Abstract
Despite preventive education, the combined consumption of alcohol and caffeine (particularly from “energy drinks”) continues to rise. Physiologic perturbations by separate intake of ethanol and caffeine have been widely documented. However, the biologic actions of the alcohol-caffeine combination and their underlying subcellular mechanisms have been scarcely studied. Using intravital microscopy on a closed-cranial window and isolated, pressurized vessels, we investigated the in vivo and in vitro action of ethanol-caffeine mixtures on cerebral arteries from rats and mice, widely recognized models to address cerebrovascular pathophysiology and pharmacology. Caffeine at concentrations found in human circulation after ingestion of one to two cups of coffee (10 µM) antagonized the endothelium-independent constriction of cerebral arteries evoked by ethanol concentrations found in blood during moderate-heavy alcohol intoxication (40–70 mM). Caffeine antagonism against alcohol was similar whether evaluated in vivo or in vitro, suggesting independence of systemic factors and drug metabolism, but required a functional endothelium. Moreover, caffeine protection against alcohol increased nitric oxide (NO•) levels over those found in the presence of ethanol alone, disappeared upon blocking NO• synthase, and could not be detected in pressurized cerebral arteries from endothelial nitric-oxide synthase knockout (eNOS−/−) mice. Finally, incubation of de-endothelialized cerebral arteries with the NO• donor sodium nitroprusside (10 µM) fully restored the protective effect of caffeine. This study demonstrates for the first time that caffeine antagonizes ethanol-induced cerebral artery constriction and identifies endothelial NO• as the critical caffeine effector on smooth muscle targets. Conceivably, situations that perturb endothelial function and/or NO• availability will critically alter caffeine antagonism of alcohol-induced cerebrovascular constriction without significantly disrupting endothelium-independent, alcohol-induced cerebral artery constriction itself.
Introduction
Caffeine and alcohol (ethyl alcohol; ethanol) are the two most widely used psychoactive agents in the world (Meredith et al., 2013; Winstock 2014). In the United States, >90% of adults consume caffeine regularly, with an average intake of >200 mg/d; that is, more caffeine than what is contained in two 6-ounce cups of coffee or five 12-ounce cans of soft drinks (Frary et al., 2005; Juliano et al., 2012). The health risks imposed on the cardiovascular system by high and/or chronic caffeine intake as well as the health hazards resulting from abrupt caffeine withdrawal are well known and reviewed elsewhere (Balster, 1998).
In turn, episodic, moderate-to-heavy ethanol intake, such as during “binging,” constitutes the primary form of excessive alcohol consumption in the United States. Indeed, ∼29% of females and 43% of males in the United States have binge drinking experience (CDC, 2014). Despite several years of preventive education, binge drinking prevalence across US college campuses has remained steadily high at >40% (McMurtrie, 2014). Numerous epidemiologic studies have portrayed the deleterious effects of alcohol on cerebrovascular health (Altura and Altura, 1984; Puddey et al., 1999; Reynolds et al., 2003; Patra et al., 2010). In particular, episodic drinking with blood alcohol levels reaching 35–80 mM is associated with increased risk for cerebrovascular ischemia (Puddey et al., 1999), stroke, and death from ischemic stroke (Reynolds et al., 2003). Cerebrovascular ischemia may result from impaired vasodilatation and/or enhanced constriction of cerebral arteries. Using a rat model that mimics human cerebral artery reactivity, we showed that ethanol (10–100 mM) constricted middle cerebral arteries both in vivo and in vitro (Liu et al., 2004; Bukiya et al., 2009, 2014).
The combined use of alcohol and caffeine, the latter as the main active ingredient of “power/energy supplements” such as chewing bars, gums, gels, and beverages (i.e., “energy drinks”) is increasing sharply, particularly among adolescent and college students. More than 50% of US college students have reported mixing energy drinks with alcohol in the past month (Malinauskas et al., 2007). Remarkably, energy drinks increase the risk of alcohol dependence (Arria et al., 2011). In addition, people who co-consume caffeine and alcohol are 3 times more likely to binge drink than drinkers who do not mix these agents (Mintel International Group, 2007), and their desire to drink alcohol is more pronounced than in subjects exposed to the same dose of alcohol alone (Marczinski, 2015). Some studies suggest that the combined use of caffeine and ethanol may increase the rate of alcohol-related injury (Reissig et al., 2009). On the other hand, the combination of these two drugs has been proposed as neuroprotection against ischemic stroke in rat models (Aronowski et al., 2003) and humans (Sacco, 2007). However, whether ethanol and caffeine actually modulate each other’s action on cerebral vessels remains unknown. More generally, in spite of increased co-consumption of alcohol and caffeine in the US population, the subcellular targets and mechanisms that are perturbed by the presence of this drug combination are mostly unknown.
Using a combination of in vivo and in vitro arterial tone determinations in a rat model and selective pharmacology, and the endothelial nitric-oxide synthase knockout (eNOS−/− KO) mouse model, our study documents that caffeine at concentrations commonly found in human circulation antagonizes constriction of cerebral arteries evoked by ethanol concentrations equivalent to blood alcohol levels found during alcohol intoxication. This action of caffeine does not require neuronal integrity, circulating factors, or systemic drug metabolism but occurs at the cerebral artery level itself. In contrast to ethanol or caffeine individual actions on middle cerebral artery diameter, which are endothelium-independent, our study further reveals that the protective action of caffeine is mediated by endothelial nitric oxide (NO•). This finding may have health-related implications, as several pathophysiologic conditions and pharmacotherapeutic interventions modify endothelial NO• levels.
Materials and Methods
Ethics.
The care of animals and experimental protocols were reviewed and approved by the Animal Care and Use Committee of the University of Tennessee Health Science Center, which is an institution accredited by the Association for Assessment and Accreditation of Laboratory Animal Care.
Measurement of Pial Arteriole Diameter In Vivo.
Adult male Sprague-Dawley rats (≈250 g) were purchased from Harlan Laboratories (Indianapolis, IN). Rats were anesthetized with ketamine hydrochloride (33 mg/kg, i.m.) and acepromazine (3.3 mg/kg, i.m.). Catheters inserted into the carotid artery were used to infuse the drugs of interest. Drugs were dissolved to final concentration in sodium saline solution (0.9% NaCl). Body temperature was maintained at 37–38°C with a heating pad. Animals were equipped with a custom-built stainless steel and glass cranial window for pial arteriole diameter monitoring in vivo. Caffeine (10 µM), ethanol (18 or 50 mM), and caffeine + ethanol mixtures were diluted in sodium saline solution, and 1 ml of this solution was infused into the cerebral circulation via the carotid artery. In all experiments where ethanol in solution was applied to tissues, solutions with urea isosmotically replacing for ethanol were used as control solution (Liu et al., 2004). Pial arteriolar diameter was monitored with a video micrometer coupled to a television camera that depicted the brain surface through the cranial window (Bukiya et al., 2013).
Measurement of Cerebral Artery Diameter In Vitro.
Adult male Sprague-Dawley rats (≈250 g) were purchased from Harlan Laboratories (Indianapolis, IN). Adult 8- to 12-week-old male homozygous eNOS (Nos3tm1Unc/J) KO (eNOS−/−) and C57BL/6 control mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Rats and mice were decapitated using a guillotine and sharp scissors, respectively. Middle cerebral arteries (<260 μm and <150 μm external diameter in rat and mouse species, respectively) were isolated and cannulated as described in our earlier work (Bukiya et al., 2013). When required, endothelium was removed by passing an air bubble into the vessel lumen for 90 seconds before vessel cannulation. The presence/absence of functional endothelium was experimentally verified by the arterial response to 10 μM acetylcholine, an endothelium-dependent vasodilator, versus the response to 10 µM sodium nitroprusside (SNP), an endothelium-independent vasodilator (Liu et al., 2004; Bukiya et al., 2007). The chamber containing the cannulated artery was continuously perfused at a rate of 3.75 ml/min with physiologic saline solution (PSS) (mM): 119 NaCl, 4.7 KCl, 1.2 KH2PO4, 1.6 CaCl2, 1.2 MgSO4, 0.023 EDTA, 11 glucose, and 24 NaHCO3. PSS was equilibrated at pH 7.4 with a 21%/5%/74% mix of O2/CO2/N2 and maintained at 35–37°C. Arteries were observed with a charge coupled device camera (Sanyo VCB-3512T; Sanyo Electric Co., Moriguchi, Japan) attached to an inverted Nikon Eclipse TS100 microscope (Nikon, Tokyo, Japan). The external diameter of the arterial wall was measured using the automatic edge-detection function of IonWizard software (IonOptics, Milton, MA) and was digitized at 1 Hz with a personal computer.
