Abstract
Ethanol produces changes in GABAA receptor trafficking and function that contribute to ethanol dependence symptomatology. Extrasynaptic γ-aminobutyric acid A receptors (GABAA-R) mediate inhibitory tonic current and are of particular interest because they are potentiated by physiologically relevant doses of ethanol. Here, we isolate GABAA α4δ receptors by western blotting in subsynaptic fractions to investigate protein kinase A (PKA) and protein kinase C (PKC) modulation of ethanol-induced receptor trafficking, while extrasynaptic receptor function is determined by measurement of tonic inhibition and responses evoked by 4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol (THIP). Rat cerebral cortical neurons were grown for 18 days in vitro and exposed to ethanol and/or PKA/PKC modulators. Ethanol exposure (1 hour) did not alter GABAA α4 receptor abundance, but it increased tonic current amplitude, an effect that was prevented by inhibiting PKA, but not PKC. Direct activation of PKA, but not PKC, increased the abundance and tonic current of extrasynaptic α4δ receptors. In contrast, prolonged ethanol exposure (4 hours) reduced α4δ receptor abundance as well as tonic current, and this effect was also PKA dependent. Finally, PKC activation by ethanol or phorbol-12,13-dibutyrate (PdBu) had no effect on extrasynaptic α4δ subunit abundance or activity. We conclude that ethanol alters extrasynaptic α4δ receptor function and expression in cortical neurons in a PKA-dependent manner, but ethanol activation of PKC does not influence these receptors. These results could have clinical relevance for therapeutic strategies to restore normal GABAergic functioning for the treatment of alcohol use disorders.
Introduction
Ethanol exposure has been shown to produce adaptations in GABAA receptor trafficking that are associated with alcohol dependence and withdrawal (Kumar et al., 2009). GABAA receptors are heteropentameric ion channels, with the majority of receptors consisting of two α (1–6), two β (1–3), and a γ (1–2) or δ subunit (Tretter and Moss, 2008). As the major inhibitory receptors in the brain, GABAA transmission involves both phasic and tonic inhibition, which are conducted by synaptic and extrasynaptic receptors, respectively (Farrant and Nusser, 2005). Previous studies have elucidated that synaptic and extrasynaptic populations of receptors are differentially regulated by ethanol. Extrasynaptic α4βδ GABAA in particular appear to be important mediators of the acute physiological effects of ethanol (Sundstrom-Poromaa et al., 2002; Wallner et al., 2003; Wei et al., 2004), though this effect is controversial (Borghese et al. 2006). In both hippocampus and cerebral cortex, extrasynaptic α4-containing receptors are down-regulated by ethanol, producing a decrease in the overall inhibitory “tone” (Liang et al., 2004; Liang et al., 2007; Carlson et al., 2014). Dysregulation of GABAergic receptors in the cerebral cortex are likely to contribute to the molecular underpinnings of decreased cognitive abilities, reduced seizure threshold, and increased anxiety observed in alcohol dependence (Koob, 2003). However, the precise mechanism for down-regulation of extrasynaptic α4-containing receptors is still unknown.
Recent studies by our laboratory have elucidated that protein kinases play a major role in facilitating the regulation of GABAA receptors after ethanol exposure (Kumar et al., 2010; Werner et al., 2011; Kumar et al., 2012). Ethanol has long been known to activate both protein kinase A (PKA) (Dohrman et al., 1996) and protein kinase C (PKC) (Messing et al., 1991), and kinases are important regulators of the behavioral effects of ethanol (Harris et al., 1995; Hodge et al., 1999; Thiele et al., 2000; Choi et al., 2008). PKCγ activation by ethanol is requisite for the increase in GABAA α4 surface expression after 4 hours of ethanol exposure in cultured cerebral cortical neurons (Werner et al., 2011). This change in surface expression was postulated to be due to an increase in synaptic α4βγ2 receptors because GABAA δ subunit surface expression remained unchanged. Interestingly, the trafficking changes mediated by PKC are mitigated by concurrent activation of PKA by ethanol both in vivo and in vitro (Kumar et al., 2012; Carlson et al., 2013). Furthermore, we found that mice lacking the PKA regulatory subunit RIIβ gene have enhanced vulnerability to reductions in extrasynaptic GABAA α4 subunit trafficking after acute ethanol challenge (Carlson et al., 2014). Taken together, this evidence suggests that PKA may play a major role in regulating extrasynaptic α4-containing GABAA receptor subtypes in the cortex.
The present study sought to clarify the role of PKA and PKC in ethanol regulation of extrasynaptic α4-containing GABAA receptor populations in cerebral cortical cultured neurons. These cells exhibit the same ethanol adaptations that have been described in the cerebral cortex of ethanol-dependent rats (Devaud et al., 1997; Kumar et al., 2003) but allow for investigation of mechanistic questions that could not be addressed in vivo. Receptor adaptations were determined using subcellular fractionation of subsynaptic membrane fractions coupled with western blot analysis (Carlson et al., 2014), while functional changes were measured using whole-cell patch clamp analysis of tonic currents. We examined the time-dependent effects of ethanol on extrasynaptic GABAA α4 receptor function and trafficking because time-dependent effects of ethanol were observed on synaptic GABAA receptors as well as ethanol activation of PKC and PKA (Kumar et al., 2012; Carlson et al., 2013).
Materials and Methods
Cultured Cerebral Cortical Neurons.
All experiments were conducted in accordance with guidelines from the National Institutes of Health and Institutional Animal Care and Use Committee at the University of North Carolina. Mixed-sex rat pups from Sprague–Dawley breeding pairs (Harlan, Indianapolis, IN) were decapitated on postnatal day 0–1. The brains were rapidly dissected, and the cerebral cortices were isolated. The cortical halves were minced into fine pieces, and the tissue was incubated in CO2-independent medium containing papain (50 U/ml; Worthington, Lakewood, NJ), l-cysteine, and DNase (both from Sigma-Aldrich, St. Louis, MO) for 30 minutes at 37°C. Tissue pieces were gently washed followed by gentle trituration in Dulbecco’s modified Eagle’s medium (GIBCO, Grand Island, NY) containing 10% horse serum, penicillin and streptomycin (Pen-Strep), and DNase. Cells used for biochemistry were plated onto poly-d-lysine–coated flasks, and cells used for electrophysiology were plated onto poly-d-lysine–coated cover slips in 12-well plates. Cells were maintained in a 5% CO2 humidified incubator.
After day 3, the cells were fed with serum-free medium containing B27 and Pen-Strep (10,000 U/ml; final concentration 50 U per flask). The medium was changed twice per week, with no more than one-third of the medium being removed during exchanges. For all experiments, Pen-Strep was removed from cultures on day 14 to prevent interactions with GABAA receptors. The cultures were maintained for 18 days before conducting experiments, as prior studies had determined that neurons at this time point show stable GABAA receptor expression, with GABAA receptor populations mirroring the electrophysiologic and pharmacologic properties and responses to ethanol of adult neurons.
Ethanol and Drug Exposure.
