Abstract
BACKGROUND AND OBJECTIVES:
Delayed detection of type 1 retinopathy of prematurity (ROP) can lead to permanent visual impairment. Providing ROP examinations is challenging because of the limited ophthalmology workforce. This study compares digital imaging–based ROP detection strategies versus serial ROP examinations.
METHODS:
We conducted an individual-level microsimulation studyof a hypothetical cohort of 650 infants with gestational age from 23 to 30 weeks. Infants were evaluated by using strategies based on indirect ophthalmoscopy or digital imaging beginning at 32 weeks’ postmenstrual age (PMA) and continuing to discharge, transfer, or 40 weeks’ PMA. ROP status and the accuracy of digital imaging were based on the e-ROP (Telemedicine Approaches to Evaluating Acute-Phase ROP) study, which enrolled high-risk infants.
RESULTS:
Within the hypothetical NICU, the strategy of ROP examinations identified an average of 45.8 cases of type 1 ROP by discharge, transfer, or 40 weeks’ PMA, and another 1.9 cases were included in the group of infants recommended to have later follow-up. Digital imaging with an ROP examination at discharge identified all 47.7 cases of type 1 ROP. On average, the ROP examination–only strategy required 1745.7 ROP examinations, whereas digital imaging with a discharge examination required 1065.5 ROP examinations and 1786.2 digital imaging sessions.
CONCLUSIONS:
Although digital imaging decreased the number of ROP examinations per infant, there was an increase in the total number of interventions (ie, ROP examinations and imaging sessions). Providing an ROP examination at the time of NICU discharge can significantly reduce the number of infants who require follow-up.
What’s Known on This Subject:
Digital retinal imaging with remote interpretation is used in some NICUs to detect retinopathy of prematurity (ROP) in at-risk infants to alleviate the shortage of ophthalmologists.
What This Study Adds:
Although digital imaging can identify significant ROP, its use can increase the total number of digital imaging and ROP examinations infants receive and prolong the follow-up period because of the inability of digital imaging to classify retina as mature.
Although most premature infants will not develop significant retinopathy of prematurity (ROP), delays in treatment can lead to severe visual impairment.1 More than 2 decades ago, a microsimulation study found that serial binocular indirect ophthalmology examinations with targeted ROP treatment not only lead to substantial gains in quality-adjusted life-years but are cost saving.2 Current guidelines3 recommend serial examinations to identify sight-threatening ROP in at-risk infants (eg, birth weight ≤1500 g, gestational age [GA] ≤30 weeks, birth weight between 1500 and 2000 g) at the recommendation of a neonatologist beginning at 31 or 32 weeks’ postmenstrual age (PMA) and stopping when no longer at risk (ie, vascularized retina, ROP regressing) or when significant ROP is identified and treated. Decreasing the number of ROP examinations that infants require would decrease the demand on the limited supply of ophthalmology specialists.4
One strategy now being adopted to reduce the number of ROP examinations is the use of digital retinal imaging with ROP examinations prompted by abnormal findings on image evaluation.5,6 Previous studies of community-based digital retinal imaging programs7 and the recently completed multicenter e-ROP (Telemedicine Approaches to Evaluating Acute-Phase ROP) study8 have found that this approach can be highly accurate even when images are obtained at the bedside and evaluated remotely by trained nonphysician personnel. Although replacing examinations with digital imaging may not decrease the infant’s stress associated with ROP evaluations (eg, both approaches often require eye speculums; for digital imaging, the camera is in contact with a gel applied to the eye), digital imaging with remote interpretation may lower overall health care costs.9,10
The current ROP detection guidelines, supported by a recent expert panel technology evaluation, allow for digital imaging as an alternative with at least 1 ROP examination before treatment or discharging infants from ROP monitoring.3,11 The goal of the present study was to evaluate outcomes associated with digital imaging compared with serial examinations for type 1 ROP detection by using microsimulation, a technique to evaluate the outcomes from competing policies by statistically modeling the experience of individuals within a hypothetical cohort.12 Unlike previous analyses,9,10 we evaluated outcomes for a range of digital imaging strategies and considered the impact of GA, PMA, and variation in length of NICU stay.
Methods
Objectives of the Analysis
The primary study objective was to estimate for each strategy the number of examinations and digital imaging sessions, the number of cases of type 1 ROP detected and the number missed, the time to diagnosis of type 1 ROP, the number of infants classified with mature retina, and the number of infants who would need further follow-up after NICU discharge or transfer or >40 weeks’ PMA if still in the NICU. Because there are few data regarding the costs of ROP examinations or digital imaging with remote interpretation, the economic assessment was a secondary outcome based on a wide range of cost estimates. We assumed that examinations would be performed by ophthalmologists and that digital images would be obtained by trained nonphysicians, with remote interpretation by trained nonphysician readers.8
Classifying ROP Examination and Digital Imaging Findings
Examination findings were classified to accommodate current treatment indications (Supplemental Information).13,14 The classifications were: immature retina; mature retina; mild ROP; type 2 ROP, which requires close follow-up because of the risk of developing type 1 ROP; or type 1 ROP, which usually indicates the need for treatment. Digital imaging findings were classified as no ROP, mild ROP, or referral-warranted retinopathy of prematurity (RW-ROP). RW-ROP, the criterion for ROP examination referral, was the presence of retinal findings consistent with type 2 or type 1 ROP. A limitation of current digital imaging is that the retina cannot be classified as mature because the full periphery is not seen. Both immature and mature retina would be classified as no ROP, creating challenges in determining when ROP evaluations can be discontinued.