Experiments were performed on arteries subjected to 60 mm Hg intravascular pressure to ensure both development and maintenance of myogenic tone (Bukiya et al., 2007). Once myogenic tone developed and artery diameter remained stable for at least 10 minutes, the artery was exposed to a high K+ solution (mM): 63.7 NaCl, 60 KCl, 1.2 KH2PO4, 1.6 CaCl2, 1.2 MgSO4, 0.023 EDTA, 11 glucose, 24 NaHCO3, pH 7.4. Drugs were applied to the artery by chamber perfusion. The effect of a given drug application was evaluated at the time point immediately before the solution was changed to drug-free PSS. At the end of each experiment, passive arterial diameter for myogenic tone determination was evaluated by exposing the vessel to Ca2+-free solution (mM): 119 NaCl, 4.7 KCl, 1.2 KH2PO4, 1.2 MgSO4, 2 EGTA, 11 glucose, 24 NaHCO3, pH 7.4.
NO• Level Assay.
Total NO• levels in homogenates of rat middle cerebral arteries from control, ethanol-exposed, and ethanol-caffeine exposed groups were determined from both oxidized forms (nitrites and nitrates) using the Nitric Oxide Fluorometric Assay Kit (Cayman Chemicals, Ann Arbor, MI), according to the manufacturer’s instructions. Fluorescence intensity was measured at excitation and emission wavelengths of 360 nm and 430 nm, respectively.
Chemicals.
Ethanol was purchased from American Bioanalytical (Natick, MA). All other chemicals were purchased from Sigma-Aldrich (St. Louis, MO). For SNP and caffeine, 1 mM stocks were prepared in PSS and further diluted in PSS to render the desirable concentration. All other chemicals were directly dissolved in PSS at their final concentration.
Data Analysis.
Final plotting, fitting, and statistical analysis of data were conducted using Origin 8.5.1 (OriginLab, Northampton, MA) and InStat 3.0 (GraphPad, La Jolla, CA). Myogenic tone was determined according to the following formula: Myogenic tone (%) = (1 − Active diameter/Passive diameter) × 100 (Adebiyi et al., 2007). Statistical analysis was conducted using either one-way analysis of variance followed by Bonferroni’s multiple comparison test or paired Student’s t test, according to experimental design. P < 0.05 was considered statistically significant. Data are expressed as mean ± S.E.M., and n = number of arteries.
Results
Caffeine Antagonized Ethanol Action on Cerebral Artery Diameter Both In Vivo and In Vitro.
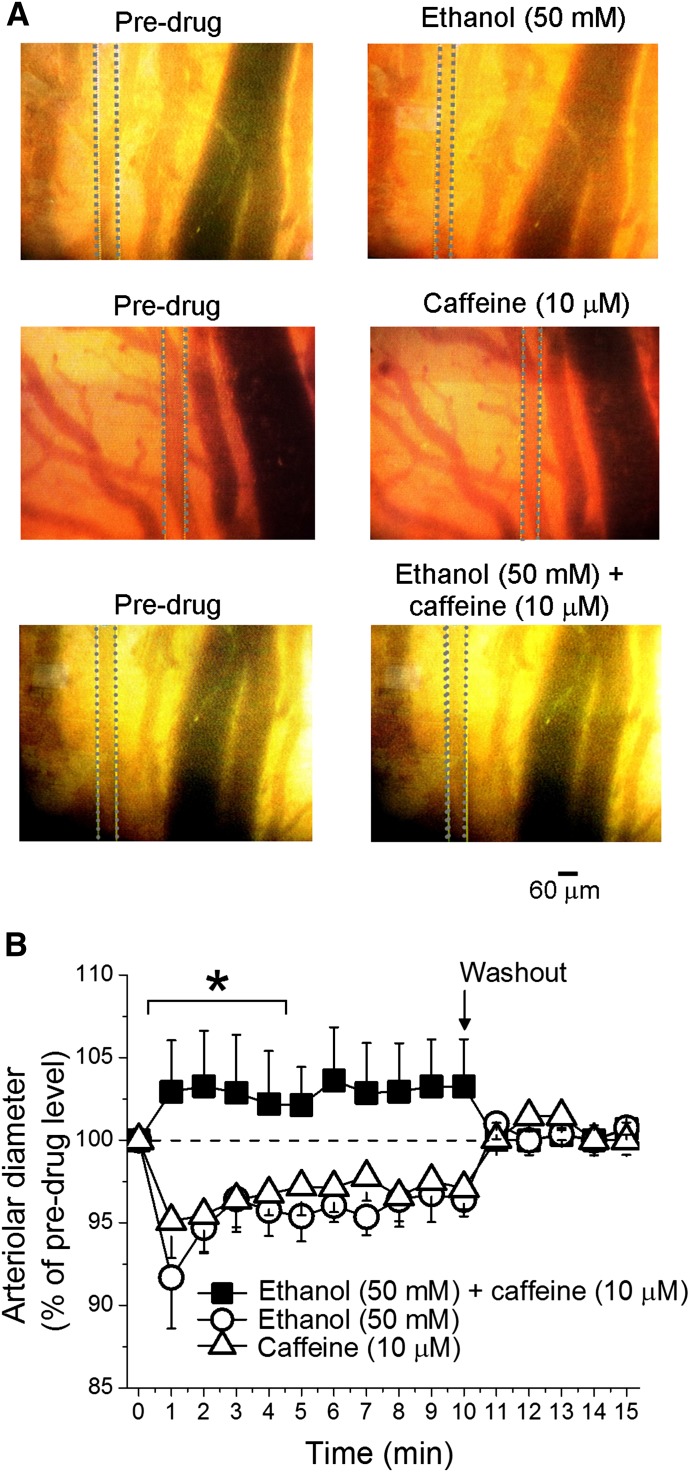
To determine the impact of caffeine-ethanol coadministration on cerebral artery diameter, we used intravital microscopy in a closed cranial window on anesthetized adult rats. As reported in animal models and humans (Altura and Altura, 1984; Reynolds et al., 2003; Bukiya et al., 2014), infusion of 50 mM ethanol into the carotid artery caused cerebral arteriole constriction (Fig. 1A, top panels), which quickly disappeared upon washout of the artery with ethanol-free PSS (Fig. 1B). It is important to note that, according to Poiseuille’s equation, blood flow is directly related to the 4th power of the vessel’s radius. Thus, the ∼5% average reduction in diameter reported here would evoke a ∼22% reduction in local blood flow.
Fig. 1.
Caffeine-ethanol mixture fails to constrict cerebral arterioles in vivo. Averaged pial arteriolar diameter (as percentage of diameter before drug application) in response to carotid artery infusion of 50 mM ethanol, 10 µM caffeine, or their combination. Each data point represents an average of no fewer than three animals. *Different from administration of 50 mM ethanol (P < 0.05).
Current in vivo findings are consistent with recent data from our group (Bukiya et al., 2014) documenting rat cerebral artery constriction after in vivo administration of ethanol with concentrations reached in human bloodstream during moderate-to-heavy alcohol intoxication (Diamond, 1992). Current results also document that rat brain arterioles constricted in response to carotid artery infusion of 10 µM caffeine (Fig. 1A. middle panels), which was fully reversible upon washout of the alkaloid (Fig. 1B). Importantly, the caffeine concentration used is found in human systemic circulation after consumption of one cup of coffee (Fausch et al., 2012).
Our results are consistent with recent data documenting reduced human cerebral blood flow after acute administration of caffeine (Vidyasagar et al., 2013; see Discussion). Remarkably, when the two agents that caused vasoconstriction applied alone (50 mM ethanol; 10 µM caffeine) were mixed immediately before administration and coinjected into the carotid artery, cerebral arteriole diameter did not differ from preinfusion levels (Fig. 1A, bottom panels). Thus, caffeine and ethanol actions on cerebral artery diameter functionally antagonized each other, raising the speculation that these two vasoactive psychostimulants may share receptors and/or downstream signaling pathways that underlie their effects on cerebral vessels.
