Abstract
Aim:
The purpose of this study was to investigate the prevalence of enteropathogenic Escherichia coli (EPEC) and shiga toxin producing E. coli (STEC) strains in healthy broilers in Iran.
Background:
STEC and EPEC strains as diarrheagenic E. coli are among the most prevalent causative agents in acute diarrhea. Domestic animals, mainly cattle and sheep, have been implicated as the principal reservoirs of these pathotypes; however their prevalence among the broilers is varied among different countries.
Patients and methods:
A total of 500 cloacal swab samples from broilers of five different poultry houses (A-E) were collected to investigate the presence of stx1, stx2, hly, eae, and bfp virulence genes among the E. coli isolates by polymerase chain reaction. The shiga toxin encoding strains were evaluated serologically to detect their interaction with a commercial antiserum against O157 antigen.
Results:
Out of the 500 collected samples, 444 E. coli strains were isolated. Three strains (0.67%) presented at least one of the studied virulence genes (stx2, hly and eae), two strains were identified as STEC (stx2+, O157:nonH7) and one as an atypical EPEC strains (eae+ bfp-).
Conclusion:
The study established the presence of STEC and atypical EPEC in healthy broilers in Iran. Poultry might serve as vectors for transmission of pathogenic E. coli to human populations.
Key Words: E. coli, O157:nonH7, STEC, Atypical EPEC, Poultry
Introduction
Shiga toxin-producing Escherichia coli (STEC) and enteropathogenic E. coli (EPEC) are considered as two diarrhoeagenic E. coli pathotypes (1). STEC, a serologically diverse group of foodborne zoonotic pathogens, is an important causative agent of haemorrhagic colitis (HC) and diarrhoea-associate haemolytic uremic syndrome (HUS) with or without neurological complications (2). Animals, especially ruminants (cattle, sheep and goat) have been implicated as the reservoirs of STEC (3). Two phage- encoded cytotoxins (Stx1 and Stx2), which are produced by stx1 and stx2 genes, mediate mainly the pathogenicity of STEC. The virulence-associated factor intimin, encoded by eae, is responsible for intimate attachment of STEC to the enterocytes, causing attaching and effacing (A⁄E) lesions in the intestinal mucosa (4). Serotype O157:H7 has been the predominant type worldwide (5). EPEC strains, as intimin-containing diarrhoeagenic E. coli, possess the ability to form attaching-and-effacing (A⁄E) lesions on intestinal cells (6). Based on the presence or absence of bundle forming pili gene (bfpA), EPEC strains are classified as typical and atypical (7). There are several reports of the prevalence and characterization of STEC in poultry (1, 8) and wild birds, including pigeon (9). However, in Iran, such information from avian species is scant. Therefore, the aim of the present study was to study the prevalence of STEC and EPEC in avian species, to analyze the stx variants (stx1 or stx2) and the typical and atypical nature of the EPEC.
Patients and Methods
Bacterial isolates
A total of 500 cloacal swab samples from avian of five different poultry houses were collected over the six-month period in 2013 in Tehran, Iran. Samples were transported to the laboratory in the Cary- Blair medium (Merck, Germany). For E. coli isolation, the swabs were immediately inoculated into MacConkey agar medium (Merck, Germany). The plates were incubated for 18–24 h at 37 °C. Single colonies with typical color and appearance of E. coli were isolated from each sample for further analysis. Characterization of the suspected E. coli isolates was performed according to conventional laboratory biochemical tests (10).
PCR for detection of stx1, stx2, eae and bfp genes
Primers used in the study are listed in Table 1. The template DNA was prepared as described by the method of Blanco et al. (11). Avian E. coli isolates were subjected to PCR for detection of stx1, stx2, hly, eae and bfp genes.
Table1.
