the methodological quality of studies in 5 domains: selection bias, information bias, detection bias, attrition bias, and other bias.11 For observational studies, an additional domain for confounding was assessed. Each domain was categorized as high, low, or unclear risk of bias. We then created an overall assessment of bias for each study. For RCTs, if both selection and information bias were low risk, the overall risk of bias was considered low. If either domain was high risk, the overall risk of bias was designated as high. For observational studies, if selection bias, information bias, and confounding were all low risk, the overall risk of bias was considered low. If any of those 3 domains was high risk, the overall risk was considered high. Otherwise, the overall risk of bias was considered unclear.
Statistical Analysis
We used the random effects estimator to assess the effect of KMC compared with conventional care on each neonatal outcome.12 For dichotomous outcomes, we report summary estimates as RR and 95% CI. For studies that did not report an RR, we calculated the RR and SE from the data if available. For continuous outcomes, we report summary estimates as MD and 95% CI. When different units or scales were used across studies, we calculated the standardized mean difference (SMD).11 When available, estimates adjusted for confounding were used rather than crude. Estimates that were presented only as medians rather than means, only as odds ratios without raw data to calculate the RR, or those with 0 cells were excluded from summary measures.13–28
We performed sensitivity analyses by restricting the analyses to RCTs and adjusted RR estimates for dichotomous outcomes and by restricting the analyses to RCTs and randomized crossover studies for continuous outcomes. To assess between-study heterogeneity, we report the I2 statistic and the P value for the Q statistic.12,29,30 The I2 statistic quantifies the amount of variation in the effect estimate attributable to between-study heterogeneity, reported as a percentage, with a higher I2 indicating more heterogeneity. We conducted subgroup analyses and metaregression for outcomes with data from ≥10 studies. We explored these predetermined subgroups: year, study type, sample size, location, country-level economy,31 country-level neonatal mortality rate in 2013,32 infant gestational age and birth weight, KMC components, KMC initiation criteria, SSC duration, and study quality classification. In metaregression analyses, we report the residual I2, indicating the amount of remaining heterogeneity in the effect estimate after adjustment for a given characteristic. The indicator method was used for missing covariate information in metaregressions.33
We assessed publication bias by visual inspection of funnel plots of effect size and SE for asymmetry and by Begg’s rank correlation and Egger’s linear regression tests.34,35 We conducted meta-analyses by using Stata statistical software (version 13.1), and risk of bias figures were created in RevMan software (version 5.3).
Results
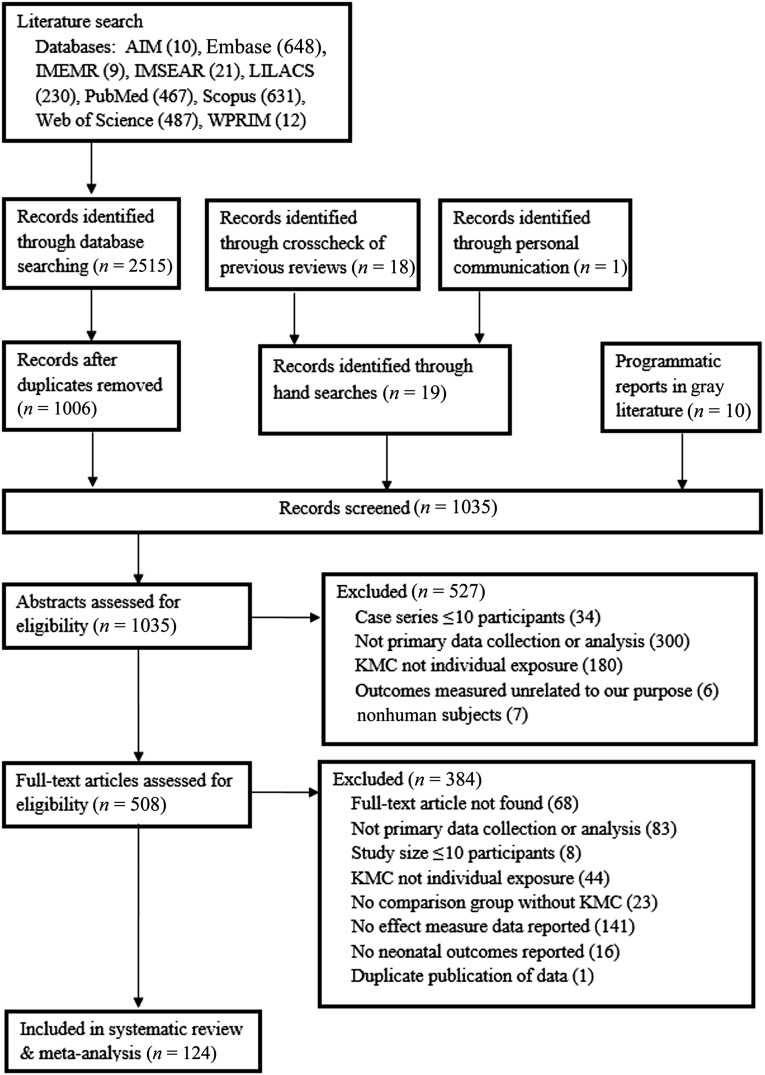
Our search identified 2515 records (Fig 1). We then identified 29 additional records related to KMC through crosscheck of reference lists, communication with an author,36 and programmatic reports. After 1006 duplicates were removed, 1035 records underwent abstract screening. Of those, 527 did not meet inclusion criteria. Full-text articles for the remaining 508 records were then assessed and of those, 384 did not meet inclusion criteria. This review and meta-analysis includes 124 studies that reported an association between KMC and ≥1 neonatal outcome. One hundred eleven (90%) were in English, 7 (6%) in Portuguese, 4 (3%) in Spanish, and 2 (2%) in Farsi. We e-mailed 8 authors to obtain additional information37–44 and received a response with data from 2.38,39
FIGURE 1.
Flow diagram for identification of included studies.
Study Characteristics
Of the 124 included studies, 110 (89%) were published between 2000 and 2014 (Table 1). Seventy-six studies (61%) had <100 participants. Fifty-five (44%) were RCTs, 8 (6%) were randomized crossover trials, and 61 (49%) were observational or nonrandomized intervention studies. Most studies (n = 113, 94%) were in middle- or high-income countries and were conducted in health facilities (n = 118, 98%).
TABLE 1.
Characteristics of Included Studies (n = 124)
| Characteristic | Number of Studies, n (%) |
|---|---|
| Year of publication | |
| 1988–1999 | 14 (11) |
| 2000–2009 | 58 (47) |
| 2010–2014 | 52 (42) |
| Sample size | |
| <50 | 43 (35) |
| 50–<100 | 33 (27) |
| 100–<200 | 21 (17) |
| ≥200 | 27 (22) |
| Study type | |
| RCT | 55 (44) |
| Cohort | 17 (14) |
| Pre–post | 23 (19) |
| Intervention trial (nonrandomized) | 8 (6) |
| Randomized crossover | 8 (6) |
| Crossover (nonrandomized) | 3 (2) |
| Case–control | 2 (2) |
| Chart review | 5 (4) |
| Facility evaluation | 2 (2) |
| Interview or survey | 1 (1) |
| Region (World Health Organization) | |
| Africa | 11 (9) |
| Americas | 50 (41) |
| Eastern Mediterranean | 11 (9) |
| Europe | 19 (16) |
| Southeast Asia | 20 (17) |
| Western Pacific | 9 (7) |
| Multiple | 1 (1) |
| Country-level economy (World Bank) | |
| Low income | 7 (6) |
| Middle income | 65 (54) |
| High income | 48 (40) |
| Multiple | 1 (1) |
| Country-level neonatal mortality ratio, deaths/1000 live births | |
| <5 | 52 (43) |
| 5–<15 | 36 (30) |
| 15–<30 | 29 (24) |
| ≥30 | 4 (3) |
| Setting | |
| Urban | 92 (90) |
| Rural | 4 (4) |
| Mixed | 6 (6) |
| Facility type | |
| NICU or step-down unit | 51 (42) |
| Health facility | 67 (55) |
| Community or population based | 3 (2) |
| Gestational age at birth | |
| Preterm, <37 wk | 34 (38) |
| Very preterm, <34 wk | 27 (30) |
| Full-term, ≥35–37 wk | 17 (19) |
| All gestational ages | 11 (12) |
| Comparison: preterm vs full term | 1 (1) |
| Birth wt | |
| LBW, ≤2500 g | 47 (58) |
| Very LBW, ≤1500 g | 15 (19) |
| Normal birth wt, ≥2500 g | 9 (11) |
| All birth weights | 10 (12) |
| KMC components | |
| SSC only | 71 (68) |
| SSC + EBF | 14 (13) |
| SSC + EBF + DC | 1 (1) |
| SSC + EBF + DC + FU | 4 (4) |
| SSC + DC | 1 (1) |
| SSC + DC + FU | 7 (7) |
| SSC + EBF + FU | 7 (7) |
| SSC initiation time | |
| Immediately after birth | 7 (8) |
| After stability criteria met | 41 (48) |
| After other criteria met | 27 (31) |
| For a painful procedure | 11 (13) |
| SSC duration promoted, h/d | |
| <2 | 38 (48) |
| 2–<4 | 14 (18) |
| 4–<9 | 6 (8) |
| 9–<12 | 0 |
| 12–<22 | 1 (1) |
| ≥22 | 20 (25) |
| Number of days of SSC promoted | |
| 1–5 | 47 (75) |
| 6–<30 | 9 (14) |
| ≥30 | 2 (3) |
| Dependent on hospital stay | 5 (8) |
DC, early discharge; EBF, exclusive breastfeeding; FU, follow-up after discharge.
