Abstract
Cocaine abuse and obesity are serious public health problems, and studies suggest that both dopamine and serotonin systems are involved in regulating the consumption of drugs and food. Lorcaserin has serotonin (5-HT)2C receptor agonist actions, is approved by the U.S. Food and Drug Administration for treating obesity, and might be effective for treating cocaine abuse. These studies characterized the pharmacokinetic and behavioral profiles of lorcaserin (intragastric administration) and determined the effectiveness of lorcaserin to alter discriminative stimulus and reinforcing effects of cocaine (intravenous administration) in rhesus monkeys. Administered acutely, lorcaserin dose-dependently increased the occurrence of yawning while decreasing spontaneous activity and operant responding for food. These effects appeared within 30–60 minutes of administration and began to dissipate by 240 minutes, a time course closely matching plasma concentrations of lorcaserin. In monkeys discriminating cocaine from saline, lorcaserin alone did not occasion cocaine-appropriate responding but shifted the cocaine dose-response curve to the right and down in two of three monkeys. When administered acutely, lorcaserin dose-dependently decreased the rate at which monkeys responded for infusions of cocaine. When administered chronically, 3.2 mg/kg lorcaserin reduced the rate of cocaine-maintained responding by 50% for the duration of a 14-day treatment period. Together, these results show that lorcaserin attenuates the discriminative stimulus effects of cocaine after acute administration and the reinforcing effects of cocaine after acute and repeated administration, consistent with the view that it might have utility in treating cocaine abuse.
Introduction
Cocaine abuse remains a significant public health problem. The National Survey on Drug Use and Health estimated that, in 2013, 600,000 individuals tried cocaine for the first time and 1.5 million Americans were considered regular users (Substance Abuse and Mental Health Services Administration, 2014). Worldwide estimates put the number of regular cocaine users at nearly 20 million (United Nations Office on Drugs and Crime, 2014). Despite longstanding efforts to develop pharmacotherapies (Mello, 1990; Roberts and Brebner, 2000; Platt et al., 2002; Dackis and O’Brien, 2003; Grabowski et al., 2004; Vocci et al., 2005; Tanda et al., 2009), there are currently no approved medications for treating cocaine abuse.
Cocaine binds to dopamine, serotonin (5-HT), and norepinephrine transporters with similar affinity, although it is thought that abuse-related effects of cocaine are mediated predominantly by its capacity to increase dopamine neurotransmission (Ritz et al., 1987). One approach for reducing these effects of cocaine is to target neurotransmitter systems and/or specific receptors that modulate (e.g., indirectly decrease or inhibit) dopamine neurotransmission. The ability of 5-HT systems to modulate dopamine activity is well documented, and mounting evidence suggests that these effects are mediated by the 5-HT2 subfamily of receptors, with agonists acting at 5-HT2A receptors stimulating dopamine release and agonists acting at 5-HT2C receptors inhibiting dopamine release within the nucleus accumbens. Conversely, antagonists of 5-HT2A or 5-HT2C receptors are known to decrease or increase dopamine neurotransmission, respectively (for review, see Howell and Cunningham, 2015).
On the basis of these effects, antagonists selective for 5-HT2A receptors and agonists selective for 5-HT2C receptors have been investigated for their ability to modify the behavioral effects of drugs of abuse, including cocaine. Although 5-HT2A receptor antagonists (e.g., SR46349B [4-[(2Z)-3-{[2-(dimethylamino)ethoxy]amino}-3-(2-fluorophenyl)prop-2-en-1-ylidene]cyclohexa-2,5-dien-1-one) and M100907 [α-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenylethyl)]-4-piperidine methanol]) inhibit the effectiveness of noncontingent cocaine injections to reinstate extinguished responding for cocaine (cocaine-primed reinstatement) at doses that do not alter the ongoing self-administration of cocaine (Fletcher et al., 2002; Filip et al., 2005; Murnane et al., 2013), 5-HT2C receptor agonists (e.g., Ro60-0175 [(S)-2(6-chloro-5-fluorindol-1-yl)-1-methylethylamine] and WAY163909 [(7bR, 10aR)-1,2,3,4,8,9,10,10a-octahydro-7bH-cyclopenta-[b][1,4]diazepino[6,7,1hi]indole]) dose-dependently inhibit the effectiveness of cocaine primes and cocaine-associated stimuli (stimulus-induced reinstatement) to reinstate extinguished responding, as well as the direct reinforcing effects of cocaine in self-administration procedures (Grottick et al., 2000; Burmeister et al., 2004; Burbassi and Cervo, 2008; Cunningham et al., 2011; Manvich et al., 2012; Rüedi-Bettschen et al., 2015). These findings suggest that 5-HT2C receptor–selective agonists might be effective for reducing cocaine abuse and for prolonging abstinence.
Lorcaserin is a selective 5-HT2C receptor agonist (18- and 100-fold selective for 5-HT2C over 5-HT2A and 5-HT2B receptors, respectively; Thomsen et al., 2008) that was approved in 2012 by the U.S. Food and Drug Administration (FDA) for the treatment of obesity. In clinical studies, lorcaserin is well tolerated and decreases food intake and body weight (Smith et al., 2009, 2010; Chan et al., 2013; Aronne et al., 2014) at doses that have low potential for producing heart valve disease (Weissman et al., 2013) or abuse (Shram et al., 2011), effects commonly associated with 5-HT2B and 5-HT2A receptor agonists, respectively (Nichols, 2004; Hutcheson et al., 2011). In addition to preclinical studies demonstrating that lorcaserin decreases food intake and body weight (Smith et al., 2008; Thomsen et al., 2008; Higgins et al., 2013b; Grottick et al., 2015), lorcaserin is also shown to decrease nicotine self-administration and the reinstatement of responding for nicotine in rats (Levin et al., 2011; Higgins et al., 2012, 2013b).
The current studies evaluated whether lorcaserin might have utility in treating cocaine abuse. Accordingly, the goals of these studies were to characterize the behavioral and pharmacokinetic profiles of lorcaserin and to assess its effectiveness to modify the discriminative stimulus and reinforcing effects of cocaine in rhesus monkeys. Because lorcaserin would be taken orally by cocaine abusers and because drug abuse is a chronic condition, these studies characterized the effects of intragastric lorcaserin. In an attempt to broadly characterize the behavioral effects of lorcaserin, time course data were collected for the effects of a range of doses of lorcaserin to alter locomotor activity and 24 distinct observable behaviors as well as food-maintained responding. To assess its potential to alter abuse-related effects of cocaine, lorcaserin was also evaluated for its effectiveness to modify plasma concentrations of cocaine, antagonize the discriminative stimulus effects of cocaine, and reduce the self-administration of cocaine; the effects of lorcaserin on cocaine self-administration were assessed after both acute and repeated (14-day) administration. Together, these results provide preclinical evidence that lorcaserin attenuates some abuse-related effects of cocaine after acute and repeated administration and suggest that lorcaserin might be effective in treating cocaine abuse.
Materials and Methods
Subjects.
A total of 16 adult rhesus monkeys were used in these studies. One male (GO) and three female rhesus monkeys (PR, IR, and LI), weighing between 6 and 9 kg, were used to characterize changes in locomotor activity and directly observable behaviors produced by lorcaserin. The male monkey (GO) and two of the female monkeys (PR and IR), along with one additional male monkey (CO; 9 kg), were used for the pharmacokinetic study. Three other male rhesus monkeys (HU, JO, and LA), weighing between 8 and 11 kg, were used to investigate the effects of lorcaserin on food-maintained responding. A separate group of three male rhesus monkeys (MU, DU, and LR), weighing between 8 and 11 kg, were used for the cocaine discrimination studies. Three male (BO, CH, and PE) and two female rhesus monkeys (DA and RO), weighing between 6 and 10 kg, were used to evaluate the effectiveness of lorcaserin to inhibit cocaine self-administration. All monkeys were housed individually on a 14-hour/10-hour light/dark cycle with free access to water in their home cage. Monkeys were fed primate chow (High Protein Monkey Diet; Harlan Teklad, Madison, WI), fresh fruit, and peanuts daily. All monkeys were maintained, and all experiments were performed, in accordance with the University of Texas Health Science Center at San Antonio Institutional Animal Care and Use Committee and the National Research Council (2011) Guide for the Care and Use of Laboratory Animals.
Surgical Preparation.
Monkeys in the drug-discrimination and self-administration studies were surgically prepared with indwelling venous catheters connected to subcutaneous vascular access ports, according to methods described elsewhere (Wojnicki et al., 1994). Briefly, monkeys were anesthetized with 10 mg/kg ketamine (subcutaneously; Fort Dodge Laboratories, Fort Dodge, IA), intubated, and then maintained on 2 l/min oxygen and isoflurane anesthesia (Butler Animal Health Supply, Grand Prairie, TX). A polyurethane catheter (SIMS Deltec Inc., St. Paul, MN) was implanted in a vein (e.g., jugular or femoral) and connected to a vascular access port (Access Technologies, Skokie, IL) that was positioned subcutaneously in the midscapular region.
Apparatus.
Accelerometers (Actical model number 1081986; Respironics Inc., Murrysville, PA) attached to collars were used to measure activity in the home cage. For experiments assessing operant behavior, monkeys were seated in commercially available chairs (model R001; Primate Products, Miami, FL) and placed in ventilated, sound-attenuating chambers. Response panels located on the front wall of each chamber contained a food cup (to which food pellets were delivered from a dispenser located outside the chamber), two response levers and two stimulus lights that could be illuminated red or green. For drug-discrimination studies, drugs were injected directly into the vascular access port, which was then flushed with 5 ml saline. For self-administration studies, cocaine was delivered intravenously by a syringe driver (Razel Scientific Instruments Inc., Stamford, CT) located outside the chamber. The syringe driver contained a 30-ml syringe that was connected to the vascular access port with a 185-cm extension set (Abbott Laboratories, Stone Mountain, GA) equipped with a 20-g Huber-point needle (Access Technologies). A computer controlled experimental events and recorded data using Med-PC IV software (Med Associates Inc., St. Albans, VT).
Collection and Storage of Samples for Pharmacokinetic Experiments.
