Abstract
Cyclooxygenase-2 (COX-2) catalyzed synthesis of prostaglandin E2 and it associates with tumor growth, infiltration, and metastasis in preclinical experiments. Known inhibitors against COX-2 exhibit toxicity. Therefore, it is of interest to screen natural compounds like flavanoids against COX-2. Molecular docking using 12 known flavanoids against COX-2 by FlexX and of ArgusLab were performed. All compounds showed a favourable binding energy of >-10 KJ/mol in FlexX and > -8 kcal/mol in ArgusLab. However, this data requires in vitro and in vivo verification for further consideration.
Keywords: COX-2, FlexX, ArgusLab, Flavonoids, Cancer
Background
More than a century ago, chronic inflammation leads to cancer development by increasing cellular proliferation [1], suggested by Virchow et al [2]. The current innovation of the inducible cyclooxygenase-2 (COX-2) gene has relight attention in the fundamental link between inflammation and cancer, and various models of carcinogenesis have been proposed involving inflammatory stimuli and COX-2 expression [3]. Cancer development in the presence of chronic inflammation involves the activation of cyclooxygenase-2 (COX-2) and other several transcription factors including NFB alpha, STAT3, activator protein-1, and hypoxia inducible factor 1 alpha [4–9]. The gene cyclooxygenase encodes two isoenzymes namely COX-1 and its inducible isoform (COX-2). The isoenzyme COX- 2 is primarily associated with inflammation [10, 11]. Under the normal conditions, COX-2 expression is low or not detected in most tissues. Conversely, its overexpression together with activation of cytosolic PLA2 by phosphorylation is a feature of inflammatory reactions. Overexpression of COX-2 occurs in breast, lung, colon, and prostate cancers [4–6]. However, recent studies representing the place of COX-2 inhibitors in the prevention of several cancer types such as colon, breast, lung and prostate cancers [12– 16]. In this context, non-steroidal antiinflammatory drugs (NSAIDs) are widely utilized for the treatment of various inflammatory conditions such as rheumatic fever, rheumatoid arthritis and osteoarthritis. However, because of NSAIDs inhibit both isoforms of cyclooxygenase (COX), their use is often accompanied by gastrointestinal side effects and renal function suppression [17, 18]. Though celecoxib and rofecoxib are two well-known selective COX-2 inhibitors belong to COXIB׳s class [19, 20]. However, the market withdrawal of some COXIBs such as rofecoxib due to increase the risk of heart attack and cardiovascular side effects [21, 22], encourages the researchers to explore new selective COX-2 inhibitors to evaluate their effects and improve the safety profiles.
In current years, several of these reviews touched the general overview for the bioactive aspect for phytochemical compounds [23– 31]. It is also well documented that phytocompounds have activity against cancer [32– 34] and COX-2 [35–38]. Therefore, in our present studies, we focused on the efficacy of natural compounds that may modulate the multistep regulation of COX-2 gene expression, we also discussed their potential as a new generation of selective COX-2 targeting agents alternative to the synthetic COX-2 inhibitors, performed by their binding pattern analysis, which is done by molecular docking analysis [39].
Methodology
Data and Databases:
The data from databases used in this study include PDB (Protein Data Bank) [40] and PubChem [41]. PubChem is a public repository of small molecules and their biological properties. Currently, it contains more than 25 million unique chemical structures and 90 million bioactivity outcomes associated with several thousand macromolecular targets [42].
Docking Tools:
The docking tools used in this study include FlexX (LeadIT 2.1.6) and ArgusLab 4.0.1. FlexX is a fully automated docking program available on LeadIT 2.1.6 package was used to dock compound into the active site of the enzymes. FlexX considers ligand flexibility by changing the conformations of the ligand in the active site, while making the protein rigid [43]. ArgusLab offers quite good on-screen molecule-building facilities, with a moderate library of useful molecules.