Among studies reporting gestational age, the majority (n = 61, 68%) were among preterm infants <37 weeks’ gestation; 17 (19%) were among full-term infants, defined as ≥37 weeks, and 12 (13%) were among infants of all gestational ages. Similarly, 47 studies (58%) were among LBW infants (≤2500 g), an additional 15 (19%) were among very LBW infants (≤1500 g), 9 (11%) were among non-LBW infants, and 10 (12%) were among infants of all birth weights. Forty-three studies (35%) did not specify infants’ birth weight, and 34 (27%) did not specify gestational age, but all studies reported either birth weight or gestational age, except for 1.45
Most studies (n = 71, 68%) defined KMC as SSC only, 14 (13%) defined KMC as SSC plus promotion of exclusive breastfeeding, 20 (19%) included an early discharge or follow-up component, and 19 (15%) did not describe the components of their KMC intervention. SSC was initiated immediately after birth in 7 studies (8%), whereas 41 (48%) had stability criteria to be met before SSC initiation, and 27 (31%) had other non–stability-related initiation criteria. Eleven studies (14%) looking at pain-related outcomes started SSC around the time of an infant procedure. Fifty-two studies (66%) promoted <4 hours of SSC per day, 20 (25%) promoted ≥22 hours per day, and few studies (n = 7, 9%) had a duration between 4 and 21 hours per day. Thirty-eight studies (31%) did not specify when SSC was initiated, and 45 studies (36%) did not report the daily duration of SSC mothers were instructed to practice. Information on duration of SSC actually practiced rather than promoted was only available in 16 studies (13%). Details of each included study are presented in Supplemental Table 16.
Meta-analysis
Summary RR estimates for dichotomous outcomes are reported in Table 2, and MD estimates for continuous outcomes are reported in Table 3.
TABLE 2.
RR and 95% CI for the Effect of KMC Compared With Conventional Care on Dichotomous Neonatal Outcomes
| Outcome | All Studies | RCT and Adjusted Observational Studies | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | RR (95% CI)a | P | Test for Heterogeneity (P) | I2, %b | n | RR (95% CI)a | p | Test for Heterogeneity (P) | I2, %b | |
| Mortality | ||||||||||
| Latest follow-up46–61 | 16 | 0.77 (0.60 to 0.99) | .05 | <.01 | 67 | 12 | 0.95 (0.73 to 1.23) | .69 | .13 | 32 |
| ≤45 d46–55,58 | 11c | 0.79 (0.57 to 1.10) | .17 | <.01 | 77 | 7 | 1.16 (0.91 to 1.47) | .23 | .29 | 18 |
| 3–12 mo46,47,56,57,59–61 | 7c | 0.59 (0.43 to 0.82) | <.01 | .63 | 0 | 6 | 0.67 (0.47 to 0.96) | .03 | .88 | 0 |
| LBW <2000 g46–54,56–61 | 15 | 0.64 (0.46 to 0.89) | .01 | <.01 | 72 | 11 | 0.86 (0.59 to 1.24) | .41 | .10 | 38 |
| All birth weights50,55 | 2 | 1.04 (0.82 to 1.33) | .73 | .83 | 0 | 1 | 1.06 (0.80 to 1.41) | .70 | — | — |
| Exclusive breastfeeding | ||||||||||
| Discharge or 40–41 wk PMA28,50,59,62–71 | 13 | 1.50 (1.26 to 1.78) | <.01 | <.01 | 93 | 8 | 1.25 (1.10 to 1.42) | <.01 | <.01 | 59 |
| 1–4 mo old45,62,63,65,69,72–74 | 8 | 1.39 (1.11 to 1.74) | .01 | .02 | 60 | 6 | 1.53 (1.08 to 2.18) | .02 | <.01 | 71 |
| Other | ||||||||||
| Infection15,27,28,48,52,53,58,60,65,67,75,76 | 12 | 0.67 (0.43 to 1.05) | .08 | <.01 | 60 | 10 | 0.60 (0.36 to 1.01) | 0.05 | <.01 | 65 |
| Sepsis15,27,28,48,52,53,58,65 | 8 | 0.53 (0.34 to 0.83) | .01 | .23 | 25 | 7 | 0.44 (0.29 to 0.66) | <.01 | .49 | 0 |
| NEC49,58,65 | 3 | 0.96 (0.45 to 2.04) | .92 | .45 | 0 | 3 | 0.96 (0.45 to 2.04) | .92 | .45 | 0 |
| Hypothermia15,18,36,48,52,58,65,77,78 | 9 | 0.22 (0.12 to 0.41) | <.01 | <.01 | 71 | 7 | 0.28 (0.15 to 0.53) | <.01 | .01 | 65 |
| Hyperthermia15,48,52 | 3 | 0.77 (0.59 to 1.01) | .06 | .88 | 0 | 3 | 0.77 (0.59 to 1.01) | .06 | .88 | 0 |
| Apnea27,46,48,52,58,65 | 6 | 0.39 (0.13 to 1.14) | .09 | .12 | 42 | 6 | 0.39 (0.13 to 1.14) | .09 | .12 | 42 |
| Hypoglycemia27,48 | 2 | 0.12 (0.05 to 0.32) | <.01 | .53 | 0 | 2 | 0.12 (0.05 to 0.32) | <.01 | .53 | 0 |
| Readmission60,74 | 2 | 0.42 (0.23 to 0.76) | <.01 | 1.00 | 0 | 1 | 0.42 (0.14 to 1.29) | .13 | — | — |
TABLE 3.
MD and 95% CI for the Effect of KMC Compared With Conventional Care on Continuous Neonatal Outcomes
| Outcome | All Studies | RCT and Randomized Crossover Studies | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | MD (95% CI)a | P | Test for Heterogeneity (P) | I2, %b | n | MD (95% CI)a | P | Test for Heterogeneity (P) | I2, %b | |
| Vital signs | ||||||||||
| Heart rate, beats/min38,65,79–91 | 15 | −0.41 (−2.25 to 1.42) | .66 | .03 | 46 | 2 | 0.04 (−1.60 to 1.68) | .96 | .60 | 0 |
| Respiratory rate, breaths/min52,65,79,81,83–90 | 12 | −3.17 (−5.15 to −1.19) | <0.01 | <.01 | 75 | 3 | −5.49 (-8.80 to −2.18) | <.01 | <.01 | 88 |
| Oxygen saturation, %52,65,79–81,83–90,92 | 14 | 0.90 (0.35 to 1.45) | <.01 | <.01 | 92 | 4 | 1.28 (0.39 to 2.17) | .01 | <.01 | 86 |
| Temperature, °C65,78–83,85–87,89,93–95 | 14 | 0.24 (0.15 to 0.33) | <.01 | <.01 | 82 | 3 | 0.24 (0.04 to 0.44) | .02 | <.01 | 91 |
| Breastfeeding initiation time, SMD48,52,96,97 | 4 | −1.07 (−2.30 to 0.17) | .09 | <.01 | 97 | 4 | −1.07 (−2.30 to 0.17) | .09 | <.01 | 97 |
| Growth | ||||||||||
| Wt change, g14,18,53,59,98 | 5 | 3.29 (−4.95 to 11.52) | .43 | .02 | 67 | 2 | 8.30 (1.16 to 15.43) | .02 | .31 | 3 |
| Wt change, g/day28,48,58,99,100 | 5 | 2.58 (−0.51 to 5.67) | .10 | <.01 | 81 | 4 | 3.04 (−1.35 to 7.43) | .18 | <.01 | 84 |
| Wt change, g/kg/day49,99 | 2 | −0.84 (−3.39 to 1.70) | .52 | .02 | 81 | 0 | — | — | — | — |
| Wt change, SMD14,18,28,48,49,53,58,59,98–100 | 11 | 0.16 (−0.08 to 0.40) | .21 | <.01 | 81 | 6 | 0.33 (−0.05 to 0.70) | .09 | <.01 | 83 |
| Length change, cm/wk18,96 | 2 | 0.15 (−0.09 to 0.39) | 0.21 | .03 | 79 | 2 | 0.15 (−0.09 to 0.39) | .21 | .03 | 79 |
| Length change, SMD18,48,58 | 3 | 0.24 (−0.02 to 0.49) | .07 | .26 | 25 | 3 | 0.24 (−0.02 to 0.49) | .07 | .26 | 25 |
| Head circumference change, cm/wk18,28,48 | 3 | 0.19 (0.01 to 0.37) | .04 | <.01 | 89 | 3 | 0.19 (0.01 to 0.37) | .04 | <.01 | 89 |
| Head circumference change, SMD18,28,48,58 | 4 | 0.61 (0.20 to 1.02) | <.01 | <.01 | 77 | 4 | 0.61 (0.20 to 1.02) | <.01 | <.01 | 77 |
| Pain | ||||||||||
| Pain score, SMD38,101–109 | 10 | −0.63 (−1.09 to −0.16) | .01 | <.01 | 89 | 8 | −0.75 (−1.28 to −0.22) | .01 | <.01 | 89 |
| Premature Infant Pain Profile score, 0–2138,103–105,107–109 | 7 | −0.83 (−1.53 to −0.13) | .02 | <.01 | 88 | 5 | −0.98 (−1.83 to −0.13) | .02 | <.01 | 91 |
| Neonatal Infant Pain Scale score, 0–738,102,106 | 3 | −1.14 (−2.34 to 0.05) | .06 | <.01 | 85 | 2 | −1.21 (−2.88 to 0.45) | .15 | <.01 | 91 |
| Neonatal Facial Coding System score, 0–10101,102 | 2 | −1.40 −3.08 to 0.28) | .10 | <.01 | 91 | 2 | −1.40 (−3.08 to 0.28) | .10 | <.01 | 91 |
| Crying duration after painful stimulus, s110–112 | 3 | −11.30(-19.79 to-2.80) | .01 | .80 | 0 | 3 | −11.30(−19.79 to −2.80) | .01 | .80 | 0 |
| Heart rate during painful stimulus, beats/min111–113 | 3 | −7.46 (−12.98 to −1.93) | .01 | .25 | 29 | 3 | −7.46 (−12.98 to −1.93) | .01 | .25 | 29 |
| Heart rate after painful stimulus, beats/min101,103,114,115 | 4 | −4.00 (−8.93 to 0.93) | .11 | <.01 | 87 | 3 | −7.52 (−8.47 to −6.58) | <.01 | .54 | 0 |
| Other | ||||||||||
| Length of hospital stay, days14,18,48,49,52,53,68,71,99,100,116,117 | 12 | −0.68 (−2.11 to 0.75) | .35 | <.01 | 95 | 5 | −0.38 (−2.99 to 2.23) | .78 | <.01 | 91 |
| Cortisol, SMD38,95,107 | 3 | −0.44 (−0.94 to 0.06) | .08 | .12 | 54 | 2 | −0.58 (−0.88 to −0.29) | <.01 | .33 | 0 |
SMD, standardized mean difference.