Monkeys were seated in restraint chairs for intragastric administration of saline or 3.2 mg/kg lorcaserin, intravenous administration of 1 mg/kg cocaine, and collection of 1 ml blood per sample by acute puncture of the saphenous vein. To monitor plasma concentrations of 3.2 mg/kg lorcaserin alone, blood was collected immediately before and 30, 60, 90, 120, and 210 minutes and 24 hours after lorcaserin administration. To determine whether lorcaserin altered plasma concentrations of cocaine, monkeys received saline or lorcaserin intragastrically and were returned to their home cage; 88 minutes later, the first blood sample was collected after monkeys were again seated in chairs. Cocaine (1 mg/kg intravenously) was then administered in the same vein, with the interval between intragastric administration of saline or lorcaserin and intravenous administration of cocaine being 90 minutes. Blood (1 ml) was collected from the opposite saphenous vein 5, 10, 15, 20, 30, 45, 60, 90, and 120 minutes after cocaine administration. All blood samples were collected in 3-ml EDTA tubes (Vacutainer; Becton, Dickson and Company, Franklin Lakes, NJ) and immediately placed on ice and shielded from light. Within 30 minutes, the tubes were centrifuged at 1000 × g for 10 minutes. Plasma was collected in polypropylene tubes containing 20 µl of a solution of 1 mg/ml sodium fluoride and 60% acetic acid and then stored at −80°C. Pharmacokinetic procedures were based on previous studies in which plasma cocaine levels were assessed in rhesus monkeys (Collins et al., 2012).
Measurement of Lorcaserin and Cocaine in Monkey Plasma.
Milli-Q water was used for preparation of all solutions (Millipore, Billerica, MA). Lorcaserin, cocaine, and deuterated cocaine (dCOC) super-stock solutions were prepared in methanol at a concentration of 1 mg/ml and stored in aliquots at −80°C. Working stock solutions (10 μg/ml) of each analyte were prepared fresh each experimental day from the super-stock solutions and used to spike the calibrator samples.
The high-pressure liquid chromatography (HPLC) system consisted of a Shimadzu SCL-10A Controller (Shimadzu Scientific Instruments, Columbia, MD), an LC-10AD pump with a FCV-10AL mixing chamber, a SIL-10AD autosampler, and an AB Sciex API 3200 tandem mass spectrometer with turbo ion spray (AB Sciex, Framingham, MA). The analytical column was an Ace Excel 3 Super C18 (3 × 75 mm, 3 µ) purchased from MacMod (Chadds Ford, PA) and was maintained at 60°C during the chromatographic runs in a Shimadzu CTO-10A column oven. Mobile phase A was 0.1% formic acid dissolved in Milli-Q water, and mobile phase B was 0.1% formic acid dissolved in acetonitrile. The flow rate of the mobile phase was 0.6 ml/min. The gradient was as follows: 0–2 minutes, 10% B to 85% B linear; 2–3.4 minutes, 85% B; 3.4–3.5 minutes, 85% B to 10% B linear; and 3.5–6 minutes, 10% B. The Q1/Q3 transitions used to quantify the analytes were 196.1→144.1 for lorcaserin, 304.1→182.2 for cocaine, and 307.2→185.3 for dCOC.
Sample Processing and Quantification of Lorcaserin and Cocaine in Plasma.
Lorcaserin and cocaine were quantified in monkey plasma. Briefly, 300 µl of calibrator and coded plasma samples were mixed with 50 µl of a 1 µg/ml dCOC solution (internal standard) and 2 ml of a mobile phase B (0.1% formic acid dissolved in acetonitrile). The samples were vortexed vigorously for 2 minutes and then centrifuged at 4925 × g for 10 minutes at 23°C. Supernatants were dried to residue under a stream of nitrogen and then the residues were dissolved in 100 µl 0.1% formic acid in water/acetonitrile (90:10). Then, 10 µl of the final samples was injected into the liquid chromatography–tandem mass spectrometry system. The ratio of the peak area of each analyte to that of the internal standard dCOC (response ratio) for each unknown sample was compared against a linear regression of calibrator response ratios at spiked concentrations of 0, 1, 10, 100, 500, and 1000 ng/ml of lorcaserin and cocaine. Final concentrations of all analytes were expressed as nanograms per milliliter of plasma.
To test the effect of lorcaserin on the pharmacokinetic profile of cocaine, four monkeys received 1 mg/kg cocaine intravenously 90 minutes after intragastric administration of either saline or 3.2 mg/kg lorcaserin. The area under the curve (AUC) was calculated for 2-hour periods after administration of cocaine, and a two-tailed, paired t test was performed to determine whether lorcaserin altered the pharmacokinetic profile of cocaine. All statistical analyses were performed using GraphPad Prism 6 software (GraphPad Software, Inc., La Jolla, CA).
Activity Monitoring and Directly Observable Behavior.
Each of four doses of lorcaserin was examined once in four monkeys. Monkeys were seated in primate chairs during the administration of saline or a dose of lorcaserin (0.32–32 mg/kg i.g.) and then immediately returned to their home cages, where activity and directly observable signs were measured. Accelerometers were attached to collars and collected activity counts in 1-minute bins continuously until they were removed from the collar. Directly observable behavior was measured 5, 15, 30, 60, 120, and 240 minutes and 24 hours after lorcaserin administration by two observers who were familiar with the behavior of these monkeys; 24 signs (Table 1) were recorded as present or absent during a 30-second observation period at each time point. One observer administered drug and therefore was not blind to treatment; however, both observers were blind to the overall purpose of the experiment and expected outcomes. The level of agreement between the two raters of directly observable signs was determined using the κ statistic, which was calculated for signs that were increased by lorcaserin; reliability between observers was considered adequate for making definite conclusions when the value of κ exceeded 0.80 (Landis and Koch, 1977; Hallgren, 2012).
TABLE 1.
Descriptions of directly observable behaviors
| Behavior | Definition |
|---|---|
| Eyes closed | 3-second minimum |
| Stereotypy | Repetitive movements (entire body or just head, often hand licking/gnawing) |
| Chewing | Vertical jaw movements |
| Licking | Tongue repetitively in contact with anything |
| Tongue movements | Tongue rolling inside mouth from cheek to cheek |
| Tongue protrusion | Sticking tongue out of the mouth for a minimum of 3 seconds |
| Biting | Teeth in contact with cage |
| Vocalization | Barking, grunting, or other sound originating from the mouth or throat |
| Tremor (body) | Shaking or trembling of body |
| Tremor (extremities) | Shaking or trembling of digits or limbs |
| Ptosis | Drooping eyelid |
| Hypersalivation | Excessive secretion of saliva exiting mouth cavity |
| Retching | Sound and movement of vomiting without vomiting |
| Emesis | Vomiting |
| Rhinorrhea | Runny nose |
| Lacrimation | Secretion of tears |
| Piloerection | Bristling (standing up) of hair |
| Scratching | Fingers or toes rapidly contacting and sliding on a part of the body |
| Yawning | Mouth opening widely and vertically |
| Tail wagging | Horizontal or vertical movement of the tail |
| Loss of balance | Falling over |
| Aggressiveness | Threatening posture and/or behavior |
| Skin color | Abnormal skin color |
| Posture | Abnormal body position |
Activity counts for the 5 minutes immediately after each observation period (e.g., minutes 6–10 after lorcaserin) were averaged for individuals and then averaged across monkeys (± 1 S.E.M.). Two-factor analysis of variance (ANOVA) with repeated measures and post hoc Holm–Sidak tests were used to determine whether activity varied as a function of lorcaserin dose or time. Data for directly observable signs are reported as the proportion of monkeys exhibiting the sign as a function of time since lorcaserin. The AUC was also calculated for each time course, and these data are reported as the mean ± 1 S.E.M. of the AUC for each dosing condition. One-factor ANOVA with repeated measures and post hoc Holm–Sidak tests were used to determine whether AUC values derived from the entire 240-minute period varied as a function of dose of lorcaserin.
Food-Maintained Responding.
Each of three doses of lorcaserin was examined once in three monkeys. Sessions lasted 2 hours and were divided into 15-minute cycles. Each cycle began with a 10-minute timeout, during which chambers were dark and responding had no programmed consequence. During the last 5 minutes of each cycle, monkeys could respond on one lever under a fixed ratio (FR) 10 schedule of food presentation. The response period was signaled by illumination of a green stimulus light; 10 consecutive responses on the lever below the illuminated light delivered a 300-mg banana-flavored food pellet. The active lever was counterbalanced across monkeys and remained the same for an individual monkey for the entire experiment. The response period ended after delivery of 10 food pellets or 5 minutes, whichever occurred first; any remaining time before the next cycle was a timeout. On separate occasions, saline or lorcaserin (1–10 mg/kg i.g.) was administered 0, 60, or 165 minutes before a 2-hour session.
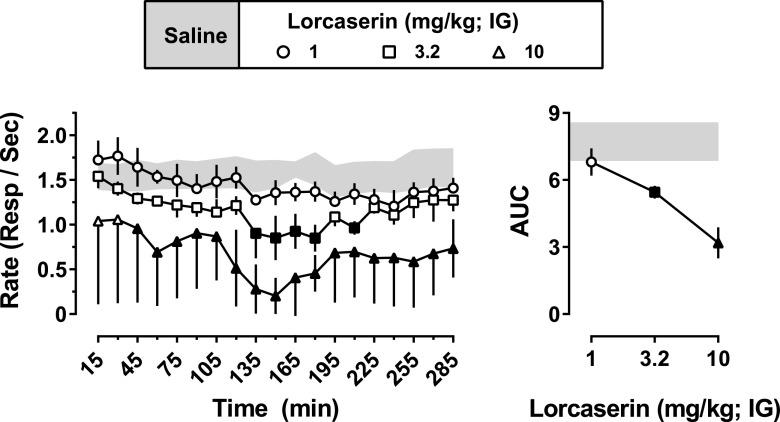
Control response rates were calculated for individual subjects by averaging response rates across cycles to obtain a mean for a session and then averaging across 10 sessions during which drug was not administered. Data represent the mean (± 1 S.E.M.) rate of responding maintained by food pellets across nineteen 5-minute response periods. Because these data represent a composite of the effects of lorcaserin across three different experimental sessions with overlapping time points, data from individual subjects were averaged for these time points (i.e., 75, 90, 105, 120, and 180 minutes) and represent the mean of the two tests, with the grouped data representing a mean across subjects. Because data were collected repeatedly after administration of saline, and because they did not differ significantly as a function of time, data shown in the figure (Fig. 3) represent the mean (± 1 S.E.M.) of the three saline tests that preceded each of the three lorcaserin tests (i.e., rates for an individual monkey represent the mean of three tests). Two-factor ANOVA with repeated measures and post hoc Holm–Sidak tests were used to determine whether food-maintained responding varied as a function of lorcaserin dose or time. One-factor ANOVA with repeated measures and post hoc Holm–Sidak tests were used to determine whether the AUC values derived from the entire 285-minute period varied as a function of the dose of lorcaserin administered.