Ligand Selection and Preparation:
For our present studies, we had selected twelve flavonoids having anticancer activity in various models and also a selective COX-2 blocker celecoxib. 3D conformer of all this compounds were downloaded from PubChem data bases in sdf format and converted in to mol2 format by open babel [44] software. Details of all compounds used in these studies are represented in the Table 1 (see supplementary material).
Protein preparation:
The crystal structure of COX-2 (pdb id : 6 COX ) enzyme was collected from protein data bank [40]. The active site of the enzyme was identified according to the giving information Kurumbail et al., 1996 [45] protein was prepared by using receptor preparing wizard available in LeadIT 2.1.6 package for FlexX Docking. Docking protocol was maintained in protein preparation for docking in ArgusLab.
Docking with FlexX:
FlexX (which is now a part of LeadIT) is a flexible docking method that uses an Incremental Construction (IC) algorithm and a pure empirical scoring function similar to the one developed by Böhm and coworkers to place ligands into the active site [46]. IC algorithms first dissect each molecule into a set of rigid fragments according to rotatable bonds, and then incrementally assemble the fragments around the binding pocket [43]. For docking studies, a receptor description file was prepared through the FlexX graphic interface. An active site was defined by selecting the residues of the protein. The active site includes protein residues around 10 Å radius sFre centered on the center of mass of the ligand. Based on energy Values, top ten ranked poses for each ligand in data set were selected for further analysis.
Docking Study with ArgusLab:
ArgusLab 4.0.1. is implemented with shape-based search algorithm. Docking has been done using “Lamarckian Genetic Algorithm Docking Engine” exhaustive search docking function of ArgusLab with grid dimension of 65 × 51 × 66. Docking precision was set to “Regular precision” and “Flexible” ligand docking mode was employed for each docking run. The stability of each docked pose was evaluated using ArgusLab energy calculations and the number of hydrogen bonds formed. For each complex, the population size is 50. The number of genes is 10 where, maximum generations are 1000 and the converged when rmsd population fitness < 1 kcal/mol. The best docking model was selected according to the lowest energy calculated by ArgusLab, and the most suitable binding conformation was selected on the basis of hydrogen bond interactions between the ligand and protein near the substrate binding site. The lowest energy poses indicate the highest binding affinity as high energy produces the unstable conformations.
Results & Discussion
As discussed earlier, COX-2 may play a role in different steps of cancer progression by increasing the proliferation of mutated cells, and thus favoring tumor promotion in addition to by affecting apoptosis, which ultimately affects the efficacy of anticancer therapies [47– 50]. Natural compounds are proved their potentiality to inhibit the key cell signaling pathways including COX-2, which is gained much attention over the last regarding years, when they are being used alone or perhaps combination with existing chemotherapeutic agents [51]. Regarding that, we tried to establish the efficacy of some natural flavonoid compounds with known anticancer activity, by analyzing their binding pattern on COX-2 enzyme with two docking routines.
Results from the computations, performed in the present work are described in Table 2 & Table 3 (see supplementary material), and their modes of binding patterns are also described below. Here, in this study, we found significant binding affinity of all flavonoids towards the COX-2 enzyme i.e. FlexX (greater than -10 KJ/mol) and ArgusLab (greater than -8 Kcal/mol). The details of protein-ligand bindings, which were generated from ArgusLab are described in Table 3 (supplementary material). The structural basis of COX-2 inhibition was illustrated by Kurumbail et al. [45], amino acid residues such as H90, R120, Q192, V349, L352, S353, Y355, L359, Y385, W387, R513, A516, F518, V523, G526, A527, L531 associated with A chain of COX-2 protein were involved for protein–ligand complementary activity [52]. There are several structural features that are considered to be important for efficient COX inhibition: (i) a carboxylate moiety that interacts with the R120 side chain; (ii) a carbonyl moiety that interacts via a hydrogen bond with the side chain of S530 and (iii) a distal aromatic ring filling a hydrophobic pocket beneath the Y385 side chain [53]. One of the keys to developing COX-2 selective drugs is the larger active site of COX-2 is partly due to a polar hydrophilic side-pocket that forms because of substitution of I523, H513 and I434 in COX-1 by V523, R513 and V434 in COX-2. V523 is less bulky than I523, which increases the volume of the active site [54, 55].