Random effects MD.
I2: variation in RR or MD attributable to heterogeneity.
Mortality
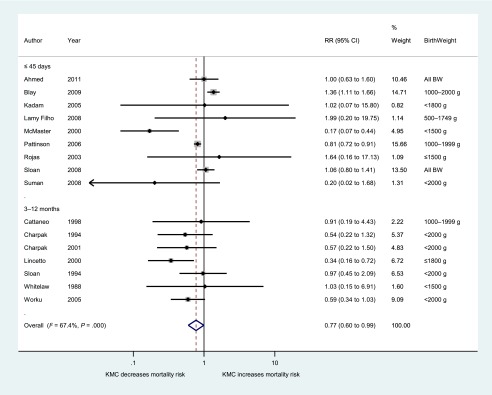
Compared with conventional care, KMC was associated with a 23% lower risk of mortality at each study’s latest follow-up time (n = 16; 95% CI, 0.60, 0.99; I2 = 67%) (Fig 2). Among 11 studies reporting mortality during the first 45 days of life, there was nonsignificant 21% decrease in mortality with KMC (95% CI, 0.57 to 1.10; I2 = 77%), whereas the 7 studies reporting mortality at 3, 6, or 12 months of age showed 41% lower mortality in the KMC groups compared with controls (95% CI, 0.43 to 0.82; I2 = 0%) (Table 2).
FIGURE 2.
Forest plot for effect of KMC compared with conventional care on mortality at latest follow-up time, grouped by follow-up time. BW, birth weight.
Among LBW newborns <2000 g, KMC decreased mortality at latest follow-up time by 36% (n = 15; 95% CI, 0.46 to 0.89; I2 = 72%). In the 2 studies of infants of all birth weights, KMC did not significantly affect mortality (RR 1.04; 95% CI, 0.82 to 1.33; I2 = 0%). Additional subgroup analyses of study characteristics and KMC components for mortality at latest follow-up are presented in Supplemental Table 4. We did not find important differences in the effect of KMC on mortality by location, country-level economy, or neonatal mortality rate. Two studies whose KMC intervention included SSC, exclusive breastfeeding, early discharge, and close follow-up showed a stronger protective effect of KMC against mortality (RR 0.43; 95% CI, 0.19 to 0.98) than studies using other KMC definitions. Similarly, when mothers were encouraged to provide SSC plus ≥1 other component, KMC was protective against mortality (n = 9; RR 0.65; 95% CI, 0.48 to 0.89), whereas studies where KMC was defined as SSC alone did not (n = 5; RR 0.71; 95% CI, 0.33 to 1.52). There was no difference in mortality between studies including promotion of exclusive breastfeeding in their KMC definition compared with those that did not.
Studies instructing mothers to start SSC after stability criteria was met showed a similarly protective effect against mortality (n = 9, RR 0.57; 95% CI, 0.34 to 0.97) as those that started SSC immediately (n = 3, RR 0.51; 95% CI, 0.33 to 0.78) (Supplemental Fig 3). Eleven studies promoting ≥22 hours of SSC per day showed a protective effect of KMC (RR 0.64; 95% CI, 0.44 to 0.92) on mortality, whereas there was no association in the 1 study promoting 4 to 8 hours per day or the 4 studies that did not define SSC duration (Supplemental Fig 4).
Breastfeeding
KMC increased the likelihood of exclusive breastfeeding at hospital discharge or 40 to 41 weeks postmenstrual age by 50% (n = 13; 95% CI, 1.26 to 1.78; I2 = 93%) (Supplemental Fig 5). KMC increased the likelihood of exclusive breastfeeding across nearly all subgroups of study, infant, and KMC characteristics (Supplemental Table 5). At 1- to 4-month follow-up, KMC increased the likelihood of exclusive breastfeeding by 39% (n = 8; 95% CI, 1.11 to 1.74; I2 = 60%) (Table 2). KMC did not have a significant impact on the MD in time to breastfeeding initiation (n = 4; SMD −1.07; 95% CI, −2.30 to 0.17; I2 = 97%) (Table 3). Several studies looked at other feeding outcomes that were too heterogeneous to combine into a summary estimate.62–64,72,77,96,118,119
Infection
Risk of infection during study follow-up was not statistically different between KMC and control groups (n = 12; RR 0.67; 95% CI, 0.43 to 1.05; I2 = 60%) (Table 2). When data were stratified by infection type, however, KMC was associated with 47% lower risk of sepsis (n = 8; 95% CI, 0.34 to 0.83; I2 = 25%) but did not have an effect on methicillin-resistant Staphylococcus aureus or other severe infections (n = 4; RR 1.00; 95% CI, 0.40 to 2.46; I2 = 77%) (Supplemental Fig 6). KMC did not have a significant effect on risk of necrotizing enterocolitis (n = 3; RR 0.96; 95% CI, 0.45 to 2.04) (Table 2). All studies that examined sepsis and necrotizing enterocolitis were among infants <2250 g at birth.
Among RCTs, KMC decreased risk of infection by 49% (n = 9; 95% CI, 0.32 to 0.81) (Supplemental Table 6). Nine studies that had stability criteria before initiating SSC showed a protective effect of KMC against infection (RR 0.50; 95% CI, 0.33 to 0.77), whereas the 2 studies that had other non–stability-related criteria before initiation did not (RR 1.00; 95% CI, 0.69 to 1.45).
Heart Rate
KMC did not have a significant effect on mean heart rate (n = 15; MD 0.41 beats per minute; 95% CI, −2.25 to 1.42; I2 = 46%) (Supplemental Fig 7). No statistical or clinically significant differences were noted in subgroup analysis of study, infant, or KMC characteristics (Supplemental Table 7).
Respiration and Oxygenation
Compared with conventional care, KMC was associated with a non–statistically significant reduction in risk of apnea among 6 studies of LBW infants <2000 g (RR 0.39; 95% CI, 0.13 to 1.14; I2 = 42%) (Table 2). On average, newborns receiving KMC had a respiratory rate 3 breaths per minute slower (n = 12; 95% CI, −5.15 to −1.19; I2 = 75%) and oxygen saturation 0.9% higher than controls (n = 14; 95% CI, 0.35 to 1.45; I2 = 92%) (Supplemental Figs 8 and 9). Across subgroup analyses, KMC was associated with lower respiratory rate and higher oxygen saturation (Supplemental Tables 8 and 9).
Temperature
Compared with conventional care, KMC was associated with 78% lower risk of hypothermia (n = 9; 95% CI, 0.12 to 0.41; I2 = 71%) and 23% lower risk of hyperthermia (n = 3; 95% CI, 0.59 to 1.01; I2 = 0%) (Table 2). Mean body temperature of infants receiving KMC was 0.24°C higher than in controls (n = 14; 95% CI, 0.15 to 0.33; I2 = 82%) (Supplemental Fig 10). This effect was similar across subgroups of study, infant, and KMC characteristics (Supplemental Table 10).
Hypoglycemia and Cortisol
KMC was strongly protective against hypoglycemia in 2 studies of LBW infants (RR 0.12; 95% CI, 0.05 to 0.32; I2 = 0%) (Table 2). Standardized mean cortisol levels were not significantly different between KMC and control groups (n = 3; SMD −0.44; 95% CI, −0.94 to 0.06; I2 = 54%) (Table 3).