Simulation Overview
This study was an individual-level microsimulation from the NICU perspective. We simulated a hypothetical cohort of 650 infants, which is the typical number of annual admissions to NICUs affiliated with children’s hospitals in the United States.15 Outcomes were also reported stratified according to GA: 22 to 25 weeks, 26 to 27 weeks, 28 to 29 weeks, and 30 weeks.
Strategies for the Detection of Type 1 ROP
Five strategies were considered. We assumed that examinations and digital imaging would be completed as scheduled.
ROP Examination Only
Current guidelines recommend that eye examinations begin at 31 weeks’ PMA for infants with a GA of <28 weeks, 32 weeks’ PMA for infants with a GA of 28 weeks, 33 weeks’ PMA for infants with a GA of 29 weeks, and 34 weeks’ PMA for infants with a GA of 30 weeks.3 However, the e-ROP study did not enroll infants with GA >32 weeks because of the low likelihood of finding significant ROP in infants in North America.8 Therefore, we modeled ROP examinations beginning at 32 weeks’ PMA for those with GA <30 weeks and at 34 weeks’ PMA for those with GA of 30 weeks. Examinations would be repeated biweekly for infants with immature retina or with mild ROP; otherwise, examinations would be repeated weekly. ROP examinations would stop with the first occurrence of the following: (1) both retinas are mature; (2) type 1 ROP is found; (3) discharge or transfer from the NICU; or (4) >40 weeks’ PMA (the end point of follow-up for the e-ROP study). In a secondary analysis, it was assumed that infants would receive an ROP examination at the time of discharge, transfer, or 40 weeks’ PMA if they were known to have immature retina according to examination in the previous week and thus otherwise would not normally be examined.
Digital Imaging
Imaging would begin at 32 or 34 weeks’ PMA. Infants classified as having no ROP would undergo digital imaging in 2 weeks and those with mild ROP in 1 week. Infants with RW-ROP would receive an ROP examination within a short period of time (eg, <72 hours), which was modeled as occurring within the same PMA. If this ROP examination identifies type 1 ROP or mature retina bilaterally, then digital imaging would stop. If type 2 ROP is found on examination, the infant would then receive weekly examinations until evaluation could stop (eg, type 1 ROP or mature retina) or digital imaging could be resumed (eg, immature retina or mild ROP). As with the ROP examination strategy, infants would be followed up in the simulation until identification of type 1 ROP or discharge, transfer, or through 40 weeks’ PMA.
Digital Imaging With Discharge ROP Examination
Those infants still being followed up in the NICU with digital imaging would receive an ROP examination instead of digital imaging during the week of discharge, transfer, or 40 weeks’ PMA.
Digital Imaging With Low-Risk Stopping Rule
This approach follows the digital imaging approach, but infants with no ROP on 2 consecutive digital imaging sessions beginning at 36 weeks’ PMA would no longer be evaluated for ROP.
Digital Imaging With Low-Risk Stopping Rule and Discharge Examination
This approach combines the low-risk stopping rule and the ROP examination for infants still followed up during the week of discharge, transfer, or 40 weeks’ PMA. Infants will only receive digital imaging in the week of discharge if it is possible that the digital imaging could lead to ending ROP follow-up based on the low-risk stopping rule; otherwise, infants receive a discharge examination. Infants who receive digital imaging during the week of discharge, transfer, or 40 weeks’ PMA that does not allow cessation of ROP follow-up also receive a discharge examination.
Simulation and Model Inputs
Each hypothetical infant was modeled by using a program developed in Visual Basic 2012 (Microsoft Corporation, Redmond, WA). We began by assigning GA probabilistically and then modeled the weekly status of the retina from 32 through 40 weeks’ PMA and the outcomes of digital imaging using data from the e-ROP study.8,16 To assure that the analysis reflected the underlying probability distributions related to the likelihood of developing ROP and the accuracy of digital imaging, a second-order simulation was conducted by replicating the cohort 10 000 times. The initial cohort simulation was based on the point estimate for each probability regarding the likelihood of developing ROP and the accuracy of the digital imaging. Each subsequent cohort simulation was based on sampling from the underlying probability distributions (Supplemental Information).
Statistical analyses were conducted by using Stata version 13 (StataCorp, College Station, TX). All analyses took into account the clustering of probabilities within each replication to generate SEs for the estimates. The Duke University School of Medicine and The Children’s Hospital of Philadelphia institutional review boards approved this study.
GA and Discharge or Transfer
Each infant was assigned GA based on the distribution of prematurity in the United States in 2012 as follows: 22 to 25 weeks, 24.1%; 26 to 27 weeks, 20.6%; 28 to 29 weeks, 31.7%; and 30 weeks, 23.6%.17 The average PMA and SD at the time of discharge or transfer18,19 decreased with increasing GA: 22 to 25 weeks’ GA had an average discharge at 40 weeks’ PMA (SD: 3); 26 to 27 weeks’ GA had an average discharge at 38 weeks’ PMA (SD: 2); 28 to 29 weeks’ GA had an average discharge at 37 weeks’ PMA (SD: 1.5); and 30 weeks’ GA had an average discharge at 36 weeks’ PMA (SD: 1). For each hypothetical infant, ROP development after discharge or transfer or >40 weeks’ PMA, whichever comes first, was not evaluated.
