Abstract
Incidence of prescription opioid abuse and overdose, often led by oxycodone, continues to increase, producing twice as many overdose deaths as heroin. Surprisingly, preclinical reports relevant to oxycodone’s abuse-related effects are relatively sparse considering its history and patient usage. The goal of this study was to characterize dose- and time-dependent effects of acute and repeated oxycodone administration in a frequency-rate intracranial self-stimulation (ICSS) procedure, an assay often predictive of drug-related reinforcing effects, in male Sprague-Dawley rats. We hypothesized that oxycodone would produce a biphasic profile of rate-increasing and rate-decreasing effects maintained by ICSS similar to μ-opioid receptor agonists. Oxycodone (0.03, 0.3, 1, and 3 mg/kg, s.c.) produced dose- and time-dependent alterations on ICSS, with the predicted biphasic profile of rate-increasing effects at lower stimulation frequencies followed by rate-decreasing effects at higher frequencies. Peak effects were observed between 30 and 60 minutes, which were reversed by naloxone pretreatment (30 minutes). Tolerance to rate-decreasing effects was observed over a 5-day period when rats were treated with 1 mg/kg oxycodone twice a day. Subsequently, the dosing regimen was increased to 3 mg/kg twice a day over 10 days, although further marked tolerance did not develop. When then challenged with 10 mg/kg naloxone, a significant suppression below baseline levels of ICSS-maintained responding occurred indicative of dependence that recovered to baseline within 5 hours. The results of this study provide the first report of acute and chronic effects of oxycodone on responding maintained by ICSS presentation and the use of ICSS-maintained responding to characterize its tolerance and dependence effects.
Introduction
Oxycodone is a semisynthetic opioid derived from the alkaloid compound thebaine found in opioid poppies. It has been used for almost 100 years in medicine, mostly for the treatment of various forms of pain (Kalso, 2005; Wightman et al., 2012). Although oxycodone has excellent efficacy as an analgesic, it also produces tolerance and dependence, similar to other opioid compounds (Mars et al., 2014). Patients treated with oxycodone for extended periods of time require larger doses to achieve desired analgesic effects, and eventually some patients report taking the drug to avoid or alleviate withdrawal symptoms and transitioning to abuse of the drug for nonmedical purposes (Jones et al., 2011a,b; Mars et al., 2014). The use and sales of oxycodone have greatly increased since the late 20th century in the United States, with sales increasing over 4-fold (Cobaugh et al., 2014) which has been mirrored by a pattern of illicit use and overdose deaths. Recent statistics show that prescription opioids cause over twice as many overdose deaths as heroin (Centers for Disease Control and Prevention, 2015). Furthermore, at least one recent study has shown that oxycodone was involved in the majority of opioid-related deaths among all opioid compounds (Hirsch et al., 2014).
Despite the rampant use of oxycodone for both medicinal and recreational reasons, while approaching a centenary of availability, there have been relatively few preclinical studies characterizing oxycodone’s abuse-related effects. In particular, there have been relatively few preclinical reports characterizing the reinforcing and dependence-producing properties of the drug. In terms of its reinforcing-related effects, oxycodone has been reported to be self-administered by laboratory animals, including rats (Beardsley et al., 2004) and mice (Zhang et al., 2009, 2015). Oxycodone also produces conditioned place preference in rats (Rutten et al., 2011) and mice (Bryant et al., 2014; Kirkpatrick and Bryant, 2014). Oxycodone dependence reports are also relatively rare. Hutchinson et al. (2009) reported that delivering 21 days of oxycodone to rats via osmotic minipumps resulted in substantial weight loss upon termination of drug administration after pump removal indicative of physical dependence. The authors reported that they did not analyze the somatic signs of oxycodone withdrawal because of the “severity of … withdrawal” it produced (Hutchinson et al., 2009). Beardsley et al. (2004) reported that oxycodone dose-dependently suppressed signs of spontaneous withdrawal in morphine-dependent monkeys and concluded that oxycodone shared cross-dependence with morphine. Also pertinent to the abuse-related effects of oxycodone is its ability to induce locomotor sensitization and its discriminative stimulus effects. Oxycodone can induce locomotor sensitization (Liu et al., 2005; Niikura et al., 2013; Bryant et al., 2014) and has heroin-like discriminative stimulus effects with a similar potency to heroin (Beardsley et al., 2004).
Intracranial self-stimulation (ICSS) is a preclinical procedure that can be predictive of abuse-related effects of drugs (Wise, 2005; Negus and Miller, 2014; Bespalov et al., 2015). During ICSS, animal subjects are trained to emit a response (e.g., lever pressing) reinforced with electrical stimulation through an electrode typically implanted in the mesolimbic dopamine reward pathway, such as the medial forebrain bundle (MFB). Drugs of abuse tend to increase or produce leftward shifts in response rates as a function of ICSS frequency, whereas drugs or other manipulations associated with presumed aversive effects (e.g., antipsychotics or drug withdrawal) tend to decrease or produce rightward shifts. Preclinical studies involving oxycodone and reward or reinforcement are limited, and there is only one that we are aware of that has assessed oxycodone in ICSS (Ewan and Martin, 2011). The primary end point of that study was to assess neuropathic pain; as such, the authors did not thoroughly characterize oxycodone in ICSS, as it was used as a control procedure.
The primary objective of this study was to test the hypothesis that oxycodone would produce a biphasic profile of rate-increasing and rate-decreasing effects maintained by ICSS similar to other μ-opioid receptor agonists such as morphine (Altarifi and Negus, 2011). The goals of this study were to assess the effects of acute and repeated oxycodone administration on ICSS, as well as to use changes in responding maintained by ICSS to infer tolerance and dependence effects. We first evaluated oxycodone in acute dose-response and time-course tests in ICSS. We then tested the effects of an opioid receptor antagonist, naloxone, for its ability to reverse oxycodone-mediated effects. We then evaluated ICSS before, during, and after repeated administration of two dose (1 and 3 mg/kg) regimens of oxycodone. Finally, we assessed the effects of precipitated withdrawal on ICSS, as well as the possible emergence of spontaneous withdrawal.
Materials and Methods
Subjects.
This study used male Sprague-Dawley rats (Jackson Laboratories, Indianapolis, IN) that weighed between 380 and 420 g at the time of surgery and had ad libitum access to food (7012 Teklad LM-485 Mouse/Rat Sterilizable Diet; Harlan, Indianapolis, IN) and water. Rats were individually housed in a temperature-controlled (20–22°C) Association for Assessment and Accreditation of Laboratory Animal Care–accredited facility and maintained on a 12-hour/12-hour light/dark cycle (0600–1800 hours lights on) with testing occurring during the light cycle. There were 23 total rats used for this study. Four subjects failed to acquire stable ICSS behavior after surgery, two rats were not included because of variable baseline ICSS throughout repeated testing, and one rat was removed for health reasons. Therefore, 16 total rats were used for data analysis. We used a mixed design because some subjects completed one experiment and some rats were used in all experiments. All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Virginia Commonwealth University Institutional Animal Care and Use Committee.
Apparatus.
ICSS testing was conducted in 12 standard, commercially obtained, rat operant conditioning chambers (model ENV-007; Med Associates, Inc., St. Albans, VT). Each chamber was equipped with retractable levers (located in each corner of the front chamber wall), two light-emitting diode stimulus lights, and a 5-W chamber house light. The outside of each chamber was equipped with a suspended electrical commutator connected to an electrical stimulator (model PHM-152; Med Associates, Inc.), as well as a tether that was connected to the electrode implant. Chambers were enclosed within sound- and light-attenuating chambers equipped with exhaust fans. MED-PC IV software (Med Associates, Inc.) was used to record lever presses and to control stimulus activation sessions.
Stereotaxic Surgery.