Hospital Stay
KMC decreased the likelihood of hospital readmission by 58% in 2 studies (95% CI, 0.23 to 0.76; I2 = 0%) (Table 2). Length of hospital stay did not differ significantly between KMC and control groups (n = 12; MD −0.68 days; 95% CI, −2.11 to 0.75; I2 = 95%) (Supplemental Fig 11, Supplemental Table 11). One study reported length of hospital and NICU stays stratified by birth weight and found shorter hospital stays in the KMC group compared with controls among infants <1500 g and in length of NICU stay among infants 1201 to 1500 g.120
Growth
Various infant growth outcomes were examined across studies. We looked at the effect of KMC on measures of weight gain individually and by combining them using the SMD (Table 3, Supplemental Fig 12). We did not find a significant association between KMC and the SMD in weight gain or body length growth. Infants receiving KMC had head circumference growth 0.19 cm per week higher than controls in 3 studies of infants <2000 g at birth (95% CI, 0.01 to 0.37; I2 = 89%). Among studies reporting weight gain outcomes, there were no important differences in the effect of KMC by subgroups of study, infant, or KMC characteristics (Supplemental Table 12). One additional study examined the risk of being malnourished, overweight, or obese at 5 to 6 years old and found no difference between the KMC and control groups.121
Pain
Several studies examined pain-related outcomes, including crying, heart rate, and pain scores during and after painful procedures (Table 3). According the Premature Infant Pain Profile scale, with a range from 0 to 21, infants receiving SSC during a painful procedure had a mean pain score 0.83 points lower than controls (n = 7; 95% CI, −1.53 to −0.13; I2 = 88%).122 Studies using the Neonatal Infant Pain Scale123 (n = 3) and the Neonatal Facial Coding System121,124 (n = 2) showed nonsignificant decreases in pain among infants receiving SSC during painful procedures compared with controls. When combined across scales using the SMD, a decrease in pain score was again noted in infants receiving SSC compared with conventional care (SMD −0.63; 95% CI, −1.09 to −0.16; I2 = 89%) (Supplemental Fig 13). This effect was similar across subgroups (Supplemental Table 13).
After a painful stimulus, infants receiving SSC cried on average 11 seconds less than control group infants (n = 3; 95% CI, −19.79 to −2.80; I2 = 0%) (Table 3). Among studies using infant heart rate during painful stimulus as a proxy pain measure, mean heart rate was 7 beats per minute slower in the SSC groups than controls (n = 3; 95% CI, −12.98 to −1.93; I2 = 29%).
Other Outcomes
A variety of other neonatal outcomes were reported in a single study or in different ways across studies that could not be combined into a summary measure. Those outcomes related to illness included retinopathy, bronchopulmonary dysplasia, regurgitation, respiratory tract disease, diarrhea, and intraventricular hemorrhage.48,49,58,60,125 Other outcomes included hyperbilirubinemia, blood pressure, stratum corneum hydration, oxygen requirement, carbon dioxide production, low-frequency/high-frequency ratio, thyroid measures, water loss, home observation of the environment, stabilization of cardiopulmonary system, and cost of care.13,44,71,79,80,89,125–139 Several studies also examined the effect of KMC on neurocognitive outcomes. These data were reported across different scales with endpoints at different ages and thus could not be combined into summary measures. They included assessments of behavior, mental and psychomotor development, reflexes, temperament, brain maturation, and sleep.16,61,77,90,91,95,110,113,115,140–151
Risk of Bias
After evaluating 5 domains of bias among the 55 RCTs, we classified 25 (45%) as overall low risk of bias, 14 (25%) as high, and 16 (29%) unclear (Supplemental Table 14; Supplemental Fig 14). When the same 5 bias domains were used plus a domain for confounding for the 69 observational studies, overall risk of bias was considered low in 29 (42%), high in 24 (35%), and unclear in 16 (23%) (Supplemental Table 15; Supplemental Fig 15). When restricted to studies with low overall risk of bias, the protective effects of KMC on mortality, exclusive breastfeeding, and infection were stronger than results obtained with all studies (Supplemental Tables 4–6). Effect estimates for continuous outcomes did not materially change when restricted to studies with low risk of bias (Supplemental Tables 7–13).
Publication Bias
We assessed publication bias for mortality at latest follow-up time, exclusive breastfeeding at discharge, and infection outcomes. No evidence of publication bias was noted for mortality by Begg’s (P = .89) or Egger’s (P = .36) tests or by visual inspection of the funnel plot (Supplemental Fig 16). Similarly, no evidence of publication bias was found for exclusive breastfeeding (Begg’s P = .25; Egger’s P = .12) or infection (Begg’s P = .45; Egger’s P = .75).
Discussion
When compared with conventional care, KMC is associated with decreased mortality among newborns who survive to receive it, particularly among LBW infants. KMC also increases likelihood of exclusive breastfeeding up to 4 months of age and decreases risk of newborn sepsis, hypothermia, hypoglycemia, and hospital readmission. Additionally, infants receiving KMC have improved vital signs, greater head circumference growth, and lower pain scores. We did not find evidence of harm related to KMC.
We found a similar magnitude in reduction of mortality risk among LBW infants exposed to KMC as in previous reviews.7,8 We did not find a significant difference in mortality in the 2 studies including all birth weights, which had not been examined in previous reviews. We noted a similar protective effect of KMC against sepsis and hypothermia, increased likelihood of exclusive breastfeeding, and lower Premature Infant Pain Profile score as described in previous work.8–10 We did not find a significant difference in length of hospital stay, which could reflect differences in study inclusion criteria and infant characteristics compared with a previous review.8 We found greater head circumference growth, but no difference in length or weight gain, with most measurements taken across the hospital stay period. Conde-Agudelo and Díaz-Rossello8 reported an increase in growth parameters for KMC-exposed infants compared with controls at latest follow-up, but as in our results, no important differences in growth measured at discharge or 40 to 41 weeks’ postmenstrual age.8
Although the improvements in respiratory rate, oxygenation, and temperature that we found associated with KMC exposure may each be of modest clinical significance, when taken together they support the hypothesis that KMC improves overall physiologic regulation in the neonate, which could have important effects on other longer-term outcomes. Lower pain measures among infants receiving KMC may also provide additional benefits for LBW infants who experience numerous injections during hospitalization.
This meta-analysis provides a comprehensive picture of the effects of KMC on neonatal health by its inclusion of all study types, outcomes, and infant populations. Therefore, we were able to look at as many studies as available for each outcome and perform sensitivity analyses. We were able to assess the effect of KMC on normal weight and term infants, albeit with limited data, and to examine several outcomes related to vital signs and procedural pain parameters that were not included in the most recent review of KMC among LBW infants.8 We also collected detailed information on study design, newborn characteristics, and KMC components to look for differences in the effect of KMC in subgroup and metaregression analyses.
How much of KMC’s effect is through SSC alone compared with KMC that includes additional components remains unclear because of the sparsity of details available on the KMC intervention practiced in many studies. When they were described, we noted heterogeneity in the definition and components of KMC and conventional care across studies. We attempted to address this limitation by performing subgroup analyses by KMC components, duration, and initiation time. The effects of KMC may be confounded with breastfeeding as a component of KMC. We explored this possibility by comparing subgroups of studies that encouraged exclusive breastfeeding as part of their intervention compared with those that did not; we did not see a consistent difference in effect.
We were limited in our ability to adequately examine the dose–response relationship between duration of SSC and neonatal outcomes because there were few studies with duration of 4 to 21 hours per day, and for any given outcome there was little variation in the SSC duration promoted across studies. We still attempted to look at the data available on SSC duration as a covariate in metaregression analyses, and we found that variation in duration did not appear to have an important impact on the effect of KMC in these data. We could not adequately assess the impact of number of days of SSC because the majority of studies promoted a similar duration of 1 to 5 days.
Conclusions
KMC is protective against a wide variety of adverse neonatal outcomes and has not shown evidence of harm. This safe, low-cost intervention has the potential to prevent many complications associated with preterm birth and may also provide benefits to full-term newborns. The consistency of these findings across study settings and infant populations provides support for widespread implementation of KMC as standard of care for newborns. Additional research is needed to determine the ideal duration and components of KMC. Successful strategies for KMC implementation in various contexts should be disseminated among clinicians and policymakers.
Acknowledgments
The authors would like to acknowledge the contributions to data abstraction and referencing by Stacie Constantian, Tobi Skotnes, Ilana Bergelson, Rodrigo Kuromoto and Eduardo Toledo. We acknowledge Kate Lobner for developing the search strategy.