Fig. 3.
Acute effects of lorcaserin on food-maintained responding. On different occasions, saline or lorcaserin (1–10 mg/kg intragastrically) was administered 0, 60, or 165 minutes before 2-hour sessions comprising 15-minute cycles (n = 3). The mean (± 1 S.E.M.) rates of responding during the last 5 minutes of each cycle are shown on the left, with the mean (± 1 S.E.M.) AUC for each time course shown on the right. Data obtained after saline administration (mean ± 1 S.E.M.) are represented by the gray-shaded areas. Black-filled symbols represent points that were significantly different from saline (P < 0.05), as determined by post hoc Holm–Sidak tests. IG, intragastric.
Cocaine Discrimination.
Three monkeys discriminated cocaine (0.178 mg/kg intravenously) from saline while responding under an FR10 schedule of food presentation. Sessions were divided into 15-minute cycles, which included a 10-minute timeout followed by a 5-minute response period during which green stimulus lights were illuminated above the two levers. A session could be as short as one cycle and as long as six cycles, depending on the training or testing conditions that were in place. Monkeys received intravenous infusions during the first minute of each cycle, and 10 consecutive responses on the infusion-appropriate lever (one lever was designated correct for cycles preceded by administration of the training dose of cocaine, whereas the other lever was designated correct for cycles preceded by administration of saline) delivered a food pellet. Response periods ended after delivery of 10 food pellets or 5 minutes, whichever occurred first; any remaining time before the next cycle was a timeout. During training sessions, cocaine could be administered prior to any of six cycles; however, that cycle was always the last cycle of the session. Saline was administered prior to any cycle that preceded administration of cocaine, or prior to all cycles if cocaine was not administered during that training session. Stimulus control was considered adequate for testing when the following criteria were satisfied for five consecutive or for six of seven training sessions: at least 80% of the total responses for each cycle occurred on the correct (infusion-appropriate) lever and fewer than 10 responses (less than 1 FR) occurred on the incorrect lever before delivery of the first food pellet for each cycle. Thereafter, test sessions were conducted every third day as long as the testing criteria were satisfied during intervening training sessions; otherwise, training continued until the criteria were satisfied for two consecutive sessions.
Test sessions were identical to training sessions except that 10 consecutive responses on either lever delivered a food pellet. Either saline or lorcaserin (0.32–10 mg/kg intragastrically) was administered 75 minutes before one of two different types of test sessions. First, the time course for lorcaserin was determined by administering saline (intravenously) during each of six cycles, thereby examining the effects of lorcaserin 90–165 minutes after administration. Second, the ability of lorcaserin to alter the discriminative stimulus effects of cocaine was studied by obtaining cocaine dose-effect curves in a single session. Saline was always administered during the first cycle, with increasing doses of cocaine administered during subsequent cycles. The first dose of cocaine was 0.032 mg/kg, which was administered during the second cycle and 90 minutes after administration of saline or lorcaserin. Thereafter, the cumulative dose of cocaine increased each cycle in one-quarter log-unit increments until at least 80% of responding occurred on the cocaine-appropriate lever or the rate of responding decreased to less than 20% of the control rate, whichever occurred first.
For individual monkeys discriminating cocaine, the percentage of total responses emitted on the drug lever during response periods was plotted as a function of dose. Control response rates are the mean of 10 training sessions during which only saline was administered and monkeys satisfied the testing criteria. For individual subjects, response rates were averaged first across cycles to obtain a mean for a session and then across sessions to obtain control rates. In subjects DU and LA, dose-response curves for cocaine administered alone or with lorcaserin were determined twice and are reported as the mean (± 1 S.E.M.); in subject MU, each dose-response curve was determined once. Although the effects of each dose of lorcaserin alone were obtained 90–165 minutes after administration, the percentage of cocaine-lever responding obtained during the first cycle (response period occurred 85–90 minutes after lorcaserin administration) is shown. Discrimination data for an individual monkey were not included when response rates were less than 20% of control.
Cocaine Self-Administration.
Five monkeys were trained to self-administer cocaine during daily 90-minute sessions. Ninety minutes prior to each session, monkeys were briefly seated in chairs and given either saline or one of four doses of lorcaserin intragastrically and returned to their home cage for the duration of the pretreatment period. During sessions, monkeys were placed in dark, ventilated, sound-attenuating chambers. One minute before the start of the session, a loading infusion was administered to fill the catheter. The session began with a noncontingent priming infusion of 0.032 mg/kg cocaine, which was delivered in conjunction with a 5-second presentation of the cocaine-associated stimulus (i.e., a red light above the active lever). Immediately after the priming infusion, a green light was illuminated above the active lever, signaling drug availability. Responding on the active lever (counterbalanced across monkeys) was reinforced by infusions of 0.032 mg/kg cocaine and a 5-second presentation of the associated stimulus according to a FR30 schedule of reinforcement. Each contingent infusion was followed by a 180-second timeout during which the chamber was dark and responses were recorded but had no scheduled consequence. Once the timeout period had elapsed, the green light was illuminated and cocaine was again available for responding under the FR30/timeout 180-second schedule of reinforcement. Responding on the inactive lever was recorded but had no scheduled consequence. Saline was occasionally substituted for 0.032 mg/kg per infusion of cocaine to ensure that responding was being maintained by the cocaine infusions.
Treatment with lorcaserin was initiated once responding for cocaine satisfied the stability criteria, defined as less than 20% difference in rates of responding across three consecutive sessions, with no increasing or decreasing trend. All five monkeys received each dose of lorcaserin (0.1–3.2 mg/kg intragastrically) for 14 consecutive days, with treatment occurring 90 minutes before the start of the self-administration session. Acute effects of lorcaserin were obtained on treatment day 1, with the effects of repeated lorcaserin treatment determined during the subsequent 13 (consecutive) days of treatment. On days in which lorcaserin was not administered, monkeys were treated daily with intragastric saline 90 minutes before cocaine (0.032 mg/kg per infusion) self-administration sessions. Evaluations of different doses of lorcaserin were separated by a minimum of seven sessions and until responding satisfied the stability criteria.
On completion of the initial dose-response curve for the effects of lorcaserin on responding maintained by 0.032 mg/kg per infusion of cocaine, four (BO, CH, PE, and DA) of the original five monkeys (three male and one female) were used to determine whether the ability of 3.2 mg/kg lorcaserin to decrease cocaine self-administration could be surmounted by increasing the unit dose of cocaine available for infusion. Similar to the dose-response determination for lorcaserin, all monkeys were initially maintained on the FR30/timeout 180-second schedule of reinforcement for 0.032 mg/kg per infusion of cocaine. Treatment with 3.2 mg/kg lorcaserin began once responding satisfied stability criteria and continued for 15 consecutive sessions. During lorcaserin treatment, the dose of cocaine available for infusion increased every five sessions from 0.032 to 0.1 to 0.32 mg/kg per infusion. Upon completion of the 15-day treatment period, saline replaced lorcaserin, and the dose of cocaine available for infusion decreased every five sessions from 0.32 to 0.1 to 0.032 mg/kg per infusion.
Acute effects of lorcaserin on cocaine self-administration represent the mean (± 1 S.E.M.) number of infusions and rates of responding obtained during the first day of treatment with each dose of lorcaserin (i.e., treatment day 1 of 14). The mean (± 1 S.E.M.) of the number of infusions and rates of responding were also reported for the first day that saline replaced lorcaserin. Because baseline performance (sessions before lorcaserin treatment began and preceded by intragastric saline) for individual monkeys did not differ across testing, these data were collapsed across tests and are reported as the mean (± 1 S.E.M.) for the baseline performance of five monkeys. One-factor ANOVA with repeated measures and post hoc Holm–Sidak tests were used to determine whether the number of cocaine infusions delivered or rates of responding varied as a function of lorcaserin dose. Data from repeated administration of lorcaserin represent the mean (± 1 S.E.M.) number of infusions and rates of responding obtained for each day of treatment, as well as the seven sessions that immediately followed lorcaserin treatment, with the gray-shaded area representing ± 1 S.E.M. of the mean number of infusions and rates of responding obtained from the seven sessions that immediately preceded treatment with lorcaserin. Data from the 14-day treatment period were analyzed by one-factor (time) ANOVA with repeated measures and post hoc Holm–Sidak tests to determine whether the effect of lorcaserin varied as a function of treatment day. Data from the dose escalation studies represent the mean (± 1 S.E.M.) number of infusions and rates of responding obtained from the five sessions comprising each treatment condition (e.g., saline pretreatment with 0.032 mg/kg per infusion of cocaine, or lorcaserin pretreatment with 0.032 mg/kg per infusion of cocaine). Two-factor ANOVA with repeated measures and post hoc Holm–Sidak tests were used to determine whether the number of cocaine infusions or response rates varied as a function of lorcaserin pretreatment or the unit dose of cocaine.
Drugs.
