Abstract
Methcathinone (MCAT) is a monoamine releaser and parent compound to a new class of designer drugs that includes the synthetic cathinones mephedrone and flephedrone. Using MCAT and a series of para-substituted (or 4-substituted) MCAT analogs, it has been previously shown that expression of abuse-related behavioral effects in rats correlates both with the volume of the para substituent and in vitro neurochemical selectivity to promote monoamine release via the dopamine (DA) versus serotonin (5-HT) transporters in rat brain synaptosomes. The present study used in vivo microdialysis to determine the relationship between these previous measures and the in vivo neurochemical selectivity of these compounds to alter nucleus accumbens (NAc) DA and 5-HT levels. Male Sprague-Dawley rats were implanted with bilateral guide cannulae targeting the NAc. MCAT and five para-substituted analogs (4-F, 4-Cl, 4-Br, 4-CH3, and 4-OCH3) produced dose- and time-dependent increases in NAc DA and/or 5-HT levels. Selectivity was determined as the dose required to increase peak 5-HT levels by 250% divided by the dose required to increase peak DA levels by 250%. This measure of in vivo neurochemical selectivity varied across compounds and correlated with 1) in vivo expression of abuse-related behavioral effects (r = 0.89, P = 0.02); 2) in vitro selectivity to promote monoamine release via DA and 5-HT transporters (r = 0.95, P < 0.01); and 3) molecular volume of the para substituent (r = −0.85, P = 0.03). These results support a relationship between these molecular, neurochemical, and behavioral measures and support a role for molecular structure as a determinant of abuse-related neurochemical and behavioral effects of MCAT analogs.
Introduction
Methcathinone (MCAT) is the β-ketone analog of methamphetamine and serves as a parent compound to abused designer drugs that include mephedrone (4-CH3 MCAT) and flephedrone (4-F MCAT) (Glennon et al., 1987; Spiller et al., 2011; De Felice et al., 2014). Like methamphetamine, MCAT functions as a monoamine transporter substrate that selectively promotes release of dopamine (DA) and norepinephrine over serotonin (5-HT) (Cozzi et al., 1999, 2013; Baumann et al., 2012). Consistent with MCAT’s Schedule I controlled substance classification in the United States, MCAT increases striatal and nucleus accumbens (NAc) DA levels as measured by in vivo microdialysis in rats (Gygi et al., 1997; Cozzi et al., 2013). Furthermore, MCAT functions as a reinforcer in nonhuman primate drug self-administration procedures (Kaminski and Griffiths, 1994) and produces an abuse-related facilitation of intracranial self-stimulation (ICSS) in rats (Bonano et al., 2015). Overall, the preclinical neurochemical and behavioral effects of MCAT are consistent with its accepted abuse liability in humans.
Results from our laboratory and others suggest that the identity of the para (or 4-position) substituent on the MCAT scaffold is a significant determinant of the abuse-related neurochemical and behavioral effects of synthetic MCAT analogs (Fig. 1). In particular, the volume of the para substituent on the MCAT scaffold appears to influence its interaction with the DA transporter (DAT) and 5-HT transporter (SERT), such that DAT prefers small-volume substituents (e.g., MCAT itself, with a hydrogen atom at the para position), whereas SERT prefers substituents with larger volumes (e.g., mephedrone, with a methyl substituent at the para position) (Bonano et al., 2015; Sakloth et al., 2015). This shift in preference from DAT to SERT with increasing volume of the para substituent is evident from in vitro studies of DAT- and SERT-mediated monoamine release in rat brain synaptosomes, and this in vitro measure of declining DAT versus SERT selectivity correlates with in vivo evidence for declining expression of abuse-related ICSS facilitation (Bauer et al., 2013; Bonano et al., 2015). For example, mephedrone (4-CH3 MCAT) has lower selectivity than MCAT to promote DAT- versus SERT-mediated monoamine release in rat brain synaptosomes and produces weaker ICSS facilitation than MCAT (Bonano et al., 2014).
Fig. 1.

Chemical structure of the MCAT pharmacophore; R was systematically varied to generate MCAT analogs.
The correlation of ICSS effects of para-substituted MCAT analogs (and other monoamine releasers) with in vitro DAT versus SERT selectivity suggests that these behavioral effects might also correlate with in vivo selectivity to promote DA versus 5-HT release. For example, it is well established that increased DA release in NAc plays a key role in mediating abuse-related effects of many classes of abused drugs (Di Chiara and Imperato, 1988). Conversely, drug-induced increases in 5-HT levels appear to oppose and limit DA-mediated effects that contribute to abuse (Czoty et al., 2002; Navailles et al., 2008; Baumann et al., 2011). The goal of the present study was to further evaluate the relationship between in vivo selectivity to increase NAc DA versus 5-HT levels and both 1) in vitro measures of DAT versus SERT selectivity and 2) expression of abuse-related behavioral effects. Toward this end, this study used an in vivo microdialysis procedure to determine the potency and time course of effects produced by MCAT and five para-substituted MCAT analogs on NAc DA and 5-HT levels in rats. We hypothesized that the selectivity, and not absolute DA or 5-HT changes, of these drugs to increase NAc DA versus 5-HT levels would correlate with our previously determined measures of both in vitro DAT versus SERT selectivity (using a rat brain synaptosome procedure) and in vivo expression of ICSS facilitation (Bonano et al., 2015; Sakloth et al., 2015). Moreover, we also hypothesized that in vivo selectivity to increase DA versus 5-HT levels would also correlate with the volume of the para substituent on the MCAT scaffold.