Glossary
- AIM
African Index Medicus
- CI
confidence interval
- IMEMR
Index Medicus for the Eastern Mediterranean Region
- IMSEAR
Index Medicus for the South-East Asian Region
- KMC
kangaroo mother care
- LBW
low birth weight
- LILACS
Latin American and Caribbean Health Sciences Information System
- MD
mean difference
- RCT
randomized controlled trial
- RR
relative risk
- SMD
standardized mean difference
- SSC
skin-to-skin contact
- WPRIM
Western Pacific Region Index Medicus
Footnotes
Dr Boundy conceptualized and designed the study, conducted the literature review, collected the data, conducted the analyses, created the tables and figures, and drafted and revised the manuscript; Dr Dastjerdi conducted the literature review, collected and cleaned the data, assisted with table and figure creation, and critically reviewed the manuscript; Dr Spiegelman contributed to the study design, statistical analyses, and data interpretation and critically reviewed the manuscript; Drs Fawzi, Missmer, and Lieberman contributed to the study design and data interpretation and critically reviewed the manuscript; Ms Kajeepeta conducted the literature review, collected the data, assisted with figure creation, and critically reviewed the manuscript;Dr Wall contributed to the conceptualization and design of the study and data interpretation and critically reviewed the manuscript; Dr Chan conceptualized and designed the study, designed the data collection instruments, conducted the literature review, coordinated and supervised data collection and analyses, and critically reviewed the manuscript; and all authors approved the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: This study was supported by the Saving Newborn Lives initiative (SNL) of Save the Children. Dr Boundy received support from training grant T32HD060454 in reproductive, perinatal, and pediatric epidemiology from the National Institute of Child Health and Human Development, National Institutes of Health, and training grant T76MC00001 from the Maternal and Child Health Bureau. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Lawn JE, Cousens S, Zupan J; Lancet Neonatal Survival Steering Team . 4 million neonatal deaths: when? Where? Why? Lancet. 2005;365(9462):891–900 [DOI] [PubMed] [Google Scholar]
- 2.Bryce J, Black RE, Victora CG. Millennium Development Goals 4 and 5: progress and challenges. BMC Med. 2013;11:225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization Preterm birth fact sheet no. 363. 2014. Available at: www.who.int/mediacentre/factsheets/fs363/en/. Accessed December 17, 2014
- 4.United Nations Children’s Fund and World Health Organization Low Birthweight: Country, Regional and Global Estimates. New York, NY: UNICEF; 2004 [Google Scholar]
- 5.Charpak N, Ruiz JG, Zupan J, et al. Kangaroo Mother Care: 25 years after. Acta Paediatr. 2005;94(5):514–522 [DOI] [PubMed] [Google Scholar]
- 6.World Health Organization Department of Reproductive Health and Research Kangaroo Mother Care: A Practical Guide. Geneva, Switzerland: World Health Organization; 2003 [Google Scholar]
- 7.Lawn JE, Mwansa-Kambafwile J, Horta BL, Barros FC, Cousens S. ‘Kangaroo mother care’ to prevent neonatal deaths due to preterm birth complications. Int J Epidemiol. 2010;39(Suppl 1):i144–i154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Conde-Agudelo A, Díaz-Rossello JL. Kangaroo mother care to reduce morbidity and mortality in low birthweight infants. Cochrane Database Syst Rev. 2011;3:CD002771. [DOI] [PubMed] [Google Scholar]
- 9.Moore ER, Anderson GC, Bergman N. Early skin-to-skin contact for mothers and their healthy newborn infants. Cochrane Database Syst Rev. 2007;3:CD003519. [DOI] [PubMed] [Google Scholar]
- 10.Johnston CC-Y, Fernandes A, Inglis D, Streiner D, Zee R. Skin-to-skin care for procedural pain in neonates. Cochrane Database Syst Rev. 2014;1:CD008435. [DOI] [PubMed] [Google Scholar]
- 11.Higgins JPT, Green S, eds. Cochrane Handbook for Systematic Reviews of Interventions. London, England: The Cochrane Collaboration; 2011 [Google Scholar]
- 12.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188 [DOI] [PubMed] [Google Scholar]
- 13.Karlsson V, Heinemann AB, Sjörs G, Nykvist KH, Agren J. Early skin-to-skin care in extremely preterm infants: thermal balance and care environment. J Pediatr. 2012;161(3):422–426 [DOI] [PubMed] [Google Scholar]
- 14.Broughton EI, Gomez I, Sanchez N, Vindell C. The cost-savings of implementing kangaroo mother care in Nicaragua. Rev Panam Salud Publica. 2013;34(3):176–182 [PubMed] [Google Scholar]
- 15.Eka Pratiwi IGAP. Soetjiningsih, Made Kardana I. Effect of kangaroo method on the risk of hypothermia and duration of birth weight regain in low birth weight infants: a randomized controlled trial. Paediatr Indones. 2009;49:253–258 [Google Scholar]
- 16.Azevedo VMGO, David RB, Xavier CC. Kangaroo mother care in preterm newborns on artificial ventilation: an evaluation of behavior patterns. Cuidado mãe canguru em recém-nascidospré-termo sob suporte ventilatório: avaliação dos estados comportamentais. Rev Bras Saude Mater Infant. 2011;11(2):133–138 [Google Scholar]
- 17.Kambarami RA, Chidede O, Kowo DT. Kangaroo care versus incubator care in the management of well preterm infants--a pilot study. Ann Trop Paediatr. 1998;18(2):81–86 [DOI] [PubMed] [Google Scholar]
- 18.Acharya N, Singh RR, Bhatta NK, Poudel P. Randomized control trial of kangaroo mother care in low birth weight babies at a tertiary level hospital. J Nepal Paediatr Soci. 2014;34(1):18–23 [Google Scholar]
- 19.Almeida CM, Almeida AFN, Forti EMP. Effects of kangaroo mother care on the vital signs of low-weight preterm newborns. Braz J Phys Ther. 2007;11(1):1–5 [Google Scholar]
- 20.Almeida H, Venancio SI, Sanches MT, Onuki D. The impact of kangaroo care on exclusive breastfeeding in low birth weight newborns. J Pediatr (Rio J). 2010;86(3):250–253 [DOI] [PubMed] [Google Scholar]
- 21.Bohnhorst B, Heyne T, Peter CS, Poets CF. Skin-to-skin (kangaroo) care, respiratory control, and thermoregulation. J Pediatr. 2001;138(2):193–197 [DOI] [PubMed] [Google Scholar]
- 22.Lyngstad LT, Tandberg BS, Storm H, Ekeberg BL, Moen A. Does skin-to-skin contact reduce stress during diaper change in preterm infants? Early Hum Dev. 2014;90(4):169–172 [DOI] [PubMed] [Google Scholar]
- 23.Priya JJ. Kangaroo care for low birth weight babies. Nurs J India. 2004;95(9):209–212 [PubMed] [Google Scholar]
- 24.Saeidi R, Asnaashari Z, Amirnejad M, Esmaeili H, Robatsangi MG. Use of “kangaroo care” to alleviate the intensity of vaccination pain in newborns. Iran J Pediatr. 2011;21(1):99–102 [PMC free article] [PubMed] [Google Scholar]
- 25.Singh A, Yadav A, Singh A. Utilization of postnatal care for newborns and its association with neonatal mortality in India: an analytical appraisal. BMC Pregnancy Childbirth. 2012;12:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Toma TS, Venâncio SI, Andretto DDA. Maternal perception of low birth weight babies before and following the implementation of the kangaroo mother care in a public hospital, in the city of São Paulo, Brazil. Percepção das mães sobre o cuidado do bebê de baixo peso antes e após implantação do método mãe-canguru em hospital público da cidade de São Paulo, Brasil. Rev Bras Saude Mater Infant. 2007;7(3):297–307 [Google Scholar]
- 27.Ghavane S, Murki S, Subramanian S, Gaddam P, Kandraju H, Thumalla S. Kangaroo Mother Care in Kangaroo ward for improving the growth and breastfeeding outcomes when reaching term gestational age in very low birth weight infants. Acta Paediatr. 2012;101(12):e545–e549 [DOI] [PubMed] [Google Scholar]
- 28.Boo NY, Jamli FM. Short duration of skin-to-skin contact: effects on growth and breastfeeding. J Paediatr Child Health. 2007;43(12):831–836 [DOI] [PubMed] [Google Scholar]
- 29.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558 [DOI] [PubMed] [Google Scholar]
- 30.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.World Bank Data: country and lending groups. 2014. Available at: http://data.worldbank.org/about/country-and-lending-groups
- 32.UN Inter-agency Group for Childhood Mortality Estimation (UNICEF, WHO, World Bank, UN DESA Population Division). Data: mortality rate, neonatal (per 1000 live births). 2014. Available at: http://data.worldbank.org/indicator/SH.DYN.NMRT
- 33.Miettinen O. Theoretical Epidemiology: Principles of Occurrence Research in Medicine. New York, NY: John Wiley & Sons; 1985 [Google Scholar]
- 34.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics. 1994;50(4):1088–1101 [PubMed] [Google Scholar]