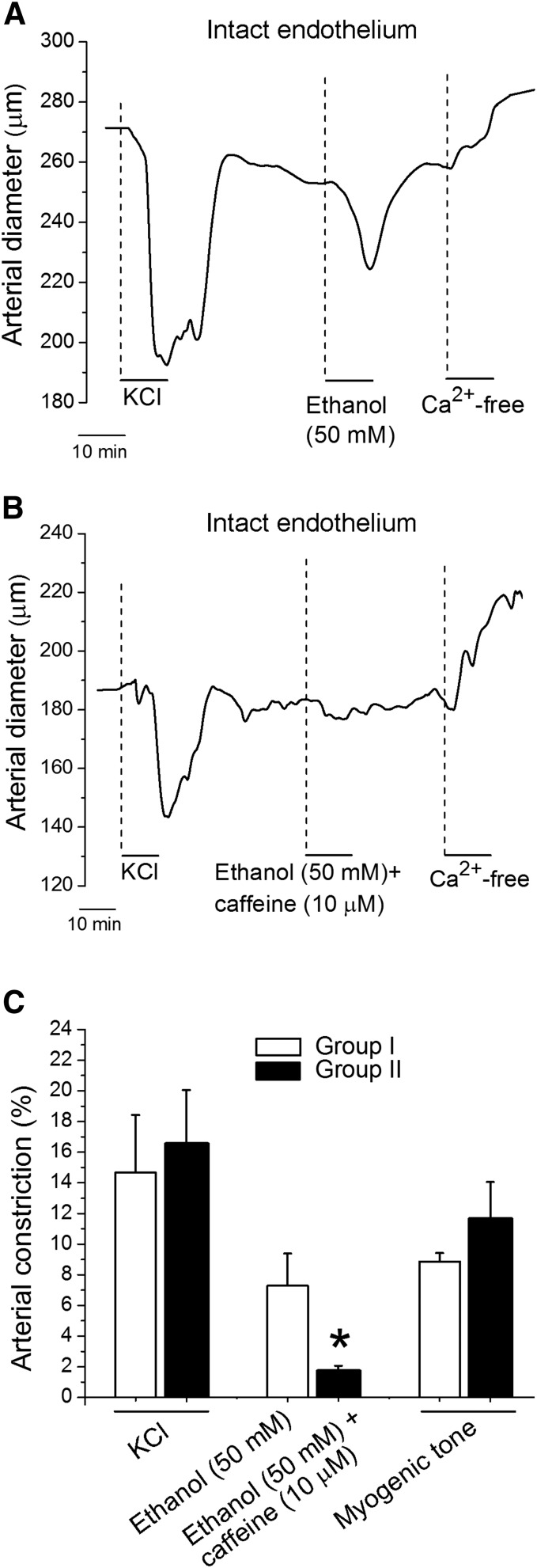
Both caffeine and alcohol are metabolized in vivo to active compounds that modify vascular function (Echeverri et al., 2010; Cahill and Redmond, 2012). In addition, it is well known that vasoactive properties of caffeine may involve modification of autonomic input, stress hormone levels, and other neural and endocrine systems (Echeverri et al., 2010). Thus, to determine whether active metabolites of ethanol, caffeine, and/or neuroendocrine regulatory factors are necessary in caffeine modulation of alcohol action on cerebral arteries, we next isolated cerebral artery segments and exposed them in vitro to each of these agents and their combination for 13 minutes. Then, drug action in intact versus de-endothelialized arteries was explored to narrow the primary target(s) involved in the caffeine-ethanol interaction. Thus, rat middle cerebral arteries were dissected out and pressurized in vitro at 60 mm Hg to develop and maintain myogenic tone (Liu et al., 2004; Bukiya et al., 2014).
As found with in vivo drug administration, 50 mM ethanol evoked a significant (P < 0.05) and fully reversible constriction of isolated, endothelium-intact, cerebral artery segments (∼8% decrease in diameter) (Fig. 2, A and C). This result confirms previous in vitro data obtained by our group by using cerebral arteries of mice and rats (Liu et al., 2004; Bukiya et al., 2009). Importantly, the combination of 50 mM ethanol and 10 µM caffeine failed to constrict cerebral arteries (Fig. 2, B and C), a result that paralleled in vivo results reported earlier (Fig. 1). Therefore, the antagonism exerted by caffeine on ethanol constriction of cerebral arteries did not seem to require systemic metabolism (largely by the liver) of either agent. Circulating mediators or intact autonomic nerve networks were not necessary either. Rather, subcellular targets and mechanisms mediating caffeine-ethanol antagonism reside within the cerebral vessels themselves.
Fig. 2.
Caffeine-ethanol mixture fails to constrict cerebral arteries in vitro. (A) Diameter trace from a pressurized middle cerebral artery from rat. After development of myogenic tone, the artery was probed with 60 mM KCl to test maximal contraction. After KCl washout, the artery chamber was perfused with either (A) 50 mM ethanol or (B) 50 mM ethanol+10 µM caffeine mixture. (C) Average change in artery diameter evoked by KCl, 50 mM ethanol, or 50 mM ethanol + 10 µM caffeine mixture. Last set of bars also depicts averaged percentage of myogenic tone. Hollow bars (group 1) represent data from arteries that were exposed to ethanol only (n = 4); black bars (group 2) reflect data from arteries that were challenged with the ethanol + caffeine mixture (n = 6). *Different from constriction by 50 mM ethanol (P < 0.05).
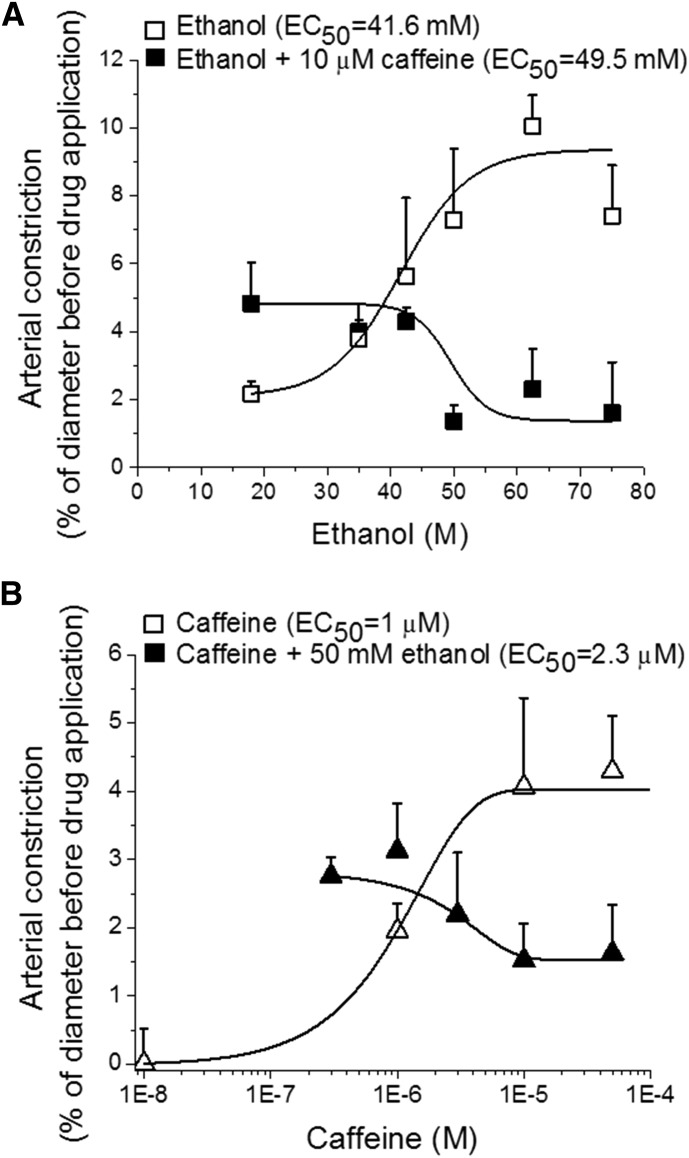
Caffeine-mediated antagonism of ethanol-induced cerebral artery constriction, however, was not observed at every ethanol concentration tested. Indeed, inspection of fitting plots included in Fig. 3A (concentration–response curve to ethanol in absence and presence of 10 µM caffeine) and Fig. 3B (concentration–response curve to caffeine in absence and presence of 50 mM ethanol) shows: 1) minor synergism between low concentrations of ethanol and caffeine to evoke cerebral artery constriction, and 2) antagonism by 10 µM caffeine occurs only at ethanol concentrations ≥45 mM (Fig. 3A), that is, ethanol concentrations between EC50 (41.6 mM) and Emax (∼100 mM) for ethanol to constrict cerebral arteries when applied alone. Conversely, antagonism against caffeine-induced cerebral constriction by 50 mM ethanol occurs only at ≥2 µM caffeine (Fig. 3B), that is, caffeine concentrations between EC50 (1 µM) and Emax (10 µM) for caffeine to constrict cerebral arteries when applied alone. Thus, for both caffeine and ethanol to antagonize each other, each drug should reach concentrations near its own Emax. Collectively, negligible constrictive synergism at low agonist concentrations with significant antagonism prevailing at higher concentrations may argue for some common, low-affinity target(s) mediating caffeine-ethanol functional interaction.
Fig. 3.
Concentration–response curves for in vitro cerebral artery constriction caused by ethanol in the presence of 10 µM caffeine (A) and by caffeine in the presence of 50 mM ethanol (B). Each data point represents the average from 3 to 7 records, each obtained from a separate artery/rat.
Caffeine-Ethanol Interaction on Cerebral Artery Diameter Required Endothelial NO Synthase.