Materials and Methods
Subjects
Adult male Sprague-Dawley rats (Harlan, Frederick, MD) weighing a minimum of 300 g at the time of surgery were individually housed and maintained on a 12-hour light/dark cycle with lights on from 6:00 AM to 6:00 PM. Rats had ad libitum access to food and water except during microdialysis experiments. Animal facilities were accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (http://www.aalas.org/), and experimental procedures were approved by the Institutional Animal Care and Use Committee in accordance with guidelines (8th edition) for the care and use of animal subjects in research (National Research Council, 2011). All efforts were made to minimize animal suffering; to reduce the number of animals used; and to use alternatives to in vivo techniques, if available.
Drugs
This study determined the neurochemical effects of MCAT and five para-substituted MCAT analogs. For MCAT, the para substituent (the 4-position on the benzene ring of the MCAT scaffold) is hydrogen (H). For the purposes of this paper, and for consistency with previous studies by Bonano et al. (2015) and Sakloth et al. (2015), MCAT analogs are designated using the nomenclature 4-R MCAT, with R being the substituent at the para position (see Fig. 1). In some cases, these compounds also have generic names or other chemical names, and in these cases, alternative names are also listed subsequently. Specifically, the following compounds were studied: MCAT (PubChem CID: 1576), 4-F MCAT (flephedrone; PubChem CID: 49853406), 4-Cl MCAT (clephedrone), 4-CH3 MCAT (mephedrone; PubChem CID: 45266826), 4-Br MCAT (brephedrone), and 4-OCH3 MCAT (methedrone; PubChem CID: 216281). MCAT and MCAT analogs were synthesized as their racemic HCl salts using previously described methods (Bonano et al., 2015). S(+)-Amphetamine hemisulfate (PubChem CID: 3007) and (±)-fenfluramine HCl (PubChem CID: 3337) (Sigma Aldrich, St. Louis, MO) were tested as comparator phenethylamine controls with selectivity for DAT and SERT, respectively (Baumann et al., 2000). All compounds were dissolved in sterile saline for i.p. injection, and all drug doses are expressed as the salt forms listed previously.
Microdialysis
Surgery.
Rats (n = 63) were anesthetized with 3.0% isoflurane in oxygen until unresponsive to toe pinch and secured in a stereotaxic apparatus (Kopf Instruments, Tujunga, CA). Guide cannulae (8 mm long, 0.5 mm outer diameter; CXG-8, Eicom, San Diego, CA) were implanted bilaterally and terminated 1 mm above the NAc (coordinates: 1.5 mm anterior to bregma; 1.8 mm lateral to midsagittal suture; 6.0 mm ventral to dura). The guide cannulae were secured to the skull using screws (Plastics One, Inc., Roanoke, VA) and orthodontic resin (Butler Schein, Dublin, OH). A dummy cannula (CXD-8, Eicom) was inserted into each guide cannula to maintain cannula patency. Animals were allowed at least seven recovery days prior to initiating microdialysis testing.
Procedure.
On test days, rats were briefly anesthetized with 3.0% isoflurane in oxygen, one of the dummy cannula was removed, and a microdialysis probe (10 mm long, CX-I-8-2, Eicom) with a 2-mm artificial cellulose cuprophan membrane (50 kDa molecular weight cutoff) at its tip was inserted into an 8-mm guide cannula such that it extended 2 mm beyond the end of the guide cannula and into the NAc. The probe was connected to a two-channel liquid swivel (TCS2-23, Eicom), and the rat was placed into an acrylic experimental cage (30 cm3). Microdialysis probes were perfused with artificial cerebrospinal fluid (147 mM NaCl, 2.8 mM KCl, 1.2 mM CaCl2, 1.2 mM MgCl2) at a rate of 1 μl/min. The mobile phase consisted of 2% methanol (EMD, Gibbstown, NJ), 100 mM phosphate buffer (Sigma Chemicals, St. Louis, MO), 500 mg/l 1-decane sodium sulfonate (TCI America, Montgomeryville, PA), and 50 mg/l EDTA-2Na+ (Dojindo Laboratories, Kumamoto, Japan).
Dialysate samples were collected into a 50-μl injector loop at 10-minute intervals using an online autoinjector (EAS-20s, Eicom) and immediately analyzed for DA and 5-HT concentrations by high-pressure liquid chromatography coupled to electrochemical detection (HTEC-500, Eicom). DA and 5-HT were separated using a C18-reverse phase column (PP-ODS II, Eicom) and detected using a graphite working electrode and an Ag+ versus AgCl reference electrode with an applied potential of +450 mV. Preliminary experiments conducted by probe immersion into a known standard concentration of DA indicated a lag time of ∼20 minutes for dialysate to traverse the tubing from the probe to the electrochemical detector at the 1 μl/min flow rate. DA and 5-HT were identified according to characteristic retention times of the standard solution and concentrations were quantified by comparison with peak heights of the standard concentration curve (0.1–100 pg per 10 μl) generated prior to drug administration in each microdialysis experiment. The lower limit of neurotransmitter detection was 0.1 pg. DA and 5-HT levels were determined to be stable after six consecutive stable baseline samples were obtained with <25% variability around the running mean of both neurotransmitters.
Testing was conducted using drug doses based on previous behavioral studies from our laboratory (Bauer et al., 2013; Bonano et al., 2014, 2015). Specifically, saline vehicle, amphetamine (0.1–1.0 mg/kg), fenfluramine (1.0–3.2 mg/kg), MCAT (0.32–3.2 mg/kg), and five para-substituted MCAT analogs [4-F, 4-Cl, 4-CH3, and 4-Br MCAT (1–10 mg/kg), and 4-OCH3 MCAT (3.2–32 mg/kg)] were administered i.p., and dialysate samples were collected for 180 minutes after drug administration. Rats were tested no more than four times (twice per cannula; at least one week between reaccessing a given site), and each site was used to test a different experimental treatment. At the completion of all experiments, rats were euthanized with CO2, and brains were removed and stored in 10% formalin. Probe placement was verified by visual inspection of cannula tracks in unstained brain sections as described previously (Bert et al., 2004; Miller et al., 2015). Only rats with correct probe placements were included in data analyses (data from n = 9 rats were discarded due to improper cannula placement).