For pharmacokinetic studies, lorcaserin standard was obtained from LC Laboratories (Woburn, MA); cocaine and dCOC standards were purchased from Sigma Chemical Company (St. Louis, MO). HPLC-grade methanol and acetonitrile were purchased from Fisher (Fair Lawn, NJ). All other reagents were HPLC grade or higher and were purchased from Sigma Chemical Company. Cocaine HCl was generously provided by the National Institutes of Health National Institute on Drug Abuse Drug Supply Program (Bethesda, MD); for behavioral studies, cocaine HCl was dissolved in physiologic saline and administered intravenously in a volume of 0.1 ml/kg. Lorcaserin HCl was purchased from MedChem Express (Princeton, NJ), dissolved in physiologic saline, and administered intragastrically (nasogastric intubation) in a volume of 0.32 ml/kg. Monkeys were seated in restraint chairs and a lubricated nasogastric feeding tube was placed in the nostril and inserted until it reached the stomach, at which point saline or lorcaserin was administered; the tube was then flushed with 10 ml saline to ensure that the entire dose was administered.
Results
Pharmacokinetics of Lorcaserin and Cocaine.
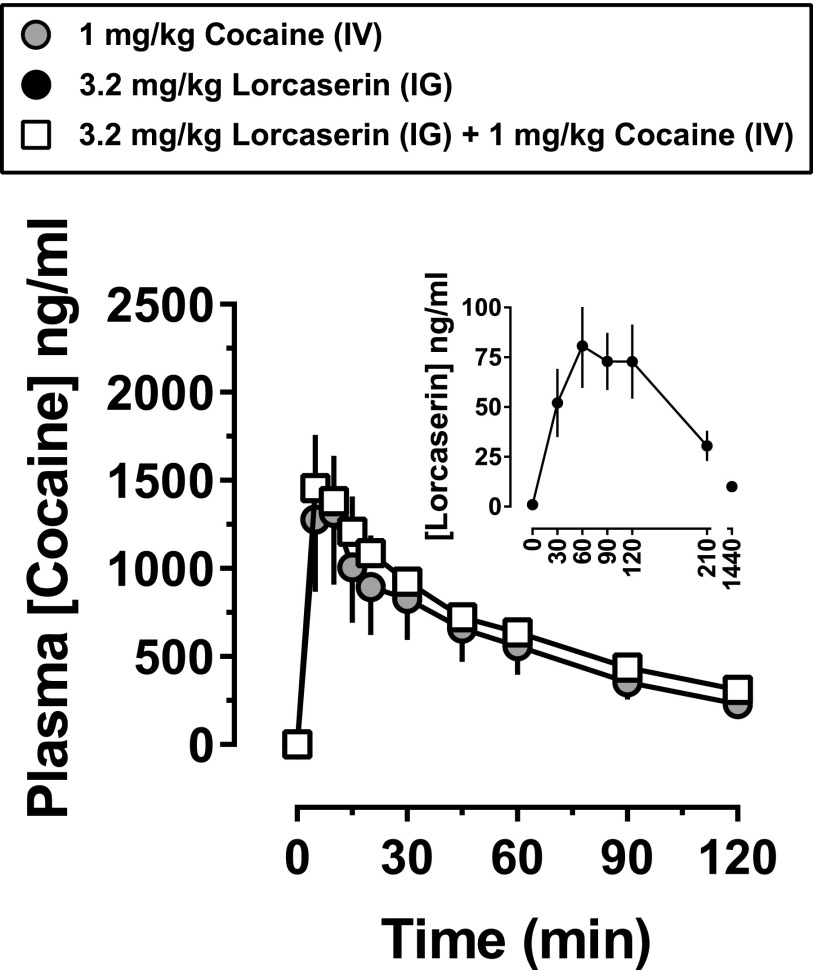
The inset in Fig. 1 shows the pharmacokinetic profile of 3.2 mg/kg lorcaserin intragastrically The concentration of lorcaserin in plasma reached a maximum of 80 ng/ml 60 minutes after administration. Plasma levels of lorcaserin were stable from 60 to 120 minutes. The pharmacokinetic profile of cocaine was evaluated twice in each monkey, once after saline administration and once after lorcaserin administration. The main panel in Fig. 1 shows the pharmacokinetic profile of 1 mg/kg cocaine administered intravenously 90 minutes after intragastric administration of either saline or 3.2 mg/kg lorcaserin; lorcaserin did not affect the pharmacokinetic profile of 1 mg/kg cocaine intravenously (two-tailed, paired t test, P = 0.15, n = 4).
Fig. 1.
Plasma concentration of lorcaserin alone, cocaine alone, and cocaine in combination with lorcaserin. Lorcaserin and cocaine were administered at doses of 3.2 mg/kg intragastrically and 1 mg/kg intravenously, respectively. The inset shows the pharmacokinetic profile of lorcaserin, administered alone and immediately after the collection of the first blood sample (0 minutes), with each filled circle representing the mean (± 1 S.E.M.) plasma concentration of lorcaserin in nanograms per milliliter (n = 4). The main panel shows the pharmacokinetic profile of cocaine 90 minutes after administration of either saline (gray circles) or lorcaserin (open squares); cocaine was administered immediately after the collection of the first blood sample (0 minutes). The symbols represent the mean (± 1 S.E.M.) plasma concentration of cocaine in nanograms per milliliter (n = 3). IG, intragastric; IV, intravenous.
Activity Monitoring and Directly Observable Behavior.
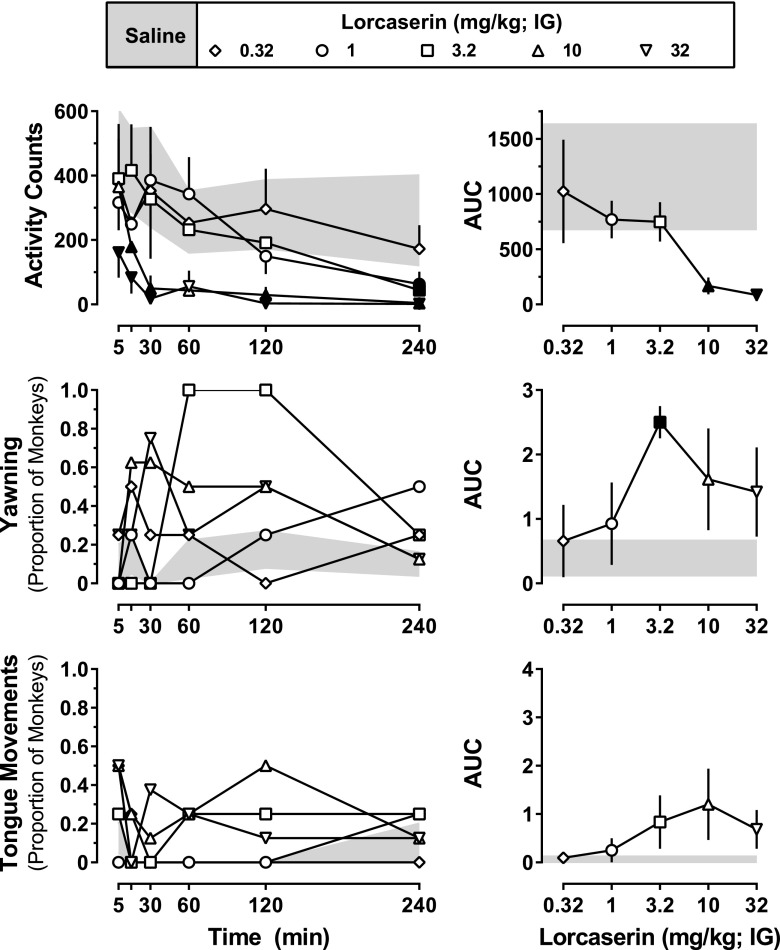
Lorcaserin (intragastric) decreased activity in the home cage in a dose- and time-dependent manner (Fig. 2, top, left; dose: F[5,15] = 5.26; P = 0.0055; time: F[5,15] = 4.48; P = 0.011). Although decreases in activity were apparent within 30 minutes of administration of 10 or 32 mg/kg lorcaserin, smaller doses (1 and 3.2 mg/kg) decreased activity only at the 240-minute time point. A similar dose-dependent decrease in activity was observed when the AUC for the entire 240-minute activity time course was analyzed (Fig. 2, top, right), with activity being significantly lower after doses of 10 or 32 mg/kg lorcaserin compared with activity after administration of saline (dose: F[5,15] = 5.49; P = 0.0045).
Fig. 2.
Acute effects of lorcaserin on activity and directly observable behaviors. A total of 24 directly observable behaviors were monitored (Table 1) before as well as 5, 15, 30, 60, 120, and 240 minutes after intragastric administration of lorcaserin (0.32–32 mg/kg) or saline (n = 4). Activity data (top left) represent the mean (± 1 S.E.M.) number of activity counts observed during 5 minutes immediately after each observation period. For yawning (middle left) and tongue movements (bottom left), the data represent the proportion of monkeys exhibiting each behavior during the 30-second period beginning at each time point. The AUC was calculated for each time course, with the mean (± 1 S.E.M.) shown in the right panels. Data obtained after saline administration (mean ± 1 S.E.M.) are represented by the gray-shaded areas. Black-filled symbols represent points that were significantly different from saline (P < 0.05), as determined by post hoc Holm–Sidak tests. IG, intragastric.
Although most of the directly observable behaviors (22 of 24) were not systematically altered by lorcaserin, yawning and tongue movements were both increased; however, the results with tongue movements failed to reach statistical significance. As shown in Fig. 2 (middle, left), lorcaserin dose-dependently increased the proportion of monkeys that yawned (dose: F[5,15] = 5.49; P = 0.0045). Similar to the effects of lorcaserin on activity, increased yawning was observed within the first 30–60 minutes after administration of lorcaserin, with all monkeys exhibiting yawning 60 and 120 minutes after receiving 3.2 mg/kg. Analysis of the AUCs derived from the entire 240-minute yawning time course assessment showed a similar dose-dependent increase in yawning (dose: F[5,15] = 5.34; P = 0.0051), with a dose of 3.2 mg/kg lorcaserin significantly increasing the proportion of monkeys yawning, compared with saline. Although it failed to reach statistical significance, lorcaserin also induced unusual tongue movements (e.g., rolling, poking cheeks, etc.); this effect appeared to be dose related in two monkeys, with three of four monkeys exhibiting some form of unusual tongue movement at larger doses. Reliability between raters of directly observable signs was determined using the κ statistic; for the two signs that were increased by lorcaserin (i.e., yawning and tongue movement), agreement between observers was found to be adequate with κ = 0.89.