The important consequence of the amino acid changes in COX- 2 is to increase the size of the NSAID-binding pocket, allowing this isoenzyme to bind bulky inhibitors more readily than COX- 1 [56]. As seen in Figure 1 and Table 3 (supplementary material), all compounds including flavonoids and celecoxib, were formed favorable bindings with COX-2 enzyme. The post docking analysis showed that the compounds taking account of celecoxib, 4', 6, 7-trihydroxyisoflavone, quercetin, quercetin-3- methyl ether, kaempferol, and luteolin formed hydrogen bonds with S530 residue. While the other flavonoids including, eridicytol and myricetin formed hydrogen bonding with R120. Moreover, hydrogen bonding between Y385 residue of COX-2 and 5-deoxykaemferol was also observed in Figure 1c.
Figure 1.
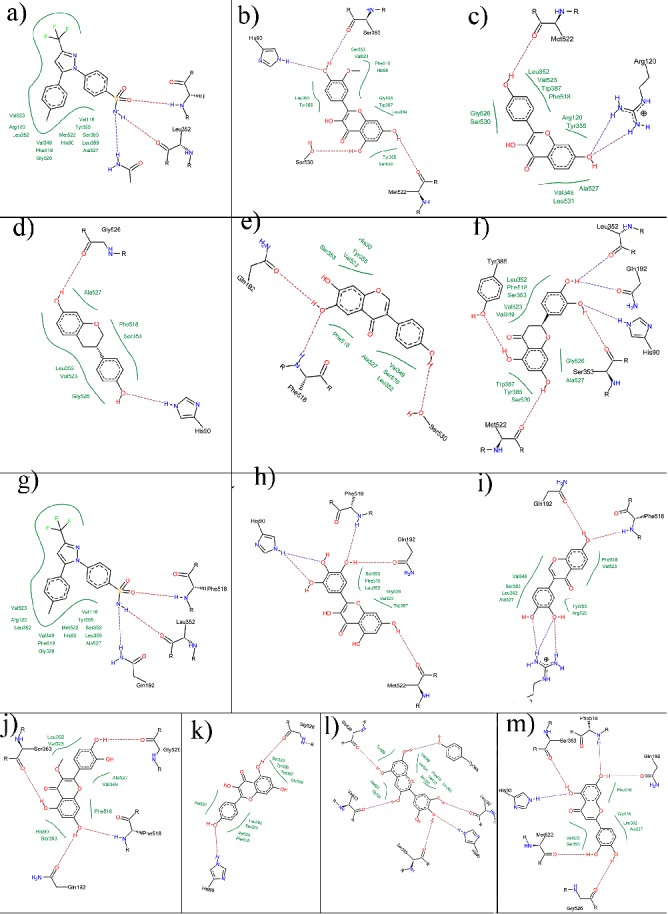
Interaction of COX-2 with a) celecoxib; b) isorhamnetin; c) 5-deoxykaempferol; d) equol; e) 4', 6, 7-trihydroxyisoflavone; f) eriodictyol; g) quercetin; h) myricetin; i) 7, 3, 4'-trihydroxyisoflavone; j) quercetin-3-methyl ether; k) kaempferol; l) delphinidin and m) luteolin by FlexX molecular simulation.
Furthermore, all compounds were found to having hydrophobic interactions with V523 and mostly with pi-alkyl and pi-sigma bonding, except delphinidin (Table 2 in in supplementary material). However, delphinidin formed pication interaction with R120 residue and hydrogen bonding with V523 (Figure 1). Generally, it is often necessary to determine, as a first step of computational drug design and discovery, the binding of a ligand to a targeted protein. The computational arrangement for predicting ligand binding occurrence, affinity, and orientation is usually denoted to as “molecular docking”, which has been a matter of rigorous research for periods [57].