Data Analysis.
The primary dependent variables were extracellular DA and 5-HT concentrations in each dialysate fraction expressed as a percent of the average of the six mean baseline concentrations before drug or vehicle administration for each experiment. The individual normalized DA and 5-HT concentrations were then averaged across rats to yield group mean results for graphical presentation. Results were analyzed for each drug dose using a repeated-measures one-way analysis of variance, with time as a fixed effect and subject as a random effect (JMP Pro 11, SAS, Cary, NC). Using this analytical method, within-subject comparisons using the Dunnet post hoc test were determined between monoamine concentrations at each time point and the 10-minute control monoamine concentration. This 10-minute time point represents a dialysis sample that was collected before drug administration, which had advanced into the cannula-to-injector-loop tubing at the time of drug administration and had reached the working electrode for analysis after drug injection due to the 20-minute lag time of tubing dead space. The criterion for statistical significance was set at P < 0.05.
Correlational Analysis.
Correlational analyses were conducted to compare selectivities of MCAT analogs to promote in vivo DA versus 5-HT release in the present study with their selectivities to promote DAT- versus SERT-mediated monoamine release in previous in vitro studies in rat brain synaptosomes (Bonano et al., 2015). For each drug in the present study, the maximum increase in mean DA and 5-HT was plotted as a function of drug dose, and log-linear regression was used to determine the drug dose producing a 250% increase in DA and 5-HT levels (ED250). In vivo neurochemical selectivity was defined as ED250 for 5-HT ÷ ED250 for DA (i.e., higher numbers indicate higher potency to increase DA versus 5-HT and correspondingly higher DA versus 5-HT selectivity), and this measure of in vivo neurochemical selectivity was compared with previous measures of in vitro neurochemical selectivity (Bonano et al., 2015) using a Pearson correlation test.
The measure of in vivo neurochemical selectivity determined in this study was also correlated to two other measures for these compounds described in previous studies (Bonano et al., 2015; Sakloth et al., 2015). First, it had been found previously that in vitro selectivity of 4-R MCAT analogs to promote monoamine release mediated by DAT versus SERT correlated with a behavioral measure of abuse-related drug effects in ICSS (Bonano et al., 2015). Accordingly, the measure of in vivo neurochemical selectivity obtained in the present study was also correlated with these previously reported measures of maximal abuse-related ICSS effects. Second, it also had been previously reported that a structural predictor of neurochemical and behavioral effects of 4-R MCAT analogs was the volume of the para substituent in the MCAT scaffold (Sakloth et al., 2015). Accordingly, the measure of in vivo neurochemical selectivity obtained in the present study was correlated with the volume of the para substituent (Sakloth et al., 2015). Correlations and statistical analyses were carried out using Prism 6.0 for Mac (GraphPad Scientific, San Diego, CA), and correlations were considered statistically significant if P < 0.05.
Results
Baseline DA and 5-HT Levels and Effects of Amphetamine and Fenfluramine.
Across all microdialysis experiments, baseline (mean ± S.E.M.) NAc extracellular DA and 5-HT levels were 0.49 ± 0.03 and 0.44 ± 0.05 nM, respectively. Supplemental Figs. 1–5 show microdialysis probe placements for all rats included in data analyses.
After saline administration, neither NAc DA nor 5-HT levels changed significantly over the 180-minute sampling period (Fig. 2). Both amphetamine and fenfluramine increased extracellular NAc DA and/or 5-HT concentrations (Fig. 2). For amphetamine, both 0.32 mg/kg (F17,89 = 12.5, P < 0.0001) and 1.0 mg/kg (F17,89 = 6.3, P < 0.0001) significantly increased DA concentrations relative to control concentrations measured at the 10-minute time point, and 1.0 mg/kg amphetamine also increased 5-HT concentrations (F17,89 = 2.9, P = 0.001). Fenfluramine did not alter DA concentrations; however, both 1.0 mg/kg (F17,89 = 2.2, P = 0.011) and 3.2 mg/kg (F17,89 = 6.8, P < 0.0001) fenfluramine significantly increased 5-HT concentrations. Both amphetamine and fenfluramine produced sequential periods of rising, peak, and declining monoamine levels during the 180-minute sampling period; however, these phases of drug effects occurred earlier for 5-HT than for DA. For example, onset and offset of significant drug effects on 5-HT occurred between 30 and 80 minutes after drug injection, whereas onset of significant amphetamine effects on DA occurred ≥50 minutes after injection and lasted until at least 160 minutes after injection. We should be re-emphasize that channeling samples directly from the probe to the high-pressure liquid chromatography system via an autoinjector imposed a 20-minute lag time between sample collection and analysis. As a result, the 30-minute time point represented the earliest opportunity for drug injection to affect NAc neurotransmitter levels.
Fig. 2.
Effects of saline vehicle, S(+)-amphetamine (0.1–1.0 mg/kg, i.p.) and (±)-fenfluramine (1.0–3.2 mg/kg, i.p.) on NAc DA and 5-HT levels expressed as a percentage of baseline neurotransmitter levels. Left panels indicate temporal changes in % baseline DA, while right panels indicate changes in % baseline 5-HT. Upward arrows indicate time of drug administration. Downward arrows indicate onset of drug effect. Filled symbols indicate statistical significance (P < 0.05) compared with 10 minute monoamine levels within a drug dose. All points show mean ± S.E.M. for seven rats (3.2 mg/kg fenfluramine) or five rats (all other treatments).
Effects of MCAT and Its Para-Substituted Analogs on NAc DA and 5-HT Levels.