Cultured cells were exposed to 50 mM ethanol, a concentration previously shown to produce changes in GABAergic inhibition highly consistent with in vivo models (Devaud et al., 1997; Kumar et al., 2003), and were placed in a plastic vapor chamber within the incubator. A beaker of 50 mM ethanol was used to maintain stable ethanol concentrations in the chamber. Control cells were exposed to an equivalent amount of water and placed in a plastic vapor chamber with a beaker containing water. Cells were exposed to drugs for either 1 or 4 hours. To examine PKA involvement, we added the PKA activator Sp-adenosine 3′,5′-cyclic monophosphothioate triethylamine (Sp-cAMP, 50 µM), or the PKA inhibitor Rp-adenosine 3′,5′-cyclic monophosphothioate triethylamine (Rp-cAMP, 50 µM) or phosphodiesterase inhibitor rolipram (10 µ) to the cell medium. These doses were chosen based on previous studies (Zhang and Pandey, 2003; Carlson et al., 2013). To examine PKC involvement, we used the PKC inhibitor calphostin C (CalC, 0.3 µM) and the activator phorbol-12,13-dibutyrate (PdBu, 100 nM), as previously described elsewhere (Kumar et al., 2010).
Fractionation.
After the experiments, the reactions were stopped by placing the flasks on ice. The cells were washed with cold phosphate-buffered saline, scraped, centrifuged at 1000g for 18 minutes, and stored at −80°C until fractionation. The cell pellets were homogenized in 0.32M d-sucrose and centrifuged at 1000g for 10 minutes. The supernatant was then centrifuged twice for 30 minutes at 12,000g. The final pellet was resuspended in phosphate-buffered saline.
For some experiments, the P2 fraction was further purified into extrasynaptic fractions according to the methods of Goebel-Goody et al. (2009). We have previously shown that this procedure produces enrichment of synaptic and extrasynaptic markers in their respective fractions (Carlson et al., 2014). The fractions were separated by 30 minutes of incubation in 0.5% Triton-X, followed by two centrifugations at 32,000g for 30 minutes. The supernatant was incubated overnight at −20°C in acetone (1:8 ratio supernatant/acetone). The resulting solution was spun twice for 30 minutes at 12,000g to produce the extrasynaptic fraction. Protein concentrations for isolated P2 or extrasynaptic fractions were calculated using a bicinchoninic acid protein assay kit (Thermo Fisher Scientific, Waltham, MA). Samples were then subjected to gel electrophoresis and western blot analysis.
Western Blot Analysis.
PKA regulatory subunit IIα (RIIα), PKA regulatory subunit IIβ (RIIβ), PKCγ, and GABAA receptor α4 and δ subunits were analyzed by western blot as described elsewhere (Kumar et al., 2010). Protein samples were subjected to SDS-PAGE using Novex Tris-Glycine (8%–16%) gels and transferred to polyvinylidene difluoride membranes (Invitrogen, Carlsbad, CA). Membranes were probed with GABAA receptor α4 (1:500; Abcam, Cambridge, MA), or δ (1:500; Millipore, Billerica, MA), PKA RIIα (1:1000; Novus, Littleton, CO), PKA RIIβ (Novus; 1:2000), and PKCγ (Novus; 1:1000) antibodies. Blots were then exposed to a β-actin antibody (1:3000; Millipore) as a loading control. Proteins were detected with enhanced chemiluminescence (GE Healthcare, Amersham, United Kingdom). Membranes were imaged using LAS-4000 (GE Healthcare), and densitometric analysis was conducted using GE ImageQuant software. Comparisons were made within blots and expressed as a percentage of averaged control values.
Electrophysiology.
Standard whole-cell voltage-clamp recordings were made with glass electrodes, fire-polished to a resistance of 2-3 MΩ and filled with internal solution (150 mM KCl, 3.1 mM MgCl2, 15 mM HEPES, 5 mM K-ATP, 5 mM EGTA, and 15 mM phosphocreatine, adjusted to pH 7.4 with KOH). The recording chamber was perfused with external solution (145 mM NaCl, 5 mM KCl, 10 mM HEPES, 2 mM CaCl2, 1 mM MgCl2, 5 mM sucrose, and 10 mM glucose, adjusted to pH 7.4 with NaOH). Drugs were diluted in external solution and applied using a U-tube apparatus. Recordings were performed at room temperature (22–23°C). The membrane potential was held at -60 mV using a patch-clamp amplifier (Axopatch 1D; Molecular Devices, Sunnyvale, CA), and data were collected with Clampex 10.2 software (Molecular Devices). Bicuculline (20 μM) was used an indicator of tonic current activity, while THIP (1 μM) was used an indicator of extrasynaptic δ-receptor agonism. Drugs were applied for 1.5 min after a 10 s baseline activity was established, followed by 30 s washout. Tonic and THIP-evoked currents were measured as the change in current amplitude in the presence of drug to an average of current baseline amplitude and post-washout.
Kinase Activity Assay.
Kinase activity was measured using the PepTag assay from Promega (Madison, WI). Assays were performed on cultured cells that were exposed to 50 mM ethanol for 1 or 4 hours and subjected to P2 fractionation procedures described earlier. Assays were conducted according to the manufacturer’s instructions.
Statistical Analysis.
All numerical data are presented as a percentage of control values and as mean ± S.E.M. Significance was determined using one-way analysis of variance (ANOVA) followed by Bonferroni correction for multiple comparisons post hoc test if there were more than two groups or Student’s t test for only two groups.
Results
Acute Ethanol Exposure Increases Tonic Current Activity in a PKA-Dependent Manner with No Effect on Extrasynaptic α4 Subunit Abundance.