The progress of a molecular docking tool typically starts with an efficient search algorithm, which places the ligand in the active site of the targeted protein in various different positions, orientations, and in flexible docking, conformations [58, 59]. However, in our study, we tried to find out the positions and orientations of flavonoids and in the active site of COX-2 enzymes. Moreover many of flavonoids compound have already proved their ability to reduce the COX-2 expressions in various cancer models (Table 1 in supplementary material). As discussed in Table 1 (supplementary material), isorhamnetin, 5-deoxykaempferol are widely present in fruit and vegetable, has the ability to suppress the UVB-induced expression of cyclooxygenase-2 (COX-2) in skin cancer [60, 61]. Another important flavonoid, quercetin belongs to the flavonoids family and consists of 3 rings and 5 hydroxyl groups found in many fruits, vegetables, leaves and grains. Early studies in various literatures suggested it has the aptitude in reducing COX-2 expression in cancer [62– 66] or noncancerous cell [67, 68]. Myricetin is a widely distributed flavonol that is found in many plants, including tea, berries, fruits, vegetables, and medicinal herbs attenuated the COX-2 expression UVB-induced skin cancer [69–71].
In previously published report, it was shown that 7, 3', 4'- trihydroxyisoflavone (7, 3', 4'-THIF) suppressed UVB-induced COX-2 expression in skin cancer [72]. It was also well known that, kaemferol inhibits COX-2 expression in inflammatory condition in both normal and cancer cells [73–75]. Furthemore delphinidin is an anthocyanidin, a primary plant pigment, already probed its ability to reduce COX-2 expression particularly in skin cancer [76, 77]. Luteolin is a common flavonoid that exists in many types of plants including fruits, vegetables, and medicinal herbs [78]. Recent studies recommended that luteolin supress the COX-2 expression in cancer [79, 80] also in non-cancerous cell [81]. Henceforth, with concerning to the low toxicity of the flavonoids toward normal cells [82, 83] Our studies are in favor of a potential use of flavonoids in adjuvant chemotherapy and COX-2 inhibition in many cancer.
Conclusion
Flavonoid modulation of COX-2 transcription may therefore be an important mechanism in anti-carcinogenesis. In the present study, docking results revealed the binding interactions between the COX-2 protein and the 12 natural flavonoids compound along with a synthetic compound where, in different docking routines, all showed a favorable binding energy greater than 10 kj/mol (FlexX) and in ArgusLab, binding energy is greater than -8 kcal/mol. However, polyphenols are a broad class of compounds with antioxidant and other health benefits. The efficacy of phytochemicals on human health is influenced by on several factors. The molecular structures of phytochemicals influence the extent to which they are altered by cooking processes and the methods by which they are fascinated by the gastrointestinal tract. Numerous actions are shared among different flavone ring-bearing molecules and their action in the complex biochemical machinery of the cell requests to be further clarified.