MCAT and its five para-substituted analogs produced significant increases in NAc DA and/or 5-HT levels (Figs. 3 and 4). Figure 3 shows effects of MCAT, 4-F MCAT, and 4-Cl MCAT. For MCAT, both 0.32 mg/kg (F17,89 = 7.5, P < 0.0001) and 1.0 mg/kg (F17,89 = 8.0, P < 0.0001) significantly increased DA concentrations; however, neither MCAT dose significantly altered 5-HT concentrations. Statistical analysis was not conducted on results with 3.2 mg/kg MCAT because this dose produced substantial locomotor activation resulting in probe dislocation in several rats, and the full 180-minute experiment was completed in only 2 rats; however, data for these two rats are included for graphical display. For 4-F MCAT, all three doses significantly increased both DA concentrations (1.0 mg/kg: F17,89 = 6.6, P < 0.0001; 3.2 mg/kg: F17,89 = 6.1, P < 0.0001; 10 mg/kg: F17,89 = 18.6, P < 0.0001) and 5-HT concentrations (1.0 mg/kg: F17,89 = 2.1, P = 0.017; 3.2 mg/kg: F17,89 = 20.1, P < 0.0001; 10 mg/kg: F17,89 = 20.0, P < 0.0001). Similarly, for 4-Cl MCAT, all three doses significantly increased DA concentrations (1.0 mg/kg: F17,89 = 5.2, P < 0.0001; 3.2 mg/kg: F17,89 = 6.0, P < 0.0001; 10 mg/kg: F17,89 = 14.5, P < 0.0001) and 5-HT concentrations (1.0 mg/kg: F17,89 = 2.1, P = 0.014; 3.2 mg/kg: F17,89 = 8.6, P < 0.0001; 10 mg/kg: F17,89 = 14.8, P < 0.0001). For all three drugs, significant increases in monoamine levels occurred earlier and dissipated more quickly for 5-HT (onset by 30 minutes, offset by 100 minutes) than for DA (onset by ≥60 minutes, offset by >180 minutes).
Fig. 3.
Effects of MCAT, 4-F MCAT (flephedrone), and 4-Cl MCAT (clephedrone) on NAc DA and 5-HT levels expressed as a percentage of baseline neurotransmitter levels. All points show mean ± S.E.M. for two rats (3.2 mg/kg MCAT) or five rats (all other treatments); other details are the same as in Fig. 2.
Fig. 4.
Effects of 4-CH3 MCAT (mephedrone), 4-Br MCAT (brephedrone), and 4-OCH3 MCAT (methedrone) on NAc DA and 5-HT levels expressed as a percentage of baseline neurotransmitter levels. All points show mean ± S.E.M. for five rats; other details are the same as in Fig. 2.
Figure 4 shows the effects of 4-CH3 MCAT, 4-Br MCAT, and 4-OCH3 MCAT. For 4-CH3 MCAT, all three doses significantly increased concentrations of DA (1.0 mg/kg: F17,89 = 2.8, P = 0.0014; 3.2 mg/kg: F17,89 = 2.4, P = 0.0054; 10 mg/kg: F17,89 = 6.4, P < 0.0001) and 5-HT (1.0 mg/kg: F17,89 = 22.1, P < 0.0001; 3.2 mg/kg: F17,89 = 14.5, P < 0.0001; 10 mg/kg: F17,89 = 34.5, P < 0.0001). For 4-Br MCAT, both 3.2 mg/kg (F17,89 = 11.4, P < 0.0001) and 10 mg/kg (F17,89 = 22.8, P < 0.0001) significantly increased DA concentrations, and all three doses significantly increased 5-HT concentrations (1.0 mg/kg: F17,89 = 2.9, P = 0.0008; 3.2 mg/kg: F17,89 = 6.9, P < 0.0001; 10 mg/kg: F17,89 = 8.5, P < 0.0001). No 4-OCH3 MCAT dose significantly altered DA concentrations; however, all three doses significantly increased 5-HT (1.0 mg/kg: F17,89 = 2.4, P < 0.0048; 10m g/kg: F17,89 = 30.4, P < 0.0001; 32 mg/kg: F17,89 = 8.0, P < 0.0001). As with the other compounds described previously, significant effects occurred earlier and dissipated more quickly for 5-HT (onset by 30 minutes, offset by 90 minutes) than for DA (onset by ≥50 minutes, offset by >180 minutes).
Correlational Analyses.