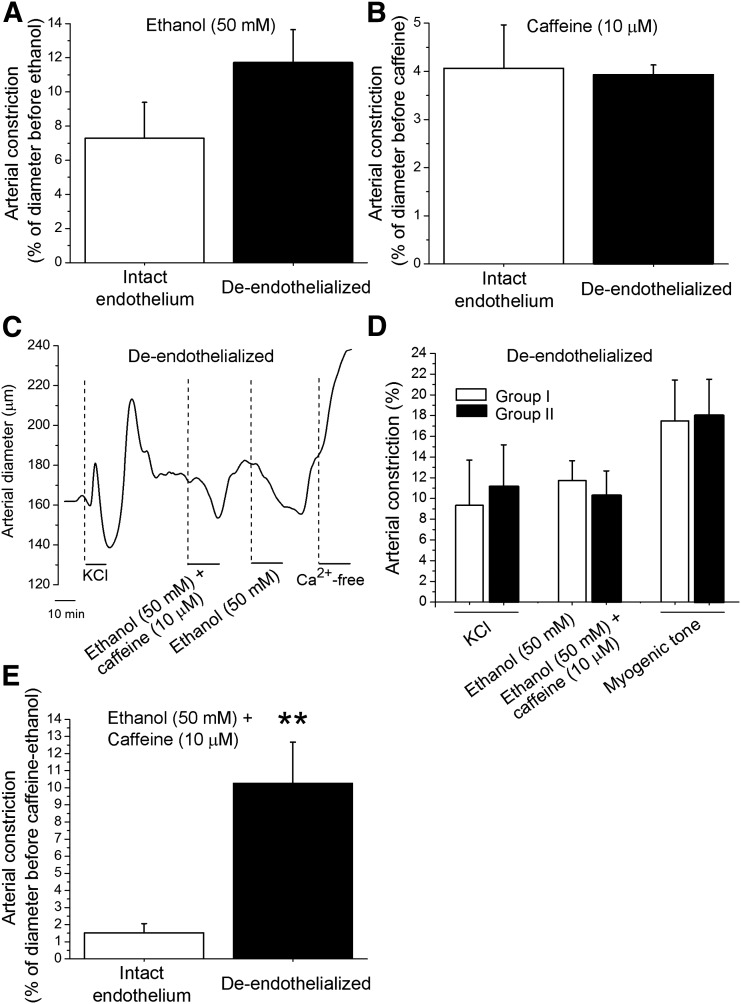
After determining that the caffeine-ethanol functional antagonism on cerebral vessels occurred at the cerebral artery itself, and considering that vascular actions of caffeine that are independent of neuroendocrine mediation may be either endothelium-dependent or directly targeted at the vascular smooth muscle (Echeverri et al., 2010), we next addressed whether caffeine antagonism of ethanol-induced cerebral artery constriction required the presence of a functional endothelium. Thus, some segments from a given artery were de-endothelized by mechanical means, the presence/absence of a functional endothelium being pharmacologically verified (Liu et al., 2004; Bukiya et al., 2009, 2014). Both ethanol (50 mM) and caffeine (10 µM) were able to constrict de-endothelialized cerebral arteries when these agents were applied separately. Moreover, ethanol (Fig. 4A) and caffeine (Fig. 4B) actions were identical in intact versus de-endothelialized cerebral arteries.
Fig. 4.
Removal of endothelium suppresses caffeine antagonism of ethanol-induced cerebral artery constriction. (A) Averaged data showing that ethanol-induced constriction is similar in arteries with intact versus denuded endothelium. (B) Averaged data showing that caffeine-induced constriction is similar in arteries with intact versus denuded endothelium. (C) Diameter trace from de-endothelialized, pressurized, middle cerebral artery of rat. After development of myogenic tone, the artery was probed with 60 mM KCl. After KCl washout, the chamber was perfused with 50 mM ethanol + 10 µM caffeine mixture or 50 mM ethanol. (D) Average change in diameter of de-endothelialized arteries when probed with KCl, 50 mM ethanol, or 50 mM ethanol + 10 µM caffeine mixture. The last set of bars also depicts averaged percentage of the myogenic tone. Hollow bars (group 1) represent data from arteries that were probed with ethanol only (n = 3); black bars (group 2) reflect data from arteries that were probed with ethanol + caffeine mixture (n = 4). (E) Average data showing that constriction of de-endothelialized arteries by the caffeine-ethanol mixture is significantly larger than that evoked in arteries with intact endothelium. **Different from constriction by caffeine-ethanol mixture in arteries with intact endothelium (P < 0.01).
These ethanol results extend previous work from our group (Liu et al., 2004; Bukiya et al., 2009) documenting that the primary targets mediating ethanol-induced constriction of cerebral arteries reside in vascular smooth muscle. Present data unveil that caffeine is also able to constrict cerebral arteries independently of neurohumoral factors or systemically formed, active metabolites that have cardio/vasoactive properties (e.g., paraxanthine, theobromine, theophylline; Bellemann and Scholz, 1974; Ried et al., 2012; Guessous et al., 2015). Thus, this result from isolated, resistance-size rat middle cerebral arteries matches those from rabbit aortic, renal, and iliac arterial strips (Yoshida et al., 1989) while differing from those in rabbit arteries and human internal mammary arteries in vitro where caffeine has been reported to evoke vasodilatation (Echeverri et al., 2008, 2010).
In sharp contrast to caffeine-induced constriction of middle cerebral arteries, caffeine-induced antagonism of ethanol-induced constriction was lost in de-endothelialized arteries. Indeed, ethanol-induced constriction of endothelium-denuded vessels in the presence of caffeine was identical to that evoked by ethanol alone (Fig. 4, C and D) and significantly larger than the constriction evoked by the caffeine-ethanol mixture in intact vessels (Fig. 4E). Collectively, these data indicate that cerebral artery endothelium is necessary for caffeine to antagonize ethanol-induced vasoconstriction. This result clearly differs from the constriction evoked by each agent alone, which does not require the presence of vascular endothelium (Fig. 4, A and B).
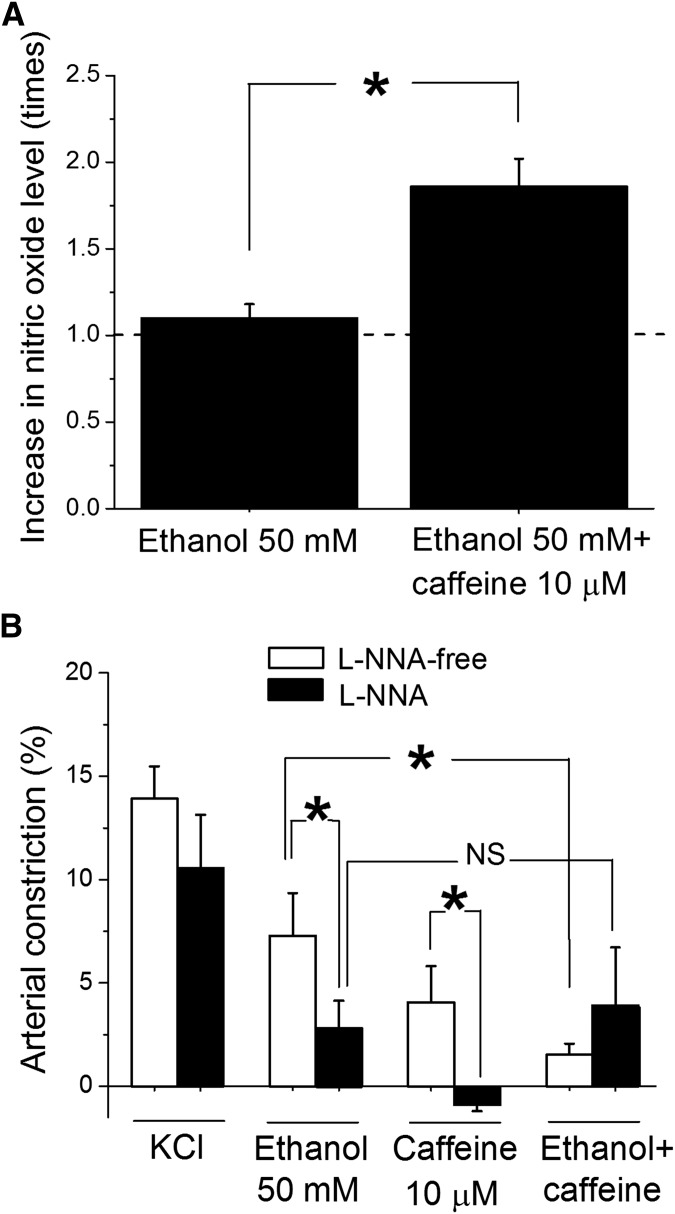
Considering that endothelium represents a major source of NO•, which is a critical regulator of cerebral artery tone (Andresen et al., 2006; Pretnar-Oblak, 2014), we measured whether NO• levels in the artery were increased by the caffeine-ethanol mixture when compared with ethanol exposure alone. Indeed, a 5- to 10-minute in vitro incubation of cerebral arteries with caffeine-ethanol resulted in a significant 2-fold increase in NO• levels when compared with those in incubation of arteries in ethanol-containing solution without caffeine (Fig. 5A). Because NO-synthase (NOS) activity is the source of NO•, we also tested the effect of caffeine-ethanol mixture on the diameter of arteries with intact endothelium in the presence of 100 µM N-omega-nitro-l-arginine (L-NNA), an effective in vitro treatment to block NOS activity (Sobey et al., 2004). L-NNA significantly (P < 0.05) reduced cerebral artery constriction evoked by either 50 mM ethanol of 10 µM caffeine alone, suggesting that NO• participates in each individual agent’s action on cerebral vessels. More important, when NOS activity was blocked, the antagonism of caffeine on ethanol-induced cerebral artery constriction was lost (Fig. 5B).