- 36.Nimbalkar SM, Patel VK, Patel DV, Nimbalkar AS, Sethi A, Phatak A. Effect of early skin-to-skin contact following normal delivery on incidence of hypothermia in neonates more than 1800 g: randomized control trial. J Perinatol. 2014;34(5):364–368 [DOI] [PubMed] [Google Scholar]
- 37.Gray L, Watt L, Blass EM Skin-to-skin contact is analgesic in healthy newborns. Pediatrics 2000;105(1 II):110–111 [DOI] [PubMed]
- 38.Mörelius E, Theodorsson E, Nelson N. Salivary cortisol and mood and pain profiles during skin-to-skin care for an unselected group of mothers and infants in neonatal intensive care. Pediatrics. 2005;116(5):1105–1113 [DOI] [PubMed] [Google Scholar]
- 39.Bigelow A, Power M, MacLellan-Peters J, Alex M, McDonald C. Effect of mother/infant skin-to-skin contact on postpartum depressive symptoms and maternal physiological stress. J Obstet Gynecol Neonatal Nurs. 2012;41(3):369–382 [DOI] [PubMed] [Google Scholar]
- 40.Ludington-Hoe SM, Hosseini R, Torowicz DL. Skin-to-skin contact (Kangaroo Care) analgesia for preterm infant heel stick. AACN Clin Issues. 2005;16(3):373–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ferber SG, Makhoul IR. Neurobehavioural assessment of skin-to-skin effects on reaction to pain in preterm infants: a randomized, controlled within-subject trial. Acta Paediatr. 2008;97(2):171–176 [DOI] [PubMed] [Google Scholar]
- 42.Campbell-Yeo M, Johnston C, Benoit B, et al. Trial of repeated analgesia with Kangaroo Mother Care (TRAKC Trial). BMC Pediatr. 2013;13:182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van Zanten HA, Havenaar AJ, Stigt HJH, Ligthart PAH, Walther FJ. The kangaroo method is safe for premature infants under 30 weeks of gestation during ventilatory support. J Neonatal Nurs. 2007;13(5):186–190 [Google Scholar]
- 44.Ludington-Hoe SM, Swinth JY. Kangaroo mother care during phototherapy: effect on bilirubin profile. Neonatal Netw. 2001;20(5):41–48 [DOI] [PubMed] [Google Scholar]
- 45.Suzuki S. Effect of early skin-to-skin contact on breast-feeding. J Obstet Gynaecol. 2013;33(7):695–696 [DOI] [PubMed] [Google Scholar]
- 46.Charpak N, Ruiz-Pelaez JG, Figueroa de C Z, Charpak Y. A randomized, controlled trial of kangaroo mother care: results of follow-up at 1 year of corrected age. Pediatrics. 2001;108(5):1072–1079 [DOI] [PubMed] [Google Scholar]
- 47.Lincetto O, Nazir AI, Cattaneo A. Kangaroo mother care with limited resources. J Trop Pediatr. 2000;46(5):293–295 [DOI] [PubMed] [Google Scholar]
- 48.Suman RP, Udani R, Nanavati R. Kangaroo mother care for low birth weight infants: a randomized controlled trial. Indian Pediatr. 2008;45(1):17–23 [PubMed] [Google Scholar]
- 49.Lamy Filho F, Silva AA, Lamy ZC, Gomes MA, Moreira ME; Grupo de Avaliação do Método Canguru; Rede Brasileira de Pesquisas Neonatais . Evaluation of the neonatal outcomes of the kangaroo mother method in Brazil. J Pediatr (Rio J). 2008;84(5):428–435 [DOI] [PubMed] [Google Scholar]
- 50.Ahmed S, Mitra SN, Chowdhury AM, Camacho LL, Winikoff B, Sloan NL. Community Kangaroo Mother Care: implementation and potential for neonatal survival and health in very low-income settings. J Perinatol. 2011;31(5):361–367 [DOI] [PubMed] [Google Scholar]
- 51.Blay S. Ghana KMC study results [program report]. 2009
- 52.Kadam S, Binoy S, Kanbur W, Mondkar JA, Fernandez A. Feasibility of kangaroo mother care in Mumbai. Indian J Pediatr. 2005;72(1):35–38 [DOI] [PubMed] [Google Scholar]
- 53.McMaster P, Haina T, Vince JD. Kangaroo care in Port Moresby, Papua New Guinea. Trop Doct. 2000;30(3):136–138 [DOI] [PubMed] [Google Scholar]
- 54.Pattinson RC, Bergh AM, Malan AF, Prinsloo R. Does kangaroo mother care save lives? J Trop Pediatr. 2006;52(6):438–441 [DOI] [PubMed] [Google Scholar]
- 55.Sloan NL, Ahmed S, Mitra SN, et al. Community-based kangaroo mother care to prevent neonatal and infant mortality: a randomized, controlled cluster trial. Pediatrics. 2008;121(5):e1047–e1059 www.pediatrics.org/cgi/content/full/121/5/e1047 [DOI] [PubMed] [Google Scholar]
- 56.Charpak N, Ruiz-Peláez JG, Charpak Y. Rey-Martinez Kangaroo Mother Program: an alternative way of caring for low birth weight infants? One year mortality in a two cohort study. Pediatrics. 1994;94(6 Pt 1):804–810 [PubMed] [Google Scholar]
- 57.Worku B, Kassie A. Kangaroo mother care: a randomized controlled trial on effectiveness of early kangaroo mother care for the low birthweight infants in Addis Ababa, Ethiopia. J Trop Pediatr. 2005;51(2):93–97 [DOI] [PubMed] [Google Scholar]
- 58.Rojas MA, Kaplan M, Quevedo M, et al. Somatic growth of preterm infants during skin-to-skin care versus traditional holding: a randomized, controlled trial. J Dev Behav Pediatr. 2003;24(3):163–168 [DOI] [PubMed] [Google Scholar]
- 59.Cattaneo A, Davanzo R, Worku B, et al. Kangaroo mother care for low birthweight infants: a randomized controlled trial in different settings. Acta Paediatr. 1998;87(9):976–985 [DOI] [PubMed] [Google Scholar]
- 60.Sloan NL, Camacho LW, Rojas EP, Stern C; Maternidad Isidro Ayora Study Team . Kangaroo mother method: randomised controlled trial of an alternative method of care for stabilised low-birthweight infants. Lancet. 1994;344(8925):782–785 [DOI] [PubMed] [Google Scholar]
- 61.Whitelaw A, Heisterkamp G, Sleath K, Acolet D, Richards M. Skin to skin contact for very low birthweight infants and their mothers. Arch Dis Child. 1988;63(11):1377–1381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Thukral A, Sankar MJ, Agarwal R, Gupta N, Deorari AK, Paul VK. Early skin-to-skin contact and breast-feeding behavior in term neonates: a randomized controlled trial. Neonatology. 2012;102(2):114–119 [DOI] [PubMed] [Google Scholar]
- 63.Marín Gabriel MA, Llana Martín I, López Escobar A, Fernández Villalba E, Romero Blanco I, Touza Pol P. Randomized controlled trial of early skin-to-skin contact: effects on the mother and the newborn. Acta Paediatr. 2010;99(11):1630–1634 [DOI] [PubMed] [Google Scholar]
- 64.Heidarzadeh M, Hosseini MB, Ershadmanesh M, Gholamitabar Tabari M, Khazaee S. The effect of kangaroo mother care (KMC) on breast feeding at the time of NICU discharge. Iran Red Crescent Med J. 2013;15(4):302–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ali SMSJ, Sharma R, Alam S. Kangaroo mother care as compared to conventional care for low birth weight babies. Dicle Med J. 2009;36(3):155–160 [Google Scholar]
- 66.Bramson L, Lee JW, Moore E, et al. Effect of early skin-to-skin mother--infant contact during the first 3 hours following birth on exclusive breastfeeding during the maternity hospital stay. J Hum Lact. 2010;26(2):130–137 [DOI] [PubMed] [Google Scholar]
- 67.Charpak N, Ruiz-Peláez JG, Figueroa de C Z, Charpak Y. Kangaroo mother versus traditional care for newborn infants [DOI] [PubMed]
- 68.Gregson S, Blacker J. Kangaroo care in pre-term or low birth weight babies in a postnatal ward. Br J Midwifery. 2011;19(9):568–577 [Google Scholar]
- 69.Hake-Brooks SJ, Anderson GC. Kangaroo care and breastfeeding of mother-preterm infant dyads 0-18 months: a randomized, controlled trial. Neonatal Netw. 2008;27(3):151–159 [DOI] [PubMed] [Google Scholar]
- 70.Niela-Vilén H, Axelin A, Salanterä S, et al. Early physical contact between a mother and her NICU-infant in two university hospitals in Finland. Midwifery. 2013;29(12):1321–1330 [DOI] [PubMed] [Google Scholar]
- 71.Wahlberg V, Affonso DD, Persson B. A retrospective, comparative study using the kangaroo method as a complement to the standard incubator care. Eur J Public Health. 1992;2(1):34–37 [Google Scholar]
- 72.Carfoot S, Williamson PR, Dickson R. The value of a pilot study in breast-feeding research. Midwifery. 2004;20(2):188–193 [DOI] [PubMed] [Google Scholar]
- 73.Ramanathan K, Paul VK, Deorari AK, Taneja U, George G. Kangaroo Mother Care in very low birth weight infants. Indian J Pediatr. 2001;68(11):1019–1023 [DOI] [PubMed] [Google Scholar]
- 74.Acosta Díaz R, Piña Borrego CE, Acosta González LR, López Fernández L. Método piel a piel. Evaluación del neurocomportamiento hasta el año de edad corregida kangaroo mother care method. Evaluation of the neurodevelopment at first year of corrected age. Rev Cubana Pediatr. 2003;75(3) [Google Scholar]
- 75.Park HK, Choi BS, Lee SJ, Son IA, Seol IJ, Lee HJ. Practical application of kangaroo mother care in preterm infants: clinical characteristics and safety of kangaroo mother care. J Perinat Med. 2014;42(2):239–245 [DOI] [PubMed] [Google Scholar]
- 76.Sakaki H, Nishioka M, Kanda K, Takahashi Y. An investigation of the risk factors for infection with methicillin-resistant Staphylococcus aureus among patients in a neonatal intensive care unit. Am J Infect Control. 2009;37(7):580–586 [DOI] [PubMed] [Google Scholar]