Supplementary material
Footnotes
Citation:Dash et al, Bioinformation 11(12): 543-549 (2015)
References
- 1.Balkwill F, Mantovani A. Lancet. 2001;357:539. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 2.Heidland A, et al. J Nephrol. 2006;19:S102. [PubMed] [Google Scholar]
- 3.Harris RE. Subcell Biochem. 2007;42:93. doi: 10.1007/1-4020-5688-5_4. [DOI] [PubMed] [Google Scholar]
- 4.De Marzo AM, et al. Nat Rev Cancer. 2007;7:256. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mantovani A, et al. Nature. 2008;454:436. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 6.Ames BN, et al. Proc Natl Acad Sci U S A. 1995;92:5258. doi: 10.1073/pnas.92.12.5258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sethi G, et al. Biosci Rep. 2012;32:1. doi: 10.1042/BSR20100136. [DOI] [PubMed] [Google Scholar]
- 8.Wang D, Dubois RN. Nat Rev Cancer. 2010;1:181. doi: 10.1038/nrc2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O׳Leary KA, et al. Mutation Research. 2004;551:245. doi: 10.1016/j.mrfmmm.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 10.Hla T, Neilson K. Proc Natl Acad Sci U S A. 1992;89:7384. doi: 10.1073/pnas.89.16.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herschman HR, et al. Cancer Metastasis Rev. 1994;13:241. doi: 10.1007/BF00666095. [DOI] [PubMed] [Google Scholar]
- 12.Firke SD, Bari SB. Bioorganic & medicinal chemistry. 2015;23:5273. doi: 10.1016/j.bmc.2015.07.070. [DOI] [PubMed] [Google Scholar]
- 13.Kawamori T, et al. Cancer Res. 1998;58:409. [PubMed] [Google Scholar]
- 14.Takkouche B, et al. J Natl Cancer Inst. 2008;100:1439. doi: 10.1093/jnci/djn324. [DOI] [PubMed] [Google Scholar]
- 15.Hernandez-Diaz S, Garcia Rodriguez LA. Int J Cancer. 2007;120:1565. doi: 10.1002/ijc.22514. [DOI] [PubMed] [Google Scholar]
- 16.Srinath P, et al. Anticancer Res. 2003;23:3923. [PubMed] [Google Scholar]
- 17.Vane JR, Botting RM. Inflamm Res. 1998;47:S78. doi: 10.1007/s000110050284. [DOI] [PubMed] [Google Scholar]
- 18.Perini R, et al. Can J Gastroenterol. 2004;18:229. doi: 10.1155/2004/890585. [DOI] [PubMed] [Google Scholar]
- 19.Consalvi S, et al. Bioorg Med Chem. 2015;23:810. doi: 10.1016/j.bmc.2014.12.041. [DOI] [PubMed] [Google Scholar]
- 20.Penning TD, et al. J Med Chem. 1997;40:1347. doi: 10.1021/jm960803q. [DOI] [PubMed] [Google Scholar]
- 21.Prasit P, et al. Bioorg Med Chem Lett. 1999;9:1773. doi: 10.1016/s0960-894x(99)00288-7. [DOI] [PubMed] [Google Scholar]
- 22.Mason RP, et al. J Cardiovasc Pharmacol. 2006;47:S7. doi: 10.1097/00005344-200605001-00003. [DOI] [PubMed] [Google Scholar]
- 23.Abuo-Rahma GE-D, et al. Eur J Med Chem. 2014;83:398. doi: 10.1016/j.ejmech.2014.06.049. [DOI] [PubMed] [Google Scholar]
- 24.Meeran SM, et al. Clin Epigenetics. 2010;1:101. doi: 10.1007/s13148-010-0011-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karikas GA. J Buon. 2010;15:627. [PubMed] [Google Scholar]
- 26.Saunders FR, Wallace HM. Plant Physiol Biochem. 2010;48:621. doi: 10.1016/j.plaphy.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 27.Sarkar FH, et al. Curr Pharm Des. 2010;6:1801. doi: 10.2174/138161210791208956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehta RG, et al. Pharm Res. 2010;27:950. doi: 10.1007/s11095-010-0085-y. [DOI] [PubMed] [Google Scholar]
- 29.Gullett NP, et al. emin Oncol. 2010;7:258. [Google Scholar]