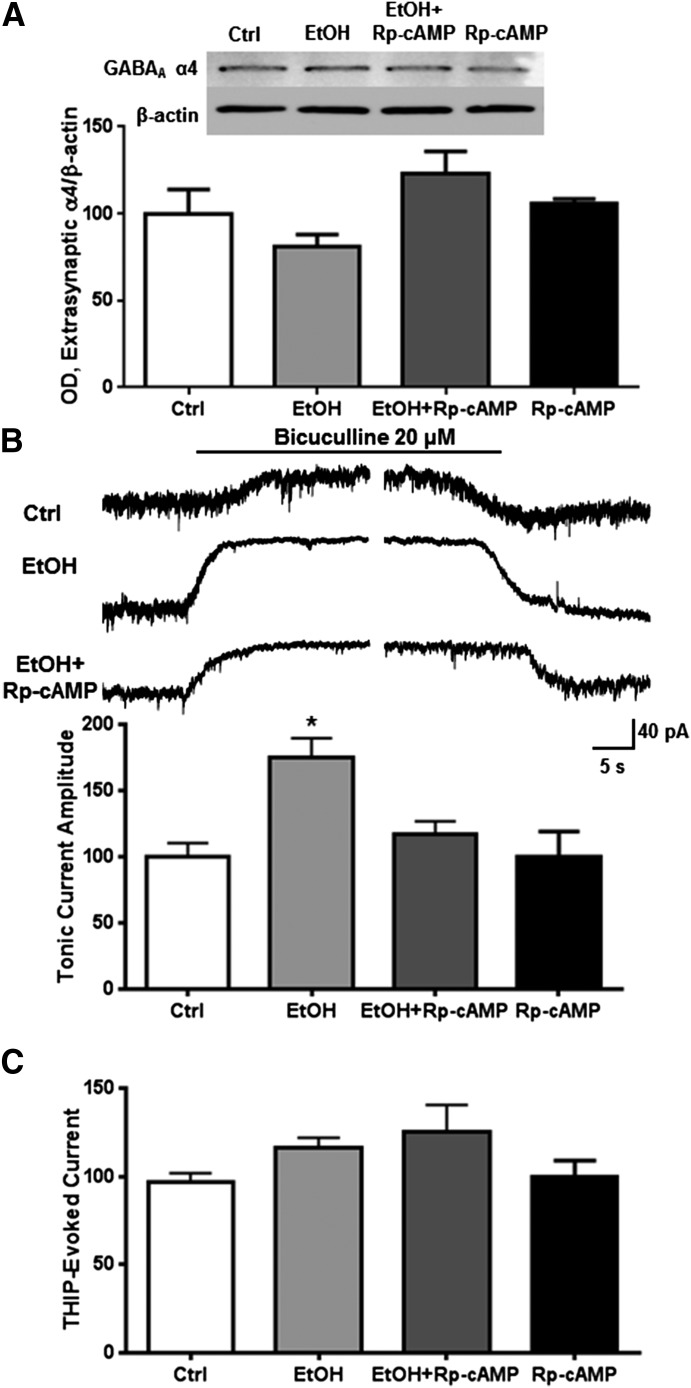
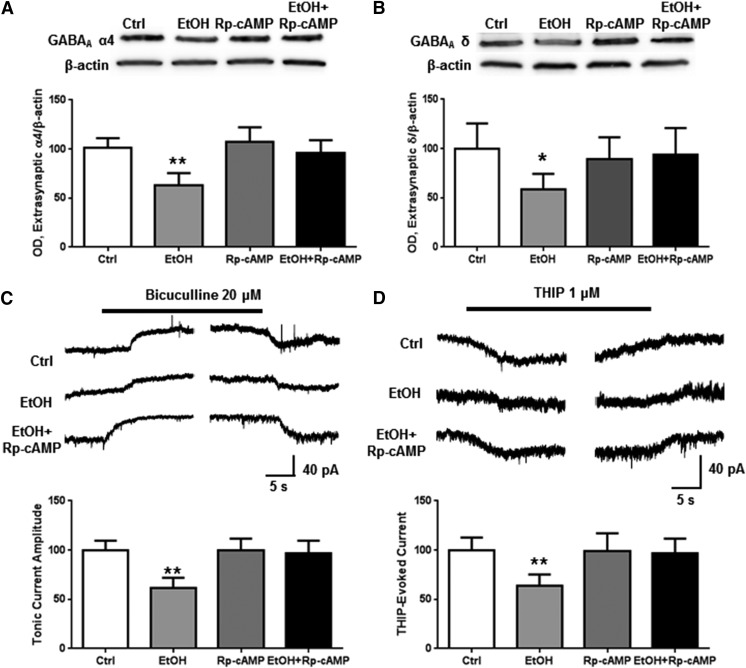
We used a subsynaptic preparation we have previously shown to enrich indicators of extrasynaptic proteins (Carlson et al., 2014) to examine the effects of ethanol exposure (50 mM) for 1 hour to determine its effect on receptor function and expression. Neither exposure to ethanol alone nor coexposure of ethanol and Rp-cAMP altered extrasynaptic abundance of the GABAA α4 subunits (Fig. 1A). However, ethanol increased the baseline tonic activity, blocked by bicuculline in neurons by 74.8% ± 15.1% (Fig. 1B; one-way ANOVA, f = 7.624, P < 0.01, Bonferroni posttest, P < 0.01, n = 5–12 per group), with no effect on current evoked by THIP (1 μM). Bicuculline effectively blocks the constitutively active baseline GABA conductance, whereas THIP shows high preference for α4β3δ receptors at 1 μM (Mortensen et al., 2010). The ethanol effect on tonic current was prevented by coexposure with Rp-cAMP. No effect was observed from ethanol or PKA inhibition on THIP-evoked current amplitude (Fig. 1C).
Fig. 1.
PKA mediates ethanol-induced increases in tonic current activity. Cortical neurons were exposed to vehicle, ethanol (50 mM), and/or Rp-cAMP (50 μM) for 1 hour followed by either subsynaptic fractionation and western blot analysis or whole-cell patch clamp recording. (A) There was no effect of ethanol and/or PKA inhibition on extrasynaptic α4 subunit abundance. (B) Ethanol exposure significantly increased bicuculline-blocked current, an effect that was prevented by inhibiting PKA. (C) There was no effect of ethanol or PKA inhibition on THIP-evoked current. * P < 0.05, one-way ANOVA Bonferroni post-test, n = 7–12.
Direct PKA Activation Increases Membrane Expression and Function of Extrasynaptic α4δ Receptors.
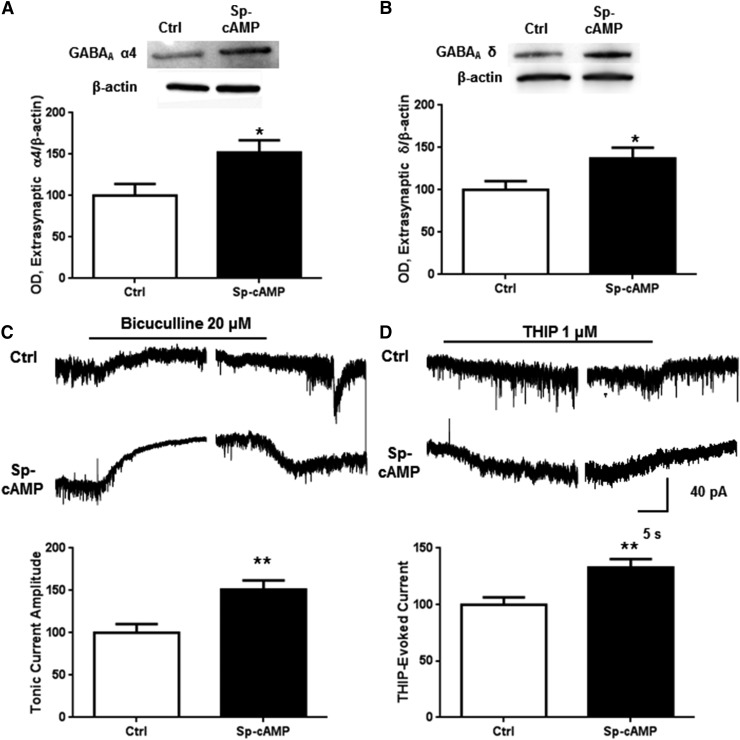
We next tested the effects of direct PKA activation on extrasynaptic GABAA α4 abundance and function. Activation of PKA by exposure to Sp-cAMP (50 µM) for 1 hour increased α4 subunit abundance in the extrasynaptic fraction by 52.4% ± 14.3% (Fig. 2A, P < 0.05, Student’s t test, n = 10). Similarly, Sp-cAMP increased δ subunit abundance by 36.6% ± 13.2% (Fig. 2B, P < 0.05, Student’s t test, n = 8). The comprehensive GABA antagonist bicuculline (20 μM) and the δ subunit-preferring agonist THIP (1 μM) were used as physiological indicators of altered tonic current activity. Tonic and THIP-evoked currents were both significantly increased after 1 hour Sp-cAMP exposure (Fig. 2, C and D; P < 0.01, Student’s t test, n = 9–13).