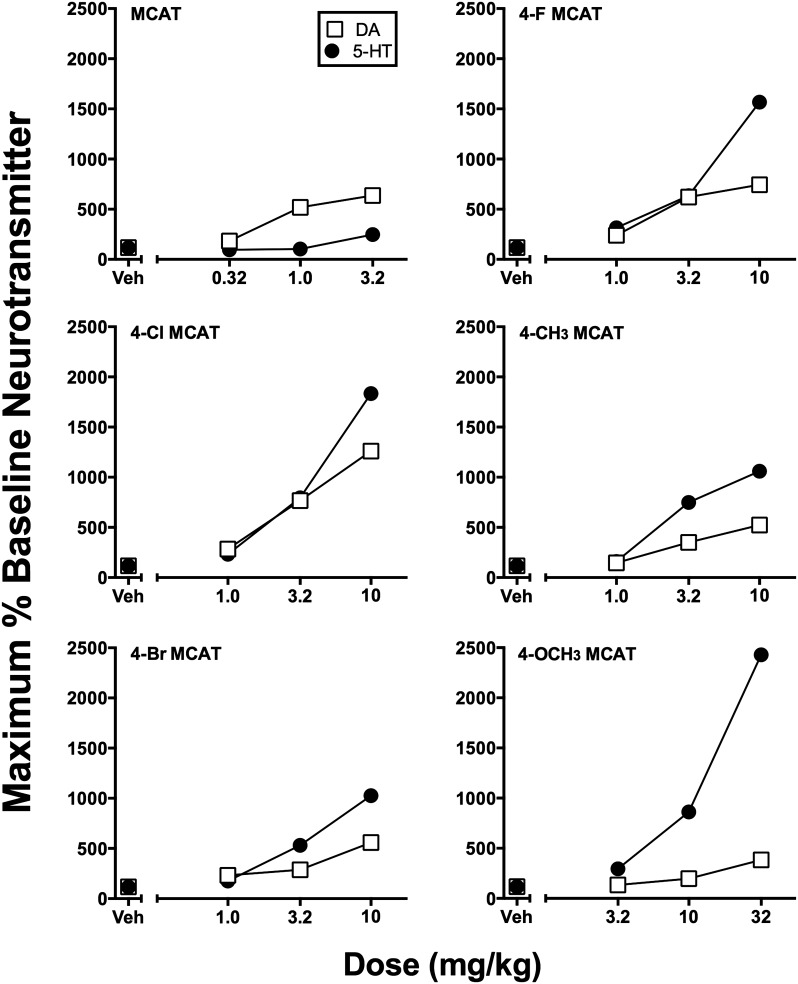
Figure 5 shows the maximal % baseline increase in DA and 5-HT levels for MCAT and its five analogs. 4-Cl MCAT (10 mg/kg) produced the largest increase (1260%) in DA levels, whereas 4-OCH3 MCAT (32 mg/kg) produced the largest increase (2428%) in 5-HT levels. The potencies and selectivities of each drug to increase NAc DA and 5-HT levels are given in Table 1. Of all the MCAT series analogs, MCAT was the most potent compound to increase DA levels and the most DA versus 5-HT selective releaser. In contrast, 4-OCH3 MCAT was the least potent compound to significantly alter DA and/or 5-HT levels, and it displayed the lowest selectivity to increase DA versus 5-HT levels.
Fig. 5.
Peak increases in NAc DA and 5-HT levels produced by each dose of MCAT and each para-substituted MCAT analog. Abscissae: Drug dose in mg/kg (log scale). Ordinates: Maximum increase in % baseline neurotransmitter levels observed at any time after each drug dose as reported in Figs. 3 and 4. Error bars are omitted for clarity.
TABLE 1.
Potency and selectivity of MCAT and its para-substituted analogs to produce a 250% increase in NAc DA and 5-HT levels (ED250 values).
| Drug |
ED250 DA |
ED250 5-HT |
Selectivity (ED250 5-HT/ ED250 DA) |
|---|---|---|---|
| mg/kg | mg/kg | ||
| MCAT | 0.37 | 4.65 | 12.56 |
| 4-F MCAT | 0.86 | 1.07 | 1.24 |
| 4-Cl MCAT | 0.93 | 1.15 | 1.23 |
| 4-Br MCAT | 1.46 | 1.30 | 0.89 |
| 4-CH3 MCAT | 1.82 | 1.12 | 0.62 |
| 4-OCH3 MCAT | 11.12 | 3.61 | 0.32 |
Figure 6 shows correlations between the present measures of in vivo neurochemical selectivity and previously reported measures (Bonano et al., 2015) of in vitro neurochemical selectivity to promote monoamine release via DAT and SERT in rat brain synaptosomes (r = 0.95, P < 0.01; Fig. 6A), and in vivo efficacy to produce abuse-related behavioral effects in an ICSS procedure in rats (r = 0.89, P = 0.02; Fig. 6B). In addition, there was an inverse correlation between in vivo neurochemical selectivity and steric volume of the para substituent (r = −0.85, P = 0.03; Fig. 6C) (Sakloth et al., 2015), such that para substituents with smaller volume (i.e., less steric bulk) were more selective to increase NAc DA relative to 5-HT levels.
Fig. 6.
Correlation of in vivo selectivity to increase NAc DA versus 5-HT with measures of in vitro selectivity to release monoamines via DAT versus SERT in rat brain synaptosomes (A), maximum abuse-related behavioral effects in an ICSS procedure in rats (B), and steric volume of the para substituent on the MCAT scaffold (C). In vitro DAT versus SERT selectivity, maximal ICSS facilitation, and physiochemical parameter volume values have been previously reported (Bonano et al., 2015; Sakloth et al., 2015).
Discussion
This study used an in vivo microdialysis procedure to compare the effects of MCAT and five para-substituted MCAT analogs on NAc DA and 5-HT levels in rats. There were two main findings. First, all MCAT analogs produced dose- and time-dependent changes in NAc DA and/or 5-HT levels. Second, the selectivity of these compounds to increase NAc DA versus 5-HT levels correlated with previously determined measures of 1) in vitro expression of DAT versus SERT selectivity in a rat brain synaptosome procedure; 2) in vivo expression of abuse-related behavioral effects using an ICSS procedure in rats; and 3) the volume of the para substituent on the MCAT scaffold. Results with this set of compounds support the utility of in vitro drug effects on monoamine release in rat brain synaptosomes to predict in vivo drug effects on monoamine release in rat NAc. These results also support the hypotheses that 1) the steric volume of the para substituent on the MCAT scaffold is one determinant of selectivity for DAT versus SERT and 2) that DAT versus SERT selectivity is a determinant of in vivo expression of abuse-related ICSS facilitation.