- 77.Mohammed El-Nagger NS, Abed El-Azim H, Mahmoud Zaki Hassan S. Effect of kangaroo mother care on premature infants’ physiological, behavioral and psychosocial outcomes in Ain Shams Maternity and Gynecological Hospital, Cairo, Egypt. Life Sci J. 2013;10(1):703–716 [Google Scholar]
- 78.Ibe OE, Austin T, Sullivan K, Fabanwo O, Disu E, Costello AM. A comparison of kangaroo mother care and conventional incubator care for thermal regulation of infants <2000 g in Nigeria using continuous ambulatory temperature monitoring. Ann Trop Paediatr. 2004;24(3):245–251 [DOI] [PubMed] [Google Scholar]
- 79.Maastrup R, Greisen G. Extremely preterm infants tolerate skin-to-skin contact during the first weeks of life. Acta Paediatr. 2010;99(8):1145–1149 [DOI] [PubMed] [Google Scholar]
- 80.Bauer J, Sontheimer D, Fischer C, Linderkamp O. Metabolic rate and energy balance in very low birth weight infants during kangaroo holding by their mothers and fathers. J Pediatr. 1996;129(4):608–611 [DOI] [PubMed] [Google Scholar]
- 81.Begum EA, Bonno M, Ohtani N, et al. Cerebral oxygenation responses during kangaroo care in low birth weight infants. BMC Pediatr. 2008;8:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bergström A, Okong P, Ransjö-Arvidson AB. Immediate maternal thermal response to skin-to-skin care of newborn. Acta Paediatr. 2007;96(5):655–658 [DOI] [PubMed] [Google Scholar]
- 83.Boju SL, Gopi Krishna M, Uppala R, Chodavarapu P, Chodavarapu R. Short spell kangaroo mother care and its differential physiological influence in subgroups of preterm babies. J Trop Pediatr. 2012;58(3):189–193 [DOI] [PubMed] [Google Scholar]
- 84.Collados-Gómez L, Aragonés-Corral B, Contreras-Olivares I, García-Feced E, Vila-Piqueras ME. [Assessing the impact of kangaroo care on preterm infant stress]. Enferm Clin. 2011;21(2):69–74 [DOI] [PubMed] [Google Scholar]
- 85.Legault M, Goulet C. Comparison of kangaroo and traditional methods of removing preterm infants from incubators. J Obstet Gynecol Neonatal Nurs. 1995;24(6):501–506 [DOI] [PubMed] [Google Scholar]
- 86.Ludington-Hoe SM, Anderson GC, Swinth JY, Thompson C, Hadeed AJ. Randomized controlled trial of kangaroo care: cardiorespiratory and thermal effects on healthy preterm infants. Neonatal Netw. 2004;23(3):39–48 [DOI] [PubMed] [Google Scholar]
- 87.Miltersteiner AR, Miltersteiner DR, Rech VV, Molle LD. Respostas fisiológicas da posição mãe-canguru em bebês pré-termos, de baixo peso e ventilando espontaneamente. Physiological responses of the kangaroo mother position in low birth weight, spontaneous ventilating premature babies. Rev Bras Saude Mater Infant. 2003;3(4):447–455 [Google Scholar]
- 88.Tenório EAM, Mota GC, Gutierres SB, et al. Avaliação dos parâmetros fisiológicos em recém-nascidos pré-termos de baixo peso antes e após a aplicação do método mãe-canguru. Evaluation of physiological parameters in preterm newborns with low weight before and after application of the kangaroo mother care. Fisioter Bras. 2010;11(1):44–48 [Google Scholar]
- 89.Lee J, Bang KS. The effects of kangaroo care on maternal self-esteem and premature infants’ physiological stability. Korean J Women Health Nurs. 2011;17(5):454–462 [DOI] [PubMed] [Google Scholar]
- 90.Messmer PR, Rodriguez S, Adams J, et al. Effect of kangaroo care on sleep time for neonates. Pediatr Nurs. 1997;23(4):408–414 [PubMed] [Google Scholar]
- 91.Feldman R, Eidelman AI. Skin-to-skin contact (Kangaroo Care) accelerates autonomic and neurobehavioural maturation in preterm infants. Dev Med Child Neurol. 2003;45(4):274–281 [DOI] [PubMed] [Google Scholar]
- 92.Sajedi F, Kashaninia Z, Rahgozar M, Noghabi FA. The effect of kangaroo care on physiologic responses to pain of an intramuscular injection in neonates. Iran J Pediatr. 2007;17(4):339–344 [DOI] [PubMed] [Google Scholar]
- 93.Heimann K, Jergus K, Abbas AK, Heussen N, Leonhardt S, Orlikowsky T. Infrared thermography for detailed registration of thermoregulation in premature infants. J Perinat Med. 2013;41(5):613–620 [DOI] [PubMed] [Google Scholar]
- 94.Ludington-Hoe SM, Nguyen N, Swinth JY, Satyshur RD. Kangaroo care compared to incubators in maintaining body warmth in preterm infants. Biol Res Nurs. 2000;2(1):60–73 [DOI] [PubMed] [Google Scholar]
- 95.Keshavarz M, Haghighi NB. Effects of kangaroo contact on some physiological parameters in term neonates and pain score in mothers with cesarean section. Koomesh. 2010;11(2):91–99 [Google Scholar]
- 96.Mahmood I, Jamal M, Khan N. Effect of mother-infant early skin-to-skin contact on breastfeeding status: a randomized controlled trial. J Coll Physicians Surg Pak. 2011;21(10):601–605 [DOI] [PubMed] [Google Scholar]
- 97.Aghdas K, Talat K, Sepideh B. Effect of immediate and continuous mother-infant skin-to-skin contact on breastfeeding self-efficacy of primiparous women: a randomised control trial. Women Birth. 2014;27(1):37–40 [DOI] [PubMed] [Google Scholar]
- 98.Ahn HY, Lee J, Shin HJ. Kangaroo care on premature infant growth and maternal attachment and post-partum depression in South Korea. J Trop Pediatr. 2010;56(5):342–344 [DOI] [PubMed] [Google Scholar]
- 99.Rodrigues MAG, Cano MAT. Estudo do ganho de peso e duração da internação do recém-nascido pré-termo de baixo peso com a utilização do método canguru. Trial gain of weight and hospital length stay of the low birth weight preterm infant in assistance for kangaroo mother care. Rev Eletrônica Enferm. 2009;8(2):185–191 [Google Scholar]
- 100.Roberts KL, Paynter C, McEwan B. A comparison of kangaroo mother care and conventional cuddling care. Neonatal Netw. 2000;19(4):31–35 [DOI] [PubMed] [Google Scholar]
- 101.Castral TC, Warnock F, Leite AM, Haas VJ, Scochi CGS. The effects of skin-to-skin contact during acute pain in preterm newborns. Eur J Pain. 2008;12(4):464–471 [DOI] [PubMed] [Google Scholar]
- 102.Chermont AG, Falcão LFM, de Souza Silva EH, de Cássia Xavier Balda R, Guinsburg R. Skin-to-skin contact and/or oral 25% dextrose for procedural pain relief for term newborn infants. Pediatrics. 2009;124(6):e1101–e1107 www.pediatrics.org/cgi/content/full/124/6/e1101 [DOI] [PubMed] [Google Scholar]
- 103.Chidambaram AG, Manjula S, Adhisivam B, Vishnu Bhat B. Effect of kangaroo mother care in reducing pain due to heel prick among preterm neonates: a crossover trial. J Matern Fetal Neonatal Med. 2014;27(5):488–490 [DOI] [PubMed] [Google Scholar]
- 104.Freire NB, Garcia JB, Lamy ZC. Evaluation of analgesic effect of skin-to-skin contact compared to oral glucose in preterm neonates. Pain. 2008;139(1):28–33 [DOI] [PubMed] [Google Scholar]
- 105.Johnston CC, Filion F, Campbell-Yeo M, et al. Kangaroo mother care diminishes pain from heel lance in very preterm neonates: a crossover trial. BMC Pediatr. 2008;8:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Marín Gabriel MA, López Escobar A, Galán Redondo M, et al. [Evaluation of pain in a neonatal intensive care unit during endocrine-metabolic tests]. An Pediatr (Barc). 2008;69(4):316–321 [DOI] [PubMed] [Google Scholar]
- 107.Mitchell AJ, Yates CC, Williams DK, Chang JY, Hall RW. Does daily kangaroo care provide sustained pain and stress relief in preterm infants? J Neonatal Perinatal Med. 2013;6(1):45–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Nanavati RN, Balan R, Kabra NS. Effect of kangaroo mother care vs expressed breast milk administration on pain associated with removal of adhesive tape in very low birth weight neonates: a randomized controlled trial. Indian Pediatr. 2013;50(11):1011–1015 [DOI] [PubMed] [Google Scholar]
- 109.Nimbalkar SM, Chaudhary NS, Gadhavi KV, Phatak A. Kangaroo Mother Care in reducing pain in preterm neonates on heel prick. Indian J Pediatr. 2013;80(1):6–10 [DOI] [PubMed] [Google Scholar]
- 110.Schneider C, Charpak N, Ruiz-Peláez JG, Tessier R. Cerebral motor function in very premature-at-birth adolescents: a brain stimulation exploration of kangaroo mother care effects. Acta Paediatr. 2012;101(10):1045–1053 [DOI] [PubMed] [Google Scholar]
- 111.Kostandy RR, Ludington-Hoe SM, Cong X, et al. Kangaroo Care (skin contact) reduces crying response to pain in preterm neonates: pilot results. Pain Manag Nurs. 2008;9(2):55–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Saeedi R, Asnaashri Z, Amirinejad M, Esmaili H. Effect of kangaroo care method on the pain intensity of vaccination in newborns. Journal of Sabzevar School of Medical Sciences. 2011;13(4):172–177 [Google Scholar]
- 113.Kashaninia Z, Sajedi F, Rahgozar M, Noghabi FA. The effect of Kangaroo Care on behavioral responses to pain of an intramuscular injection in neonates. J Spec Pediatr Nurs. 2008;13(4):275–280 [DOI] [PubMed] [Google Scholar]