Cost Estimates and Cost Outcome Measures
Cost estimates were based on expected allowed charges for an initial inpatient consultation and subsequent examinations (Supplemental Information).20 The baseline estimated cost of an ROP examination was $104 for the initial examination and $74 for subsequent examinations, with a range of $79 to $312 for the first consultation and $56 to $222 for subsequent ROP examinations. The baseline estimated cost of digital imaging with remote interpretation was $52, with a range of $39 to $156. The cost analysis is from the health care system perspective, reflecting those costs that could accrue in the NICU, and do not reflect the cost of ROP treatment or any services provided after discharge, transfer, or 40 weeks’ PMA. For each ROP detection strategy, we considered the total cost across the hypothetical NICU and the total costs stratified according to GA. As a primary measure of cost-effectiveness, the average cost per infant for each case of type 1 ROP detected was evaluated. Most infants will not develop ROP that requires treatment. Because follow-up after NICU discharge or transfer can be logistically challenging, the average cost per infant for each case in which ROP monitoring has been completed was also evaluated. ROP monitoring is completed for an infant when follow-up is no longer required (eg, mature retina according to ROP examination or considered low-risk according to the specific digital imaging rule). In the analysis, an infant could develop type 1 ROP, but it could have been missed because the infant was classified as no longer requiring monitoring.
Results
Hypothetical Cohort
Table 1 summarizes the average characteristics of the hypothetical cohort of 650 infants. The average GA was 27 weeks, and infants were in the NICU on average 10 weeks before discharge or transfer. An average of 47.7 infants (7.3%) developed type 1 ROP, and 278.0 infants (42.8%) had mature retina by the time of discharge, transfer, or 40 weeks’ PMA. The likelihood of developing type 1 ROP dropped sharply with increasing GA, with type 1 ROP being a rare event in those with GA of 30 weeks (P < .001). Type 1 ROP generally first occurred at <37 weeks’ PMA. On average, those with a younger GA developed type 1 ROP at a slightly older PMA (P < .001).
TABLE 1.
Characteristics of the Hypothetical Cohort of 650 Infants According to GA
| Characteristic | GA, wk | |||
|---|---|---|---|---|
| 22–25 | 26–27 | 28–29 | 30 | |
| Distribution | 24.1% | 20.6% | 31.7% | 23.6% |
| Discharge, average PMA, wk | 39.5 | 37.5 | 36.5 | 35.5 |
| Type 1 ROP | ||||
| Proportion | 25.3% | 4.3% | 1.1% | <0.01% |
| Average PMA, wk | 36.5 | 36.3 | 35.8 | NAa |
Analysis included the distribution, average PMA at discharge, the proportion developing type 1 ROP by discharge, and the average PMA at which type 1 ROP developed among those who did so by discharge. NA, not applicable.
Too few cases to reliably estimate.
Average Expected Outcomes for the Hypothetical Cohort
For each ROP detection strategy across the hypothetical cohort, Table 2 lists the average expected number of interventions (ie, ROP examinations, digital imaging sessions, total number of sessions) and the expected outcomes (ie, type 1 ROP identified; follow-up needed after discharge, transfer, or 40 weeks’ PMA; percentage of those who need follow-up after discharge or transfer) for each strategy across the hypothetical cohort. Table 3 lists the expected costs across the hypothetical cohort with the baseline estimate and range of costs. Across each strategy, most (>90%) of the infants needing follow-up for ROP had been discharged or transferred instead of having a PMA >40 weeks.
TABLE 2.
Expected Outcomes for Each ROP Detection Strategy in the Hypothetical Cohort of 650 Infants According to Discharge, Transfer, or 40 Weeks’ PMA
| Strategy | No. of Interventions | Outcomes | ||||
|---|---|---|---|---|---|---|
| ROP Examinations | Digital Imaging Sessions | Total | No. of Cases of Type 1 ROP Detected in NICU | No. (%) With Follow-up Needed | % Discharge or Transferred | |
| ROP examination only | 1745.7 | 0 | 1745.7 | 45.8 | 356.7 (54.9) | 90.9 |
| Digital imaging alone | 544.1 | 2125.1 | 2669.2 | 46.9 | 601.1 (92.5) | 92.0 |
| Digital imaging + discharge examination | 1065.5 | 1786.2 | 2851.7 | 47.7 | 324.8 (50.0) | 90.3 |
| Digital imaging + low-risk stopping rule | 543.1 | 2102.3 | 2645.4 | 46.7 | 496.9 (76.4) | 93.6 |
| Digital imaging + low-risk stopping rule + discharge examination | 963.1 | 1853.1 | 2816.2 | 47.5 | 308.6 (47.5) | 90.3 |
Analysis included the number of ROP examinations and digital imaging sessions. It also included the number of cases of type 1 ROP detected; the number and percentage who needed follow-up after discharge, transfer, or 40 weeks’ PMA; and the percentage of those who needed follow-up who were discharged or transferred.