Consistent with their in vitro DAT versus SERT selectivity profiles (Rothman et al., 2001), S(+)-amphetamine produced a selective increase in NAc DA versus 5-HT levels, whereas fenfluramine produced a selective increase in NAc 5-HT versus DA levels. These results are consistent with previous microdialysis studies in both rats (Baumann et al., 2000; Kehr et al., 2011) and nonhuman primates (Laruelle et al., 1997; Murnane et al., 2010; Sawyer et al., 2012), and confirm the sensitivity of this microdialysis procedure to changes in NAc DA and 5-HT levels produced by agents that have been extensively evaluated. Also consistent with a previous MCAT microdialysis study (Cozzi et al., 2013), MCAT displayed a greater potency to increase NAc DA versus 5-HT levels. The present study extends these previous findings by examining a longer time course of MCAT-induced DA and 5-HT effects and using an i.p. rather than an i.v. route of administration. Also consistent with previous microdialysis results, the addition of a para-methyl group, 4-CH3 MCAT (mephedrone), significantly altered in vivo DA versus 5-HT selectivity, such that 4-CH3 MCAT preferentially increased 5-HT versus DA levels (Kehr et al., 2011; Baumann et al., 2012; Wright et al., 2012). The present 4-CH3 MCAT results extended these previous findings by determining neurochemical effects via the i.p. route of administration. In summary, these MCAT and 4-CH3 MCAT results provide the empirical framework for interpreting the effects of other para-substituted MCAT analogs and allow for the subsequent correlation with other in vitro neurochemical and in vivo behavioral measures.
The present results extended previous MCAT and 4-CH3 MCAT findings by determining the in vivo neurochemical effects of four other para-substituted MCAT analogs, which to the best of our knowledge have not been previously reported. Relative to MCAT, the addition of a para-halogen (-F, -Cl, or -Br) produced qualitatively similar neurochemical effects across all three substituents by slightly lowering the potency to increase DA levels and raising the potency to increase 5-HT levels. The result of these changes was a net decrease in DA versus 5-HT selectivity. Addition of the 4-OCH3 and largest-volume substituent to the MCAT scaffold (i.e., methedrone) produced a further decrease in potency to increase DA levels. Relative to 4-CH3 and 4-halogenated analogs, 4-OCH3 MCAT also displayed reduced potency to increase 5-HT levels; however, the decline in potency to release DA was larger than the decline in potency to release 5-HT, and as a result overall DA versus 5-HT selectivity was lower for 4-OCH3 MCAT than for any other compound examined.
A major goal of the present study was to determine the degree to which in vivo measures of DA versus 5HT selectivity for these MCAT analogs might correlate with in vitro measures of their selectivity to promote monoamine release via DAT and SERT in a rat brain synaptosome preparation (Bonano et al., 2015), and a summary of these correlations is shown in Fig. 7. The high and significant correlation between selectivity measures in these in vitro and in vivo procedures supports the utility of this in vitro procedure to predict in vivo neurochemical results. Moreover, selectivity of these MCAT analogs in both in vitro and in vivo neurochemical procedures correlates both with 1) the volume of the para substituent on the MCAT scaffold, and 2) the efficacy to produce abuse-related behavioral effects in an ICSS procedure (Bonano et al., 2015; Sakloth et al., 2015). Given the positive correlation between ICSS facilitation and other preclinical and clinical measures of abuse potential (Negus and Miller, 2014), the present results provide evidence for a mechanism whereby molecular features of a drug molecule (in this case the steric volume of the para substituent of the MCAT scaffold) can determine neurochemical selectivity to act at DAT and SERT and ultimately influence behavioral measures of abuse potential.
Fig. 7.
Correlation summaries of MCAT and its five para-substituted analogs between the steric volume, in vitro DAT versus SERT selectivity in a rat brain synaptosome procedure, in vivo DA versus 5-HT selectivity in an in vivo microdialysis procedure, and maximal facilitation in an ICSS procedure. Correlations between the physiochemical parameter volume, in vitro DAT versus SERT selectivity, and maximal ICSS facilitation have been previously reported (Bonano et al., 2015; Sakloth et al., 2015).
The present results with MCAT analogs also agree with studies that examined para-substituted analogs of either amphetamine or methamphetamine. For example, both 4-F amphetamine (para-fluroamphetamine, also known as PAL-303) and 4-CH3 amphetamine (para-methylamphetamine, also known as PAL-313) were more potent to increase NAc 5-HT versus DA levels (Baumann et al., 2011). Furthermore, 4-OCH3 methamphetamine (para-methoxymethamphetamine) had a DA versus 5-HT selectivity ratio of 0.28 (Matsumoto et al., 2014), which is consistent with the DA versus 5-HT selectivity ratio of 0.32 for 4-OCH3 MCAT in the present study. Unfortunately, only DA levels have been reported for 4-Br amphetamine (Zsilla et al., 1981) and 4-Cl amphetamine (Hiramatsu and Cho, 1990; Johnson et al., 1990), thus precluding a direct comparison of in vivo selectivities between para-substituted amphetamine or methamphetamine analogs and the present results. Nonetheless, these results suggest that neurochemical and behavioral effects associated with para substitutions to the MCAT scaffold may also generalize to effects associated with effects of para-substituted analogs of amphetamine and methamphetamine.
In the present study, all compounds were administered i.p. to match the route of administration used in previous behavioral studies with these compounds in an ICSS procedure of abuse-potential assessment (Bauer et al., 2013; Bonano et al., 2014, 2015). A striking outcome using this route of administration was that DA effects displayed both a slower onset and longer duration than 5-HT effects, and this dissociation in time course of drug effects on DA and 5-HT may be related to route of administration. For example, i.v. 4-CH3 MCAT produced coincident peak DA and 5-HT effects within 20 minutes (Baumann et al., 2011), whereas DA effects were more delayed compared with 5-HT effects after s.c. administration (Kehr et al., 2011; Wright et al., 2012), and DA effects were even more delayed after i.p. administration (present study). Moreover, the relatively slow onset of DA effects after i.p. administration of MCAT analogs in this study agrees with the slow onset of DA effects reported in previous studies of amphetamine and other para-halogenated or para-methoxy phenethylamines administered by the i.p. route (Marona-Lewicka et al., 1995; Matsumoto et al., 2014). The reason for the apparent route of administration–dependent differences in DA versus 5-HT time course effects remains to be elucidated.