Surgical procedures for implanting electrodes in rats were similar to those previously reported (Carlezon and Chartoff, 2007). Rats were anesthetized with isoflurane inhalation (approximately 3%) and continued delivery during surgical procedures. Bipolar twisted stainless steel electrodes (0.125-mm single wire diameter; Plastics One, Roanoke, VA) were implanted into the right MFB of the rats using coordinates similar to those reported by Carlezon and Chartoff (2007): 2.8 mm posterior of the bregma, 1.7 mm lateral from the midline, and 8.8 mm from the skull. After the hole for the electrode was drilled, three holes were bored into the skull surrounding the area the electrode would be placed and anchoring screws were secured. Finally, an electrode was inserted and fixed to the skull using dental cement. Rats were given postoperative care for 5 days after surgery, including treatment with bacitracin and physical and behavioral health monitoring (e.g., checking weight, physical appearance, etc.), and they were introduced to operant chambers 1 week after surgery.
ICSS.
After recovery, rats began lever-press training. Training procedures were similar to previous conditions we reported when using mice, except rats were used in this study (Wiebelhaus et al., 2015). Rats were trained to lever press on a fixed-ratio 1 (FR1) schedule of reinforcement, in which each lever press resulted in a 0.5-second train of square-wave cathodal pulses (0.1-millisecond pulse duration) at a frequency of 158 Hz that were accompanied by the activations of the stimulus lights and the offset of the house light. Amplitudes of stimulation were adjusted for each rat on an individual basis to produce maximal responding. Rats were exposed to daily 30- to 60-minute FR1 training sessions until they maintained stable responding at above a threshold response rate (i.e., ≥35 responses per minute). Stimulation amplitudes were adjusted individually between 55 and 300 μA to maintain maximal rates before testing began.
After stable responding was maintained, rats began frequency-rate training. During frequency-rate training, rats were presented with and allowed to respond for a series of 10 descending stimulation frequencies at 0.05 log unit increments (158 to 56 Hz; i.e., 2.2 to 1.75 log Hz), in contrast with only being exposed to one frequency (158 Hz) during FR1 training. During each frequency presentation within a series, rats experienced a 5-second time-out followed by 5 seconds of five noncontingent stimulation of a scheduled frequency accompanied by the flashing of the stimulus lights and the offset of the house lights, which in turn was followed by a 60-second period during which they were allowed to lever press for additional stimulations. The stimulation frequency was then decreased by 0.05 log units, and the next frequency presentation began. The series of frequency presentations were repeated between three and five times per training session. Current amplitudes were individually adjusted as needed for each subject to maintain ≥ 50% baseline maximal responding in at least three and fewer than eight frequencies before experiments began. The first series of frequency presentations each day was considered a daily “warm-up” period and data were excluded from all analyses. The next two series were averaged together to create the baseline response curve for that day. After a given treatment, rats were again allowed to respond for at least two series of frequencies, which were averaged together for analysis. Rats were considered eligible for testing when the total responses during baseline varied by less than 20% for 3 consecutive days of assessment, response rates during vehicle/saline tests were between 80% and 120% of baseline responding for at least two consecutive assessments, and frequency-rate curves were considered curvilinear (via visual analysis).
On test days, rats were placed in the chambers and allowed to respond during the “warm-up” and baseline phases. Immediately after baseline responding, rats were removed from operant chambers, placed in their home cages, and injected with drug or vehicle. After the selected pretreatment time, rats were returned to the operant chambers for testing during an additional series of frequency presentations considered the test series. Initially, injections of vehicle were administered to ensure that injections did not significantly affect the frequency-rate curves before beginning dose-response tests. Vehicle tests were also conducted after completion of dose-response tests to ensure that conditioned effects did not develop over time, and that baselines remained stable throughout the course of the experiment. Rats were exposed to oxycodone doses in ascending order, except for 0.03 mg/kg, which was tested last. This study used a mixed design, wherein some subjects completed only one drug curve and other subjects completed several tests.
Experimental Design.
Rats were initially tested at several time points (5, 30, 60, 180, and 300 minutes) after subcutaneous administrations of oxycodone (0.03, 0.3, 1, and 3 mg/kg). Doses were tested in ascending order, except 0.03 mg/kg, which was tested last. A separate group of subjects were tested with naloxone (1, 3, and 10 mg/kg; 40-minute pretreatment time) to characterize effects of naloxone on ICSS in opiate-naive rats. Rats were then tested with 3 mg/kg naloxone plus 1 mg/kg oxycodone (40-minute and 30-minute pretreatment times, respectively) to determine whether the effects of oxycodone were mediated through opioid receptors. The dose and pretreatment time of oxycodone and naloxone for antagonism tests were chosen to evaluate ICSS facilitating and attenuating effects produced by oxycodone at a single time point, as well taking into account the relatively short half-life of naloxone (Ngai et al., 1976). After time-course testing and naloxone antagonism, rats began the repeated dosing component of studies and were first assessed using ICSS with vehicle given 30 and 60 minutes before the start of the session (day 0). The next day, rats were administered oxycodone (1 mg/kg) twice daily (10:00 AM and 6:00 PM) for 5 consecutive days after daily baseline sessions (days 1–5). Rats were tested with ICSS after the initial oxycodone injection each day. After 5 days of 1 mg/kg treatment, rats began receiving 3 mg/kg oxycodone twice daily (10:00 AM and 6:00 PM) for 10 consecutive days (days 6–15). ICSS was assessed 30 and 60 minutes after the initial injection each day. On day 16, rats responded during an ICSS baseline session and then were given 1 mg/kg oxycodone to determine the effects of repeated drug administration on oxycodone-facilitated ICSS. After the 1 mg/kg oxycodone challenge (30- and 60-minute pretreatment test times), rats were administered naloxone (10 mg/kg) to precipitate withdrawal (2 hours after oxycodone injection). Rats were subsequently tested 5, 30, 60, 180, and 300 minutes after naloxone administration to characterize the time course of withdrawal effects. After over 2 weeks of drug and training abstinence, subjects were assessed in ICSS again to evaluate baseline stability. Rats were then tested at the same time points with naloxone (10 mg/kg) and vehicle, to serve as a control and determine whether a high dose of naloxone had any effects on ICSS at any time point.
Drugs.
Oxycodone HCl (Mallinckrodt, Hazelwood, MO), and naloxone HCl (Sigma-Aldrich, St. Louis, MO) were dissolved in 0.9% saline (General Laboratory Products, Yorkville, IL). All drugs were administered subcutaneously at a volume equivalent to 1 ml/kg body weight.
Data Analysis.
The independent variable measured in the ICSS studies was stimulations per minute during each frequency trial. ICSS response data were expressed and analyzed using three different methods. In the first method, data were normalized to the highest response rate recorded during the baseline frequency-rate curve generated for each individual rat. This transformation allowed data to be expressed as the percent maximum control rate that normalized individual response rates across subjects. These data were then used to generate the frequency-rate curves, which were assessed using repeated-measures, two-way analyses of variance (ANOVAs) (treatment × frequency) between either vehicle and drug curves or baseline and treatment curves depending on the condition being tested. When significant interactions in the ANOVA were determined, Holm–Sidak tests were used for post hoc analyses, to analyze differences at specific frequencies between treatment conditions.
A second method was used to conduct between-dose comparisons and to provide summary data. The total number of stimulations during the test series was divided by the total number of responses during the baseline series and multiplied by 100 to produce percent baseline stimulations (Negus et al., 2010). This effectively collapsed all of the separate frequency stimulation rates into an overall stimulation count and allowed for comparison of data between days/treatments. This calculation was performed for all doses of each drug and vehicle and those values were analyzed using repeated-measures one-way ANOVAs. Dunnett’s post hoc tests were conducted contingent upon significant ANOVAs to compare treatment groups with vehicle or baseline controls.
