Abstract
Aliskiren has been found to reduce chronic injury and steatosis in the liver of methionine-choline-deficient (MCD) diet-fed mice. This study investigated whether aliskiren has an anti-steatotic effect in HFD-fed mice, which are more relevant to human patients with non-alcoholic fatty liver disease than MCD mice. Mice fed with 4-week normal chow or HFD randomly received aliskiren (50 mg/kg/day) or vehicle via osmotic minipumps for further 4 weeks. Aliskiren reduced systemic insulin resistance, hepatic steatosis, epididymal fat mass and increased gastrocnemius muscle glucose transporter type 4 levels with lower tissue angiotensin II levels in the HFD-fed mice. In addition, aliskiren lowered nuclear peroxisome proliferator-activated receptor gamma and its down-signaling molecules and increased cytochrome P450 4A14 and carnitine palmitoyltransferase 1A (CPT1a) in liver. In epididymal fat, aliskiren inhibited expressions of lipogenic genes, leading to decrease in fat mass, body weight, and serum levels of leptin and free fatty acid. Notably, in the gastrocnemius muscle, aliskiren increased phosphorylation of insulin receptor substrate 1 and Akt. Based on these beneficial effects on liver, peripheral fat and skeletal muscle, aliskiren is a promising therapeutic agent for patients with NAFLD.
Nonalcoholic fatty liver disease (NAFLD) is rapidly emerging as the most prevalent hepatic disorder in the Western world1. Some patients with NAFLD progress to non-alcoholic steatohepatitis, cirrhosis2, end-stage liver disease, and hepatocellular carcinoma3. There are currently no approved effective medications for the treatment of NAFLD, and the effectiveness of non-pharmacological treatments in this patient population is insufficient4. Thus, there is an urgent medical need for pharmacological agents for this disease.
Inhibition of the renin angiotensin system has been shown to improve NAFLD in animal studies5,6,7. However, the efficacy of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers on human NAFLD is uncertain8. Genetic depletion of renin, the rate-determining enzyme for the generation of angiotensin, has been shown to result in resistance to diet-induced obesity and a reduction in fatty liver in mice5. Recently, aliskiren, the first renin inhibitor to be approved for clinical use, has been reported to reduce hepatic steatosis. Kishina et al.9 reported that aliskiren reduced hepatic steatosis in Shionogi-ob/ob mice, however the underlying mechanism to reduce steatosis was not elucidated and insulin resistance was not measured. We recently found that aliskiren attenuated hepatic steatosis by up-regulating fatty acid oxidation-related genes with increasing systemic insulin sensitivity in methionine-choline-deficient (MCD) diet-fed mice10. However, MCD mice have been reported to show better insulin sensitivity than normal mice11. This characteristic of MCD mice is in contrast to clinical patients with NAFLD, who often exhibit increased insulin resistance. Hence, it is still preliminary to translate the results from MCD mice or Shionogi-ob/ob mice to clinical use in patients with NAFLD.
Aliskiren has been demonstrated to improve insulin resistance in transgenic Ren2 rats, obese Zucker rats, streptozotocin-induced diabetic rats, and db/db mice12,13,14,15,16 by improving skeletal muscle glucose transport activity13,15,16 and pancreas islet function12. However, the effect of aliskiren on liver steatosis was not fully investigated in these studies. Therefore, this study aimed to evaluate the possible anti-steatotic effects and mechanisms of the chronic administration of aliskiren in a mouse model with hepatic steatosis and systemic insulin resistance which is relevant to clinical patients. Moreover, we also examined the epididymal fat and gastrocnemius muscle to investigate whether there was any effect of aliskiren on these two important tissues which contribute important roles to lipid metabolism.
Results
Aliskiren decreased lipid accumulation in liver
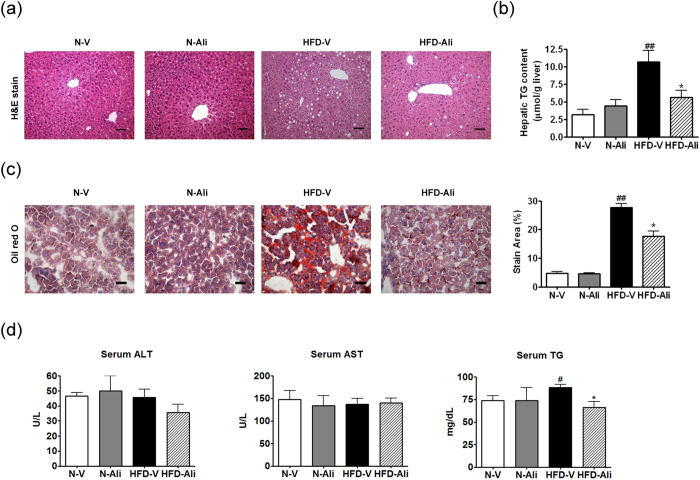
The H&E and Oil Red O stains show that aliskiren decreased hepatic steatosis in the HFD-Ali group compared to the HFD-V group (Fig. 1a,c). The hepatic and serum triglyceride contents were significantly reduced after aliskiren treatment (Fig. 1b,d). There were no differences in the serum levels of alanine aminotransferase and aspartate aminotransferase among the groups (Fig. 1d).
Figure 1. Aliskiren reduced hepatic steatosis in the mice fed with a high fat diet.