The present results also afforded an opportunity to compare potency and time course of monoamine releaser effects in rats on in vivo NAc DA and 5-HT levels (present study) and previously published behavioral effects in our ICSS procedure (Bauer et al., 2013; Bonano et al., 2014, 2015). The abuse-related facilitation of low ICSS rates by monoamine releasers is thought to be mediated primarily by increases in DA, whereas abuse-limiting depression of high ICSS rates by these compounds is thought to be mediated primarily by increases in 5-HT (Wise, 1998; Negus and Miller, 2014). However, different relationships were observed between microdialysis measures of NAc DA and ICSS facilitation on the one hand and NAc 5HT and ICSS depression on the other hand. Specifically, DA-selective monoamine releasers such as amphetamine and MCAT displayed a lower potency, slower onset, and longer duration of action to increase NAc DA levels than to facilitate ICSS. Conversely, serotonin-selective releasers such as fenfluramine and 4-OCH3 MCAT displayed a similar or higher potency, similar rate of onset, and shorter duration of action to increase NAc 5-HT levels than to depress ICSS. Thus, microdialysis measures of NAc DA served as a lagging and less-sensitive indicator than ICSS facilitation of monoamine releaser-induced DA effects, whereas measures of NAc 5-HT were a leading and more-sensitive indicator than ICSS depression of monoamine releaser-induced 5-HT effects.
In conclusion, the in vivo neurochemical effects of MCAT and five para-substituted MCAT analogs support the hypothesis that selectivity of monoamine releasers to elevate DA versus 5-HT levels via the respective monoamine transporters is a significant contributor to the abuse-related behavioral effects of monoamine releasers in ICSS studies. Although potency differences were noted, all six compounds examined produced similar maximal increases in extracellular DA levels. Thus, the in vivo selectivity differences observed in the present study were mostly driven by decreased potency to increase extracellular 5-HT levels. Furthermore, the present results provide an in vivo neurochemical connection with previous in vitro neurochemical and behavioral effects of these compounds (Bauer et al., 2013; Bonano et al., 2015). Given the purported direct and indirect connections between serotonergic pathways and the mesolimbic DA pathway (Alex and Pehek, 2007), the present results provide further neuropharmacological evidence for the abuse-limiting effects of 5-HT.
Supplementary Material
Abbreviations
- Da
dopamine
- DAT
dopamine transporter
- 5-HT
serotonin
- ICSS
intracranial self-stimulation
- MCAT
methcathinone
- NAc
nucleus accumbens
- SERT
serotonin transporter
Authorship Contributions
Participated in research design: Suyama, Glennon, Negus, Banks.
Conducted experiments: Suyama, Lazenka.
Contributed new reagents or analytic tools: Sakloth, Kolanos.
Performed data analysis: Suyama, Banks.
Wrote or contributed to the writing of the manuscript: Suyama, Lazenka, Glennon, Negus, Banks.
Footnotes
The research reported in this publication was supported by the National Institutes of Health National Institute on Drug Abuse [Grants R01DA033930, F30DA037649, and T32DA007027]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
 This article has supplemental material available at jpet.aspetjournals.org.
This article has supplemental material available at jpet.aspetjournals.org.
References
- Alex KD, Pehek EA. (2007) Pharmacologic mechanisms of serotonergic regulation of dopamine neurotransmission. Pharmacol Ther 113:296–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer CT, Banks ML, Blough BE, Negus SS. (2013) Use of intracranial self-stimulation to evaluate abuse-related and abuse-limiting effects of monoamine releasers in rats. Br J Pharmacol 168:850–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Dersch CM, Brockington A, Rice KC, Rothman RB. (2000) Effects of phentermine and fenfluramine on extracellular dopamine and serotonin in rat nucleus accumbens: therapeutic implications. Synapse 36:102–113. [DOI] [PubMed] [Google Scholar]
- Baumann MH, Ayestas MA, Jr, Partilla JS, Sink JR, Shulgin AT, Daley PF, Brandt SD, Rothman RB, Ruoho AE, Cozzi NV. (2012) The designer methcathinone analogs, mephedrone and methylone, are substrates for monoamine transporters in brain tissue. Neuropsychopharmacology 37:1192–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumann MH, Clark RD, Woolverton WL, Wee S, Blough BE, Rothman RB. (2011) In vivo effects of amphetamine analogs reveal evidence for serotonergic inhibition of mesolimbic dopamine transmission in the rat. J Pharmacol Exp Ther 337:218–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bert L, Favale D, Jego G, Greve P, Guilloux JP, Guiard BP, Gardier AM, Suaud-Chagny MF, Lestage P. (2004) Rapid and precise method to locate microdialysis probe implantation in the rodent brain. J Neurosci Methods 140:53–57. [DOI] [PubMed] [Google Scholar]
- Bonano JS, Banks ML, Kolanos R, Sakloth F, Barnier ML, Glennon RA, Cozzi NV, Partilla JS, Baumann MH, Negus SS. (2015) Quantitative structure-activity relationship analysis of the pharmacology of para-substituted methcathinone analogues. Br J Pharmacol 172:2433–2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonano JS, Glennon RA, De Felice LJ, Banks ML, Negus SS. (2014) Abuse-related and abuse-limiting effects of methcathinone and the synthetic “bath salts” cathinone analogs methylenedioxypyrovalerone (MDPV), methylone and mephedrone on intracranial self-stimulation in rats. Psychopharmacology (Berl) 231:199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzi NV, Brandt SD, Daley PF, Partilla JS, Rothman RB, Tulzer A, Sitte HH, Baumann MH. (2013) Pharmacological examination of trifluoromethyl ring-substituted methcathinone analogs. Eur J Pharmacol 699:180–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozzi NV, Sievert MK, Shulgin AT, Jacob P, III, Ruoho AE. (1999) Inhibition of plasma membrane transporters by beta-ketoamphetamines. Eur J Pharmacol 381:63–69. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Ginsburg BC, Howell LL. (2002) Serotonergic attenuation of the reinforcing and neurochemical effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther 300:831–837. [DOI] [PubMed] [Google Scholar]