Fig. 2.
PKA activation increases extrasynaptic α4 subunit abundance and function. Cortical neurons were exposed to vehicle or Sp-cAMP (50 μM) for 1 hour followed by either subsynaptic fractionation and western blot analysis or whole-cell patch clamp recording. Exposure to Sp-cAMP for 1 hour caused an increase in (A) extrasynaptic α4 and (B) δ subunit abundance as well as (C) tonic current blocked by bicuculline (20 μM) or (D) THIP-evoked (1 μM) current. *P < 0.05, **P < 0.01, Student’s t test, n = 7–13.
PKC Is Not Involved in Ethanol Regulation of Extrasynaptic GABAA α4 Receptors.
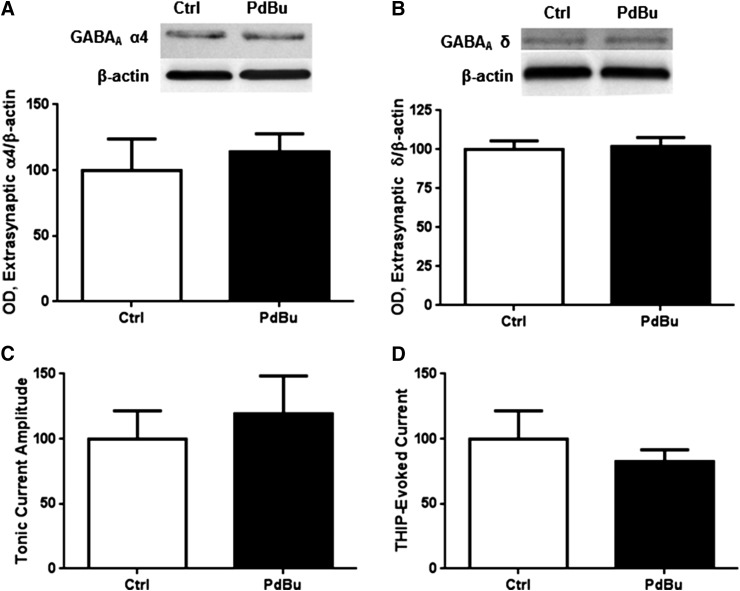
Given that our prior work indicates that PKA and PKC have antagonistic actions on GABAA receptor α1 subunit abundance (Werner et al., 2011; Carlson et al., 2013), we investigated the effect of PKC activity on extrasynaptic GABAA α4 abundance and function. Although PKCγ is not directly increased in the subsynaptic fraction (Table 1), we cannot discount downstream or non-site-specific effects of PKC. Activation of PKC for 1 hour using PdBu (100 nM) had no effect on extrasynaptic α4 (Fig. 3A) or δ subunit abundance (Fig. 3B). PKC has previously been shown to regulate synaptic α4 current in cortical neurons (Werner et al., 2011), so we investigated whether PKC activation altered tonic current. PdBu exposure did not alter tonic current (Fig. 3C) or THIP-evoked current amplitude (Fig. 3D).
TABLE 1.
Kinase abundance in the subsynaptic fraction after 1 and 4 hours of ethanol exposure.
Values expressed as percentage relative to control.
| Protein | 1-h Ethanol | 4-h Ethanol |
|---|---|---|
| PKA RIIα | 122.0 ± 3.5a | 97.3 ± 5.9 |
| PKA RIIβ | 126.2 ± 4.1a | 102.8 ± 6.3 |
| PKCγ | 104.5 ± 6.7 | 96.4 ± 5.1 |
P < 0.01, compared with control, Student’s t test, n = 4.
Fig. 3.
PKC activation does not alter extrasynaptic α4 subunit abundance or function. Cortical neurons were exposed to vehicle or PdBu (100 nM) for 1 hour followed by subsynaptic fractionation and western blot analysis. (A) PdBu exposure did not alter extrasynaptic α4 or (B) extrasynaptic δ subunit abundance, or (C) tonic or (D) THIP-evoked current.
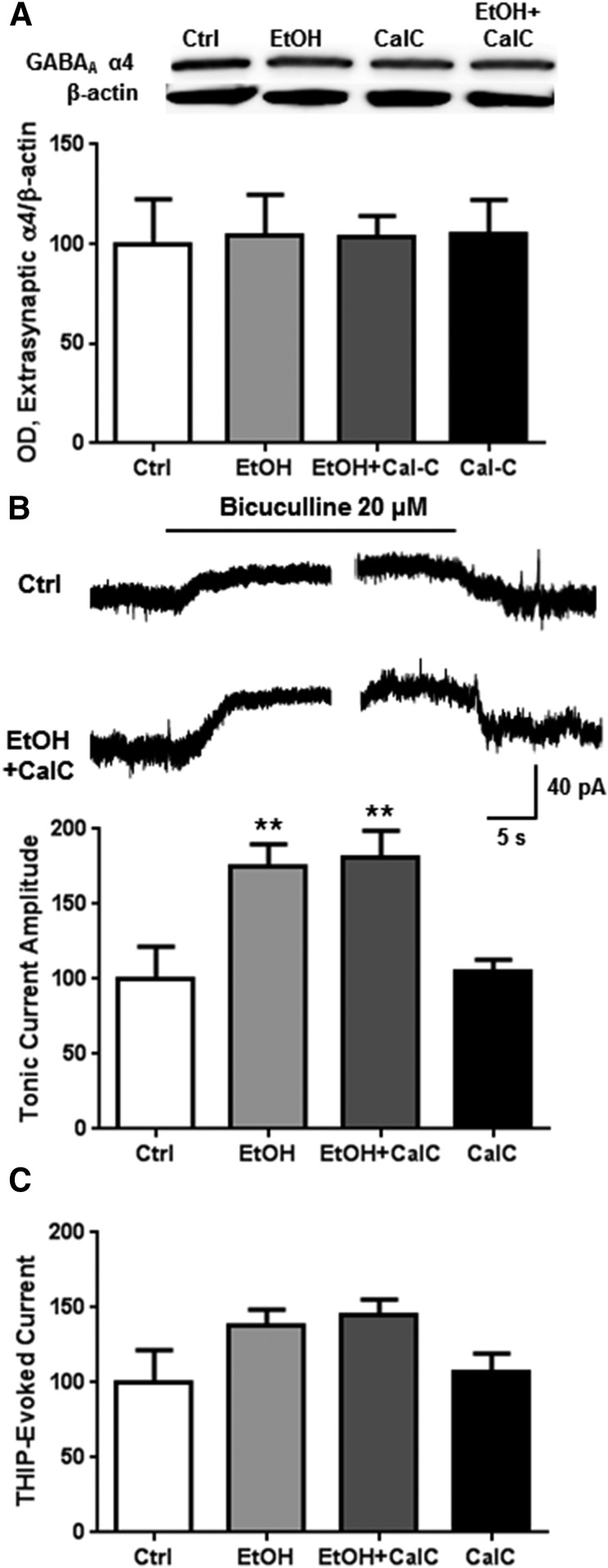
We investigated the inhibition of PKC in the presence of ethanol to determine whether PKC might be involved in the effects of ethanol on extrasynaptic GABAA α4 receptors. Inhibition of PKC by CalC (300 nM) in the presence of ethanol had no effect on extrasynaptic GABAA α4 subunit abundance (Fig. 4A). Similar to our previous data (Fig. 1B), ethanol exposure increased tonic current, an effect that was not prevented by coexposure with the PKC inhibitor CalC (Fig. 4B; one-way ANOVA, f = 6.6, P < 0.01, Bonferroni posttest, P < 0.01, n = 6–9). Ethanol alone or coexposure of ethanol with CalC did not alter THIP-evoked current amplitude (Fig. 4C).