Food-Maintained Responding.
In monkeys responding under an FR10 schedule of food presentation, mean control response rate (± 1 S.E.M.), averaged across three monkeys, was 1.57 ± 0.19 responses per second. Lorcaserin decreased rates of responding in a dose- and time-dependent manner across the nineteen 5-minute response periods comprising the 285-minute time course (Fig. 3, left; dose: F[3,6] = 21.0; P = 0.0014; time: F[18,36] = 2.34; P = 0.015). A dose of 10 mg/kg produced a sustained decrease in responding from 45 to 285 minutes after drug administration, whereas a dose of 3.2 mg/kg decreased responding only from 135 to 210 minutes after drug administration. Analysis of AUC values for the entire 285-minute period revealed a dose-dependent decrease in response rate (F[3,6] = 18.7; P = 0.0019) with significant reductions observed after administration of 3.2 and 10 mg/kg lorcaserin (Fig. 3, right).
Cocaine Discrimination.
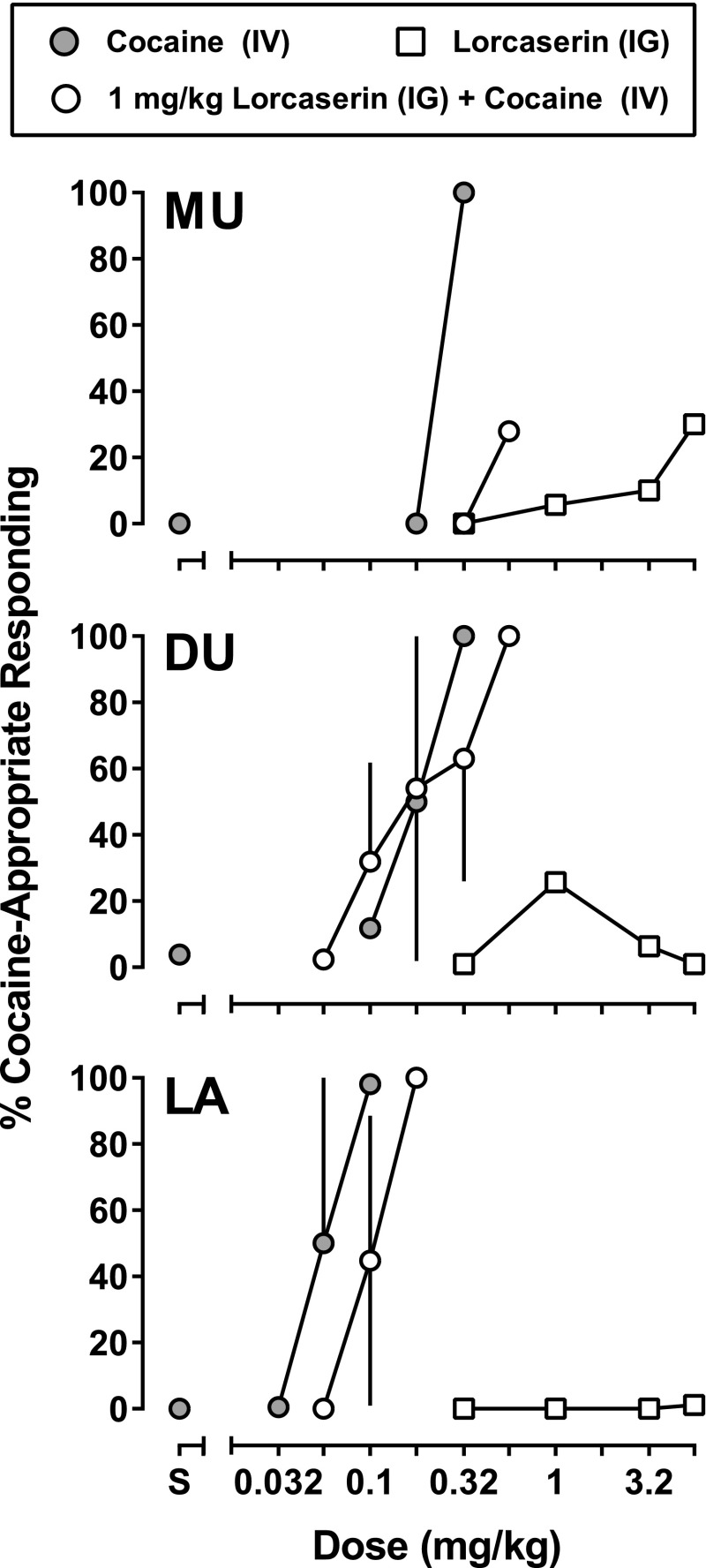
The mean control response rate was 1.17 ± 0.23 responses per second in three monkeys discriminating cocaine while responding under an FR10 schedule of food presentation. All three monkeys responded exclusively on the saline-associated lever after receiving saline and responded progressively more on the drug-associated lever with the administration of increasing doses of cocaine (Fig. 4). A cumulative dose of 0.1 mg/kg cocaine produced responding exclusively on the cocaine-associated lever in one monkey (LA) and increased response rates in that monkey (data not shown). A larger cumulative dose of cocaine (0.32 mg/kg) was needed to produce responding exclusively on the cocaine-associated lever in the other two monkeys (MU and DU), and this dose decreased response rates in both monkeys. Fig. 4 also shows data for lorcaserin alone (0.32, 1, 3.2, and 5.6 mg/kg intragastrically) administered 75 minutes before the first of six test cycles (85 minutes before the start and 90 minutes before the end of the first response period); in subsequent cycles, monkeys responded predominantly on the saline-associated lever (105–165 minutes) with the average cocaine-lever responding never exceeding 35%. When administered 75 minutes before the first cycle, the smallest dose of lorcaserin increased response rates to 130% and 117% of control in DU and MU, respectively, and decreased response rates to 68% of control in LA; the effects of larger doses on response rates varied across monkeys and across doses (data not shown). In subsequent cycles, response rates varied as time since lorcaserin administration increased, although the changes were not consistent across monkeys or across cycles.
Fig. 4.
Acute effects of lorcaserin in three monkeys (MU, DU, and LA) discriminating 0.178 mg/kg cocaine intravenously from saline. Gray-filled circles represent the mean (± 1 S.E.M.) percentage of responding that occurred on the cocaine-appropriate lever during test sessions in which saline was administered 90 minutes before saline (S) or the first dose of cocaine. Open squares represent the percentage of responding on the cocaine-associated lever during tests in which lorcaserin was administered before sessions (details available in the Materials and Methods). Open circles represent the mean (± 1 S.E.M.) percentage of responding on the cocaine-associated lever during tests in which 1 mg/kg lorcaserin was administered 75 minutes before cumulative doses of cocaine. IG, intragastric; IV, intravenous.
When administered 75 minutes before a cocaine test session (90 minutes before the first dose of cocaine), 1 mg/kg lorcaserin shifted the cocaine dose-response curve downward or rightward in two of the three monkeys (MU and LA) without affecting the curve in a third monkey (DU); in DU, evidence for attenuation of the discriminative stimulus effects of cocaine by this dose of lorcaserin was apparent only at the largest dose of cocaine (i.e., the dose that produced exclusive choice of the cocaine-appropriate lever under control conditions). A dose of 1 mg/kg lorcaserin decreased responding in DU and had little effect on response rates in the other two monkeys; under these treatment conditions, cocaine decreased rates in MU and DU and did not change rates in LA up to the dose that produced responding on the cocaine-associated lever. A larger dose of lorcaserin (3.2 mg/kg; data not shown) markedly decreased response rates in all monkeys; the rate-decreasing effect was most evident in MU and on one of two occasions in DU, when response rates were decreased to < 20% of control. In LA, 3.2 mg/kg lorcaserin decreased responding to 0.32 responses per second (67% of control), and in the presence of this dose of lorcaserin, cocaine produced no more than 24% responding on the cocaine-associated lever up to a dose that eliminated responding (0.56 mg/kg).
Cocaine Self-Administration.
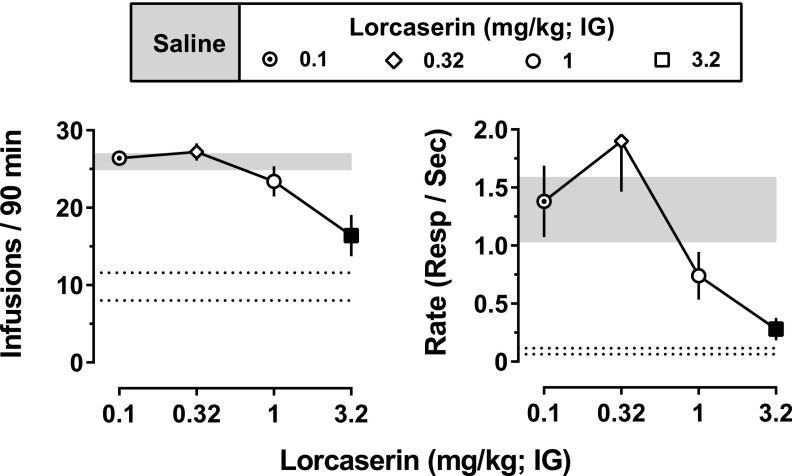
During baseline sessions preceded by saline (intragastric), 0.032 mg/kg per infusion of cocaine maintained high rates of responding (average of 1.31 ± 0.27 responses per second) in all monkeys with monkeys receiving an average of 25.9 ± 0.9 infusions per session (Fig. 5; shaded areas). Upon substituting saline for cocaine, responding decreased in all monkeys (0.09 ± 0.03 responses per second) with an average of 9.8 ± 1.8 infusions of saline received during the first session in which saline was available (Fig. 5, area between dotted lines). When administered acutely 90 minutes before the session, lorcaserin decreased the number of cocaine infusions received (F[4,16] = 11.37; P = 0.0001) and rates of responding (F[4,16] = 7.03; P = 0.0018) in a dose-dependent manner with significant decreases in both measures observed at 3.2 mg/kg lorcaserin (filled symbols, Fig. 5).