A third method analyzed baselines over the course of the experiments expressing percent day 0 baseline stimulations. The calculation was identical to calculation of the percent baseline stimulations, except all days were normalized to day 0 baselines to determine the effect of repeated oxycodone treatment over time. Comparisons were also analyzed using a repeated-measures one-way ANOVA. A Dunnett’s post hoc test was conducted contingent upon a significant ANOVA to compare baseline days during repeated oxycodone dosing to the day 1 baseline levels. All subjects grouped together for repeated-measures ANOVAs received all manipulations included for each separate analysis.
Results
Effects of Oxycodone on ICSS: Dose Response and Time Course.
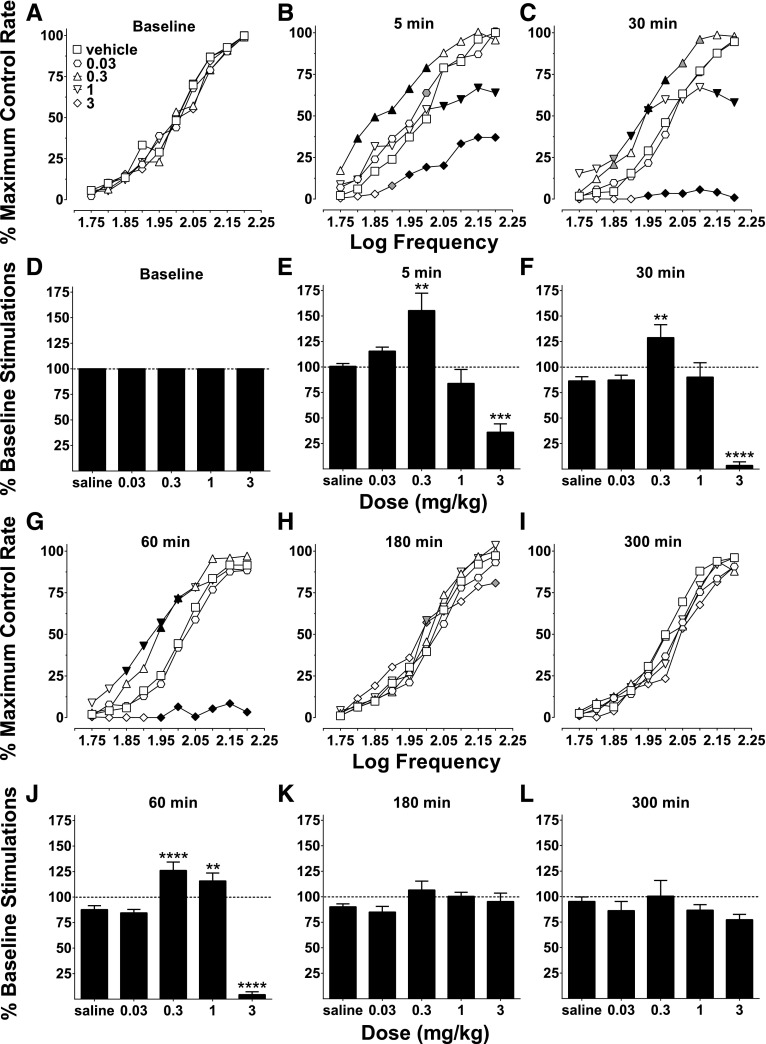
Oxycodone produced dose- and time-dependent effects on ICSS (Fig. 1). Baseline frequency response curves were stable across time, and there were no differences among these curves across oxycodone doses tested (Fig. 1A), and total baseline stimulations were thus used to normalize responding during various time points (Fig. 1D). At 5 minutes, 0.03 facilitated ICSS at one frequency and 0.3 mg/kg oxycodone increased ICSS response rates at five intermediate (1.8–2.0 log Hz) frequencies (Fig. 1B). By contrast, 1 mg/kg oxycodone had little effect at frequencies up to 2.0 log Hz and attenuated responding at the highest four frequencies (2.05 to 2.2 log Hz), and 3 mg/kg oxycodone reduced response rates at seven frequencies (1.9–2.2 log Hz). Percent baseline stimulation results at 5 minutes were similarly affected, because 0.3 mg/kg facilitated ICSS and 3 mg/kg attenuated ICSS (Fig. 1E). At 30 minutes, a similar profile of effects occurred as was observed at 5 minutes, with 0.03 mg/kg oxycodone having little systematic effect, 0.3 mg/kg generally facilitating ICSS, 1 mg/kg first increasing and then decreasing ICSS as frequency was increased, and 3 mg/kg reducing ICSS (Fig. 1C). These same effects on ICSS were captured with percent baseline stimulations except for 1 mg/kg, which did not affect this measure, probably because of the biphasic nature of the responding (facilitation of low frequencies, and attenuation of high frequencies; Fig. 1F). At 60 minutes, 0.3 and 1 mg/kg oxycodone facilitated ICSS at intermediate frequencies, whereas 3 mg/kg continued to attenuate ICSS (Fig. 1G); these effects were evident on percent baseline stimulations as well (Fig. 1J). At 180 minutes, responding began to return to vehicle levels; 0.3 and 3 mg/kg oxycodone significantly facilitated frequency-rate responding at 2.0 log Hz and 3 mg/kg attenuated ICSS at 2.2 log Hz, whereas all other doses/frequencies did not differ from vehicle responding (Fig. 1H). Percent baseline stimulations at 180 minutes were generally similar to frequency-rate results in that there were no differences from vehicle (saline) responding (Fig. 1K). At 300 minutes, frequency-rate and percent baseline stimulations had returned to vehicle levels, and there were no differences of any dose compared with vehicle (Fig. 1, I and L).
Fig. 1.
Oxycodone dose- and time-dependent effects on ICSS. Frequency-rate response curves (A–C and G–I) and percent baseline stimulations (D–F and J–L) for the effects of oxycodone with vehicle (saline) at multiple time points (baseline, 5, 30, 60, 180, and 300 minutes). (A) There were no differences between baseline frequency-rate response curves for any dose, as there was no effect of baseline day (F4, 28 = 2.18, P = 0.10) or an interaction between treatment baselines and frequency (F36, 252 = 1.21, P = 0.21). (B) Frequency-rate analysis at 5 minutes showed a main effect of oxycodone dose (F4, 28 = 23.75, P < 0.0001) and an interaction between dose and frequency (F36, 252 = 5.37, P < 0.0001). (C) At 30 minutes, there was a main effect of dose (F4, 28 = 30.16, P < 0.0001) and an interaction between dose and frequency (F36, 252 = 12.44, P < 0.0001). (D) Percent baseline responding was used to normalize baselines for each dose. (E) Percent baseline stimulation analysis showed differential effects of oxycodone at 5 minutes (F4, 28 = 20.42, P < 0.0001). (F) Percent baseline stimulation analysis also showed effects of oxycodone at 30 minutes (F4, 28 = 28.56, P < 0.0001). (G) At 60 minutes, there was a main effect of dose (F4, 28 = 64.11, P < 0.0001) and an interaction between dose and frequency (F36, 252 = 10.83, P < 0.0001). (H) At 180 minutes, there was no main effect of dose (F4, 28 = 1.15, P = 0.35), but there was an interaction between dose and frequency (F36, 252 = 1.85, P < 0.01). (I) At 300 minutes, there was no effect of dose (F4, 28 = 1.15, P = 0.35) or an interaction between dose and frequency (F36, 252 = 0.88, P = 0.66). (J) There was also an effect on percent baseline stimulations at 60 minutes (F4, 28 = 84.67, P < 0.0001). (K) There was no effect on percent baseline stimulations at 180 minutes (F4, 28 = 1.81, P = 0.15). (L) There were also no effects on percent baseline stimulations at 300 minutes (F4, 28 = 1.23, P = 0.32). Frequency-rate response values represent the mean normalized response rate (percent of maximum control responding) across 10 frequency presentations (1.75–2.20 log/Hz). Percent baseline stimulations represent the total number of responses across all test frequency presentations expressed as a percentage of each individual subject’s total baseline responses. All values represent mean ± S.E.M. (error bars excluded from frequency-rate panels) of eight rats. For frequency-rate panels, significant differences compared with vehicle at the respective frequencies are denoted by filled symbols (gray: P < 0.05; black: P < 0.01). Significant differences for percent baseline stimulations panels are denoted by asterisks (**P < 0.01; ***P < 0.001; ****P < 0.0001).