Fig. 4.
PKC is not involved in 1-hour ethanol regulation of tonic current activity. Cortical neurons were exposed to vehicle, ethanol (50 mM), and/or CalC (0.3 μM) for 1 hour followed by subsynaptic fractionation and western blot analysis or whole-cell patch clamp recording. (A) There was no change in extrasynaptic GABAA α4 abundance at 1 hour for any of the exposures. (B) Ethanol and ethanol + CalC treatment increased bicuculline-blocked tonic current activity, but (C) had no effect on THIP-evoked current. ** P < 0.01, one-way ANOVA Bonferroni post-test, n = 5–9.
PKA Is Involved in Functional and Biochemical Changes in Extrasynaptic GABAA α4 after 4-Hour Ethanol Exposure.
We next investigated the role of PKA in mediating ethanol adaptations after 4 hours of ethanol exposure, a paradigm that recapitulates in cultured cortical neurons the changes seen after chronic ethanol exposure in vivo (Kumar et al., 2010; Werner et al., 2011). Ethanol produced a 37.2% ± 5.2% decrease in extrasynaptic GABAA α4 subunits (Fig. 5A; one-way ANOVA, f = 14.51, P < 0.0001, Bonferroni posttest, P < 0.01, n = 6 per group) and 41.3% ± 6.4% decrease in GABAA δ subunits (Fig. 5B; one-way ANOVA, f = 3.818, P < 0.05, Bonferroni posttest, P < 0.05, n = 6–7 per group), and both effects were prevented by coexposure with Rp-cAMP. Similarly, 4 hours of ethanol exposure decreased tonic current by 38.7% ± 6.3% (Fig. 5B; one-way ANOVA, f = 17.63, P < 0.0001, Bonferroni posttest, P < 0.01, n = 6–7 per group) and THIP-induced current by 36.3% ± 5.4% (Fig. 5C; one-way ANOVA, f = 10.18, P < 0.001, Bonferroni posttest, P < 0.01, n = 7 per group). Both effects were prevented by coexposure with Rp-cAMP.
Fig. 5.
Four-hour ethanol exposure causes a decrease in extrasynaptic α4 subunit abundance and function through a PKA-dependent mechanism. Cortical neurons were exposed to vehicle, ethanol (50 mM) and/or Rp-cAMP (50 μM) for 4 hours followed by either extrasynaptic fractionation and western blot analysis or whole-cell patch clamp recording. Ethanol caused a decrease in extrasynaptic GABAA receptor (A) α4 and (B) δ subunit abundance, (C) tonic current blocked by bicuculline, and (D) THIP-evoked current, all of which were prevented by Rp-cAMP coexposure. *P < 0.05, ** P < 0.01, one-way ANOVA Bonferroni post-test, n = 7-12.
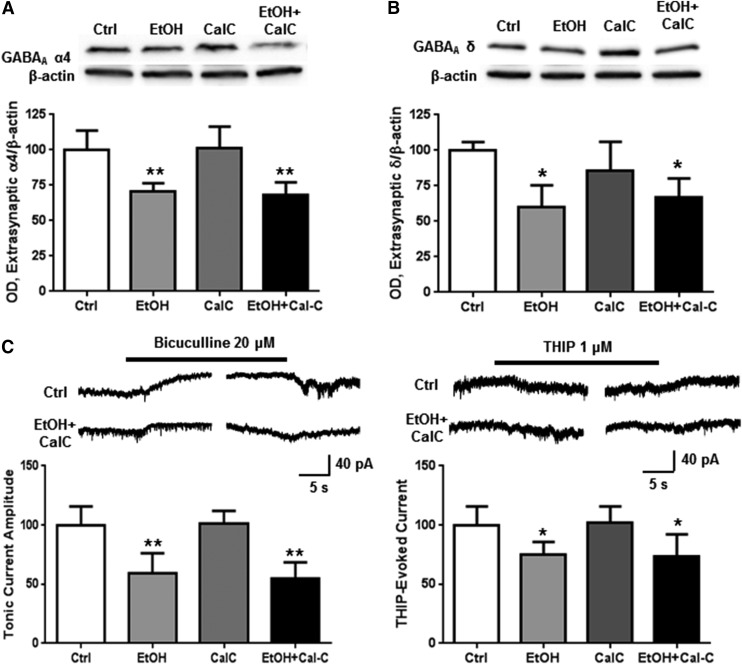
PKC is known to be active in cultured cortical neurons after 4-hour ethanol exposure (Kumar et al., 2010; Werner et al., 2011), so we investigated whether PKC might also be involved in the decreased abundance and functioning of extrasynaptic GABAA α4 subunits at this time point. Coexposure with CalC failed to prevent ethanol-induced decreases in extrasynaptic GABAA α4 (Fig. 6A; one-way ANOVA, f = 14.53, P < 0.0001, Bonferroni posttest, P < 0.01, n = 7 per group) or δ (Fig. 6B; one-way ANOVA, f = 8.802, P < 0.01, Bonferroni posttest, P < 0.01, n = 5–6 per group) subunit levels, tonic current (Fig. 6C; one-way ANOVA, f = 18.64, P < 0.0001, Bonferroni posttest, P < 0.01, n = 6–7 per group), or THIP-evoked current amplitude (Fig. 6D; one-way ANOVA, f = 6.454, P < 0.01, Bonferroni posttest, P < 0.05, n = 6–7 per group).
Fig. 6.