Fig. 5.
Acute effects of lorcaserin on cocaine self-administration. Plotted are the mean (± 1 S.E.M.) number of infusions received of 0.032 mg/kg cocaine (left) and mean (± 1 S.E.M.) rates of responding (right) during sessions in which lorcaserin (0.1–3.2 mg/kg intragastrically) was administered as a 90-minute pretreatment (n = 5). Data obtained during baseline cocaine self-administration sessions preceded by saline (mean ± 1 S.E.M.) are represented by the gray-shaded areas. Black-filled symbols represent values that are significantly different from baseline (P < 0.05), as determined by post hoc Holm–Sidak tests. Dotted lines represent the mean (± 1 S.E.M.) number of infusions and rate of responding during a single session in which saline was substituted for cocaine. IG, intragastric.
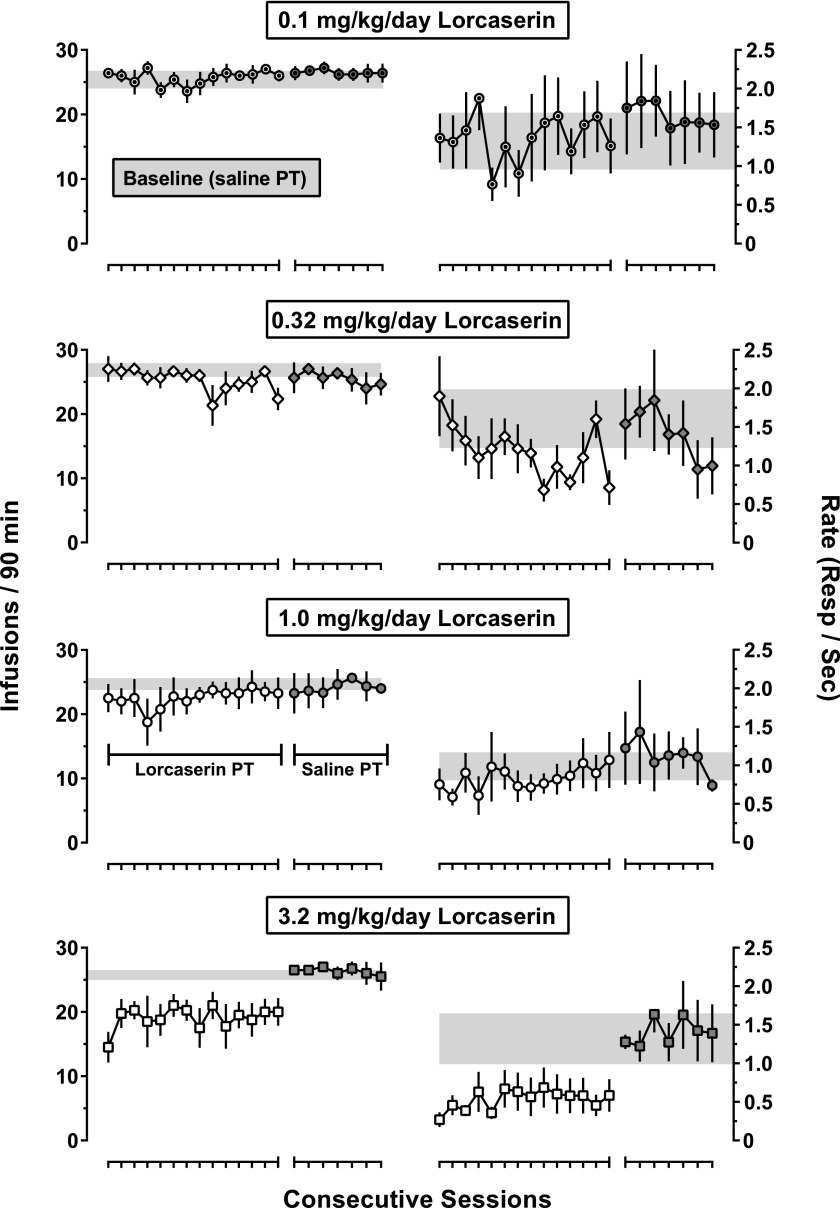
Similar to the effects obtained with the acute administration of lorcaserin, daily administration of 3.2 mg/kg lorcaserin decreased the number of cocaine infusions received as well as response rates across a 14-day treatment period (Fig. 6, bottom two panels); daily administration of smaller doses of lorcaserin (0.1–1.0 mg/kg per day) did not systematically alter cocaine self-administration (Fig. 6, top six panels). One-factor ANOVA failed to reveal any significant difference in the number of infusions delivered or response rates across the 14-day treatment period for any dose of lorcaserin. Upon discontinuation of daily treatment (i.e., 24 hours after the last dose of lorcaserin), both rates of responding and the number of cocaine infusions received returned to control (i.e., before lorcaserin treatment) values, where they remained for at least seven sessions (Fig. 6, gray symbols).
Fig. 6.
Effects of repeated lorcaserin administration on cocaine self-administration. Plotted are the mean (± 1 S.E.M.) number of infusions delivered of 0.032 mg/kg cocaine (left; n = 5) and the mean (± 1 S.E.M.) rates of responding (right) during 14 consecutive sessions in which lorcaserin (0.1–3.2 mg/kg intragastrically) was administered as a 90-minute pretreatment (open symbols). Gray-filled symbols represent data obtained during the block of seven consecutive sessions that immediately followed lorcaserin treatment, of which saline was administered 90 minutes beforehand. Gray-shaded areas represent the mean (± 1 S.E.M.) for the average number of cocaine infusions (left) and rates of responding (right) during the seven baseline sessions that immediately preceded lorcaserin treatment. PT, pretreatment; Resp, response.
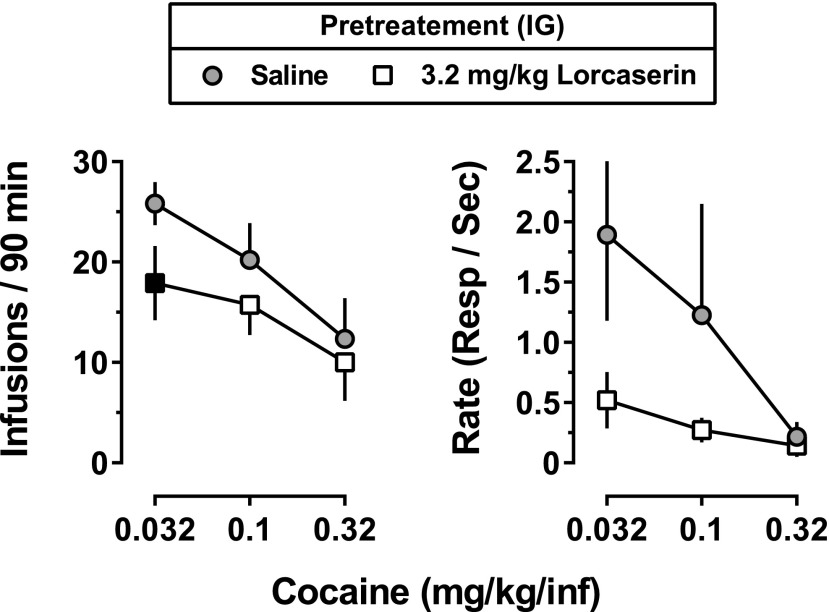
To assess whether the effects of lorcaserin in decreasing cocaine self-administration could be surmounted by larger unit doses of cocaine, on a separate occasion, monkeys were again treated with 3.2 mg/kg lorcaserin 90 minutes before daily sessions for 15 days; however, unlike the initial study that examined only 0.032 mg/kg per infusion of cocaine, the unit dose of cocaine increased by one-half log unit every five sessions, from 0.032 to 0.1 to 0.32 mg/kg per infusion. When saline was administered 90 minutes before sessions, increasing the unit dose of cocaine produced a dose-dependent decrease in the number of infusions and response rates (gray circles, Fig. 7). There was a main effect of lorcaserin on the number of cocaine infusions delivered, and this effect was dependent on the unit dose of cocaine (Fig. 7; lorcaserin dose: F[1,3] = 89.57; P = 0.0025; cocaine dose: F[2,6] = 15.09; P = 0.046). Although lorcaserin decreased average rates of responding maintained by 0.032 or 0.1 mg/kg per infusion of cocaine, these decreases were not statistically significant (possibly because of the variability among monkeys in rates of cocaine-maintained responding under baseline conditions).
Fig. 7.
Effects of increasing the unit dose of cocaine available for self-administration during 15 consecutive sessions, each preceded by administration of 3.2 mg/kg lorcaserin intragastrically Gray-filled circles represent the mean (± 1 S.E.M.) for the average number of cocaine infusions delivered and rates of responding during five consecutive sessions in which saline was administered as a 90-minute pretreatment (n = 4). Open squares represent the mean (± 1 S.E.M.) for the average number of cocaine infusions and rates of responding during five consecutive sessions preceded by 3.2 mg/kg lorcaserin (90-minute pretreatment). Black-filled symbols represent points that were significantly different from baseline (P < 0.05), as determined by post hoc Holm–Sidak tests. IG, intragastric; Resp, response.
Discussion
The results of these studies demonstrate the following: lorcaserin decreases ongoing cocaine self-administration, the effects of lorcaserin on cocaine self-administration are not surmountable when larger unit doses of cocaine are made available, tolerance to lorcaserin does not develop over 14 days of once-daily treatment, and attenuation of the effects of cocaine by lorcaserin is likely attributable to pharmacodynamic, and not pharmacokinetic, mechanisms. Importantly, these effects of lorcaserin were observed at a dose that also produced yawning, a putative 5-HT2C receptor–mediated effect, but was smaller than doses that were required to produce large disruptions in food-maintained responding or spontaneous activity. Taken together, these findings in nonhuman primates suggest that lorcaserin, a drug with agonist activity at 5-HT2C receptors that was recently approved by the FDA for the treatment of obesity, might also be effective for treating cocaine abuse and addiction.