Effects of Naloxone and Naloxone Antagonism of Oxycodone on ICSS.
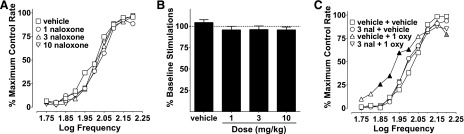
To assess the involvement of opioid receptors, naloxone was administered before oxycodone. Naloxone (1–10 mg/kg) did not affect frequency-rate or percent baseline stimulation ICSS responding (Fig. 2, A and B). Naloxone (3 mg/kg) blocked the effects produced by 1 mg/kg oxycodone on ICSS, because the only treatment that significantly differed from vehicle plus vehicle was vehicle plus 1 mg/kg oxycodone (Fig. 2C).
Fig. 2.
Naloxone effects on ICSS and antagonism of oxycodone (1 mg/kg). (A) Naloxone (1–10 mg/kg) did not produce any effects on ICSS frequency-rate curves; there was no effect of dose (F3, 18 = 2.90, P = 0.06) and no interaction between dose and frequency (F27, 162 = 1.16, P = 0.31). (B) Naloxone did not produce any effects on percent baseline stimulations either (F3, 18 = 1.75, P = 0.19). (C) Administration of naloxone (3 mg/kg) blocks effects on ICSS produced by 1 mg/kg oxycodone. Vehicle plus oxycodone was the only treatment that was significantly different from vehicle plus vehicle; there was no main effect of treatment (F3, 18 = 2.10, P = 0.14), but there was a significant interaction between treatment and frequency (F27, 162 = 2.51, P < 0.001). Significant differences for frequency-rate curves (A and C) are denoted by filled symbols (gray: P < 0.05; black: P < 0.01). All values represent means ± S.E.M., and error bars on frequency-rate curves are omitted for clarity (n = 7). oxy, oxycodone.
Effects of Repeated Oxycodone (1 and 3 mg/kg) on ICSS.
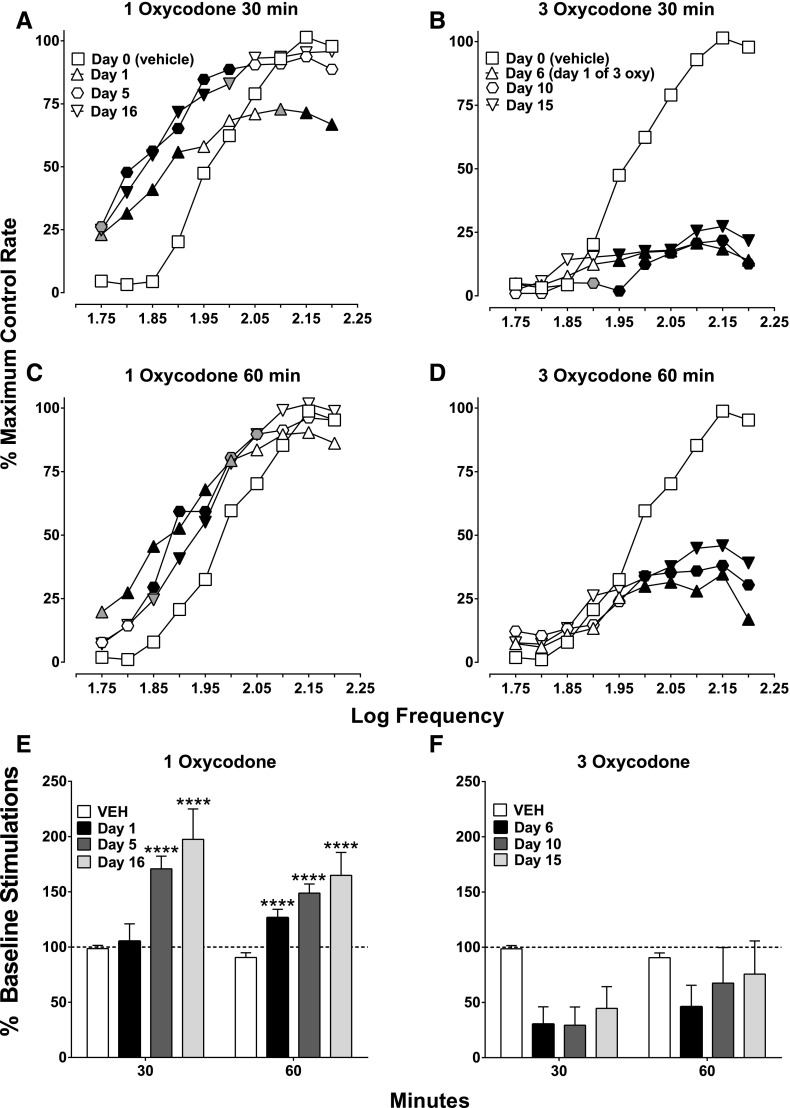
Repeated oxycodone administration (1 mg/kg, twice a day for 5 days) resulted in tolerance development to the rate-decreasing effects observed at high frequencies at 30 minutes post-treatment, while maintaining facilitation at low frequencies (Fig. 3A). This pattern of effect was preserved after subsequent treatment with 10 days of 3 mg/kg oxycodone administration twice a day (see day 16, Fig. 3A). The facilitation of ICSS produced by 1 mg/kg oxycodone at 60 minutes was maintained after repeated dosing of both 1 and 3 mg/kg (15 days total twice-daily dosing; Fig. 3C). Tolerance to the rate-decreasing effects at 30 minutes is also shown with percent baseline stimulations, because day 1 did not differ from saline; however, as exposure to oxycodone continued, responding was facilitated relative to vehicle (Fig. 3E). Tests at 60 minutes showed that 1 mg/kg facilitated ICSS throughout repeated dosing (Fig. 3E). Tolerance to the rate-decreasing effects of 3 mg/kg oxycodone did not develop when given repeatedly (twice a day for 10 days, immediately after 5 days of 1 mg/kg; Fig. 3, B and D). The attenuation of ICSS produced by 3 mg/kg was maintained throughout testing at both 30 and 60 minutes (Fig. 3, B and D, respectively). Percent baseline stimulation analysis did not result in a significant ANOVA due to high between-subject variability, but it showed a clear attenuation of ICSS similar to that of frequency-rate results (Fig. 3F).
Fig. 3.