PKC is not involved in decreased extrasynaptic α4 abundance and function after 4-hour ethanol. Cortical neurons were exposed to vehicle, ethanol (50 mM), and/or CalC (0.3 μM) for 4 hours followed by subsynaptic fractionation and western blot analysis or whole-cell patch clamp recording. There was a decrease in extrasynaptic GABAA receptor (A) α4 and (B) δ subunit abundance, (C) tonic current blocked by bicuculline, and (D) THIP-evoked current in the ethanol and ethanol + CalC-exposed groups for all experiments. *P < 0.05, **P < 0.01, one-way ANOVA Bonferroni post-test, n = 6–7.
Ethanol Increases PKA Activity after 1 Hour of Exposure but Not 4 Hours.
We previously found that PKA regulatory subunits and PKCγ are enriched in P2 membrane fractions after 1 hour of ethanol exposure, but only PKCγ is increased after 4 hours (Kumar et al., 2010; Carlson et al., 2013). The PKA regulatory subunits RIIα and RIIβ were both increased in the subsynaptic fraction after 1 hour of ethanol exposure (P < 0.01, Student’s t test, n = 4), but PKCγ levels were unaffected (Table 1). Protein levels for all three kinase subunits were unaffected by 4 hours of ethanol exposure.
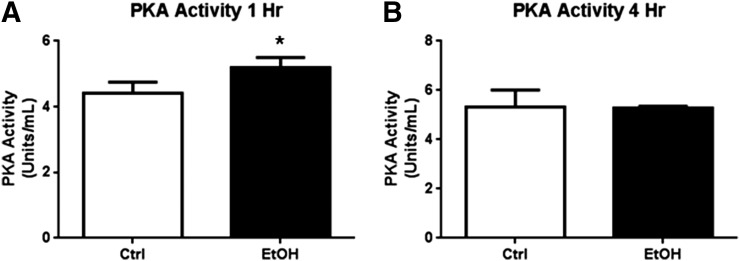
Because the PKA regulatory subunits show increased abundance in the extrasynaptic membrane fractions after 1 hour of ethanol exposure but not 4 hours of ethanol, we next assayed the level of PKA kinase activity at these time points to determine the functional significance of the contrasting effect of 1-hour versus 4-hour ethanol exposure. Ethanol increased PKA kinase activity after 1 hour by 17.6% ± 4.3% (Fig. 7A; Student’s t test, P < 0.05, n = 3) but not after 4 hours of ethanol exposure (Fig. 7B).
Fig. 7.
PKA activity is increased by 1-hour but not 4-hour ethanol exposure. Cortical neurons were exposed to ethanol (50 mM) followed by P2 fractionation and PepTag kinase activity assay. (A) PKA activity was increased after 1-hour ethanol exposure, but (B) not 4-hour ethanol exposure. *P < 0.05, Student’s t test, n = 3.
Discussion
Our study demonstrates that ethanol modulation of extrasynaptic GABAA α4 and δ subunits is PKA dependent and that PKA has direct but independent actions on the function and expression of these receptors in cortical neurons. Conversely, PKC activation by ethanol is not involved in the regulation of extrasynaptic α4 and δ subunits in cortical neurons. The present results provide further characterization of the subcellular mechanisms through which alcohol regulates GABAergic trafficking, which should have implications for cortical circuit functioning and could provide targets for restoring normal GABAA functioning during ethanol withdrawal.
Interestingly, although activation of PKA and PKC by ethanol has directly opposing effects on synaptic α1 receptors (Carlson et al., 2013), our results suggest that PKC does not play a role in regulating the extrasynaptic α4δ receptor population. This finding is consistent with previous studies in our laboratory that showed no effect of PKC activation on δ subunit expression in P2 membrane fractions (Werner et al., 2011). However, this result contrasts with previous studies that have found that PKC has a role in regulating extrasynaptic α4 receptor populations in the thalamus and hippocampus (Choi et al., 2008; Abramian et al., 2010; Bright and Smart, 2013). This discrepancy could be explained by a difference in regulation by brain region, cell type, or the slice preparation. One study found PKCδ enhanced tonic GABA currents in mouse hippocampal and thalamic slices (Choi et al., 2008); however, PKCδ was not found to associate with GABAA α4 receptors after chronic ethanol in the cerebral cortex (Kumar et al., 2002). Further, PKCδ is not detectable by western blot in our cerebral cortical cultures (unpublished results), which could also explain the lack of an effect of PKC on tonic current. Another study found brief PKC activation reduced tonic current activity in the hippocampus and thalamus only at physiologic temperatures (Bright and Smart, 2013). As the electrophysiologic recordings in our study were made at room temperature, we cannot rule out a temperature-specific effect of PKC on tonic GABA currents. The Bright and Smart (2013) study was also performed at 10 minutes of PKC activation, whereas our study found no effects at either 1 or 4 hours, so it is possible that there are also time-dependent effects. Other studies in cerebellar granule cells have found that differences in PKC activity can either enhance or inhibit tonic current in the presence of ethanol with variations attributable to genotype, suggesting PKC regulation is complex (Kaplan et al., 2013).
A decrease in tonic current inhibition after ethanol exposure is believed to be linked to many of the deleterious symptoms associated with alcohol withdrawal, such as increased seizure susceptibility (Liang et al., 2004). Dysfunctions in extrasynaptic GABAA receptors have also been linked to a variety of pathologic conditions including epilepsy, traumatic brain injury, depression, and schizophrenia (Whissell et al., 2015). Thus, PKA activation may provide a method for restoring tonic current functioning to normal levels during ethanol withdrawal as well as a variety of disease states. Indeed, the loss of PKA activation at 4 hours may suggest that restoration of PKA activation could reverse the effect of ethanol at this time point. Recent studies finding a reduction in drinking of rodents coexposed to the phosphodiesterase inhibitor rolipram further underscore the potential therapeutic relevance of manipulating PKA signaling in the treatment of alcohol use disorders (Hu et al., 2011; Wen et al., 2012).
The finding that acute ethanol exposure increased tonic current activity is consistent with previous studies in other brain regions (Jia et al., 2007; Santhakumar et al., 2007; Shen et al., 2011; Herman et al., 2013); however, to our knowledge this is the first report of increased tonic current inhibition after acute ethanol treatment in cortical neurons. If the direct involvement of PKA in mediating this increase is generalized to other brain regions, this finding could have broad implications for a potential role of PKA in ethanol effects on these receptors.
Surprisingly, whereas direct PKA activation increased abundance of both the extrasynaptic α4 and δ subunits corresponding with an increase in both tonic current and THIP-evoked current, ethanol activation of PKA did not appear to increase THIP-evoked current, despite the increase in tonic current. One explanation for this discrepancy is that ethanol did not alter receptor abundance after 1 hour of exposure. Alternatively, some of the ethanol-induced increase in tonic current may be mediated by α1 receptors.