Fig. 5.
NOS activity is critical for caffeine to antagonize ethanol-induced cerebral artery constriction. (A) Averaged data showing increased NO• tissue levels after artery in vitro incubation with caffeine-ethanol mixture (n = 7) when compared with incubation with ethanol alone (n = 7) or untreated samples (dashed line) (n = 7). *Different from treatment by 50 mM ethanol (P < 0.05). (B) Averaged data showing that the antagonism exerted by caffeine presence on ethanol action is lost in the presence of 100 µM L-NNA. Hollow bars represent data from arteries perfused with L-NNA-free solution (n = 6); black bars show data from arteries perfused with 100 µM L-NNA (n = 4). *Different from constriction by 50 mM ethanol on L-NNA solution (P < 0.05); NS, not statistically significant.
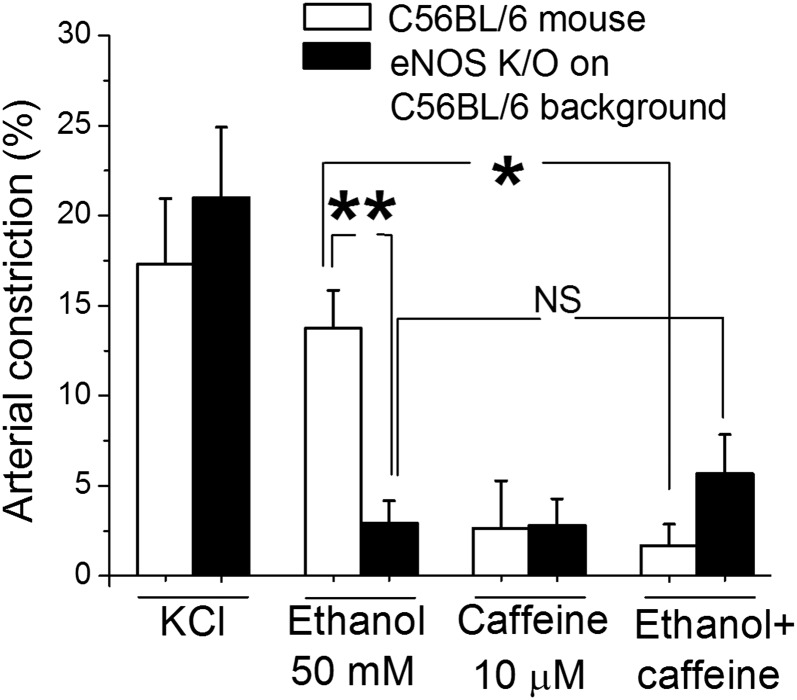
Collectively, the results from Figs. 4 and 5 strongly suggest that the eNOS–NO• pathway mediates or at least contributes to caffeine antagonism of ethanol-induced cerebrovascular constriction. To test this hypothesis, we next addressed the role of eNOS in caffeine-ethanol interaction on cerebral artery diameter by evaluating drug actions on cerebral arteries from eNOS knockout (KO−/−) mice. Data showed that caffeine action on eNOS KO−/− mouse was similar to that on wild-type mice whereas ethanol-induced constriction was milder than that in wild-type mice. More importantly, cerebral artery constriction by the mixture caffeine-ethanol in eNOS KO−/− mice was undistinguishable from the constriction evoked by 50 mM ethanol alone (Fig. 6). This result unveils a critical role for eNOS in caffeine antagonism of ethanol-induced constriction of cerebral arteries.
Fig. 6.
Genetic ablation of eNOS suppresses caffeine antagonism of ethanol-induced cerebral artery constriction. Averaged data showing that the antagonism exerted by caffeine presence on ethanol action is lost in eNOS KO−/− mice while preserved in wild-type (C57BL/6) mice. Hollow bars present data from arteries from C57BL/6 mice (n = 4); black bars show data from arteries from eNOS KO−/− mice on a C57BL/6 background (n = 4). *Different from constriction by 50 mM ethanol in arteries from control (P<0.05); **(P<0.01); NS, not significant.
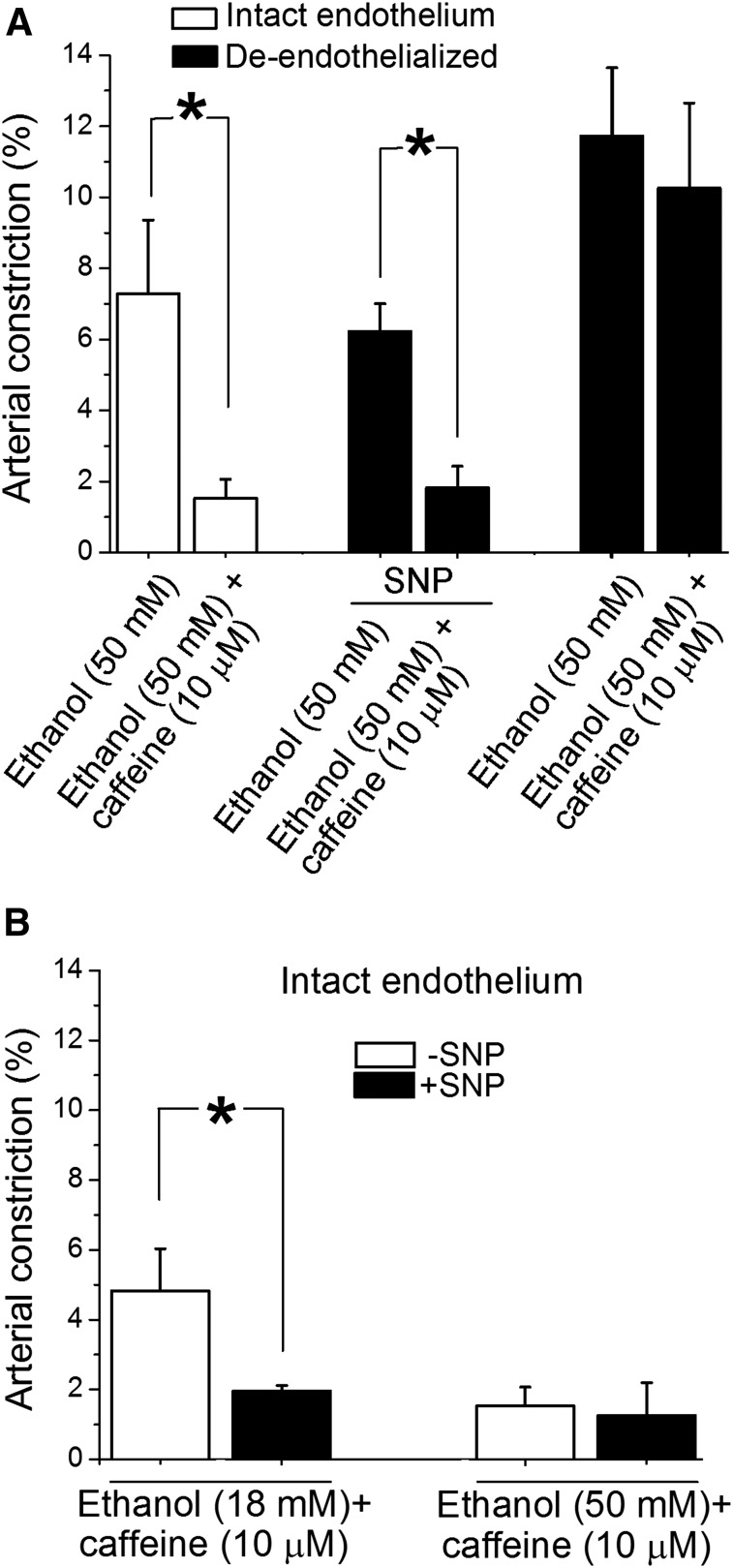
Caffeine Antagonism on Ethanol-Induced Cerebral Artery Constriction Was Mediated by NO• Itself.