One goal of these studies was to characterize the behavioral effects of lorcaserin administered intragastrically: from ineffective doses, to doses that significantly alter behavior. Lorcaserin was well tolerated up to a dose of 32 mg/kg and produced dose- and time-dependent effects on spontaneous activity, yawning, tongue movements, and food-maintained responding. Yawning began to emerge within 30 minutes of administration of 3.2 mg/kg with yawning observed in all monkeys 60 and 120 minutes after drug administration. The time course for yawning induced by 3.2 mg/kg lorcaserin closely matched plasma drug concentrations after administration of this dose, including the lack of behavioral effect and disappearance of lorcaserin in blood 24 hours after administration. These findings are consistent with a role for 5-HT2C receptors in mediating the induction of yawning by drugs acting on 5-HT mechanisms (Pomerantz et al., 1993; Collins and Eguibar, 2010). In addition to yawning, larger doses of lorcaserin also decreased spontaneous activity and food-maintained responding in all monkeys and produced oral-facial stereotypies (i.e., abnormal tongue movements) in a subset of monkeys. Taken together, these findings suggest that intragastric administration of 3.2 mg/kg lorcaserin results in plasma drug concentrations that are sufficient to produce putative 5-HT2C receptor–mediated effects without markedly disrupting spontaneous or operant behavior.
Based on the behavioral and pharmacokinetic profiles of lorcaserin, a 90-minute pretreatment time was used to evaluate the effectiveness of lorcaserin to alter behavioral effects of cocaine that are related to its abuse, including discriminative stimulus effects and reinforcing effects. When administered alone over a wide range of doses, lorcaserin failed to substantially increase responding on the cocaine-associated lever. Although these are the first studies to evaluate lorcaserin in animals trained to discriminate cocaine, they are in agreement with previous studies in rats discriminating MK212 (6-chloro-2-[1-piperazinyl]-pyrazine), a less selective 5-HT3/2C/2B receptor agonist (Callahan and Cunningham, 1995), and suggest that 5-HT2C receptor agonists, such as lorcaserin, do not share discriminative stimulus effects with cocaine. When administered before cocaine, 1 mg/kg lorcaserin shifted the cocaine dose-response curve downward or rightward in two of three monkeys. Although modest, these shifts in dose-response functions are similar in magnitude to results reported for combinations of MK212 and cocaine in rats and suggest that lorcaserin does not enhance, but rather attenuates, the discriminative stimulus effects of cocaine after intragastric administration. Although these studies were not designed to elucidate the mechanism(s) by which lorcaserin inhibits the effects of cocaine, given that lorcaserin did not alter the pharmacokinetic properties of cocaine, it is likely that these effects are mediated by its actions at 5-HT2C receptors. Consistent with this notion is the finding that 5-HT2C receptor antagonists (e.g., SDZ SER-082 [(+)-cis-4,5,7a,8,9,10,11,11a-octahydro-7H-10-methylindolo[1,7-bc][2,6]-naphthyridine fumarate]) can enhance the discriminative stimulus effects of cocaine (Filip et al., 2006) and that 5-HT2C receptor agonists and antagonists appear to modify the effects of cocaine by inhibiting and enhancing dopamine transmission, respectively (for reviews, see Bubar and Cunningham, 2006, 2008).
Unlike typical pharmacotherapies for drug abuse, which often target the primary site of action of the abused drug (e.g., methadone and naltrexone, both acting at μ-opioid receptors, for treating opioid addiction), it is also possible to reduce abuse-related effects through actions at sites other than the primary site of action of the abused drug. Because of their role in modulating dopamine neurotransmission, 5-HT2C receptors have received considerable attention as a potential target for treating cocaine abuse (Higgins et al., 2013a; Higgins and Fletcher, 2015; Howell and Cunningham, 2015); however, the relative lack of highly selective compounds has limited a full characterization of the effectiveness of 5-HT2C receptor agonists to reduce cocaine self-administration. Lorcaserin is 18- and 100-fold selective for 5-HT2C over 5-HT2A and 5-HT2B receptors, respectively (Thomsen et al., 2008). In the current studies, lorcaserin dose-dependently decreased rates of responding and number of cocaine infusions delivered with significant decreases observed for both measures after intragastric administration of 3.2 mg/kg lorcaserin. It is unlikely that this suppression of cocaine self-administration reflects a leftward shift in the cocaine dose-response curve insofar as lorcaserin shifts the cocaine self-administration dose-response curve to the right in monkeys responding under a progressive ratio schedule (unpublished observations). Taken together, these findings are consistent with the acute effects of the 5-HT2C receptor agonists WAY163909 and Ro60-0175 in rats and squirrel monkeys, respectively (Cunningham et al., 2011; Manvich et al., 2012; Rüedi-Bettschen et al., 2015), and provide further support for the notion that targeting 5-HT2C receptors could provide an effective strategy for treating cocaine addiction.
In addition to assessing the acute effects of lorcaserin, these studies also evaluated the effectiveness of lorcaserin across 14 days of repeated treatment as well as whether the effectiveness of lorcaserin is surmounted when larger unit doses of cocaine are made available. Lorcaserin not only retained its effectiveness to decrease cocaine self-administration across the entire 14-day treatment period, but it also continued to decrease cocaine self-administration even when larger unit doses of cocaine were available. A similar downward shift in the dose-response curve for cocaine self-administration has been reported for squirrel monkeys treated acutely with Ro60-0175 (Manvich et al., 2012). These findings are important because they suggest that individuals would not become tolerant to the effects of lorcaserin during long-term treatment and they would be unlikely to overcome the effects of lorcaserin by taking more cocaine.
In summary, these studies characterized the behavioral and pharmacokinetic profile of the clinically used 5-HT2C receptor agonist, lorcaserin, and evaluated its effectiveness to reduce the discriminative stimulus and reinforcing effects of cocaine in rhesus monkeys. The main findings of these studies are as follows. First, lorcaserin dose-dependently increased 5-HT2C receptor–mediated behaviors over a time course that closely matched its pharmacokinetic profile and at doses smaller than those that disrupted other spontaneous or operant behavior. Second, acute administration of lorcaserin attenuated the discriminative stimulus effects of cocaine in two of three monkeys and dose-dependently inhibited cocaine self-administration in all monkeys, at doses smaller than those that produced comparable reductions in food-maintained responding. Finally, lorcaserin retained its effectiveness to decrease cocaine self-administration over a range of cocaine doses and over 14 days of once-daily treatment. Together, these findings provide further support for the hypothesis that activation of 5-HT2C receptors can decrease abuse-related effects of cocaine and suggest that lorcaserin, an FDA-approved drug with 5-HT2C receptor agonist properties, should be further evaluated as a candidate medication for cocaine abuse and addiction.
Acknowledgments
The authors gratefully acknowledge the contributions and leadership of Dr. Roger D. Porsolt in the design and planning of these studies and his authorship of the original grant application. The authors also thank Marlisa Jacobs, Nicole Garcia, Mark Garza, Marissa McCarthy, Chris Robinson, and Crystal Taylor for their technical assistance.
Abbreviations
- ANOVA
analysis of variance
- AUC
area under the curve
- dCOC
deuterated cocaine
- FDA
Food and Drug Administration
- 5-HT
serotonin
- FR
fixed ratio
- HPLC
high-pressure liquid chromatography
- M100907
α-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenylethyl)]-4-piperidine methanol
- MK-212
6-chloro-2-[1-piperazinyl]-pyrazine
- Ro60-0175
(S)-2(6-chloro-5-fluorindol-1-yl)-1-methylethylamine
- SDZ SER-082
(+)-cis-4,5,7a,8,9,10,11,11a-octahydro-7H-10-methylindolo[1,7-bc][2,6]-naphthyridine fumarate
- SR46349B
4-[(2Z)-3-{[2-(dimethylamino)ethoxy]amino}-3-(2-fluorophenyl)prop-2-en-1-ylidene]cyclohexa-2,5-dien-1-one
- WAY163909
(7bR, 10aR)-1,2,3,4,8,9,10,10a-octahydro-7bH-cyclopenta-[b][1,4]diazepino[6,7,1hi]indole
Authorship Contributions
Participated in research design: Collins, Gerak, Javors, France.
Conducted experiments: Collins, Gerak, Javors.
Performed data analysis: Collins, Gerak, Javors.
Wrote or contributed to the writing of the manuscript: Collins, Gerak, Javors, France.
Footnotes
This research was supported by the National Institutes of Health National Institute on Drug Abuse [Grants U01DA034992 and K05DA017918]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the National Institute on Drug Abuse.