Recently a novel form of tonic current activity has been characterized that is mediated by α1βγ receptors (Herman et al., 2013), known to be up-regulated by ethanol activation of PKA (Carlson et al., 2013). An additional explanation is that ethanol-induced PKA activation may alter the conformation of α4δ receptors in a manner that does not support enhanced function by THIP. Further, ethanol is a known positive allosteric modulator of GABAA-Rs and may potentiate GABAA-Rs at the dose used in the current study. Finally, ethanol may be acting on other intracellular pathways whose activity is masking an effect of PKA on THIP-evoked current (Freund and Palmer, 1997; Marutha Ravindran and Ticku, 2006). It is possible that ethanol is acting via kinase or phosphatase pathways whose actions have not been clearly defined within our experimental parameters (Kittler and Moss, 2003). Future studies aimed at further characterization of both the altered tonic current as well as the activity of other kinase and phosphatase pathways could resolve this issue.
The finding that PKA mediates an increase in tonic current after 1 hour of ethanol exposure was unsurprising, as PKA has been shown to mediate increased spontaneous channel opening of GABAA α4 receptors (Tang et al., 2010); however, the observation that PKA activity mediates a decrease in extrasynaptic GABAA α4 abundance and function after 4 hours of ethanol exposure was unexpected. There are two likely explanations for the discrepancy observed between increased activity at 1-hour but decreased activity at the 4-hour time point. It is possible that activation of PKA by ethanol at the 1-hour time point leads to activation of other pathways downstream of PKA, which leads to the alterations in GABAergic functioning. It is also possible that the observed decrease in abundance and function may be caused by a compensatory decrease after sustained increases caused by PKA at earlier time points. Future studies investigating other subcellular pathways, including other kinases, phosphatases, or phosphodiesterases could clarify this issue.
Overall, the present results further underscore the relevance of PKA signaling in modulating the GABAergic effects of ethanol. The data suggest that PKA activity may enhance GABAergic inhibition that is down-regulated by chronic ethanol exposure. Thus, this pathway could provide an important target for treatments aimed at restoring normal GABAA receptor functioning after chronic alcohol misuse. Given that extrasynaptic GABAA α4 receptors are altered in a variety of pathologic conditions (Whissell et al., 2015), the present results could have broad implications in treating disease states.
Acknowledgments
The authors thank Todd K. O’Buckley, Raechel McKinley, and Danielle Morrow for technical assistance.
Abbreviations
- ANOVA
analysis of variance
- CalC
calphostin-C
- GABAA-R
γ-aminobutyric acid A receptor
- PdBu
phorbol-12,13-dibutyrate
- Pen-Strep
penicillin and streptomycin
- PKA
protein kinase A
- PKC
protein kinase C
- RIIα
protein kinase A regulatory subunit IIα
- RIIβ
protein kinase A regulatory subunit IIβ
- Rp-cAMP
Rp-adenosine 3′,5′-cyclic monophosphothioate triethylamine
- Sp-cAMP
Sp-adenosine 3′,5′-cyclic monophosphothioate triethylamine
- THIP
4,5,6,7-tetrahydroisoxazolo[5,4-c]pyridin-3-ol
Authorship Contributions
Participated in research design: Carlson, Bohnsack, Morrow.
Conducted experiments: Carlson, Bohnsack, Patel.
Performed data analysis: Carlson, Bohnsack.
Wrote or contributed to the writing of the manuscript: Carlson, Bohnsack, Morrow.
Footnotes
This work was supported by National Institutes of Health National Institute on Alcohol Abuse and Alcoholism [Grants P60-AA11605, T32-AA007573] and the UNC Bowles Center for Alcohol Studies.
References
- Abramian AM, Comenencia-Ortiz E, Vithlani M, Tretter EV, Sieghart W, Davies PA, Moss SJ. (2010) Protein kinase C phosphorylation regulates membrane insertion of GABAA receptor subtypes that mediate tonic inhibition. J Biol Chem 285:41795–41805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghese CM, Stórustovu Si, Ebert B, Herd MB, Belelli D, Lambert JJ, Marshall G, Wafford KA, Harris RA. (2006) The δ subunit of γ-aminobutyric acid type A receptors does not confer sensitivity to low concentrations of ethanol. J Pharmacol Exp Ther 316:1360–1368. [DOI] [PubMed] [Google Scholar]
- Bright DP, Smart TG. (2013) Protein kinase C regulates tonic GABA(A) receptor-mediated inhibition in the hippocampus and thalamus. Eur J Neurosci 38:3408–3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SL, Kumar S, Werner DF, Comerford CE, Morrow AL. (2013) Ethanol activation of protein kinase A regulates GABAA α1 receptor function and trafficking in cultured cerebral cortical neurons. J Pharmacol Exp Ther 345:317–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson SL, O’Buckley TK, Thomas R, Thiele TE, Morrow AL. (2014) Altered GABAA receptor expression and seizure threshold following acute ethanol challenge in mice lacking the RIIβ subunit of PKA. Neurochem Res 39:1079–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DS, Wei W, Deitchman JK, Kharazia VN, Lesscher HM, McMahon T, Wang D, Qi ZH, Sieghart W, Zhang C, et al. (2008) Protein kinase Cδ regulates ethanol intoxication and enhancement of GABA-stimulated tonic current. J Neurosci 28:11890–11899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devaud LL, Fritschy J-M, Sieghart W, Morrow AL. (1997) Bidirectional alterations of GABA(A) receptor subunit peptide levels in rat cortex during chronic ethanol consumption and withdrawal. J Neurochem 69:126–130. [DOI] [PubMed] [Google Scholar]
- Dohrman DP, Diamond I, Gordon AS. (1996) Ethanol causes translocation of cAMP-dependent protein kinase catalytic subunit to the nucleus. Proc Natl Acad Sci USA 93:10217–10221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. (2005) Variations on an inhibitory theme: phasic and tonic activation of GABA(A) receptors. Nat Rev Neurosci 6:215–229. [DOI] [PubMed] [Google Scholar]
- Freund RK, Palmer MR. (1997) Beta adrenergic sensitization of gamma-aminobutyric acid receptors to ethanol involves a cyclic AMP/protein kinase A second-messenger mechanism. J Pharmacol Exp Ther 280:1192–1200. [PubMed] [Google Scholar]
- Goebel-Goody SM, Davies KD, Alvestad Linger RM, Freund RK, Browning MD. (2009) Phospho-regulation of synaptic and extrasynaptic N-methyl-d-aspartate receptors in adult hippocampal slices. Neuroscience 158:1446–1459. [DOI] [PubMed] [Google Scholar]