Effects of repeated oxycodone (1 and 3 mg/kg): 1 mg/kg was given twice daily for 5 consecutive days, immediately followed by 10 consecutive days of 3 mg/kg. ICSS was evaluated at 30 and 60 minutes each day after the morning treatment. (A, C, and E) Effects of repeated 1 mg/kg oxycodone at 30 and 60 minutes on days 1, 5, and 16 of repeated dosing. (B, D, and F) Effects of repeated 3 mg/kg oxycodone on days 6, 10, and 15 of repeated dosing. (A and B) There was a significant effect on ICSS frequency-rate responding after repeated dosing with oxycodone (1 and 3 mg/kg) at 30 minutes, because there was a main effect of treatment day for both doses (1 mg/kg: F3, 24 = 6.73, P < 0.01; 3 mg/kg: F3, 24 = 16.49, P < 0.0001, respectively) and an interaction between treatment day and frequency for both doses (1 mg/kg: F27, 216 = 5.30, P < 0.0001; 3 mg/kg: F27, 216 = 16.48, P < 0.0001). (C and D) There was a significant effect of treatment day with 1 mg/kg oxycodone at 60 minutes (F3, 24 = 5.56, P < 0.01); although there was no effect of treatment day for 3 mg/kg (F3, 24 = 2.97, P = 0.05), there was an interaction between treatment day and frequency for 1 and 3 mg/kg (1 mg/kg: F27, 216 = 2.67, P < 0.0001; 3 mg/kg: F27, 216 = 6.91, P < 0.0001). (E) Effects of repeated 1 mg/kg oxycodone on percent baseline stimulations. There was no effect of time point (30 or 60 minutes; F1, 8 = 3.42, P = 0.10) for 1 mg/kg oxycodone, whereas there was an effect of treatment day (1, 5, or 16; F3, 24 = 13.34, P < 0.0001) and an interaction between treatment day and time point (F3, 24 = 3.97, P < 0.0001). (F) Effects of repeated 3 mg/kg oxycodone on percent baseline stimulations. There was no effect of time point (F1, 8 = 2.36, P = 0.16), there was an effect of day (F3, 24 = 3.96, P < 0.05), and there was no interaction between the two variables (F3, 24 = 1.22, P = 0.32). Significant differences for frequency-rate curves (A–D) are denoted by filled symbols (gray: P < 0.05; black: P < 0.01) and by asterisks for the percent baseline stimulations (E and F; ****P < 0.0001). All values represent means ± S.E.M., and error bars on frequency-rate curves are omitted for clarity (n = 9). oxy, oxycodone; VEH, vehicle.
Effects of Naloxone-Precipitated Oxycodone Withdrawal on ICSS.
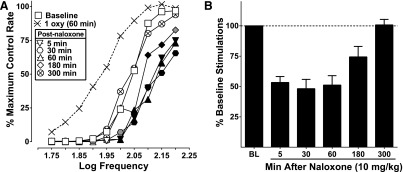
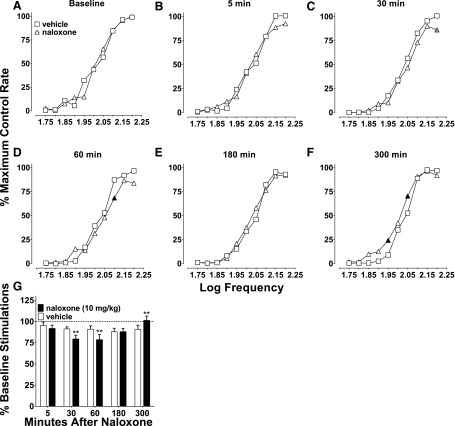
Naloxone (10 mg/kg) precipitated withdrawal in subjects repeatedly administered oxycodone (1 then 3 mg/kg twice a day across 15 days). On day 16, 2 hours after the final 1 mg/kg oxycodone test, subjects were administered naloxone and ICSS was measured at various time points. Naloxone significantly attenuated frequency-rate ICSS compared with daily baseline between 5 and 180 minutes after administration (Fig. 4A). Similarly, percent baseline stimulations were also attenuated (Fig. 4B). ICSS responding was reduced by 50%–75% between 5 and 180 minutes after naloxone administration (inferential statistics not conducted because of the absence of a chronic vehicle group). Responding returned to daily baseline levels 300 minutes after naloxone was given.
Fig. 4.
Effects of naloxone-precipitated withdrawal after 15 days of twice-daily oxycodone (day 16). (A) After 15 days of repeated oxycodone, naloxone (10 mg/kg) attenuated ICSS, and there was an effect of naloxone (F5, 35 = 7.35, P < 0.0001) and an interaction between naloxone time point and frequency (F45, 315 = 1.43, P < 0.05) between 5 and 180 minutes after administration compared with daily baseline responding. Oxycodone (1 mg/kg) was given 2 hours prior to naloxone and is represented by the hashed-line with X symbols (also shown for reference in Fig. 3, A and C) and is not included in statistical analysis. (B) Percent baseline stimulations for the same data set; no stats were conducted on these data because there was no saline control point to use for comparison, but responding is reduced to approximately 50% of daily baseline through 60 minutes after naloxone administration and 75% at 180 minutes, responding returns to baseline at 300 minutes similar to frequency-rate data. Significant differences for frequency-rate curves (A) are denoted by filled symbols (gray: P < 0.05, black: P < 0.01). All values represent means ± S.E.M., and error bars on frequency-rate curves are omitted for clarity (n = 9). oxy, oxycodone.
Time Course of Naloxone Effects.
To serve as a control for the 10 mg/kg naloxone-precipitated withdrawal data, naloxone was tested at the same time points (5–300 minutes) given alone and compared with vehicle effects. There were no differences between frequency-rate baselines before corresponding saline and naloxone treatment (Fig. 5A). There were also no differences between the two treatments at 5 minutes and 180 minutes (Fig. 5, B and E). ICSS was mildly attenuated by naloxone at 30 and 60 minutes, because only one frequency was reduced at each time point (Fig. 5, C and D), and was mildly facilitated at 300 minutes (Fig. 5F). Analysis of percent baseline stimulations showed identical effects as frequency-rate curves (Fig. 5G). Overall, naloxone (10 mg/kg) had modest effects on ICSS.
Fig. 5.
Effects of naloxone (10 mg/kg) over time (5–300 minutes). (A) There were no differences between vehicle (saline) and naloxone (10 mg/kg) baseline responding, because there was no main effect of treatment (F1, 7 = 0.00, P = 0.99) and no interaction between treatment and frequency (F9, 63 = 1.78, P = 0.09). (B) At 5 minutes, there was no effect of naloxone (F1, 7 = 0.19, P = 0.18), and there was an interaction between naloxone treatment and frequency (F9, 63 = 2.09, P < 0.05); however, post hoc tests showed no differences at any frequency. (C) At 30 minutes, there was a main effect of naloxone (F1, 7 = 8.51, P < 0.05) and an interaction (F9, 63 = 2.07, P < 0.05). (D) 60 minutes after administration, there was a main effect of naloxone treatment (F1, 7 = 6.72, P < 0.05) and an interaction between treatment and frequency (F9, 63 = 3.65, P < 0.01). (E) At 180 minutes, there was no effect of naloxone (F1, 7 = 0.76, P = 0.41) and no interaction between naloxone and frequency (F9, 63 = 1.24, P = 0.29). (F) At 300 minutes, there was an effect of naloxone treatment (F1, 7 = 10.52, P < 0.05) and an interaction between treatment and frequency (F9, 63 = 2.82, P < 0.01). (G) Percent baseline stimulations from the same data set. There was a main effect of time point (F4, 28 = 3.47, P < 0.05) and no main effect of naloxone treatment (F1, 7 = 0.57, P = 0.47); however, there was an interaction between time point and naloxone treatment (F4, 28 = 6.78, P < 0.001). Significant differences for frequency-rate curves (A–F) are denoted by filled symbols (gray: P < 0.05; black: P < 0.01) and by asterisks for the percent baseline stimulations (G; **P < 0.01). All values represent means ± S.E.M., and error bars on frequency-rate curves are omitted for clarity (n = 8).
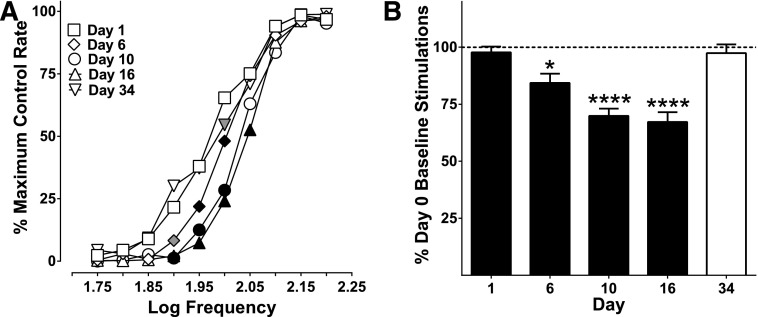
Effects of Repeated Oxycodone on Baseline ICSS Responding: Potential Spontaneous Withdrawal Effects.