TABLE 3.
Baseline and Range of Estimates of the Average Expected Total Cost of Evaluating the Hypothetical Cohort of 650 Infants for ROP Through the Time of Discharge for Each of the Detection Strategies, the Cost per Case of Type 1 ROP Detected, and the Cost per Case of ROP Monitoring Completed by the Time of Discharge, Transfer, or 40 Weeks’ PMA
| Strategy | Total Cost (Range), $ | Cost per Case (Range), $ | |
|---|---|---|---|
| Type 1 ROP Detected | ROP Monitoring Completed | ||
| ROP examination only | 148 552 (112 609–445 656) | 3243 (2459–9730) | 506 (384–1519) |
| Digital imaging alone | 156 514 (117 753–469 542) | 3347 (2511–10 012) | 3201 (2408–9602) |
| Digital imaging + discharge examination | 191 102 (144 183–573 309) | 4006 (3023–12 019) | 588 (443–1763) |
| Digital imaging + low-risk stopping rule | 155 235 (116 793–465 704) | 3324 (2501–9972) | 1014 (763–3042) |
| Digital imaging + low-risk stopping rule + discharge examination | 184 219 (138 922–552 658) | 3878 (2925–11 635) | 540 (407–1619) |
Each strategy detected nearly all cases of type 1 ROP by the time of discharge, transfer, or 40 weeks’ PMA. However, only 1 strategy (ie, digital imaging with a discharge examination) detected all cases. The cases not detected by the ROP examination–only strategy occurred among infants with scheduled follow-up who did not have an examination in the week of discharge, transfer, or 40 weeks’ PMA; that is, they had mild ROP discovered by ROP examination and were scheduled for a repeat ROP examination in 2 weeks but were discharged during this period with follow-up ROP examination scheduled, leading to a potential delay in diagnosis or missed case if the follow-up examinations did not occur. Modifying the ROP examination–only strategy to include examinations at the time of discharge, transfer, or 40 weeks’ PMA if known to have immature retina or mild ROP in the previous week (and therefore normally not examined) would lead to the detection of all cases of type 1 ROP that had developed and decrease the number of infants needing follow-up by an average of 31.9 to 324.8 infants. However, this approach would require, on average, an additional 167.8 examinations across the hypothetical cohort, which is a 9.6% increase.
The low-risk stopping rule decreased the number of infants who require follow-up. However, in contrast to the ROP examination–only strategy, the low-risk stopping rule was associated with potentially missing cases of type 1 ROP with no scheduled follow-up. Across the hypothetical cohort, both digital imaging strategies with the low-risk stopping rule had an ∼20% risk of missing 1 case of type 1 ROP. Among the cases of type 1 ROP identified, the likelihood that any of the strategies would delay identification of type 1 ROP by >1 week was <0.001%. Among the digital imaging strategies, the proportion of infants needing ROP follow-up ranged from 47.5% for digital imaging with the low-risk stopping rule and discharge examination to 92.5% for digital imaging of all.
Across the hypothetical cohort, the ROP examination–only strategy had the fewest number of total interventions, and digital imaging with a discharge ROP examination was the strategy with the greatest total number of interventions (Table 2). Switching from the ROP examination–only strategy to digital imaging with a discharge ROP examination would decrease the total number of examinations by 39% but also increase the total number of interventions by 63%. As a result, using the baseline estimates of cost, digital imaging with a discharge ROP examination was $42 580 more than the ROP examination–only strategy across the hypothetical cohort, which is a 29% increase. As modeled, the total cost of the ROP examination–only strategy is a function of only the cost of an ROP examination; however, the total cost of the digital imaging with a discharge ROP examination is a function of the cost of a ROP examination and of obtaining and interpreting digital images (Fig 1). The least expensive strategy from the perspective of the health care system (including NICU costs for the detection of type 1 ROP within the hypothetical cohort using the baseline estimates of costs) was digital imaging with the low-risk stopping rule. However, that strategy was associated with a 20% risk of missing 1 case of type 1 ROP not scheduled for follow-up ROP examinations from the hypothetical cohort of 650 infants.
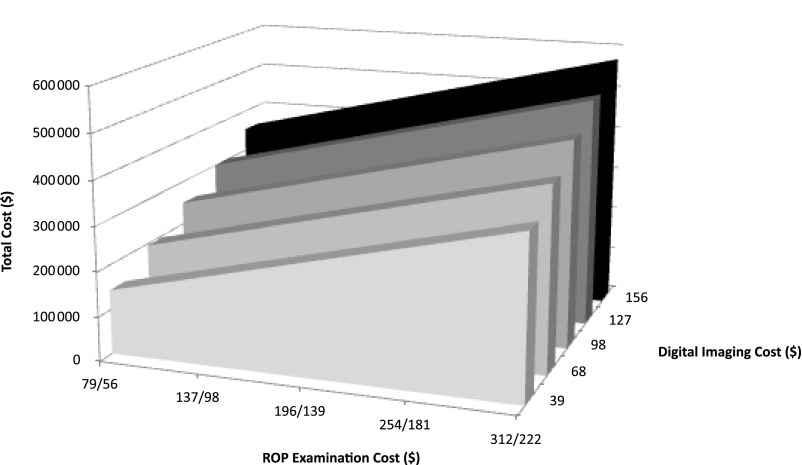
FIGURE 1.