- Harris RA, McQuilkin SJ, Paylor R, Abeliovich A, Tonegawa S, Wehner JM. (1995) Mutant mice lacking the gamma isoform of protein kinase C show decreased behavioral actions of ethanol and altered function of gamma-aminobutyrate type A receptors. Proc Natl Acad Sci USA 92:3658–3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herman MA, Contet C, Justice NJ, Vale W, Roberto M. (2013) Novel subunit-specific tonic GABA currents and differential effects of ethanol in the central amygdala of CRF receptor-1 reporter mice. J Neurosci 33:3284–3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodge CW, Mehmert KK, Kelley SP, McMahon T, Haywood A, Olive MF, Wang D, Sanchez-Perez AM, Messing RO. (1999) Supersensitivity to allosteric GABA(A) receptor modulators and alcohol in mice lacking PKCε. Nat Neurosci 2:997–1002. [DOI] [PubMed] [Google Scholar]
- Hu W, Lu T, Chen A, Huang Y, Hansen R, Chandler LJ, Zhang HT. (2011) Inhibition of phosphodiesterase-4 decreases ethanol intake in mice. Psychopharmacology (Berl) 218:331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia F, Pignataro L, Harrison NL. (2007) GABAA receptors in the thalamus: α4 subunit expression and alcohol sensitivity. Alcohol 41:177–185. [DOI] [PubMed] [Google Scholar]
- Kaplan JS, Mohr C, Rossi DJ. (2013) Opposite actions of alcohol on tonic GABA(A) receptor currents mediated by nNOS and PKC activity. Nat Neurosci 16:1783–1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittler JT, Moss SJ. (2003) Modulation of GABAA receptor activity by phosphorylation and receptor trafficking: implications for the efficacy of synaptic inhibition. Curr Opin Neurobiol 13:341–347. [DOI] [PubMed] [Google Scholar]
- Koob GF. (2003) Alcoholism: allostasis and beyond. Alcohol Clin Exp Res 27:232–243. [DOI] [PubMed] [Google Scholar]
- Kumar S, Kralic JE, O’Buckley TK, Grobin AC, Morrow AL. (2003) Chronic ethanol consumption enhances internalization of α1 subunit-containing GABAA receptors in cerebral cortex. J Neurochem 86:700–708. [DOI] [PubMed] [Google Scholar]
- Kumar S, Porcu P, Werner DF, Matthews DB, Diaz-Granados JL, Helfand RS, Morrow AL. (2009) The role of GABA(A) receptors in the acute and chronic effects of ethanol: a decade of progress. Psychopharmacology (Berl) 205:529–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Ren Q, Beckley JH, O’Buckley TK, Gigante ED, Santerre JL, Werner DF, Morrow AL. (2012) Ethanol activation of protein kinase A regulates GABA(A) receptor subunit expression in the cerebral cortex and contributes to ethanol-induced hypnosis. Front Neurosci 6:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Sieghart W, Morrow AL. (2002) Association of protein kinase C with GABA(A) receptors containing α1 and α4 subunits in the cerebral cortex: selective effects of chronic ethanol consumption. J Neurochem 82:110–117. [DOI] [PubMed] [Google Scholar]
- Kumar S, Suryanarayanan A, Boyd KN, Comerford CE, Lai MA, Ren Q, Morrow AL. (2010) Ethanol reduces GABAA α1 subunit receptor surface expression by a protein kinase Cγ-dependent mechanism in cultured cerebral cortical neurons. Mol Pharmacol 77:793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang J, Cagetti E, Olsen RW, Spigelman I. (2004) Altered pharmacology of synaptic and extrasynaptic GABAA receptors on CA1 hippocampal neurons is consistent with subunit changes in a model of alcohol withdrawal and dependence. J Pharmacol Exp Ther 310:1234–1245. [DOI] [PubMed] [Google Scholar]
- Liang J, Suryanarayanan A, Abriam A, Snyder B, Olsen RW, Spigelman I. (2007) Mechanisms of reversible GABAA receptor plasticity after ethanol intoxication. J Neurosci 27:12367–12377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marutha Ravindran CR, Ticku MK. (2006) Tyrosine kinase phosphorylation of GABAA receptor subunits following chronic ethanol exposure of cultured cortical neurons of mice. Brain Res 1086:35–41. [DOI] [PubMed] [Google Scholar]
- Messing RO, Petersen PJ, Henrich CJ. (1991) Chronic ethanol exposure increases levels of protein kinase C δ and ε and protein kinase C-mediated phosphorylation in cultured neural cells. J Biol Chem 266:23428–23432. [PubMed] [Google Scholar]
- Mortensen M, Ebert B, Wafford K, Smart TG. (2010) Distinct activities of GABA agonists at synaptic- and extrasynaptic-type GABAA receptors. J Physiol 588:1251–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santhakumar V, Wallner M, Otis TS. (2007) Ethanol acts directly on extrasynaptic subtypes of GABAA receptors to increase tonic inhibition. Alcohol 41:211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Lindemeyer AK, Spigelman I, Sieghart W, Olsen RW, Liang J. (2011) Plasticity of GABAA receptors after ethanol pre-exposure in cultured hippocampal neurons. Mol Pharmacol 79:432–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundstrom-Poromaa I, Smith DH, Gong QH, Sabado TN, Li X, Light A, Wiedmann M, Williams K, Smith SS. (2002) Hormonally regulated α4β2δ GABA(A) receptors are a target for alcohol. Nat Neurosci 5:721–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X, Hernandez CC, Macdonald RL. (2010) Modulation of spontaneous and GABA-evoked tonic α4β3δ and α4β3γ2L GABAA receptor currents by protein kinase A. J Neurophysiol 103:1007–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele TE, Willis B, Stadler J, Reynolds JG, Bernstein IL, McKnight GS. (2000) High ethanol consumption and low sensitivity to ethanol-induced sedation in protein kinase A-mutant mice. J Neurosci 20:RC75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tretter V, Moss SJ. (2008) GABA(A) receptor dynamics and constructing GABAergic Synapses. Front Mol Neurosci 1:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner M, Hanchar HJ, Olsen RW. (2003) Ethanol enhances α 4 β 3 δ and α 6 β 3 δ γ-aminobutyric acid type A receptors at low concentrations known to affect humans. Proc Natl Acad Sci USA 100:15218–15223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Faria LC, Mody I. (2004) Low ethanol concentrations selectively augment the tonic inhibition mediated by delta subunit-containing GABAA receptors in hippocampal neurons. J Neurosci 24:8379–8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen RT, Zhang M, Qin WJ, Liu Q, Wang WP, Lawrence AJ, Zhang HT, Liang JH. (2012) The phosphodiesterase-4 (PDE4) inhibitor rolipram decreases ethanol seeking and consumption in alcohol-preferring Fawn-Hooded rats. Alcohol Clin Exp Res 36:2157–2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner DF, Kumar S, Criswell HE, Suryanarayanan A, Fetzer JA, Comerford CE, Morrow AL. (2011) PKCγ is required for ethanol-induced increases in GABA(A) receptor α4 subunit expression in cultured cerebral cortical neurons. J Neurochem 116:554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whissell PD, Lecker I, Wang DS, Yu J, Orser BA. (2015) Altered expression of δGABAA receptors in health and disease. Neuropharmacology 88:24–35. [DOI] [PubMed] [Google Scholar]
- Zhang H, Pandey SC. (2003) Effects of PKA modulation on the expression of neuropeptide Y in rat amygdaloid structures during ethanol withdrawal. Peptides 24:1397–1402. [DOI] [PubMed] [Google Scholar]