Baselines before, during, and after repeated dosing were analyzed to determine whether there were any rightward shifts, or attenuation of ICSS, suggestive of spontaneous withdrawal effects indicative of physical dependence. After 5 days of repeated oxycodone (1 mg/kg), the baseline was already shifted to the right compared with the day 1 baseline (before oxycodone dosing). Baselines continued shifting to the right during and after 3 mg/kg dosing and returned to day 1 baseline responding after a 2.5-week period of drug abstinence (day 34), with responding at only one frequency (2.0 Hz) attenuated from day 1 baseline. These shifts in baseline responding to the right, with subsequent recovery after prolonged (18-day) abstinence, suggest that the emergence of spontaneous withdrawal effects occurred between the previous day’s oxycodone administration and the next morning’s administration.
Discussion
In this study, we found that oxycodone had biphasic effects (facilitating and attenuating) on ICSS as a function of dose and pretreatment time. In general, 0.3 mg/kg oxycodone facilitated ICSS or had no effect, whereas 1 mg/kg attenuated ICSS at the earliest time point (5 minutes), attenuated high frequencies and facilitated low frequencies at 30 minutes (similar to the effect of moderate morphine doses on ICSS in the literature; Altarifi and Negus, 2011), and produced only facilitation at 60 minutes, and 3 mg/kg oxycodone either attenuated ICSS or had no effect at all time points.
To our knowledge, there has been only one other published study in which oxycodone was evaluated for its effects on ICSS. Ewan and Martin (2011) evaluated several different opioid compounds, including oxycodone, specifically for their effects on pain-suppressed ICSS. The study’s focus was on a neuropathic pain state rather than opioid effects on ICSS alone; thus, oxycodone was only assessed at one time point, and the only dependent measure reported was reduction in EF50 (frequency at which rats emitted 50% of maximal responding). The authors did show a facilitating effect of oxycodone on ICSS with 1 and 3 mg/kg at 60 minutes. The effect of 1 mg/kg oxycodone was similar to that in our study, whereas Ewan and Martin showed a facilitating effect and our study showed an attenuating effect of 3 mg/kg at 60 minutes. There are several procedural differences to consider that could explain these seemingly disparate results. First, Ewan and Martin used Fisher 344 rats, whereas this study used Sprague-Dawley rats. Strain differences can produce profound differences in drug effects in experimental assays including ICSS (Fish et al., 2012). Second, the previous study examined ICSS in the ventral tegmental area of the brain rather than the MFB. Third, oxycodone was administered intraperitoneally in the previous study, rather than subcutaneously as in this study. Intraperitoneal administration allows for more first-pass liver metabolism than does subcutaneous administration, thus affecting central nervous system levels differently by identical doses. Fourth, the data were analyzed in a different manner in the previous study with ICSS, as the authors used regression analysis to generate EF50 values and assessed shifts in the curve.
In this study, the effects of 1 mg/kg oxycodone on ICSS at 30 minutes were blocked by pretreatment with naloxone (3 mg/kg), showing that opioid receptors are necessary for oxycodone to augment ICSS. Naloxone given alone did not affect ICSS, which is consistent with the available literature (Borowski and Kokkinidis, 1992; Altarifi et al., 2012). This indicates that exogenously administered agonists of the opioid system can influence ICSS but, observing that naloxone has no effects, suggests that endogenous opioids are not a critical component involved in ICSS behavior, at least that involving the mesolimbic system.
Interestingly, within 5 days of repeated administration of 1 mg/kg oxycodone, rats developed tolerance to the rate-decreasing effects observed at high frequencies of stimulation, although not affecting facilitation produced at lower frequencies. These results are similar to those produced by morphine in frequency-rate ICSS procedures (Altarifi and Negus, 2011). In apparent contrast with morphine doses up to 18 mg/kg given twice a day for 7 days (Altarifi and Negus, 2011), the rate-decreasing effects of 3 mg/kg oxycodone remained constant even after 10 subsequent days of twice-daily administration. This was surprising considering that humans report having to administer more oxycodone over time to maintain a consistent drug effect or prevent withdrawal symptoms (Jones et al., 2011a,b; Mars et al., 2014). However, it remains unknown whether an extended regimen and/or more frequent injections would be effective in producing tolerance to the attenuating effects of 3 mg/kg oxycodone. Indeed, two subjects did show evidence of becoming tolerant to 3 mg/kg oxycodone in our study. Another consideration is that subjects in the morphine study by Altarifi and Negus (2011) were exposed to a longer escalating dose regimen (28 days total with chronic doses ranging between 3.2 and 18 mg/kg morphine). Future studies to determine whether the attenuating effects of 3 mg/kg oxycodone or higher can be tolerated with different dosing schedules would be of interest. Overall, our results suggest that (at least with short dosing regimens of oxycodone) behavioral effects persist at higher doses.
Some of the most provocative findings of this study involve the apparent withdrawal effects observed. After 15 days of oxycodone twice a day, we were able to observe withdrawal effects indicative of dependence. Naloxone (10 mg/kg) produced a profound attenuation (at up to five frequencies with reductions up to 50% baseline stimulations) of ICSS that persisted for approximately 3 hours after its administration (Fig. 4). Importantly, 10 mg/kg naloxone tested in the same group of rats after an extended drug washout period had little to no effect relative to that during precipitated withdrawal (Fig. 5). There were modest reductions in ICSS responding compared with vehicle at 30 and 60 minutes and slight facilitation of ICSS at 300 minutes in the absence of a recent oxycodone history (Fig. 5); however, these differences were relatively negligible compared with attenuation produced by the same dose of naloxone (10 mg/kg) given after repeated oxycodone administration (Fig. 4). The precipitated withdrawal effect observed (i.e., attenuation of ICSS) was interesting, because somatic signs characteristic of opioid withdrawal (e.g., wet dog shakes, diarrhea) were not observed, suggesting that ICSS may be a more sensitive assay to assess oxycodone withdrawal. The observation that ICSS-maintained operant behavior may be more sensitive than conventional observational measures to a drug’s abstinence is consistent with other reports documenting the greater sensitivity of operant behavior in this regard involving opiates, nicotine, cannabinoids, and dissociative anesthetics (Beardsley et al., 1986; Beardsley and Balster, 1987; Bespalov et al., 1999; Vann et al., 2006; Desai et al., 2013).
Especially interesting was the observation of what were likely spontaneous withdrawal effects emerging between the afternoon and the next morning’s oxycodone injections, which involved approximately 16 hours of oxycodone abstinence. As shown in Fig. 6, baseline ICSS began to shift to the right (i.e., attenuate) after only 5 days of repeated (twice-daily) treatment with oxycodone. As days of repeated oxycodone administration progressed, baselines became increasingly attenuated. Given the likelihood that the repeated dosing regimen used was sufficient to produce dependence resulting in spontaneous withdrawal effects, administering chronic oxycodone while conducting ICSS testing could be a useful assay to evaluate determinants for augmenting or attenuating the dependence effects of oxycodone. Accordingly, such procedures may be of value for evaluating potential medications for treating oxycodone withdrawal.
Fig. 6.
Baselines before, during, and after repeated oxycodone administration. (A) Baselines significantly shifted to the right during repeated dosing but recovered after a period of drug abstinence. There was a significant effect of baseline day (F4, 32 = 17.57, P < 0.0001) and an interaction between baseline day and frequency (F36, 288 = 3.13, P < 0.0001). (B) Percent day 0 baseline stimulations were also reduced during repeated administration of oxycodone and returned to day 1 baseline responding after a period of drug abstinence (day 34), shown as the white bar (F4, 32 = 22.87, P < 0.0001). Significant differences for frequency-rate curves (A) are denoted by filled symbols (gray: P < 0.05; black: P < 0.01) and by asterisks for the percent baseline stimulations (B; *P < 0.05; ****P < 0.0001). All values represent means ± S.E.M., and error bars on frequency-rate curves are omitted for clarity (n = 9).