Nucleotide sequences of primers that used in PCR for detection of E. coli pathotypes
| Target gene | Primer | Sequence(5'-3') | Size (bp) | Reference |
|---|---|---|---|---|
| stx1 | Stx1F | 5׳ GAAGAGTCCGTGGGATTACG | 130 | (12) |
| Stx1R | 5׳ AGCGATGCAGCTATTAATA | |||
| stx2 | Stx2F | 5׳ GGATGCATCTCTGGTCATTG | 478 | (13) |
| Stx2R | 5׳ CTTCGGTATCCTATTCCCGG | |||
| hly | HlyF | 5׳ AGCTGCAAGTGCGGGTCTG | 569 | (14) |
| HlyR | 5׳ TACGGGTTATGCCTGCAAGTTCAC | |||
| eae | EaeF | 5 ׳TCAATGCAGTTCCGTTATCAGTT | 482 | (15) |
| EaeR | 5׳ GTAAAGTCCGTTACCCCAACCTG | |||
| bfp | BfpF | 5׳ CACCGTTACCGCAGGTGTGA | 450 | (16) |
Phenotypic identification of E. coli O157 strains
Ability of sorbitol fermentation of Shiga toxin encoding strains was evaluated on Sorbitol MacConkey agar medium. A Commercial serologic test was used for the detection of O157 serotype (Baharafshan, Iran) according to the manufacturers’ instruction manual (17).
Results
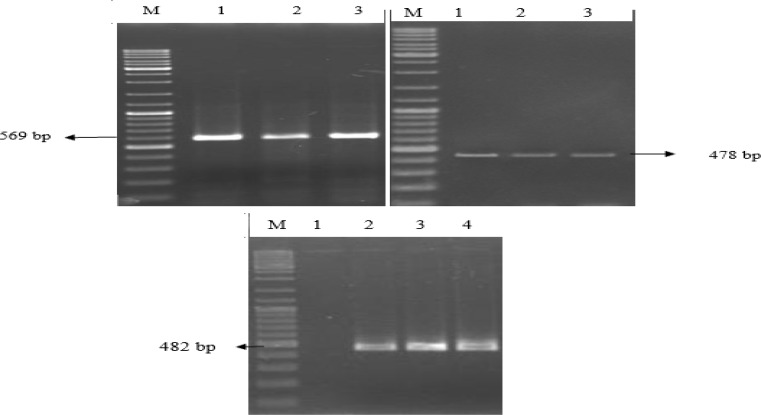
Out of the 500 collected samples, about 88.8% of the samples showed positive culture results for E. coli. Most of the isolates were related to lactose positive E. coli strains (98.6%), therefore the poultry house E included the most E. coli isolate lactose negative (3/6). Three of 444 E. coli isolates showed the presence of one of the studied virulence genes, among them two strains belonged to STEC and one to EPEC. Both of two STEC isolates were harbored stx2 and hly, but lacked eae virulence gene. None of the eae+ EPEC isolates harbored bfp and were designated as atypical (eae+/bfp-) (Figure 1). Serotyping of the Shiga toxin encoding strains showed that they belonged to O157 serotype and were identified as O157:nonH7 strains. While the EPEC strain showed a positive reaction with the sorbitol fermentation test, both the STEC strains failed to ferment sorbitol.
Figure 1.
Detection of hly (top left), stx2 (top right), and eae (bottom) genes in E. coli isolates from poultry. Lane M: DNA ladder mix, Lane 2: Positive control for each genes
Discussion
EPEC and STEC are foodborne pathogens that produce potentially fatal infant diarrhea and bloody diarrhea / hemolytic uremic syndrome, respectively (18, 19). In the present study, two and one strains of E. coli from cloacal swabs of broilers identified as STEC and atypical-EPEC, respectively. Transmission of these pathogenic E. coli strains from poultry to human through consumption of inadequate cooked products is problematic. Compared with studies that performed on cattle and sheep, limited numbers of surveys analyzed the prevalence of STEC and EPEC from avian E. coli pathotypes (20, 21). Contrary of data exist to correlate carriage of pathogenic E. coli in poultry and infection of human. In a study that was performed in Iran in 2014, EPEC and STEC pathotypes were detected from 12 carcasses of broiler chickens (22). Farooq et al. showed the presence of these pathotypes in avian species in India. Among 212 faecal samples, nine (4.24%) isolates were STEC, and 33 (15.56%) were EPEC (23).