- De Felice LJ, Glennon RA, Negus SS. (2014) Synthetic cathinones: chemical phylogeny, physiology, and neuropharmacology. Life Sci 97:20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. (1988) Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA 85:5274–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glennon RA, Yousif M, Nalman N, Kalix P. (1987) Methcathinone: A new and potent amphetamine-like agent. Pharmacol Biochem Behav 26:547–551. [DOI] [PubMed] [Google Scholar]
- Gygi MP, Fleckenstein AE, Gibb JW, Hanson GR. (1997) Role of endogenous dopamine in the neurochemical deficits induced by methcathinone. J Pharmacol Exp Ther 283:1350–1355. [PubMed] [Google Scholar]
- Hiramatsu M, Cho AK. (1990) Enantiomeric differences in the effects of 3,4-methylenedioxymethamphetamine on extracellular monoamines and metabolites in the striatum of freely-moving rats: an in vivo microdialysis study. Neuropharmacology 29:269–275. [DOI] [PubMed] [Google Scholar]
- Johnson MP, Huang XM, Oberlender R, Nash JF, Nichols DE. (1990) Behavioral, biochemical and neurotoxicological actions of the alpha-ethyl homologue of p-chloroamphetamine. Eur J Pharmacol 191:1–10. [DOI] [PubMed] [Google Scholar]
- Kaminski BJ, Griffiths RR. (1994) Intravenous self-injection of methcathinone in the baboon. Pharmacol Biochem Behav 47:981–983. [DOI] [PubMed] [Google Scholar]
- Kehr J, Ichinose F, Yoshitake S, Goiny M, Sievertsson T, Nyberg F, Yoshitake T. (2011) Mephedrone, compared with MDMA (ecstasy) and amphetamine, rapidly increases both dopamine and 5-HT levels in nucleus accumbens of awake rats. Br J Pharmacol 164:1949–1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laruelle M, Iyer RN, al-Tikriti MS, Zea-Ponce Y, Malison R, Zoghbi SS, Baldwin RM, Kung HF, Charney DS, Hoffer PB, et al. (1997) Microdialysis and SPECT measurements of amphetamine-induced dopamine release in nonhuman primates. Synapse 25:1–14. [DOI] [PubMed] [Google Scholar]
- Marona-Lewicka D, Rhee GS, Sprague JE, Nichols DE. (1995) Psychostimulant-like effects of p-fluoroamphetamine in the rat. Eur J Pharmacol 287:105–113. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Maeno Y, Kato H, Seko-Nakamura Y, Monma-Ohtaki J, Ishiba A, Nagao M, Aoki Y. (2014) 5-hydroxytryptamine- and dopamine-releasing effects of ring-substituted amphetamines on rat brain: a comparative study using in vivo microdialysis. Eur Neuropsychopharmacol 24:1362–1370. [DOI] [PubMed] [Google Scholar]
- Miller LL, Leitl MD, Banks ML, Blough BE, Negus SS. (2015) Effects of the triple monoamine uptake inhibitor amitifadine on pain-related depression of behavior and mesolimbic dopamine release in rats. Pain 156:175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murnane KS, Fantegrossi WE, Godfrey JR, Banks ML, Howell LL. (2010) Endocrine and neurochemical effects of 3,4-methylenedioxymethamphetamine and its stereoisomers in rhesus monkeys. J Pharmacol Exp Ther 334:642–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navailles S, Moison D, Cunningham KA, Spampinato U. (2008) Differential regulation of the mesoaccumbens dopamine circuit by serotonin2C receptors in the ventral tegmental area and the nucleus accumbens: an in vivo microdialysis study with cocaine. Neuropsychopharmacology 33:237–246. [DOI] [PubMed] [Google Scholar]
- Negus SS, Miller LL. (2014) Intracranial self-stimulation to evaluate abuse potential of drugs. Pharmacol Rev 66:869–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothman RB, Baumann MH, Dersch CM, Romero DV, Rice KC, Carroll FI, Partilla JS. (2001) Amphetamine-type central nervous system stimulants release norepinephrine more potently than they release dopamine and serotonin. Synapse 39:32–41. [DOI] [PubMed] [Google Scholar]
- Sakloth F, Kolanos R, Mosier PD, Bonano JS, Banks ML, Partilla JS, Baumann MH, Negus SS, Glennon RA. (2015) Steric parameters, molecular modeling and hydropathic interaction analysis of the pharmacology of para-substituted methcathinone analogues. Br J Pharmacol 172:2210–2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawyer EK, Mun J, Nye JA, Kimmel HL, Voll RJ, Stehouwer JS, Rice KC, Goodman MM, Howell LL. (2012) Neurobiological changes mediating the effects of chronic fluoxetine on cocaine use. Neuropsychopharmacology 37:1816–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiller HA, Ryan ML, Weston RG, Jansen J. (2011) Clinical experience with and analytical confirmation of “bath salts” and “legal highs” (synthetic cathinones) in the United States. Clin Toxicol (Phila) 49:499–505. [DOI] [PubMed] [Google Scholar]
- Wise RA. (1998) Drug-activation of brain reward pathways. Drug Alcohol Depend 51:13–22. [DOI] [PubMed] [Google Scholar]
- Wright MJ, Jr, Angrish D, Aarde SM, Barlow DJ, Buczynski MW, Creehan KM, Vandewater SA, Parsons LH, Houseknecht KL, Dickerson TJ, et al. (2012) Effect of ambient temperature on the thermoregulatory and locomotor stimulant effects of 4-methylmethcathinone in Wistar and Sprague-Dawley rats. PLoS One 7:e44652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsilla G, Knoll B, Knoll J. (1981) The action of single and repeated doses of p-bromo-methamphetamine on the monoamine content and turnover rate in rat brain. Neuropharmacology 20:833–838. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.