Results of this study using rats may translate to the human condition. Patients report withdrawal symptoms even after short periods (roughly 2 weeks) of therapeutic oxycodone use (Cobaugh et al., 2014). Although people may not always experience the full spectrum of the physical signs of oxycodone withdrawal, they still may be inflicted with those unpleasant enough to promote escalation of self-dosing, with the drug obtained either through legitimate or illegitimate channels. ICSS has been proposed as a preclinical model that can be used to model affective-like withdrawal symptoms, because many drugs, including morphine (Schaefer and Michael, 1986; Easterling et al., 2000; Liu and Schulteis, 2004; Altarifi and Negus, 2011; Holtz et al., 2015), nicotine (Epping-Jordan et al., 1998; Cryan et al., 2003; Kenny and Markou, 2005; Igari et al., 2014; Manbeck et al., 2014; Qi et al., 2015), ethanol (Schulteis et al., 1995; Chester et al., 2006; Rylkova et al., 2009; Boutros et al., 2014), and cocaine (Markou and Koob, 1991; Stoker and Markou, 2011), produce decreases in ICSS after either spontaneous or precipitated withdrawal. Furthermore, withdrawal from nicotine and morphine has been associated with decreased ventral tegmental area dopaminergic activity, which correlates with ICSS deficits (Liu and Jin, 2004; Kaufling and Aston-Jones, 2015). Accordingly, this procedure could be used to evaluate potential therapeutics for treating opioid withdrawal.
Overall, the results of this study indicate that oxycodone facilitates ICSS in a dose- and time-dependent manner. Subjects became tolerant to the rate-attenuating effects of 1 mg/kg oxycodone at high frequencies in a relatively short time (5 days); however, the same subjects did not become tolerant to the rate-attenuating effects produced by a higher dose (3 mg/kg) over a longer period of time (10 days). This suggests that certain effects of oxycodone may be resistant to tolerance development. Naloxone (10 mg/kg) produced signs of withdrawal in ICSS after repeated oxycodone dosing, while having limited effects of its own after an extended abstinence period. Baseline ICSS responding was attenuated after repeated oxycodone administration, suggesting that it may be used as an assay to measure spontaneous oxycodone withdrawal effects indicative of dependence. On the basis of these results, ICSS can be used to investigate both the abuse- and dependence-related effects of oxycodone.
Acknowledgments
The authors thank Aaron D. Loveland and Lea T. Moisa for technical contributions to the work presented in this article.
Abbreviations
- ANOVA
analysis of variance
- FR1
fixed-ratio 1
- ICSS
intracranial self-stimulation
- MFB
medial forebrain bundle
Authorship Contributions
Participated in research design: Wiebelhaus, Walentiny, Beardsley.
Conducted experiments: Wiebelhaus.
Performed data analysis: Wiebelhaus.
Wrote or contributed to the writing of the manuscript: Wiebelhaus, Walentiny, Beardsley.
Footnotes
This research was supported by the National Institutes of Health National Institute on Drug Abuse [Contracts HHSN271201200003C (to J.M.W., D.M.W., and P.M.B.) and HHSN271201400035C (to P.M.B.)]. Additional support was provided by Virginia Commonwealth University [Grant PHTX292321].
References
- Altarifi AA, Miller LL, Negus SS. (2012) Role of µ-opioid receptor reserve and µ-agonist efficacy as determinants of the effects of µ-agonists on intracranial self-stimulation in rats. Behav Pharmacol 23:678–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altarifi AA, Negus SS. (2011) Some determinants of morphine effects on intracranial self-stimulation in rats: dose, pretreatment time, repeated treatment, and rate dependence. Behav Pharmacol 22:663–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beardsley PM, Aceto MD, Cook CD, Bowman ER, Newman JL, Harris LS. (2004) Discriminative stimulus, reinforcing, physical dependence, and antinociceptive effects of oxycodone in mice, rats, and rhesus monkeys. Exp Clin Psychopharmacol 12:163–172. [DOI] [PubMed] [Google Scholar]
- Beardsley PM, Balster RL. (1987) Behavioral dependence upon phencyclidine and ketamine in the rat. J Pharmacol Exp Ther 242:203–211. [PubMed] [Google Scholar]
- Beardsley PM, Balster RL, Harris LS. (1986) Dependence on tetrahydrocannabinol in rhesus monkeys. J Pharmacol Exp Ther 239:311–319. [PubMed] [Google Scholar]
- Bespalov AY, Balster RL, Beardsley PM. (1999) N-Methyl-D-aspartate receptor antagonists and the development of tolerance to the discriminative stimulus effects of morphine in rats. J Pharmacol Exp Ther 290:20–27. [PubMed] [Google Scholar]
- Bespalov AY, Beardsley PM, Todtenkopf MS. (2015) Utility of intracranial self-stimulation in the assessment of the abuse liability of new pharmaceuticals, in Nonclinical Assessment of Abuse Potential for New Pharmaceuticals (Compton DC, Hudzik TJ, Markgraf CG. eds) pp 197–213, Elsevier, New York. [Google Scholar]
- Borowski TB, Kokkinidis L. (1992) Long-term influence of d-amphetamine on mesolimbic brain-stimulation reward: comparison to chronic haloperidol and naloxone effects. Pharmacol Biochem Behav 43:1–15. [DOI] [PubMed] [Google Scholar]
- Boutros N, Semenova S, Markou A. (2014) Adolescent intermittent ethanol exposure diminishes anhedonia during ethanol withdrawal in adulthood. Eur Neuropsychopharmacol 24:856–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant CD, Guido MA, Kole LA, Cheng R. (2014) The heritability of oxycodone reward and concomitant phenotypes in a LG/J × SM/J mouse advanced intercross line. Addict Biol 19:552–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Chartoff EH. (2007) Intracranial self-stimulation (ICSS) in rodents to study the neurobiology of motivation. Nat Protoc 2:2987–2995. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (2015) Underlying Cause of Death, 1999-2013, National Center for Health Statistics, Hyattsville, MD. [Google Scholar]
- Chester JA, Rausch EJ, June HL, Froehlich JC. (2006) Decreased reward during acute alcohol withdrawal in rats selectively bred for low alcohol drinking. Alcohol 38:165–172. [DOI] [PubMed] [Google Scholar]
- Cobaugh DJ, Gainor C, Gaston CL, Kwong TC, Magnani B, McPherson ML, Painter JT, Krenzelok EP. (2014) The opioid abuse and misuse epidemic: implications for pharmacists in hospitals and health systems. Am J Health Syst Pharm 71:1539–1554. [DOI] [PubMed] [Google Scholar]
- Cryan JF, Bruijnzeel AW, Skjei KL, Markou A. (2003) Bupropion enhances brain reward function and reverses the affective and somatic aspects of nicotine withdrawal in the rat. Psychopharmacology (Berl) 168:347–358. [DOI] [PubMed] [Google Scholar]
- Desai RI, Thakur GA, Vemuri VK, Bajaj S, Makriyannis A, Bergman J. (2013) Analysis of tolerance and behavioral/physical dependence during chronic CB1 agonist treatment: effects of CB1 agonists, antagonists, and noncannabinoid drugs. J Pharmacol Exp Ther 344:319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Easterling KW, Plovnick RM, Holtzman SG. (2000) Acute opioid but not benzodiazepine dependence in rats responding for intracranial self-stimulation. Psychopharmacology (Berl) 148:263–271. [DOI] [PubMed] [Google Scholar]
- Epping-Jordan MP, Watkins SS, Koob GF, Markou A. (1998) Dramatic decreases in brain reward function during nicotine withdrawal. Nature 393:76–79. [DOI] [PubMed] [Google Scholar]