After establishing the critical role of eNOS in caffeine antagonism of ethanol-induced constriction of cerebral arteries, we set out to determine whether eNOS itself or its product NO• mediated caffeine-ethanol interaction on cerebral arteries. Figure 7A data confirmed that endothelial removal resulted in loss of caffeine antagonism of ethanol action on cerebral artery diameter (compare the difference between the two white columns on the left with the difference between the two black columns on the right). More importantly, Fig. 7A data demonstrated that caffeine-induced protection against ethanol-mediated constriction was fully restored when de-endothelialized arteries were treated with an NO• donor: in the presence of 10 μM SNP, constriction by the caffeine-ethanol mixture was significantly smaller (P < 0.05) than that evoked by ethanol alone (compare the difference between the two black columns in the middle with the differences between the other two pairs of columns). Thus, the presence of eNOs per se was not critical for the effect of caffeinated ethanol on cerebral artery diameter. Rather, caffeine-induced protection against ethanol-induced cerebral artery constriction seemed to depend on NO• availability to vascular smooth muscle (see Discussion).
Fig. 7.
NO• availability is required for caffeine to antagonize ethanol-induced cerebral artery constriction. (A) Averaged data demonstrating that in de-endothelialized arteries, caffeine antagonism of ethanol action is restored in the presence of the NO• donor, SNP (10 µM). *Different from constriction by 50 mM ethanol in corresponding experimental group (P < 0.05). (B) Averaged data showing that in arteries with intact endothelium, SNP treatment (10 µM) evokes caffeine antagonism of vasoconstriction by 18 mM but fails to further increase the antagonistic action of caffeine in presence of 50 mM ethanol. *Different from constriction by caffeine-ethanol mixture in absence of SNP (P < 0.05).
Interestingly, NO• donor treatment (10 µM SNP) of arteries with intact endothelium resulted in caffeine-induced antagonism against constriction by 18 mM ethanol (Fig. 7B), this antagonism not being evident in the absence of SNP (Fig. 3A). NO• donor treatment, however, failed to further enhance caffeine antagonism against constriction by 50 mM ethanol (Fig. 7B). These results seemed to indicate that the NO•-mediated, protective mechanism of caffeine antagonism against constriction by ethanol reached a ceiling effect in the presence of high ethanol concentrations.
Discussion
Our current study documents for the first time that concentrations of caffeine found in human blood circulation after ingestion of one to two cups of coffee (Fausch et al., 2012) antagonize the cerebral artery constriction evoked by moderate-to-high ethanol concentrations (≥45 mM; Fig. 3A) obtained in human circulation during moderate-heavy alcohol drinking (Diamond, 1992). This phenomenon has been evaluated both in vivo and in vitro with rodent (mouse and rat) arterioles and resistance-size, middle cerebral arteries, which control local blood flow (Faraci et al., 1987). Similarities between our in vivo and in vitro data are consistent with the finding that caffeine does not significantly alter the metabolism of alcohol in the body (Ferreira et al., 2006).
Middle cerebral arteries are widely recognized as valid models to understand pathophysiology and pharmacology of human cerebral arteries, including alcohol vasoactive properties (reviewed by Zhang et al., 1993; see also Bake et al., 2012). The similarities between our in vivo and in vitro findings indicate that circulating, paracrine, and neural factors, all known to be altered by circulating alcohol or caffeine (Jackson, 1991; Varani et al., 1999; Fleming et al., 2001; Corti et al., 2002; Lane et al., 2002), do not play a primary role in mediating caffeine modulation of alcohol action on cerebral arteries. Furthermore, our results obtained after administering drugs via carotid artery (from which the middle cerebral artery originates) and the in vitro data strongly suggest that the phenomenon under study does not rely on modifications in the systemic (mainly hepatic; Roe, 1979; Tassaneeyakul et al., 1994) metabolism of either alcohol or caffeine.
As we found with the effect of either ethanol or caffeine on middle cerebral artery diameter, caffeine-ethanol interaction was sustained in isolated, cerebral artery segments. However, our data reveal that the tissue source and pathways underlying the caffeine-alcohol interaction are distinct from the primary source underlying individual drug action on cerebral artery diameter, as follows.
First, our current results confirm previous studies by our group and others (Zhang et al., 1993; Liu et al., 2004; Bukiya et al., 2014) reporting that intoxicating levels of ethanol (10–100 mM) constrict rat middle cerebral arteries, an effect that does not depend on an intact endothelium (Figs. 4A). Indeed, alcohol action on these arteries is primarily mediated by two pathways that converge on β1 subunit-containing potassium channels of the BKCa type located in the arterial myocyte plasmalemma: 1) direct ethanol inhibition of BKCa steady-state activity (Liu et al., 2004; Bukiya et al., 2009); and 2) indirect modulation of this channel via suppression of ryanodine receptors (RyR)-generated calcium sparks (Liu et al., 2004), which likely results from ethanol inhibition of type 2 ryanodine receptors (Ye et al., 2014) located in the sarcoplasmic reticulum of the cerebral artery myocyte.
Second, our current results demonstrate that concentrations of caffeine commonly reached in human circulation constrict middle cerebral arteries in a largely endothelium-independent manner, as ethanol does. The literature on caffeine effect on arterial diameter is highly heterogeneous, with both dilatation and constriction being reported. These variant outcomes have been mainly attributed to differences across vascular nets and species, adenosine levels, length of caffeine administration, and differential involvement of adenosine receptors and/or endothelium- versus smooth muscle-driven mechanisms (reviewed in Frishman et al., 2003; Addicott et al., 2009; Echeverri et al., 2010; Higgins and Babu, 2013). Our data from isolated arterial segments exposed in vitro for a few minutes to caffeine argue against involvement of long-term changes in adenosine levels or receptors in caffeine-induced constriction of cerebral arteries. Rather, mechanisms of action appear to involve downstream targets.
The isolated nature of the arterial segment in our experiments also argues against the need of intact coupling in the neurovascular triad (vessel-neuron-glia; Pelligrino et al., 2010) for caffeine action on middle cerebral arteries. Remarkably, our in vivo and in vitro data from rat middle cerebral, resistance-size arteries are consistent with several in vivo studies in humans finding decreased cerebral blood flow after acute caffeine consumption (Mathew and Wilson, 1985; Cameron et al., 1990; Lunt et al., 2004; Addicott et al., 2009; Vidyasagar et al., 2013).
The most novel aspect of our study is that caffeine antagonizes ethanol action via endothelial NO•: the caffeine-ethanol interaction is distinct from each agent’s action alone, which is primarily endothelium-independent. Our finding may have practical consequences; while alcohol-induced cerebral artery constriction would remain largely uncompromised, caffeine protection against alcohol would be drastically reduced under conditions that impair endothelial function and NO• availability, such as aging (Scioli et al., 2014), arterial hypertension (Hall et al., 2012), and obesity (Dorrance et al., 2014). Conversely, caffeine action against alcohol-induced vasoconstriction could be potentiated in the presence of NO• donors (as shown here from in vitro data showing SNP impacting the caffeine-18 mM ethanol interaction) that are widely used in clinical practice to treat various pathophysiologic conditions (Miller and Megson, 2007).
Interestingly, the caffeine-ethanol mixture (often called “caffeinol”) has been found to be neuroprotective in a rat cerebral ischemia/stroke model: administration of caffeinol (0.65 g/kg ethanol and 10 mg/kg caffeine) up to 120 minutes after induction of ischemic stroke by a 3-hour-long middle cerebral/common carotid artery occlusion significantly reduced infarct volume (Strong et al., 2000). Caffeinol has also been reported to evoke a decrease in brain infarct volume and facilitate transient behavioral improvement, in spite of the fact that ethanol alone aggravated ischemic brain damage (Aronowski et al., 2003). Conceivably, the neuroprotection by caffeinol reported from neuropathologic and behavioral findings may have (at least partially) an arterial basis, a possibility directly inferred from our current results. In light of recent attempts to use caffeinol as a neuroprotective intervention against cerebral ischemia (Sacco, 2007), systematic future studies to precisely identify the possible multiple molecular targets and their relative contribution to caffeine-ethanol interaction on cerebral arteries acquire paramount importance.
Our data underscore the importance of intact endothelium, NO• levels, and NO• availability in caffeine-induced antagonism of alcohol action. Caffeine has been reported to increase availability of endothelial NO• in a series of events that include activation of RyR, increased binding of calcium to calmodulin, and complexing calcium-calmodulin to eNOS, leading to increased enzymatic activity of the latter (reviewed in Echeverri et al., 2010).