However, the absence of STEC in chicken was established in different studies (24-26). It has already been established that typical EPEC are rarely isolated from animals, while atypical EPEC strains are isolated from both animals and humans. Detection of atypical EPEC in the present study is in agreement with the observations of Krause et al. who reported the presence of atypical EPEC in poultry in Germany (28). We reported that two STEC strains possessed stx2, which is consistent with the finding of Ghanbarpour et al. (2011) who revealed that 4.5% from healthy broilers in Iran harbored stx2 (29). Detection of stx2 in our study is in accordance with the observations of other investigations in pigeon who reported the isolation of Stx2 producing E. coli with the exception that belonged to O45, O18 and O75 serogroups (30, 31). However, in our study these strains belonged to serogroup O157. Doyle and Schoeni in 1987 reported the presence of E. coli O157 in poultry meat for the first time and stated that the organism is not a rare contamination of fresh meat and poultry (32). Similarly, verotoxin producing E. coli strains of serogroup O157 were detected among poultry in the Netherlands (33).
To the best of our knowledge, this is the first report of the presence of E. coli O157 serogroupe in healthy broilers in Iran. Seeking the environmental sources of these infections was the main limitation of this study that should be considered for a better control of their transmission.
In conclusion, our findings provide an initial data about the carriage of STEC and atypical-EPEC in healthy poultry in Iran. Therefore, poultry might serve as vectors for transmission of pathogenic E. coli strains to the human populations. The study of EPEC and E. coli O157 contamination at different stages of the slaughtering process in a chicken processing plant is a practical suggestion for more precise tracking of foodborne pathogens in the human food chain.
Acknowledgment
This study was part of Fatemeh Doregiraee PhD thesis and financially supported by the Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences and Department of Microbiology and Immunology, faculty of Veterinary Medicine, Tehran University, Iran.
References
- 1.Jafari F, Garcia-Gil LJ, Salmanzadeh-Ahrabi S, Shokrzadeh L, Aslani MM, Pourhoseingholi MA, et al. Diagnosis and prevalence of enteropathogenic bacteria in children less than 5 years of age with acute diarrhea in Tehran children's hospitals. J Infect. 2009;58:21–27. doi: 10.1016/j.jinf.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 2.Islam MA, Heuvelink AE, de Boer E, Sturm PD, Beumer RR, Zwietering MH, et al. Shiga toxin-producing Escherichia coli isolated from patients with diarrhoea in Bangladesh. J Med Microbiol. 2007;56:380–85. doi: 10.1099/jmm.0.46916-0. [DOI] [PubMed] [Google Scholar]
- 3.La Ragione RM, Best A, Woodward MJ, Wales AD. Escherichia coli O157:H7 colonization in small domestic ruminants. FEMS Microbiol Rev. 2009;33:394–410. doi: 10.1111/j.1574-6976.2008.00138.x. [DOI] [PubMed] [Google Scholar]
- 4.Moraa A, Blancoa JE, Blancoa M, Alonsob MP, Dhabia G, Echeitac A, et al. Antimicrobial resistance of Shiga toxin (verotoxin)-producing Escherichia coli O157:H7 and non-O157 strains isolated from humans, cattle, sheep and food in Spain. Res Microbiol. 2005;156:793–806. doi: 10.1016/j.resmic.2005.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Tarr PI, Gordon CA, Chandler WL. Shiga toxinproducing Escherichia coli and haemolytic uremic syndrome. Lancet. 2005;365:1073–86. doi: 10.1016/S0140-6736(05)71144-2. [DOI] [PubMed] [Google Scholar]