Expected total cost of digital imaging with a discharge ROP examination for the hypothetical cohort of 650 infants across the range of estimates regarding costs of an ROP examination (initial/subsequent) and digital imaging.
Average Expected Outcomes and Costs According to GA
Figure 2 illustrates the average expected outcomes per infant according to GA for 2 strategies: ROP examination–only and digital imaging with an ROP discharge examination. Table 4 presents the corresponding costs. Across GA, both strategies had a similar likelihood of identifying type 1 ROP and of needing follow-up. However, regardless of GA, the total number of interventions was greater for digital imaging with an ROP discharge examination. Because of the low likelihood that an infant with GA of 30 weeks would develop type 1 ROP, the total cost per case of type 1 ROP identified among such infants was more than $150 million.
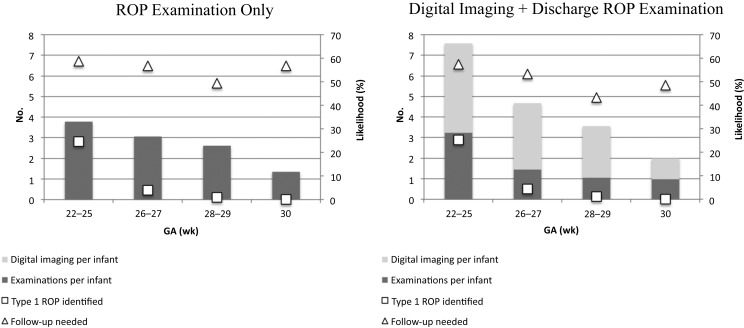
FIGURE 2.
Average expected outcomes across the simulations per infant according to GA, including the number or ROP examinations, the number of digital imaging sessions, and the likelihood of identifying type 1 ROP or of needing follow-up after discharge, transfer, or 40 weeks’ PMA.
TABLE 4.
Detection Strategies and the Total Expected Cost Per Infant, the Cost Per Case of Type 1 ROP Detected, and the Cost Per Case of ROP Monitoring Completed According to Time of Discharge, Transfer, or 40 Weeks’ PMA, Stratified According to GA
| GA | ROP Examination Only | Digital Imaging + Discharge ROP Examination | ||||
|---|---|---|---|---|---|---|
| Cost per (Range), $ | Cost per (Range), $ | |||||
| Infant | Type 1 | Screening Completed | Infant | Type 1 | ROP Monitoring Completed | |
| 22 to 25 wk | 309 (234–927) | 1260 (954–3781) | 748 (566–2244) | 496 (374–1486) | 1960 (1478–5871) | 1162 (876–3842) |
| 26 to 27 wk | 256 (194–767) | 6374 (4830–19 096) | 591 (448–1771) | 305 (230–914) | 7046 (5314–21 116) | 653 (492–1957) |
| 28 to 29 wk | 223 (169–669) | 22 658 (17 171–67 973) | 440 (334–1321) | 238 (180–715) | 21 638 (16 160–64 193) | 420 (317–1261) |
| 30 wk | 130 (99–389) | >150 milliona | 301 (229–900) | 154 (117–462) | >150 milliona | 299 (227–897) |
Too few cases to estimate with greater certainty.
Discussion
Early detection of sight-threatening ROP (ie, type 1) allows for the timely delivery of potentially sight-saving treatment. The challenge is how best to detect cases that would benefit from treatment. Although the current guidelines for ROP detection are inefficient because all at-risk infants require serial ROP examinations even though the risk of developing significant ROP for most is low, a single missed case can lead to a lifetime of preventable blindness. Digital retinal imaging has been proposed as a solution. By using trained nonphysicians to capture images for remote interpretation by nonphysician readers, the need for ophthalmologists could be decreased. This approach could be of significant benefit in communities with limited access to ophthalmologists who perform ROP examinations. However, digital imaging does not obviate the need for ROP examinations to identify significant ROP because infants with abnormal digital imaging findings require confirmatory examinations to consider the need for treatment. Furthermore, because digital imaging alone cannot be used to determine whether infants are no longer at risk for ROP because the retina is mature in both eyes, infants either need to be followed up with serial digital imaging until they reach an age at which the risk of developing significant ROP is very low or they must undergo an ROP examination to identify mature retina in both eyes to confidently stop monitoring for significant ROP. Use of digital imaging alone in higher level NICUs could increase the burden of ROP monitoring on lower level NICUs; these NICUs receive transferred infants because they would not have had a transfer examination to evaluate for mature retinas. Lower level NICUs using imaging to evaluate transferred infants would need to arrange for discharge eye examinations to detect mature retina or assure that potentially at-risk infants receive ophthalmology follow-up monitoring, thereby increasing the burden on families and providers.