Considering that after endothelium removal the vast majority of remaining cellular content is provided by vascular smooth muscle cells (Lee, 1995), it is fair to assume advance that the final targets of endothelial NO• that mediate caffeine antagonism against ethanol reside within the vascular myocyte. Then, caffeine-induced relaxation of vascular smooth muscle via endothelium/NO•-independent processes such as caffeine direct inhibition of phosphodiesterases (Ahn et al., 1988), MLC kinase (Ozaki et al., 1990), inositol-1,4,5-trisphosphate receptors (Missiaen et al., 1994), or voltage-gated calcium channels (Hughes et al., 1990) are unlikely primary mechanisms. However, it should be underscored that caffeine-induced vasodilatation via NO•-dependent pathways is not a simple scenario but involves multiple targets and mechanisms.
It has been fairly well established (Brian et al., 1996; Garland et al., 2011) that vasodilatation in response to endothelium-generated NO• may require direct targeting of several smooth muscle receptors by NO• itself (among these, RyR and BK channels, both regulated by ethanol; Dopico et al., 2014; Ye et al., 2014). Moreover, endothelium-generated NO• caused by caffeine can positively feed-back via calcium-induced calcium release on the endothelial cell, leading to further NO• availability (Echeverri et al., 2010). The relative contribution of each and all these NO• effectors in caffeine protection against alcohol action on cerebral arteries requires further systematic experimentation.
In conclusion, our study clearly demonstrates for the first time that caffeine antagonizes ethanol-induced cerebral artery constriction and identifies endothelial NO• as the critical mediator of this caffeine action, an identification that may have direct health-related and therapeutic implications.
Acknowledgments
The authors thank Maria T. Asuncion-Chin and Shivantika Bisen, Department of Pharmacology, College of Medicine, UTHSC, for technical assistance, and David L. Armbruster for careful editing of the manuscript.
Abbreviations
- Emax
concentration of a ligand needed to reach its maximal effect
- eNOS
endothelial nitric-oxide synthase
- KO
knockout
- L-NNA
N-omega-nitro-L-arginine
- NO•
nitric oxide
- NOS
nitric oxide synthase
- PSS
physiologic saline solution
- RyR
ryanodine receptors
- SNP
sodium nitroprusside
Authorship Contributions
Participated in research design: Bukiya, Chang, Kuntamallappanavar, Dopico.
Conducted experiments: Chang, Fedinec, Kuntamallappanavar, Bukiya.
Performed data analysis: Bukiya, Chang, Kuntamallappanavar.
Wrote or contributed to the writing of the manuscript: Bukiya, Chang, Leffler, Dopico.
Footnotes
This work was supported by the National Institutes of Health National Institute on Alcohol Abuse and Alcoholism [Grant R37-AA11560 (to A.M.D.)] and the National Heart, Lung, and Blood Institute [Grants R01-HL104631 (to A.M.D.), R01-HL42851, and R01-HL34059 (to C.W.L.)].
References
- Addicott MA, Yang LL, Peiffer AM, Burnett LR, Burdette JH, Chen MY, Hayasaka S, Kraft RA, Maldjian JA, Laurienti PJ. (2009) The effect of daily caffeine use on cerebral blood flow: how much caffeine can we tolerate? Hum Brain Mapp 30:3102–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adebiyi A, Zhao G, Cheranov SY, Ahmed A, Jaggar JH. (2007) Caveolin-1 abolishment attenuates the myogenic response in murine cerebral arteries. Am J Physiol Heart Circ Physiol 292:H1584–H1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn HY, Karaki H, Urakawa N. (1988) Inhibitory effects of caffeine on contractions and calcium movement in vascular and intestinal smooth muscle. Br J Pharmacol 93:267–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altura BM, Altura BT. (1984) Alcohol, the cerebral circulation and strokes. Alcohol 1:325–331. [DOI] [PubMed] [Google Scholar]
- Andresen J, Shafi NI, Bryan RM., Jr (2006) Endothelial influences on cerebrovascular tone. J Appl Physiol (1985) 100:318–327. [DOI] [PubMed] [Google Scholar]
- Aronowski J, Strong R, Shirzadi A, Grotta JC. (2003) Ethanol plus caffeine (caffeinol) for treatment of ischemic stroke: preclinical experience. Stroke 34:1246–1251. [DOI] [PubMed] [Google Scholar]
- Arria AM, Caldeira KM, Kasperski SJ, Vincent KB, Griffiths RR, O’Grady KE. (2011) Energy drink consumption and increased risk for alcohol dependence. Alcohol Clin Exp Res 35:365–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bake S, Tingling JD, Miranda RC. (2012) Ethanol exposure during pregnancy persistently attenuates cranially directed blood flow in the developing fetus: evidence from ultrasound imaging in a murine second trimester equivalent model. Alcohol Clin Exp Res 36:748–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balster RL. (1998) Drug abuse, in Human Pharmacology: Molecular to Clinical, 3rd ed. (Brody TM, Larner J, Minneman KP, eds) p 447–459, Mosby-Year Book, St. Louis, MO. [Google Scholar]
- Bellemann P, Scholz H. (1974) Relationship between theophylline uptake and inotropic effect in the guinea-pig heart. Br J Pharmacol 52:265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brian JE, Jr, Faraci FM, Heistad DD. (1996) Recent insights into the regulation of cerebral circulation. Clin Exp Pharmacol Physiol 23:449–457. [DOI] [PubMed] [Google Scholar]
- Bukiya A, Dopico AM, Leffler CW, Fedinec A. (2014) Dietary cholesterol protects against alcohol-induced cerebral artery constriction. Alcohol Clin Exp Res 38:1216–1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukiya AN, Liu J, Dopico AM. (2009) The BK channel accessory beta1 subunit determines alcohol-induced cerebrovascular constriction. FEBS Lett 583:2779–2784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukiya AN, Liu J, Toro L, Dopico AM. (2007) Beta1 (KCNMB1) subunits mediate lithocholate activation of large-conductance Ca2+-activated K+ channels and dilation in small, resistance-size arteries. Mol Pharmacol 72:359–369. [DOI] [PubMed] [Google Scholar]
- Bukiya AN, McMillan JE, Fedinec AL, Patil SA, Miller DD, Leffler CW, Parrill AL, Dopico AM. (2013) Cerebrovascular dilation via selective targeting of the cholane steroid-recognition site in the BK channel β1-subunit by a novel nonsteroidal agent. Mol Pharmacol 83:1030–1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill PA, Redmond EM. (2012) Alcohol and cardiovascular disease—modulation of vascular cell function. Nutrients 4:297–318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron OG, Modell JG, Hariharan M. (1990) Caffeine and human cerebral blood flow: a positron emission tomography study. Life Sci 47:1141–1146. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) (2014) Fact sheets—binge drinking. CDC, Alcohol and Public Health, http://www.cdc.gov/alcohol/fact-sheets/binge-drinking.htm.