- 30.Chen J, Xu X. Nutr Cancer. 2010;62:1. [Google Scholar]
- 31.Huang J, et al. Curr Drug Targets. 2011;12:1925. doi: 10.2174/138945011798184155. [DOI] [PubMed] [Google Scholar]
- 32.Bishayee A, et al. Front Biosci. 2011;16:980. doi: 10.2741/3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Callahan R, Hurvitz S. Curr Opin Obstet Gynecol. 2011;23:37. doi: 10.1097/gco.0b013e3283414e87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pan MH, et al. Mol Nutr Food Res. 2011;5:32. [Google Scholar]
- 35.Olejnik A, et al. Postepy Hig Med Dosw. 2010;64:175. [PubMed] [Google Scholar]
- 36.Cerella C, et al. Biochem Pharmacol. 2010;80:1801. doi: 10.1016/j.bcp.2010.06.050. [DOI] [PubMed] [Google Scholar]
- 37.Romagnolo DF, et al. Inflamm Allergy Drug Targets. 2010;9:181. doi: 10.2174/187152810792231922. [DOI] [PubMed] [Google Scholar]
- 38.Tahanian E, et al. Drug Des Devel Ther. 2011;5:299. doi: 10.2147/DDDT.S19931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dash R, et al. Bioinformation. 2014;10:562. doi: 10.6026/97320630010562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berman HM, et al. Nucleic Acids Res. 2000;28:235. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang Y, et al. Nucleic Acids Res. 2014;42:5. doi: 10.1093/nar/gkt1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li Q, et al. Drug Discov Today. 2010;15:1052. doi: 10.1016/j.drudis.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rarey M, et al. Mol Bio. 1996;26:470. doi: 10.1006/jmbi.1996.0477. [DOI] [PubMed] [Google Scholar]
- 44.O'Boyle NM, et al. J Cheminform. 2011;3:1758. [Google Scholar]
- 45.Kurumbail RG, et al. Nature. 1996;384:644. doi: 10.1038/384644a0. [DOI] [PubMed] [Google Scholar]
- 46.Bohm HJ, et al. J Comput Aided Mol Des. 1998;12:309. doi: 10.1023/a:1007999920146. [DOI] [PubMed] [Google Scholar]
- 47.Chan MW, et al. Oncol Rep. 2007;18:1557. [PubMed] [Google Scholar]
- 48.Johnson GE, et al. Apoptosis. 2008;13:790. doi: 10.1007/s10495-008-0212-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Palayoor ST, et al. Clin Cancer Res. 2005;11:6980. doi: 10.1158/1078-0432.CCR-05-0326. [DOI] [PubMed] [Google Scholar]
- 50.Philip M, et al. Semin Cancer Biol. 2004;14:433. doi: 10.1016/j.semcancer.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 51.Sobolewski C, et al. Int J Cell Biol. 2010;2010:215158. doi: 10.1155/2010/215158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Krishna PS, et al. SpringerPlus. 2013;2:172. doi: 10.1186/2193-1801-2-172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Llorens O, et al. J Mol Graph Model. 2002;20:359. doi: 10.1016/s1093-3263(01)00135-8. [DOI] [PubMed] [Google Scholar]
- 54.Llorens O, et al. Bioorg Med Chem Lett. 1999;9:2779. doi: 10.1016/s0960-894x(99)00481-3. [DOI] [PubMed] [Google Scholar]
- 55.Michaux C, et al. Mini Rev Med Chem. 2004;4:603. doi: 10.2174/1389557043403756. [DOI] [PubMed] [Google Scholar]
- 56.DeWitt DL, et al. Molecular Pharmacology. 1999;55:625. [PubMed] [Google Scholar]
- 57.Kitchen DB, et al. Nat Rev Drug Discov. 2004;3:935. doi: 10.1038/nrd1549. [DOI] [PubMed] [Google Scholar]
- 58.Ferrara P, et al. J Med Chem. 2004;47:3032. doi: 10.1021/jm030489h. [DOI] [PubMed] [Google Scholar]
- 59.Wang R, et al. J Med Chem. 2003;46:2287. doi: 10.1021/jm0203783. [DOI] [PubMed] [Google Scholar]