- Ewan EE, Martin TJ. (2011) Rewarding electrical brain stimulation in rats after peripheral nerve injury: decreased facilitation by commonly abused prescription opioids. Anesthesiology 115:1271–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fish EW, Robinson JE, Krouse MC, Hodge CW, Reed C, Phillips TJ, Malanga CJ. (2012) Intracranial self-stimulation in FAST and SLOW mice: effects of alcohol and cocaine. Psychopharmacology (Berl) 220:719–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirsch A, Proescholdbell SK, Bronson W, Dasgupta N. (2014) Prescription histories and dose strengths associated with overdose deaths. Pain Med 15:1187–1195. [DOI] [PubMed] [Google Scholar]
- Holtz NA, Radke AK, Zlebnik NE, Harris AC, Carroll ME. (2015) Intracranial self-stimulation reward thresholds during morphine withdrawal in rats bred for high (HiS) and low (LoS) saccharin intake. Brain Res 1602:119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchinson MR, Lewis SS, Coats BD, Skyba DA, Crysdale NY, Berkelhammer DL, Brzeski A, Northcutt A, Vietz CM, Judd CM, et al. (2009) Reduction of opioid withdrawal and potentiation of acute opioid analgesia by systemic AV411 (ibudilast). Brain Behav Immun 23:240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igari M, Alexander JC, Ji Y, Qi X, Papke RL, Bruijnzeel AW. (2014) Varenicline and cytisine diminish the dysphoric-like state associated with spontaneous nicotine withdrawal in rats. Neuropsychopharmacology 39:455–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Sullivan MA, Manubay J, Vosburg SK, Comer SD. (2011a) The subjective, reinforcing, and analgesic effects of oxycodone in patients with chronic, non-malignant pain who are maintained on sublingual buprenorphine/naloxone. Neuropsychopharmacology 36:411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones JD, Vosburg SK, Manubay JM, Comer SD. (2011b) Oxycodone abuse in New York City: characteristics of intravenous and intranasal users. Am J Addict 20:190–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalso E. (2005) Oxycodone. J Pain Symptom Manage 29 (Suppl):S47–S56. [DOI] [PubMed] [Google Scholar]
- Kaufling J, Aston-Jones G. (2015) Persistent adaptations in afferents to ventral tegmental dopamine neurons after opiate withdrawal. J Neurosci 35:10290–10303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenny PJ, Markou A. (2005) Conditioned nicotine withdrawal profoundly decreases the activity of brain reward systems. J Neurosci 25:6208–6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick SL, Bryant CD. (2014) Behavioral architecture of opioid reward and aversion in C57BL/6 substrains. Front Behav Neurosci 8:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Schulteis G. (2004) Brain reward deficits accompany naloxone-precipitated withdrawal from acute opioid dependence. Pharmacol Biochem Behav 79:101–108. [DOI] [PubMed] [Google Scholar]
- Liu YL, Liang JH, Yan LD, Su RB, Wu CF, Gong ZH. (2005) Effects of l-tetrahydropalmatine on locomotor sensitization to oxycodone in mice. Acta Pharmacol Sin 26:533–538. [DOI] [PubMed] [Google Scholar]
- Liu ZH, Jin WQ. (2004) Decrease of ventral tegmental area dopamine neuronal activity in nicotine withdrawal rats. Neuroreport 15:1479–1481. [DOI] [PubMed] [Google Scholar]
- Manbeck KE, Shelley D, Schmidt CE, Harris AC. (2014) Effects of oxytocin on nicotine withdrawal in rats. Pharmacol Biochem Behav 116:84–89. [DOI] [PubMed] [Google Scholar]
- Markou A, Koob GF. (1991) Postcocaine anhedonia. An animal model of cocaine withdrawal. Neuropsychopharmacology 4:17–26. [PubMed] [Google Scholar]
- Mars SG, Bourgois P, Karandinos G, Montero F, Ciccarone D. (2014) “Every ‘never’ I ever said came true”: transitions from opioid pills to heroin injecting. Int J Drug Policy 25:257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Miller LL. (2014) Intracranial self-stimulation to evaluate abuse potential of drugs. Pharmacol Rev 66:869–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negus SS, Morrissey EM, Rosenberg M, Cheng K, Rice KC. (2010) Effects of kappa opioids in an assay of pain-depressed intracranial self-stimulation in rats. Psychopharmacology (Berl) 210:149–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngai SH, Berkowitz BA, Yang JC, Hempstead J, Spector S. (1976) Pharmacokinetics of naloxone in rats and in man: basis for its potency and short duration of action. Anesthesiology 44:398–401. [DOI] [PubMed] [Google Scholar]
- Niikura K, Ho A, Kreek MJ, Zhang Y. (2013) Oxycodone-induced conditioned place preference and sensitization of locomotor activity in adolescent and adult mice. Pharmacol Biochem Behav 110:112–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi X, Guzhva L, Ji Y, Bruijnzeel AW. (2015) Chronic treatment with the vasopressin 1b receptor antagonist SSR149415 prevents the dysphoria associated with nicotine withdrawal in rats. Behav Brain Res 292:259–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutten K, van der Kam EL, De Vry J, Tzschentke TM. (2011) Critical evaluation of the use of extinction paradigms for the assessment of opioid-induced conditioned place preference in rats. Pharmacology 87:286–296. [DOI] [PubMed] [Google Scholar]
- Rylkova D, Shah HP, Small E, Bruijnzeel AW. (2009) Deficit in brain reward function and acute and protracted anxiety-like behavior after discontinuation of a chronic alcohol liquid diet in rats. Psychopharmacology (Berl) 203:629–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer GJ, Michael RP. (1986) Changes in response rates and reinforcement thresholds for intracranial self-stimulation during morphine withdrawal. Pharmacol Biochem Behav 25:1263–1269. [DOI] [PubMed] [Google Scholar]
- Schulteis G, Markou A, Cole M, Koob GF. (1995) Decreased brain reward produced by ethanol withdrawal. Proc Natl Acad Sci USA 92:5880–5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker AK, Markou A. (2011) Withdrawal from chronic cocaine administration induces deficits in brain reward function in C57BL/6J mice. Behav Brain Res 223:176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann RE, Balster RL, Beardsley PM. (2006) Dose, duration, and pattern of nicotine administration as determinants of behavioral dependence in rats. Psychopharmacology (Berl) 184:482–493. [DOI] [PubMed] [Google Scholar]
- Wiebelhaus JM, Grim TW, Owens RA, Lazenka MF, Sim-Selley LJ, Abdullah RA, Niphakis MJ, Vann RE, Cravatt BF, Wiley JL, et al. (2015) Δ9-tetrahydrocannabinol and endocannabinoid degradative enzyme inhibitors attenuate intracranial self-stimulation in mice. J Pharmacol Exp Ther 352:195–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman R, Perrone J, Portelli I, Nelson L. (2012) Likeability and abuse liability of commonly prescribed opioids. J Med Toxicol 8:335–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. (2005) Forebrain substrates of reward and motivation. J Comp Neurol 493:115–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Brownstein AJ, Buonora M, Niikura K, Ho A, Correa da Rosa J, Kreek MJ, Ott J. (2015) Self administration of oxycodone alters synaptic plasticity gene expression in the hippocampus differentially in male adolescent and adult mice. Neuroscience 285:34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Picetti R, Butelman ER, Schlussman SD, Ho A, Kreek MJ. (2009) Behavioral and neurochemical changes induced by oxycodone differ between adolescent and adult mice. Neuropsychopharmacology 34:912–922. [DOI] [PMC free article] [PubMed] [Google Scholar]