- 6.Kaper JB. Defining EPEC. Rev Microbiol. 1996;27:130–33. [Google Scholar]
- 7.Blanco M, Schumacher S, Tasara T, Zweifel C, Blanco JE, Dahbi G, et al. Serotypes, intimin variants and other virulence factors of eae positive Escherichia coli strains isolated from healthy cattle in Switzerland. Identification of a new intimin variant gene (eae-eta2) BMC Microbiol. 2005;5:23–41. doi: 10.1186/1471-2180-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pilipcinec E, Tkacikova L, Naas HT, Cabadaj R, Mikula I. Isolation of verotoxigenic Escherichia coli O157 from poultry. Folia Microbiol. 1999;44:455–56. doi: 10.1007/BF02903722. [DOI] [PubMed] [Google Scholar]
- 9.Morabito S, Dell’Omo G, Agrimi U, Schmidt H, Karch H, Cheasty T, et al. Detection and characterization of Shiga toxin-producing Escherichia coli in feral pigeons. Vet Microbiol. 2001;82:275–83. doi: 10.1016/s0378-1135(01)00393-5. [DOI] [PubMed] [Google Scholar]
- 10.Odonkor ST, Ampofo JK. Escherichia coli as an indicator of bacteriological quality of water: an overview. Microbiol Res. 2013;4:1–7. [Google Scholar]
- 11.Blanco M, Blanco JE, Mora A, Dahbi G, Alonso MP, Gonzalez EA, et al. Serotypes, virulence genes, and intimin types of shiga toxin (verotoxin)-producing Escherichia coli isolates from cattle in Spain and identification of a new intimin variant gene (eae- ) J Clin Microbiol. 2004;42:645–51. doi: 10.1128/JCM.42.2.645-651.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bopp DJ, Sauders BD, Waring AL, Ackelsberg J, Dumas N, Braun-Howland E, et al. Detection, isolation, and molecular subtyping of Escherichia coli O157:H7 and Campylobacter jejuni associated with a large waterborne outbreak. J Clin Microbiol. 2003;41:174–80. doi: 10.1128/JCM.41.1.174-180.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rashid M, Kotwal S, Malik M, Singh M. Prevalence, genetic profile of virulence determinants and multidrug resistance of Escherichia coli isolates from foods of animal origin. Vet World. 2013;6:139. [Google Scholar]
- 14.Wang G, Clark CG, Rodgers FG. Detection in Escherichia coli of the genes encoding the major virulence factors, the genes defining the O157:H7 serotype, and components of the type 2 Shiga toxin family by multiplex PCR. J Clin Microbiol. 2002;40:3613–19. doi: 10.1128/JCM.40.10.3613-3619.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stanilova S, Rusenova N, Petrova D, Stoyanchev T. Polymerase chain reaction assay for detection of enteropathogenic Escherichia coli strains in meat. Trakia J Sci. 2011;9:51–57. [Google Scholar]
- 16.Amisano G, Fornasero S, Migliaretti G, Caramello S, Tarasco V, Savino F. Diarrheagenic Escherichia coli in acute gastroenteritis in infants in North-West Italy. New Microbiol. 2011;34:45–51. [PubMed] [Google Scholar]
- 17.Kateete DP, Kimani CN, Katabazi FA, Okeng A, Okee MS, Nanteza A, et al. Identification of Staphylococcus aureus: DNase and Mannitol salt agar improve the efficiency of the tube coagulase test. Ann Clin Microbiol Antimicrob. 2010;9:23. doi: 10.1186/1476-0711-9-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alonso MZ, Padola NL, Parma AE, Lucchesi PMA. Enteropathogenic Escherichia coli contamination at different stages of the chicken slaughtering process. Poult Science. 2011;90:2638–41. doi: 10.3382/ps.2011-01621. [DOI] [PubMed] [Google Scholar]
- 19.Hunt JM. Shiga toxin-producing Escherichia coli (STEC) Clin Lab Med. 2010;30:21–45. doi: 10.1016/j.cll.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pilipcinec E, Tkacikova L, Naas HT, Cabadaj R, Mikula I. Isolation of verotoxigenic Escherichia coli O157 from poultry. Folia Microbiol. 1999;44:455–56. doi: 10.1007/BF02903722. [DOI] [PubMed] [Google Scholar]