- 60.Dou W, et al. J Nutr Biochem. 2014;25:923. doi: 10.1016/j.jnutbio.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ribeiro D, et al. Inflammation. 2014;38:858. doi: 10.1007/s10753-014-9995-x. [DOI] [PubMed] [Google Scholar]
- 62.Mutoh M, et al. Jpn J Cancer Res. 2000;91:686. doi: 10.1111/j.1349-7006.2000.tb01000.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Xiao X, et al. PLoS One. 2011;6:8. doi: 10.1371/journal.pone.0023505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Banerjee T, et al. Prostaglandins Leukot Essent Fatty Acids. 2002;66:485. doi: 10.1054/plef.2002.0387. [DOI] [PubMed] [Google Scholar]
- 65.Al-Fayez M, et al. Cancer Chemother Pharmacol. 2006;58:816. doi: 10.1007/s00280-006-0228-3. [DOI] [PubMed] [Google Scholar]
- 66.Lee Y-K, et al. Exp Mol Med. 2009;41:201. doi: 10.3858/emm.2009.41.3.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.O'Leary KA, et al. Mutat Res. 2004;551:245. doi: 10.1016/j.mrfmmm.2004.01.015. [DOI] [PubMed] [Google Scholar]
- 68.de Pascual-Teresa S, et al. J Nutr. 2004;134:552. doi: 10.1093/jn/134.3.552. [DOI] [PubMed] [Google Scholar]
- 69.Kang NJ, et al. Ann N Y Acad Sci. 2011;1229:124. doi: 10.1111/j.1749-6632.2011.06122.x. [DOI] [PubMed] [Google Scholar]
- 70.Lee KM, et al. J Agric Food Chem. 2007;55:9678. doi: 10.1021/jf0717945. [DOI] [PubMed] [Google Scholar]
- 71.Gutierrez-Venegas G, et al. Cell Mol Biol Lett. 2014;19:126. doi: 10.2478/s11658-014-0186-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee DE, et al. J Biol Chem. 2011;286:14246. doi: 10.1074/jbc.M110.147348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Garcia-Mediavilla V, et al. Eur J Pharmacol. 2007;557:221. doi: 10.1016/j.ejphar.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 74.Lee KM, et al. Biochem Pharmacol. 2010;80:2042. doi: 10.1016/j.bcp.2010.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wall, et al. Oxid Med Cell Longev. 2013;485201:5. doi: 10.1155/2013/485201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kwon JY, et al. Carcinogenesis. 2009;30:1932. doi: 10.1093/carcin/bgp216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kang NJ, et al. Cancer Prev Res. 2008;1:522. doi: 10.1158/1940-6207.CAPR-08-0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lin Y, et al. Curr Cancer Drug Targets. 2008;8:634. doi: 10.2174/156800908786241050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Eun-Jeong G, Jae-Chang J. Cancer prevention research. 2012;17:218. [Google Scholar]
- 80.Kim JE, et al. J Pharmacol Exp Ther. 2011;338:1013. doi: 10.1124/jpet.111.179200. [DOI] [PubMed] [Google Scholar]
- 81.Harris GK, et al. J Nutr. 2006;136:1517. doi: 10.1093/jn/136.6.1517. [DOI] [PubMed] [Google Scholar]
- 82.Spagnuolo C, et al. Ann N Y Acad Sci. 2012;95:103. doi: 10.1111/j.1749-6632.2012.06599.x. [DOI] [PubMed] [Google Scholar]
- 83.Tsuji PA, et al. Nutr Cancer. 2013;65:1014. doi: 10.1080/01635581.2013.809127. [DOI] [PubMed] [Google Scholar]
- 84.Hynes NE, Lane HA. Nat Rev Cancer. 2005;5:341. doi: 10.1038/nrc1609. [DOI] [PubMed] [Google Scholar]
- 85.Joannou GE, et al. J Steroid Biochem Mol Biol. 1995;54:167. doi: 10.1016/0960-0760(95)00131-i. [DOI] [PubMed] [Google Scholar]
- 86.Northrop JP, et al. J Biol Chem. 1993;268:2917. [PubMed] [Google Scholar]