References
- Aronne L, Shanahan W, Fain R, Glicklich A, Soliman W, Li Y, Smith S. (2014) Safety and efficacy of lorcaserin: a combined analysis of the BLOOM and BLOSSOM trials. Postgrad Med 126:7–18. [DOI] [PubMed] [Google Scholar]
- Bubar MJ, Cunningham KA. (2006) Serotonin 5-HT2A and 5-HT2C receptors as potential targets for modulation of psychostimulant use and dependence. Curr Top Med Chem 6:1971–1985. [DOI] [PubMed] [Google Scholar]
- Bubar MJ, Cunningham KA. (2008) Prospects for serotonin 5-HT2R pharmacotherapy in psychostimulant abuse. Prog Brain Res 172:319–346. [DOI] [PubMed] [Google Scholar]
- Burbassi S, Cervo L. (2008) Stimulation of serotonin2C receptors influences cocaine-seeking behavior in response to drug-associated stimuli in rats. Psychopharmacology (Berl) 196:15–27. [DOI] [PubMed] [Google Scholar]
- Burmeister JJ, Lungren EM, Kirschner KF, Neisewander JL. (2004) Differential roles of 5-HT receptor subtypes in cue and cocaine reinstatement of cocaine-seeking behavior in rats. Neuropsychopharmacology 29:660–668. [DOI] [PubMed] [Google Scholar]
- Callahan PM, Cunningham KA. (1995) Modulation of the discriminative stimulus properties of cocaine by 5-HT1B and 5-HT2C receptors. J Pharmacol Exp Ther 274:1414–1424. [PubMed] [Google Scholar]
- Chan EW, He Y, Chui CSL, Wong AYS, Lau WCY, Wong ICK. (2013) Efficacy and safety of lorcaserin in obese adults: a meta-analysis of 1-year randomized controlled trials (RCTs) and narrative review on short-term RCTs. Obes Rev 14:383–392. [DOI] [PubMed] [Google Scholar]
- Collins GT, Brim RL, Noon KR, Narasimhan D, Lukacs NW, Sunahara RK, Woods JH, Ko MC. (2012) Repeated administration of a mutant cocaine esterase: effects on plasma cocaine levels, cocaine-induced cardiovascular activity, and immune responses in rhesus monkeys. J Pharmacol Exp Ther 342:205–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins GT, Eguibar JR. (2010) Neurophamacology of yawning. Front Neurol Neurosci 28:90–106. [DOI] [PubMed] [Google Scholar]
- Cunningham KA, Fox RG, Anastasio NC, Bubar MJ, Stutz SJ, Moeller FG, Gilbertson SR, Rosenzweig-Lipson S. (2011) Selective serotonin 5-HT(2C) receptor activation suppresses the reinforcing efficacy of cocaine and sucrose but differentially affects the incentive-salience value of cocaine- vs. sucrose-associated cues. Neuropharmacology 61:513–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dackis C, O’Brien C. (2003) Glutamatergic agents for cocaine dependence. Ann N Y Acad Sci 1003:328–345. [DOI] [PubMed] [Google Scholar]
- Filip M, Bubar MJ, Cunningham KA. (2006) Contribution of serotonin (5-HT) 5-HT2 receptor subtypes to the discriminative stimulus effects of cocaine in rats. Psychopharmacology (Berl) 183:482–489. [DOI] [PubMed] [Google Scholar]
- Filip M, Frankowska M, Zaniewska M, Gołda A, Przegaliński E. (2005) The serotonergic system and its role in cocaine addiction. Pharmacol Rep 57:685–700. [PubMed] [Google Scholar]
- Fletcher PJ, Grottick AJ, Higgins GA. (2002) Differential effects of the 5-HT(2A) receptor antagonist M100907 and the 5-HT(2C) receptor antagonist SB242084 on cocaine-induced locomotor activity, cocaine self-administration and cocaine-induced reinstatement of responding. Neuropsychopharmacology 27:576–586. [DOI] [PubMed] [Google Scholar]
- Grabowski J, Shearer J, Merrill J, Negus SS. (2004) Agonist-like, replacement pharmacotherapy for stimulant abuse and dependence. Addict Behav 29:1439–1464. [DOI] [PubMed] [Google Scholar]
- Grottick AJ, Fletcher PJ, Higgins GA. (2000) Studies to investigate the role of 5-HT(2C) receptors on cocaine- and food-maintained behavior. J Pharmacol Exp Ther 295:1183–1191. [PubMed] [Google Scholar]
- Grottick AJ, Whelan K, Sanabria EK, Behan DP, Morgan M, Sage C. (2015) Investigating interactions between phentermine, dexfenfluramine, and 5-HT2C agonists, on food intake in the rat. Psychopharmacology (Berl) 232:1973–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallgren KA. (2012) Computing inter-rater reliability for observational data: an overview and tutorial. Tutor Quant Methods Psychol 8:23–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins GA, Fletcher PJ. (2015) Therapeutic potential of 5-HT2C receptor agonists for addictive disorders. ACS Chem Neurosci 6:1071–1088. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Sellers EM, Fletcher PJ. (2013a) From obesity to substance abuse: therapeutic opportunities for 5-HT2C receptor agonists. Trends Pharmacol Sci 34:560–570. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Silenieks LB, Lau W, de Lannoy IAM, Lee DKH, Izhakova J, Coen K, Le AD, Fletcher PJ. (2013b) Evaluation of chemically diverse 5-HT₂c receptor agonists on behaviours motivated by food and nicotine and on side effect profiles. Psychopharmacology (Berl) 226:475–490. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Silenieks LB, Rossmann A, Rizos Z, Noble K, Soko AD, Fletcher PJ. (2012) The 5-HT2C receptor agonist lorcaserin reduces nicotine self-administration, discrimination, and reinstatement: relationship to feeding behavior and impulse control. Neuropsychopharmacology 37:1177–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell LL, Cunningham KA. (2015) Serotonin 5-HT2 receptor interactions with dopamine function: implications for therapeutics in cocaine use disorder. Pharmacol Rev 67:176–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson JD, Setola V, Roth BL, Merryman WD. (2011) Serotonin receptors and heart valve disease--it was meant 2B. Pharmacol Ther 132:146–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis JR, Koch GG. (1977) The measurement of observer agreement for categorical data. Biometrics 33:159–174. [PubMed] [Google Scholar]
- Levin ED, Johnson JE, Slade S, Wells C, Cauley M, Petro A, Rose JE. (2011) Lorcaserin, a 5-HT2C agonist, decreases nicotine self-administration in female rats. J Pharmacol Exp Ther 338:890–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manvich DF, Kimmel HL, Howell LL. (2012) Effects of serotonin 2C receptor agonists on the behavioral and neurochemical effects of cocaine in squirrel monkeys. J Pharmacol Exp Ther 341:424–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello NK. (1990) Preclinical evaluation of the effects of buprenorphine, naltrexone and desipramine on cocaine self-administration. NIDA Res Monogr 105:189–195. [PubMed] [Google Scholar]
- Murnane KS, Winschel J, Schmidt KT, Stewart LM, Rose SJ, Cheng K, Rice KC, Howell LL. (2013) Serotonin 2A receptors differentially contribute to abuse-related effects of cocaine and cocaine-induced nigrostriatal and mesolimbic dopamine overflow in nonhuman primates. J Neurosci 33:13367–13374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Research Council (2011) Guide for the Care and Use of Laboratory Animals, 8th ed, National Academies Press, Washington, DC. [Google Scholar]
- Nichols DE. (2004) Hallucinogens. Pharmacol Ther 101:131–181. [DOI] [PubMed] [Google Scholar]
- Platt DM, Rowlett JK, Spealman RD. (2002) Behavioral effects of cocaine and dopaminergic strategies for preclinical medication development. Psychopharmacology (Berl) 163:265–282. [DOI] [PubMed] [Google Scholar]
- Pomerantz SM, Hepner BC, Wertz JM. (1993) 5-HT1A and 5-HT1C/1D receptor agonists produce reciprocal effects on male sexual behavior of rhesus monkeys. Eur J Pharmacol 243:227–234. [DOI] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. (1987) Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science 237: 1219–1223. [DOI] [PubMed] [Google Scholar]
- Roberts DC, Brebner K. (2000) GABA modulation of cocaine self-administration. Ann N Y Acad Sci 909:145–158. [DOI] [PubMed] [Google Scholar]
- Rüedi-Bettschen D, Spealman RD, Platt DM. (2015) Attenuation of cocaine-induced reinstatement of drug seeking in squirrel monkeys by direct and indirect activation of 5-HT2C receptors. Psychopharmacology (Berl) 232:2959–2968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shram MJ, Schoedel KA, Bartlett C, Shazer RL, Anderson CM, Sellers EM. (2011) Evaluation of the abuse potential of lorcaserin, a serotonin 2C (5-HT2C) receptor agonist, in recreational polydrug users. Clin Pharmacol Ther 89:683–692. [DOI] [PubMed] [Google Scholar]
- Smith BM, Smith JM, Tsai JH, Schultz JA, Gilson CA, Estrada SA, Chen RR, Park DM, Prieto EB, Gallardo CS, et al. (2008) Discovery and structure-activity relationship of (1R)-8-chloro-2,3,4,5-tetrahydro-1-methyl-1H-3-benzazepine (Lorcaserin), a selective serotonin 5-HT2C receptor agonist for the treatment of obesity. J Med Chem 51:305–313. [DOI] [PubMed] [Google Scholar]
- Smith SR, Prosser WA, Donahue DJ, Morgan ME, Anderson CM, Shanahan WR, APD356-004 Study Group (2009) Lorcaserin (APD356), a selective 5-HT(2C) agonist, reduces body weight in obese men and women. Obesity (Silver Spring) 17:494–503. [DOI] [PubMed] [Google Scholar]
- Smith SR, Weissman NJ, Anderson CM, Sanchez M, Chuang E, Stubbe S, Bays H, Shanahan WR, Behavioral Modification and Lorcaserin for Overweight and Obesity Management (BLOOM) Study Group (2010) Multicenter, placebo-controlled trial of lorcaserin for weight management. N Engl J Med 363:245–256. [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration (2014) Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H–48, HHS Publication No. (SMA) 14-48, Substance Abuse and Mental Health Services Administration, Rockville, MD. [Google Scholar]
- Tanda G, Newman AH, Katz JL. (2009) Discovery of drugs to treat cocaine dependence: behavioral and neurochemical effects of atypical dopamine transport inhibitors. Adv Pharmacol 57:253–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen WJ, Grottick AJ, Menzaghi F, Reyes-Saldana H, Espitia S, Yuskin D, Whelan K, Martin M, Morgan M, Chen W, et al. (2008) Lorcaserin, a novel selective human 5-hydroxytryptamine2C agonist: in vitro and in vivo pharmacological characterization. J Pharmacol Exp Ther 325:577–587. [DOI] [PubMed] [Google Scholar]
- United Nations Office on Drugs and Crime (2014) World Drug Report 2014 (United Nations publication, Sales No. E.14.XI.7), United Nations Office on Drugs and Crime, Vienna, Austria. [Google Scholar]
- Vocci FJ, Acri J, Elkashef A. (2005) Medication development for addictive disorders: the state of the science. Am J Psychiatry 162:1432–1440. [DOI] [PubMed] [Google Scholar]
- Weissman NJ, Sanchez M, Koch GG, Smith SR, Shanahan WR, Anderson CM. (2013) Echocardiographic assessment of cardiac valvular regurgitation with lorcaserin from analysis of 3 phase 3 clinical trials. Circ Cardiovasc Imaging 6:560–567. [DOI] [PubMed] [Google Scholar]
- Wojnicki FH, Bacher JD, Glowa JR. (1994) Use of subcutaneous vascular access ports in rhesus monkeys. Lab Anim Sci 44:491–494. [PubMed] [Google Scholar]