- 21.Morabito S, Dell’Omo G, Agrimi U, Schmidt H, Karch H, Cheasty T, et al. Detection and characterization of Shiga toxin-producing Escherichia coli in feral pigeons. Vet Microbiol. 2001;82:275–83. doi: 10.1016/s0378-1135(01)00393-5. [DOI] [PubMed] [Google Scholar]
- 22.Bagheri M, Ghanbarpour R, Alizade H. Shiga toxin and beta-lactamases genes in Escherichia coli phylotypes isolated from carcasses of broiler chickens slaughtered in Iran. Int J Food Microbiol. 2014;177:16–20. doi: 10.1016/j.ijfoodmicro.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 23.Farooq S, Hussain I, Mir MA, Bhat MA, Wani SA. Isolation of atypical enteropathogenic Escherichia coli and Shiga toxin 1 and 2f-producing Escherichia coli from avian species in India. Lett Appl Microbiol. 2009;48:692–97. doi: 10.1111/j.1472-765X.2009.02594.x. [DOI] [PubMed] [Google Scholar]
- 24.Wani SA, Samanta I, Bhat MA, Nishikawa Y. Investigation of Shiga toxin-producing Escherichia coli in avian species in India. Lett Appl Microbiol. 2004;39:389–94. doi: 10.1111/j.1472-765X.2004.01586.x. [DOI] [PubMed] [Google Scholar]
- 25.Kobayashi H, Pohjanvirta T, Pelkonan S. Prevalence and characteristics of intimin- and Shiga toxin producing Escherichia coli from gulls, pigeons and broilers in Finland. J Vet Med Sci. 2002;64:1071–73. doi: 10.1292/jvms.64.1071. [DOI] [PubMed] [Google Scholar]
- 26.Schroeder CM, White DG, Ge B, Zhang Y, McDermott PF, Ayers S, et al. Isolation of antimicrobial resistant Escherichia coli from retail meats purchased in Greater Washington, DC, USA. Int J Food Microbiol. 2003;85:197–202. doi: 10.1016/s0168-1605(02)00508-1. [DOI] [PubMed] [Google Scholar]
- 27.Aktan I, Sprigings KA, La Ragione RM, Faulkner LM, Paiba GA, Woodward MJ. Characterization of attaching-effacing Escherichia coli isolated from animals at slaughter in England and Wales. Vet Microbiol. 2004;102:43–53. doi: 10.1016/j.vetmic.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 28.Krause G, Zimmermann S, Beutin L. Investigation of domestic animals and pets as a reservoir for intimin- (eae) gene positive Escherichia coli types. Vet Microbiol. 2005;106:87–95. doi: 10.1016/j.vetmic.2004.11.012. [DOI] [PubMed] [Google Scholar]
- 29.Ghanbarpour R, Sami M, Salehi M, Ouromiei M. Phylogenetic background and virulence genes of Escherichia coli isolates from colisepticemic and healthy broiler chickens in Iran. Trop Anim Health Prod. 2011;43:153–7. doi: 10.1007/s11250-010-9667-2. [DOI] [PubMed] [Google Scholar]
- 30.Schmidt H, Scheef J, Morabito S, Caprioli A, Wielar LH, Karch H. A new Shiga toxin 2 variant (stx2f) from Escherichia coli isolated from pigeons. Appl Environ Microbiol. 2000;66:1205–8. doi: 10.1128/aem.66.3.1205-1208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morabito S, Dell’Omo G, Agrimi U, Schmidt H, Karch H, Cheasty T, et al. Detection and characterization of Shiga toxin-producing Escherichia coli in feral pigeons. Vet Microbiol. 2001;82:275–83. doi: 10.1016/s0378-1135(01)00393-5. [DOI] [PubMed] [Google Scholar]
- 32.Doyle MP, Schoeni JL. Isolation of Escherichia coli O157:H7 from retail fresh meats and poultry. Appl Environ Microbiol. 1987;53:2394–96. doi: 10.1128/aem.53.10.2394-2396.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Heuvelink A, Zwartkruis-Nahuis JT, van den Biggelaar FL, van Leeuwen WJ, de Boer E. Isolation and characterization of verocytotoxin-producing Escherichia coli O157 from slaughter pigs and poultry. Int J Food Microbiol. 1999;52:67–75. doi: 10.1016/s0168-1605(99)00119-1. [DOI] [PubMed] [Google Scholar]