- 87.Suzuki T, et al. J Virol. 1994;68:3527. doi: 10.1128/jvi.68.6.3527-3535.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hynes NE, Boulay A. J Mammary Gland Biol Neoplasia. 2006;11:53. doi: 10.1007/s10911-006-9012-6. [DOI] [PubMed] [Google Scholar]
- 89.Hwang MK, et al. Int J Biochem Cell Biol. 2009;41:1592. doi: 10.1016/j.biocel.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 90.Huang MT, et al. Cancer Res. 1997;57:2623. [PubMed] [Google Scholar]
- 91.Kavanagh KT, et al. J Cell Biochem. 2001;82:387. doi: 10.1002/jcb.1164. [DOI] [PubMed] [Google Scholar]
- 92.Jung SK, et al. Carcinogenesis. 2010;31:911. doi: 10.1093/carcin/bgp221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Widyarini S, et al. Photochem Photobiol. 2005;81:32. doi: 10.1562/2004-06-02-RA-183. [DOI] [PubMed] [Google Scholar]
- 94.Lampe JW. J Nutr. 2010;140:26. doi: 10.3945/jn.109.118323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Antunes-Ricardo M, et al. Plant Foods Hum Nutr. 2014;69:331. doi: 10.1007/s11130-014-0438-5. [DOI] [PubMed] [Google Scholar]
- 96.Kim JE, et al. Cancer Prev Res. 2011;4:582. doi: 10.1158/1940-6207.CAPR-11-0032. [DOI] [PubMed] [Google Scholar]
- 97.Saud SM, et al. Cancer Res. 2013;73:5473. doi: 10.1158/0008-5472.CAN-13-0525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Li J, et al. Carcinogenesis. 2012;33:459. doi: 10.1093/carcin/bgr281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li J, et al. Mol Carcinog. 2013;52:134. doi: 10.1002/mc.21839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rubio S, et al. Carcinogenesis. 2007;28:2105. doi: 10.1093/carcin/bgm131. [DOI] [PubMed] [Google Scholar]
- 101.Lee HS, et al. J Cancer Prev. 2014;19:161. doi: 10.15430/JCP.2014.19.3.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sak K, et al. Pharmacogn Rev. 2014;8:122. doi: 10.4103/0973-7847.134247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yao K, et al. Cancer Prev Res. 2014;7:958. doi: 10.1158/1940-6207.CAPR-14-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Arul N, Cho YY. Front Oncol. 2013;3:201. doi: 10.3389/fonc.2013.00201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.van Zanden JJ, et al. Biochem Pharmacol. 2004;67:1607. doi: 10.1016/j.bcp.2003.12.032. [DOI] [PubMed] [Google Scholar]
- 106.Markaverich BM, et al. J Steroid Biochem Mol Biol. 2010;122:219. doi: 10.1016/j.jsbmb.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Bai L, et al. Mol Pharmacol. 2012;81:549. doi: 10.1124/mol.111.076653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pratheeshkumar P, et al. PLoS One. 2012;7:31. [Google Scholar]
- 109.Lim do Y, et al. BMC Gastroenterol. 2012;12:12. [Google Scholar]
- 110.Byun S, et al. Cancer Res. 2010;70:2415. doi: 10.1158/0008-5472.CAN-09-4093. [DOI] [PubMed] [Google Scholar]
- 111.Lee KM, et al. Cancer Prev Res. 2010;3:454. doi: 10.1158/1940-6207.CAPR-09-0137. [DOI] [PubMed] [Google Scholar]
- 112.Lee DE, et al. arcinogenesis. 2011;32:629. doi: 10.1093/carcin/bgr008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Backhus LM, et al. J Thorac Cardiovasc Surg. 2006;132:297. doi: 10.1016/j.jtcvs.2005.10.060. [DOI] [PubMed] [Google Scholar]
- 114.Sandler AB, Dubinett SM. Semin Oncol. 2004;31:45. doi: 10.1053/j.seminoncol.2004.03.045. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