This study has several important implications for clinical practice. Digital imaging can be used within the NICU setting to identify type 1 ROP; however, it is less efficient than providing serial ROP examinations without digital imaging when considering the total number of interventions (ie, digital imaging sessions, ROP examinations) and the need for follow-up after NICU discharge or transfer is considered. Digital retinal imaging without a discharge or transfer ROP examination substantially increases the total number of interventions (ie, digital imaging sessions, ROP examinations) that infants receive and the number of infants who require follow-up after NICU discharge or transfer. Although addition of the low-risk stopping rule to digital imaging decreases the need for follow-up, it is unlikely to be adopted because of the small risk of missing type 1 ROP. Digital imaging with a discharge ROP examination identified all cases of type 1 ROP and decreased the need for follow-up after NICU discharge or transfer, but it still led to a greater number of interventions compared with using only serial examinations to identify significant ROP. Overall, this analysis suggests that NICUs which choose to adopt digital imaging recognize that this approach could lead to an increase in the total number of interventions required; these NICUs should consider including an ROP examination at the time of discharge or transfer to a lower acuity NICU to decrease the need for follow-up.
One of the striking findings of the present analysis was the large health care cost of identifying type 1 ROP, regardless of strategy, among infants with 30 weeks’ GA. Although the e-ROP study targeted infants at high risk for developing significant ROP based on birth weight <1251 g, no cases of type 1 ROP were identified among infants with 30 weeks’ GA. We were unable to evaluate the current recommendations that infants with GA >30 weeks’ or birth weight >1500 g with an unstable clinical course also undergo evaluation for ROP because of the lack of such subjects in the e-ROP study.8 Because of the lifetime consequences of blindness due to missed ROP, the current recommendations might not change, even though the absolute risk is low.
As with all decision-analytic modeling, limitations are related to the simplifying assumptions and the data available for model inputs. Because most of the probabilities come from the e-ROP study,8 which specifically included high-risk infants, the model might overestimate the likelihood of type 1 ROP for typical NICUs in the United States. We did not model specific retinal findings (eg, zone of involvement) leading to the classification of type 2 or type 1 ROP because of the lack of statistical power to adequately model this level of granularity and because of the complexity it would add to the underlying model. We assumed that ROP evaluation would not begin until 32 weeks’ PMA for those with <30 weeks’ GA because of the high likelihood of finding immature retina earlier; this approach would slightly underestimate the number of examinations or digital imaging sessions according to the current recommendations. The analysis assumed that examinations and digital imaging would always be successfully completed on time and that the examination always reflected the ROP status of the eye. We did not consider other benefits of digital imaging beyond screening for ROP, such as having objective and permanent documentation of the retinal status or by using imaging to inform families about the infant’s eye status. Any harms associated with examinations or digital imaging were also not considered. We assumed that all of the hypothetical infants would be at risk for ROP and did not consider the impact of comorbid conditions. Costs were estimated as wide ranges based on expected allowed charges according to Medicare data; this approach likely underestimated the true costs of examinations and of digital imaging and interpretation but likely overestimated actual payments received because most Medicaid programs pay less than Medicare. Contractual payments for providing ROP services or facility support of imaging services were not included.
Future research should focus on developing strategies to efficiently identify infants no longer at risk for acute-phase ROP. Digital imaging would be of much greater value if it were possible to completely image the peripheral retina to identify those infants with complete vascularization of the retina who no longer need follow-up; however, the optical properties of current technology are insufficient. Combining ROP detection by using digital imaging with other risk stratification strategies (eg, postnatal weight gain21,22) or biomarkers may be an important approach to targeting those infants at greatest risk.
Acknowledgments
Members of the e-ROP Study Cooperative Group are as follows.
Office of Study Chair
The Children’s Hospital of Philadelphia: Graham E. Quinn, MD, MSCE (Principal Investigator); Kelly Wade, MD, PhD; Agnieshka Baumritter, MS; Trang B. Duros; and Lisa Erbring.
Clinical Sites
Baltimore, Maryland–Johns Hopkins University: Michael X. Repka, MD (Site Principal Investigator); Jennifer A. Shepard, CRNP; David Emmert; and C. Mark Herring.
Boston, Massachusetts–Boston Children’s Hospital: Deborah VanderVeen, MD (Site Principal Investigator); Suzanne Johnston, MD; Carolyn Wu, MD; Jason Mantagos, MD; Danielle Ledoux, MD; Tamar Winter, RN, BSN, IBCLC; Frank Weng; and Theresa Mansfield, RN.
Columbus, Ohio–Nationwide Children’s Hospital and The Ohio State University Hospital: Don L. Bremer, MD (Site Principal Investigator); Mary Lou McGregor, MD; Catherine Olson Jordan, MD; David L. Rogers, MD; Rae R. Fellows, MEd, CCRC; Suzanne Brandt, RN-C, BSN; and Brenda Mann, RN-C, BSN.
Durham, North Carolina–Duke University: David Wallace, MD (Site Principal Investigator); Sharon Freedman, MD; Sarah K. Jones; Du Tran-Viet; and Rhonda “Michelle” Young.
Louisville, Kentucky–University of Louisville: Charles C. Barr, MD (Site Principal Investigator); Rahul Bhola, MD; Craig Douglas, MD; Peggy Fishman, MD; Michelle Bottorff; Brandi Hubbuch, RN, MSN, NNP-BC; and Rachel Keith, PhD.