- 114.Cong X, Ludington-Hoe SM, McCain G, Fu P. Kangaroo Care modifies preterm infant heart rate variability in response to heel stick pain: pilot study. Early Hum Dev. 2009;85(9):561–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Neu M, Robinson J. Maternal holding of preterm infants during the early weeks after birth and dyad interaction at six months. J Obstet Gynecol Neonatal Nurs. 2010;39(4):401–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Gathwala G, Singh B, Balhara B. KMC facilitates mother baby attachment in low birth weight infants. Indian J Pediatr. 2008;75(1):43–47 [DOI] [PubMed] [Google Scholar]
- 117.Tuoni C, Scaramuzzo RT, Ghirri P, Boldrini A, Bartalena L. Kangaroo Mother Care: four years of experience in very low birth weight and preterm infants. Minerva Pediatr. 2012;64(4):377–383 [PubMed] [Google Scholar]
- 118.Bier JAB, Ferguson AE, Morales Y, et al. Comparison of skin-to-skin contact with standard contact in low-birth-weight infants who are breast-fed. Arch Pediatr Adolesc Med. 1996;150(12):1265–1269 [DOI] [PubMed] [Google Scholar]
- 119.Furman L, Minich N, Hack M. Correlates of lactation in mothers of very low birth weight infants. Pediatrics. 2002;109(4):e57 www.pediatrics.org/cgi/content/full/109/4/e57 [DOI] [PubMed] [Google Scholar]
- 120.Tessier R, Cristo M, Velez S, et al. Kangaroo mother care and the bonding hypothesis. Pediatrics. 1998;102(2):e17 www.pediatrics.org/cgi/content/full/102/2/e17 [DOI] [PubMed] [Google Scholar]
- 121.Acosta Díaz R, Brito Miliáns L, Miliáns Uriarte RD, Morera Betancourt O. Método piel a piel. Repercusión sobre el desarrollo físico-intelectual a la edad preescolar. Kangaroo mother care method. Impact on the physical and intellectual development at preschool age. Rev Cubana Pediatr. 2003;75(3) [Google Scholar]
- 122.Stevens B, Johnston C, Petryshen P, Taddio A. Premature Infant Pain Profile: development and initial validation. Clin J Pain. 1996;12(1):13–22 [DOI] [PubMed] [Google Scholar]
- 123.Lawrence J, Alcock D, McGrath P, Kay J, MacMurray SB, Dulberg C. The development of a tool to assess neonatal pain. Neonatal Netw. 1993;12(6):59–66 [PubMed] [Google Scholar]
- 124.Grunau RV, Craig KD. Pain expression in neonates: facial action and cry. Pain. 1987;28(3):395–410 [DOI] [PubMed] [Google Scholar]
- 125.Christensson K, Bhat GJ, Amadi BC, Eriksson B, Höjer B. Randomised study of skin-to-skin versus incubator care for rewarming low-risk hypothermic neonates. Lancet. 1998;352(9134):1115. [DOI] [PubMed] [Google Scholar]
- 126.Cong X, Cusson RM, Walsh S, Hussain N, Ludington-Hoe SM, Zhang D. Effects of skin-to-skin contact on autonomic pain responses in preterm infants. J Pain. 2012;13(7):636–645 [DOI] [PubMed] [Google Scholar]
- 127.Dageville C, Pignol J, De Smet S. Very early neonatal apparent life-threatening events and sudden unexpected deaths: incidence and risk factors. Acta Paediatr. 2008;97(7):866–869 [DOI] [PubMed] [Google Scholar]
- 128.Mitchell AJYC, Yates C, Williams K, Hall RW. Effects of daily kangaroo care on cardiorespiratory parameters in preterm infants. J Neonatal Perinatal Med. 2013;6(3):243–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Abouelfettoh A, Ludington-Hoe SM, Burant CJ, Visscher MO. Effect of skin-to-skin contact on preterm infant skin barrier function and hospital-acquired infection. J Clin Med Res. 2011;3(1):36–46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Tessier R, Charpak N, Giron M, Cristo M, de Calume ZF, Ruiz-Peláez JG. Kangaroo Mother Care, home environment and father involvement in the first year of life: a randomized controlled study. Acta Paediatr. 2009;98(9):1444–1450 [DOI] [PubMed] [Google Scholar]
- 131.Vesel L, ten Asbroek AH, Manu A, et al. Promoting skin-to-skin care for low birthweight babies: findings from the Ghana Newhints cluster-randomised trial. Trop Med Int Health. 2013;18(8):952–961 [DOI] [PubMed] [Google Scholar]
- 132.Bergman NJ, Linley LL, Fawcus SR. Randomized controlled trial of skin-to-skin contact from birth versus conventional incubator for physiological stabilization in 1200- to 2199-gram newborns. Acta Paediatr. 2004;93(6):779–785 [DOI] [PubMed] [Google Scholar]
- 133.Azevedo VM, Xavier CC, Gontijo FO. Safety of Kangaroo Mother Care in intubated neonates under 1500 g. J Trop Pediatr. 2012;58(1):38–42 [DOI] [PubMed] [Google Scholar]
- 134.Carbasse A, Kracher S, Hausser M, et al. Safety and effectiveness of skin-to-skin contact in the NICU to support neurodevelopment in vulnerable preterm infants. J Perinat Neonatal Nurs. 2013;27(3):255–262 [DOI] [PubMed] [Google Scholar]
- 135.Entringer AP, Gomes MA, Pinto M, Caetano R, Magluta C, Lamy ZC. [Cost analysis of hospital care for newborns at risk: comparison of an Intermediate Neonatal Care Unit and a Kangaroo Unit]. Cad Saude Publica. 2013;29(6):1205–1216 [PubMed] [Google Scholar]
- 136.Nimbalkar SM, Tandon R, Chaudhary NS. Reduced duration of CPAP in preterm babies receiving kangaroo care within an hour of birth: randomized trial. Arch Dis Child. 2012;97:A512 [Google Scholar]
- 137.Samra NM, El Taweel A, Cadwell K. The effect of kangaroo mother care on the duration of phototherapy of infants re-admitted for neonatal jaundice. J Matern Fetal Neonatal Med. 2012;25(8):1354–1357 [DOI] [PubMed] [Google Scholar]
- 138.Weller A, Rozin A, Goldstein A, et al. Longitudinal assessment of pituitary-thyroid axis and adrenal function in preterm infants raised by ‘kangaroo mother care’. Horm Res. 2002;57(1-2):22–26 [DOI] [PubMed] [Google Scholar]
- 139.Anderson GC, Chiu SH, Dombrowski MA, Swinth JY, Albert JM, Wada N. Mother-newborn contact in a randomized trial of kangaroo (skin-to-skin) care. J Obstet Gynecol Neonatal Nurs. 2003;32(5):604–611 [DOI] [PubMed] [Google Scholar]
- 140.Constantinou JC, Adamson-Macedo EN, Stevenson DK, Mirmiran M, Fleisher BE. Effects of skin-to-skin holding on general movements of preterm infants. Clin Pediatr (Phila). 1999;38(8):467–471 [DOI] [PubMed] [Google Scholar]
- 141.Acosta Díaz R, Vara Cuesta OL, Fuentes Guerra JM, Iglesias Castro D, Piloña Ruiz S. Método piel a piel. Evaluación clínica-humoral durante el primer año de edad corregida Kangaroo mother care method. Clinical and humoral assessment during the first year of corrected age. Rev Cubana Pediatr. 2003;75(3) [Google Scholar]
- 142.Ohgi S, Fukuda M, Moriuchi H, et al. Comparison of kangaroo care and standard care: behavioral organization, development, and temperament in healthy, low-birth-weight infants through 1 year. J Perinatol. 2002;22(5):374–379 [DOI] [PubMed] [Google Scholar]
- 143.Kaffashi F, Scher MS, Ludington-Hoe SM, Loparo KA. An analysis of the kangaroo care intervention using neonatal EEG complexity: a preliminary study. Clin Neurophysiol. 2013;124(2):238–246 [DOI] [PubMed] [Google Scholar]
- 144.Saeidi R, Tafazoli M, Robatsangi MG. Kangaroo mother care for infantile colic: a randomized clinical trial. Tehran Univ Med J. 2010;67(12):870–875 [Google Scholar]
- 145.Tessier R, Cristo MB, Velez S, et al. Kangaroo mother care: a method for protecting high-risk low-birth weight and premature infants against developmental delay. Infant Behav Dev. 2003;26(3):384–397 [Google Scholar]
- 146.Scher MS, Ludington-Hoe S, Kaffashi F, Johnson MW, Holditch-Davis D, Loparo KA. Neurophysiologic assessment of brain maturation after an 8-week trial of skin-to-skin contact on preterm infants. Clin Neurophysiol. 2009;120(10):1812–1818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Morgan BE, Horn AR, Bergman NJ. Should neonates sleep alone? Biol Psychiatry. 2011;70(9):817–825 [DOI] [PubMed] [Google Scholar]
- 148.Neu M, Robinson J, Schmiege SJ. Influence of holding practice on preterm infant development. MCN Am J Matern Child Nurs. 2013;38(3):136–143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Bera A, Ghosh J, Singh AK, Hazra A, Mukherjee S, Mukherjee R. Effect of kangaroo mother care on growth and development of low birthweight babies up to 12 months of age: a controlled clinical trial. Acta Paediatr. 2014;103(6):643–650 [DOI] [PubMed] [Google Scholar]
- 150.Miles R, Cowan F, Glover V, Stevenson J, Modi N. A controlled trial of skin-to-skin contact in extremely preterm infants. Early Hum Dev. 2006;82(7):447–455 [DOI] [PubMed] [Google Scholar]
- 151.Andrade ISND, Guedes ZCF. Sucção do recém-nascido prematuro: comparação do método mãe-canguru com os cuidados tradicionais. Suckling of the premature newborn child: comparison between the kangaroo mother method with traditional care. Rev Bras Saude Mater Infant. 2005;5(1):61–69 [Google Scholar]