- Corti R, Binggeli C, Sudano I, Spieker L, Hänseler E, Ruschitzka F, Chaplin WF, Lüscher TF, Noll G. (2002) Coffee acutely increases sympathetic nerve activity and blood pressure independently of caffeine content: role of habitual versus nonhabitual drinking. Circulation 106:2935–2940. [DOI] [PubMed] [Google Scholar]
- Diamond I. (1992) Alcoholism and alcohol abuse, in Cecil Textbook of Medicine (Wyngaarden JB, Smith LH, Plum F, eds) pp 44–47, Saunders, Philadelphia. [Google Scholar]
- Dopico AM, Bukiya AN, Martin GE. (2014) Ethanol modulation of mammalian BK channels in excitable tissues: molecular targets and their possible contribution to alcohol-induced altered behavior. Front Physiol 5:466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorrance AM, Matin N, Pires PW. (2014) The effects of obesity on the cerebral vasculature. Curr Vasc Pharmacol 12:462–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Echeverri D, Buitrago L, Delgadillo A, Beltrán M, Montes F. (2008) In-vitro vasodilator effect of caffeine in atherosclerotic aorta rabbits. Clin Investig Arterioscler 20:41–47. [Google Scholar]
- Echeverri D, Montes FR, Cabrera M, Galán A, Prieto A. (2010) Caffeine’s vascular mechanisms of action. Int J Vasc Med 2010:834060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faraci FM, Mayhan WG, Werber AH, Heistad DD. (1987) Cerebral circulation: effects of sympathetic nerves and protective mechanisms during hypertension. Circ Res 61:II102–II106. [PubMed] [Google Scholar]
- Fausch K, Uehlinger D, Jakob S, Pasch A. (2012) Haemodialysis in massive caffeine intoxication. Clin Kidney J 5:150–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira SE, de Mello MT, Pompéia S, de Souza-Formigoni ML. (2006) Effects of energy drink ingestion on alcohol intoxication. Alcohol Clin Exp Res 30:598–605. [DOI] [PubMed] [Google Scholar]
- Fleming M, Mihic SJ, Harris RA. (2001) Ethanol, in The Pharmacological Basis of Therapeutics, 10th ed. (Hardman JG, Limbird LE, Goodman Gilman A eds) pp 429–445, McGraw-Hill Medical, New York. [Google Scholar]
- Frary CD, Johnson RK, Wang MQ. (2005) Food sources and intakes of caffeine in the diets of persons in the United States. J Am Diet Assoc 105:110–113. [DOI] [PubMed] [Google Scholar]
- Frishman WH, Del Vecchio A, Sanal S, Ismail A. (2003) Cardiovascular manifestations of substance abuse: part 2: alcohol, amphetamines, heroin, cannabis, and caffeine. Heart Dis 5:253–271. [DOI] [PubMed] [Google Scholar]
- Garland CJ, Hiley CR, Dora KA. (2011) EDHF: spreading the influence of the endothelium. Br J Pharmacol 164:839–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guessous I, Pruijm M, Ponte B, Ackermann D, Ehret G, Ansermot N, Vuistiner P, Staessen J, Gu Y, Paccaud F, et al. (2015) Associations of ambulatory blood pressure with urinary caffeine and caffeine metabolite excretions. Hypertension 65:691–696. [DOI] [PubMed] [Google Scholar]
- Hall JE, Granger JP, do Carmo JM, da Silva AA, Dubinion J, George E, Hamza S, Speed J, Hall ME. (2012) Hypertension: physiology and pathophysiology. Compr Physiol 2:2393–2442. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Babu KM. (2013) Caffeine reduces myocardial blood flow during exercise. Am J Med 126:730.e1–730.e8. [DOI] [PubMed] [Google Scholar]
- Hughes AD, Hering S, Bolton TB. (1990) The action of caffeine on inward barium current through voltage-dependent calcium channels in single rabbit ear artery cells. Pflugers Arch 416:462–466. [DOI] [PubMed] [Google Scholar]
- Jackson EK. (1991) Adenosine: a physiological brake on renin release. Annu Rev Pharmacol Toxicol 31:1–35. [DOI] [PubMed] [Google Scholar]
- Juliano LM, Evatt DP, Richards BD, Griffiths RR. (2012) Characterization of individuals seeking treatment for caffeine dependence. Psychol Addict Behav 26:948–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane JD, Pieper CF, Phillips-Bute BG, Bryant JE, Kuhn CM. (2002) Caffeine affects cardiovascular and neuroendocrine activation at work and home. Psychosom Med 64:595–603. [DOI] [PubMed] [Google Scholar]
- Lee RM. (1995) Morphology of cerebral arteries. Pharmacol Ther 66:149–173. [DOI] [PubMed] [Google Scholar]
- Liu P, Xi Q, Ahmed A, Jaggar JH, Dopico AM. (2004) Essential role for smooth muscle BK channels in alcohol-induced cerebrovascular constriction. Proc Natl Acad Sci USA 101:18217–18222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lunt MJ, Ragab S, Birch AA, Schley D, Jenkinson DF. (2004) Comparison of caffeine-induced changes in cerebral blood flow and middle cerebral artery blood velocity shows that caffeine reduces middle cerebral artery diameter. Physiol Meas 25:467–474. [DOI] [PubMed] [Google Scholar]
- Malinauskas BM, Aeby VG, Overton RF, Carpenter-Aeby T, Barber-Heidal K. (2007) A survey of energy drink consumption patterns among college students. Nutr J 6:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marczinski CA. (2015) Can energy drinks increase the desire for more alcohol? Adv Nutr 6:96–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathew RJ, Wilson WH. (1985) Caffeine induced changes in cerebral circulation. Stroke 16:814–817. [DOI] [PubMed] [Google Scholar]
- McMurtrie B. (2014) Why colleges haven’t stopped binge drinking. Chron High Educ Dec 2, http://chronicle.com/interactives/alcohol_binge. [Google Scholar]
- Meredith SE, Juliano LM, Hughes JR, Griffiths RR. (2013) Caffeine use disorder: a comprehensive review and research agenda. J Caffeine Res 3:114–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MR, Megson IL. (2007) Recent developments in nitric oxide donor drugs. Br J Pharmacol 151:305–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mintel International Group (2007) Energy Drinks, Mintel International Group Ltd, Chicago. [Google Scholar]
- Missiaen L, Parys JB, De Smedt H, Himpens B, Casteels R. (1994) Inhibition of inositol trisphosphate-induced calcium release by caffeine is prevented by ATP. Biochem J 300:81–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki H, Kasai H, Hori M, Sato K, Ishihara H, Karaki H. (1990) Direct inhibition of chicken gizzard smooth muscle contractile apparatus by caffeine. Naunyn Schmiedebergs Arch Pharmacol 341:262–267. [DOI] [PubMed] [Google Scholar]
- Patra J, Taylor B, Irving H, Roerecke M, Baliunas D, Mohapatra S, Rehm J. (2010) Alcohol consumption and the risk of morbidity and mortality for different stroke types—a systematic review and meta-analysis. BMC Public Health 10:258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelligrino DA, Xu HL, Vetri F. (2010) Caffeine and the control of cerebral hemodynamics. J Alzheimers Dis 20 (Suppl 1):S51–S62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pretnar-Oblak J. (2014) Cerebral endothelial function determined by cerebrovascular reactivity to L-arginine. BioMed Res Int 2014:601515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puddey IB, Rakic V, Dimmitt SB, Beilin LJ. (1999) Influence of pattern of drinking on cardiovascular disease and cardiovascular risk factors—a review. Addiction 94:649–663. [DOI] [PubMed] [Google Scholar]
- Reissig CJ, Strain EC, Griffiths RR. (2009) Caffeinated energy drinks—a growing problem. Drug Alcohol Depend 99:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds K, Lewis B, Nolen JD, Kinney GL, Sathya B, He J. (2003) Alcohol consumption and risk of stroke: a meta-analysis. JAMA 289:579–588. [DOI] [PubMed] [Google Scholar]
- Ried K, Sullivan TR, Fakler P, Frank OR, Stocks NP. (2012) Effect of cocoa on blood pressure. Cochrane Database Syst Rev 8:CD008893. [DOI] [PubMed] [Google Scholar]
- Roe D. (1979) Alcohol and the Diet, Avi, Westport, CT. [Google Scholar]
- Sacco RL. (2007) Alcohol and stroke risk: an elusive dose-response relationship. Ann Neurol 62:551–552. [DOI] [PubMed] [Google Scholar]
- Scioli MG, Bielli A, Arcuri G, Ferlosio A, Orlandi A. (2014) Ageing and microvasculature. Vasc Cell 6:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobey CG, Weiler JM, Boujaoude M, Woodman OL. (2004) Effect of short-term phytoestrogen treatment in male rats on nitric oxide-mediated responses of carotid and cerebral arteries: comparison with 17β-estradiol. J Pharmacol Exp Ther 310:135–140. [DOI] [PubMed] [Google Scholar]
- Strong R, Grotta JC, Aronowski J. (2000) Combination of low dose ethanol and caffeine protects brain from damage produced by focal ischemia in rats. Neuropharmacology 39:515–522. [DOI] [PubMed] [Google Scholar]
- Tassaneeyakul W, Birkett DJ, McManus ME, Tassaneeyakul W, Veronese ME, Andersson T, Tukey RH, Miners JO. (1994) Caffeine metabolism by human hepatic cytochromes P450: contributions of 1A2, 2E1 and 3A isoforms. Biochem Pharmacol 47:1767–1776. [DOI] [PubMed] [Google Scholar]
- Varani K, Portaluppi F, Merighi S, Ongini E, Belardinelli L, Borea PA. (1999) Caffeine alters A2A adenosine receptors and their function in human platelets. Circulation 99:2499–2502. [DOI] [PubMed] [Google Scholar]
- Vidyasagar R, Greyling A, Draijer R, Corfield DR, Parkes LM. (2013) The effect of black tea and caffeine on regional cerebral blood flow measured with arterial spin labeling. J Cereb Blood Flow Metab 33:963–968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstock AR. (2014) The Global Drug Survey 2014 findings: reflections on the results of the world’s biggest ever drug survey by Dr Adam Winstock Global Drug Survey , http://www.globaldrugsurvey.com/facts-figures/the-global-drug-survey-2014-findings/.
- Ye Y, Jian K, Jaggar JH, Bukiya AN, Dopico AM. (2014) Type 2 ryanodine receptors are highly sensitive to alcohol. FEBS Lett 588:1659–1665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida M, Ueda S, Machida J, Ikegami K. (1989) Effects of caffeine on the vascular smooth muscles isolated from two-kidney, one-clip renovascular hypertension in rabbits. Urol Int 44:147–151. [DOI] [PubMed] [Google Scholar]
- Zhang A, Altura BT, Altura BM. (1993) Ethanol-induced contraction of cerebral arteries in diverse mammals and its mechanism of action. Eur J Pharmacol 248:229–236. [DOI] [PubMed] [Google Scholar]