Minneapolis, Minnesota–University of Minnesota: Erick D. Bothun, MD (Site Principal Investigator); Inge DeBecker, MD; Jill Anderson, MD; Ann Marie Holleschau, BA, CCRP; Nichole E. Miller, MA, RN, NNP; and Darla N. Nyquist, MA, RN, NNP.
Oklahoma City, Oklahoma–University of Oklahoma: R. Michael Siatkowski, MD (Site Principal Investigator); Lucas Trigler, MD; Marilyn Escobedo, MD; Karen Corff, MS, ARNP, NNP-BC; Michelle Huynh, MS, ARNP; and Kelli Satnes, MS, ARNP, NNP-BC.
Philadelphia, Pennsylvania–Children’s Hospital of Philadelphia: Monte D. Mills, MD (Site Principal Investigator); Will Anninger, MD; Gil Binenbaum, MD MSCE; Graham Quinn, MD, MSCE; Karen A. Karp, BSN; and Denise Pearson, COMT.
San Antonio, Texas–University of Texas Health Science Center: Alice Gong, MD (Site Principal Investigator); John Stokes, MD; Clio Armitage Harper, MD; Laurie Weaver; Carmen McHenry, BSN; Kathryn Conner; Rosalind Heemer; and Elnora Cokley, RN-C.
Salt Lake City, Utah–University of Utah: Robert Hoffman, MD (Site Principal Investigator); David Dries, MD; Katie Jo Farnsworth; Deborah Harrison, MS; Bonnie Carlstrom; and Cyrie Ann Fry, CRA, OCT-C.
Nashville, Tennessee–Vanderbilt University: David Morrison, MD (Site Principal Investigator); Sean Donahue, MD; Nancy Benegas, MD; Sandy Owings, COA, CCRP; Sandra Phillips, COT, CRI; and Scott Ruark.
Calgary, Alberta, Canada–Hospital of the Foothills Medical Center: Anna Ells, MD, FRCS (Site Principal Investigator); Patrick Mitchell, MD; April Ingram; and Rosie Sorbie, RN.
Data Coordinating Center
University of Pennsylvania School of Medicine: Gui-shuang Ying, PhD (Data Coordinating Center Principal Investigator); Maureen Maguire, PhD; Mary Brightwell-Arnold, BA, SCP; Maxwell Pistilli, MS; Kathleen McWilliams, CCRP; and Sandra Harkins.
Image Reading Center
University of Pennsylvania School of Medicine: Ebenezer Daniel, MBBS, MS, MPH (Imaging Reading Center Principal Investigator); E. Revell Martin; Candace R. Parker Ostroff; Krista Sepielli; Eli Smith; and Claressa Whearry.
Expert Readers
Antonio Capone, MD (The Vision Research Foundation, Royal Oak, MI); G. Baker Hubbard, MD (Emory University School of Medicine, Atlanta, GA); and Anna Ells, MD (University of Calgary Medical Center, Calgary, AB, Canada).
Image Data Management Center
Inoveon Corporation, Oklahoma City, Oklahoma: Peter Lloyd Hildebrand, MD (Data Management Principal Investigator); Kerry Davis; G. Carl Gibson; and Regina Hansen.
Data Management and Oversight Committee
David C. Musch, PhD, MPH (Chair); Stephen P. Christiansen, MD; Ditte J. Hess, CRA; Steven M. Kymes, PhD; Srinivas R. Sadda, MD; and Ryan Spaulding, PhD.
National Eye Institute
Eleanor B. Schron, PhD, RN, FAAN.
Glossary
- GA
gestational age
- PMA
postmenstrual age
- ROP
retinopathy of prematurity
- RW-ROP
referral-warranted retinopathy of prematurity
Footnotes
Dr Kemper conceptualized and designed the study, and drafted the initial manuscript; Dr Prosser assisted in development of the decision-analytic model, helped interpret the findings, and reviewed and revised the manuscript; Dr Wade provided clinical insight into the design of the decision-analytic model, helped gather data, assisted in the interpretation of findings, and reviewed and revised the manuscript; Dr Repka assisted in the assignment of costs and the development of the cost analyses, helped interpret findings, and reviewed and revised the manuscript; Dr Ying assisted in the development and interpretation of the statistical analyses, helped interpret findings, and reviewed and revised the manuscript; Ms Baumritter assisted in obtaining the data, helped develop the decision-analytic model, participated in the interpretation of the findings, and reviewed the revised manuscript; and Dr Quinn oversaw the data collection, helped conceptualize and design the study, participated in the interpretation of findings, and reviewed the revised manuscript. All authors approved the final manuscript as submitted.
This trial has been registered at www.clinicaltrials.gov (identifier NCT01264276).
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Supported by a National Institutes of Health grant (U10EY017014). Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Blencowe H, Lawn JE, Vazquez T, Fielder A, Gilbert C. Preterm-associated visual impairment and estimates of retinopathy of prematurity at regional and global levels for 2010. Pediatr Res. 2013;74(suppl 1):35–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Javitt J, Dei Cas R, Chiang YP. Cost-effectiveness of screening and cryotherapy for threshold retinopathy of prematurity. Pediatrics. 1993;91(5):859–866 [PubMed] [Google Scholar]
- 3.Fierson WM; American Academy of Pediatrics Section on Ophthalmology; American Academy of Ophthalmology; American Association for Pediatric Ophthalmology and Strabismus; American Association of Certified Orthoptists . Screening examination of premature infants for retinopathy of prematurity. Pediatrics. 2013;131(1):189–195 [DOI] [PubMed] [Google Scholar]
- 4.Kemper AR, Freedman SF, Wallace DK. Retinopathy of prematurity care: patterns of care and workforce analysis. J AAPOS. 2008;12(4):344–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fijalkowski N, Zheng LL, Henderson MT, Wallenstein MB, Leng T, Moshfeghi DM. Stanford University Network for Diagnosis of Retinopathy of Prematurity (SUNDROP): four-years of screening with telemedicine. Curr Eye Res. 2013;38(2):283–291 [DOI] [PubMed] [Google Scholar]
- 6.Weaver DT, Murdock TJ. Telemedicine detection of type 1 ROP in a distant neonatal intensive care unit. J AAPOS. 2012;16(3):229–233 [DOI] [PubMed] [Google Scholar]
- 7.Fijalkowski N, Zheng LL, Henderson MT, et al. Stanford University Network for Diagnosis of Retinopathy of Prematurity (SUNDROP): five years of screening with telemedicine. Ophthalmic Surg Lasers Imaging Retina. 2014;45(2):106–113 [DOI] [PubMed] [Google Scholar]
- 8.Quinn GE, Ying GS, Daniel E, et al. ; e-ROP Cooperative Group . Validity of a telemedicine system for the evaluation of acute-phase retinopathy of prematurity. JAMA Ophthalmol. 2014;132(10):1178–1184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jackson KM, Scott KE, Graff Zivin J, et al. Cost-utility analysis of telemedicine and ophthalmoscopy for retinopathy of prematurity management. Arch Ophthalmol. 2008;126(4):493–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castillo-Riquelme MC, Lord J, Moseley MJ, Fielder AR, Haines L. Cost-effectiveness of digital photographic screening for retinopathy of prematurity in the United Kingdom. Int J Technol Assess Health Care. 2004;20(2):201–213 [DOI] [PubMed] [Google Scholar]
- 11.Fierson WM, Capone A Jr; American Academy of Pediatrics Section on Ophthalmology; American Academy of Ophthalmology, American Association of Certified Orthoptists . Telemedicine for evaluation of retinopathy of prematurity. Pediatrics. 2015;135(1). Available at: www.pediatrics.org/cgi/content/full/135/1/e238 [DOI] [PubMed] [Google Scholar]
- 12.Zucchelli E, Jones AM, Rice N. The evaluation of health policies through dynamic microsimulation methods. Int J Microsimulation. 2012;5:2–20 [Google Scholar]
- 13.Early Treatment For Retinopathy Of Prematurity Cooperative Group . Revised indications for the treatment of retinopathy of prematurity: results of the early treatment for retinopathy of prematurity randomized trial. Arch Ophthalmol. 2003;121(12):1684–1694 [DOI] [PubMed] [Google Scholar]
- 14.Good WV, Hardy RJ, Dobson V, et al. ; Early Treatment for Retinopathy of Prematurity Cooperative Group . Final visual acuity results in the early treatment for retinopathy of prematurity study. Arch Ophthalmol. 2010;128(6):663–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murthy K, Dykes FD, Padula MA, et al. The Children’s Hospitals Neonatal Database: an overview of patient complexity, outcomes and variation in care. J Perinatol. 2014;34(8):582–586 [DOI] [PubMed] [Google Scholar]
- 16.Kemper AR, Wade KC, Hornik CP, Ying GS, Baumritter A, Quinn GE; Telemedicine Approaches to Evaluating Acute-phase Retinopathy of Prematurity (e-ROP) Study Cooperative Group . Retinopathy of prematurity risk prediction for infants with birth weight less than 1251 grams. J Pediatr. 2015;166(2):257–61.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Centers for Disease Control and Prevention CDC WONDER. Available at: http://wonder.CDC.gov. Accessed September 19, 2014
- 18.Stoll BJ, Hansen NI, Bell EF, et al. ; Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network . Neonatal outcomes of extremely preterm infants from the NICHD Neonatal Research Network. Pediatrics. 2010;126(3):443–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shah P, Yoon EW, Chan P, and the Members of the Annual Report Review Committee The Canadian Neonatal Network Annual Report, 2013. Available at: www.canadianneonatalnetwork.org/portal/. Accessed September 19, 2014
- 20.Centers for Medicare & Medicaid Services Physician fee schedule: overview. Available at: www.cms.gov/apps/physician-fee-schedule/overview.aspx. Accessed June 1, 2015
- 21.Hellström A, Hård AL, Engström E, et al. Early weight gain predicts retinopathy in preterm infants: new, simple, efficient approach to screening. Pediatrics. 2009;123(4). Available at: www.pediatrics.org/cgi/content/full/123/4/e638 [DOI] [PubMed] [Google Scholar]
- 22.Binenbaum G, Ying G-S, Quinn GE, et al. The CHOP postnatal weight gain, birth weight, and gestational age retinopathy of prematurity risk model. Arch Ophthalmol. 2012;130(12):1560–1565 [DOI] [PubMed] [Google Scholar]