(a) Hematoxylin and eosin stains (H&E stain) of liver, scale bar = 100 μm, (b) Hepatic triglyceride (TG) content, (c) Oil Red O stain and the quantification of hepatic steatosis, scale bar = 50 μm, (d) The serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST) and triglyceride (TG) in all groups. N-V: mice fed with a normal diet (n = 6); N-Ali: mice fed with a normal diet and treated with aliskiren (n = 6); HFD-V: mice fed with a high fat diet; HFD-Ali (n = 7): mice fed with a high fat diet and treated with aliskiren (n = 7). #p < 0.05 vs. the N-V group, ##p < 0.01 vs. the N-V group; *p < 0.05 vs. the HFD-V group.
Aliskiren ameliorated systemic insulin resistance
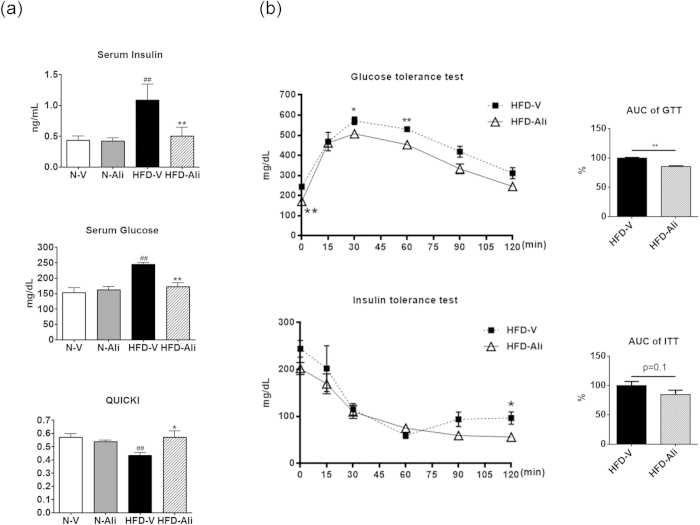
The HFD-V mice exhibited higher serum levels of insulin and glucose and lower levels of quantitative insulin sensitivity check index (QUICKI) than the N-V group (Fig. 2a). Aliskiren treatment significantly decreased serum levels of insulin and glucose and increased QUICKI levels in the HFD-Ali group when compared to the HFD-V group. The HFD-Ali mice had a significant improvement in the glucose tolerance test and a trend of improvement in insulin tolerance test when compared with the HFD-V mice (Fig. 2b). It indicates that aliskiren treatment improves systemic insulin resistance in the HFD-fed mice.
Figure 2. Aliskiren improved insulin sensitivity in the mice fed with a high fat diet.
(a) The levels of insulin and glucose in serum and quantitative insulin sensitivity check index (QUICKI), a measurement of insulin sensitivity derived from the formula 1/(log insulin.glucose) from all groups. N-V: mice fed with a normal diet (n = 6); N-Ali: mice fed with a normal diet and treated with aliskiren (n = 6); HFD-V: mice fed with a high fat diet; HFD-Ali (n = 7): mice fed with a high fat diet and treated with aliskiren (n = 7). (b) Glucose tolerance test (GTT) and insulin tolerance test (IGG) in a set of HFD-V and HFD-Ali mice (n = 4 in each group). The area of the blood glucose response profile curve from each mouse is summed to obtain the area under the curve (AUC). The relative area values of the HFD-Ali group are expressed as a percentage relative to the average AUC of the HFD-V group, which is defined as 100%. ##P < 0.01 vs. N-V; *P < 0.05 vs. HFD-V; **P < 0.01 vs. HFD-V group.
Aliskiren inactivated the PPARγ signaling pathway in the liver
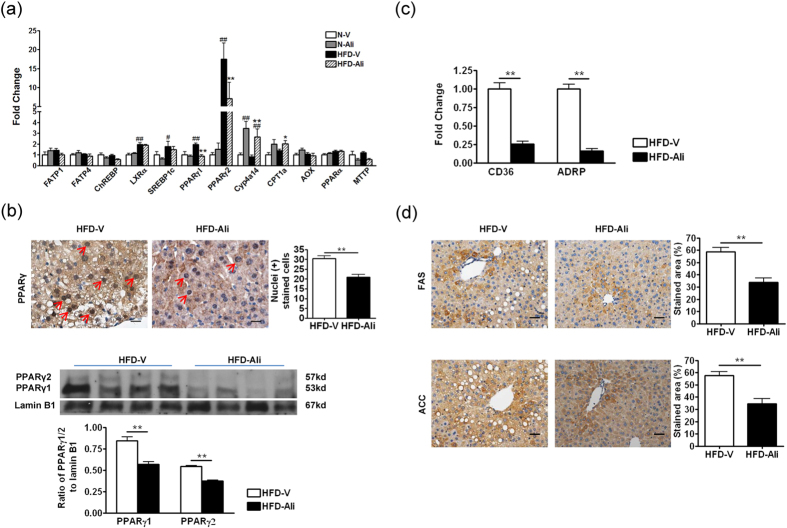
To elucidate which genes are involved in the anti-steatotic effect of aliskiren, the transcript expressions of the genes regulating hepatic lipid metabolism were measured by quantitative RT-PCR. Compared to the N-V mice, the HFD-V mice had significantly higher expressions of liver X receptor alpha (LXRα), sterol regulatory element binding protein-1c, and peroxisome proliferators activated receptor gamma 1/2 (PPARγ1/2) in the liver (Fig. 3a). Aliskiren treatment significantly decreased the hepatic expression levels of PPARγ1/2 (Fig. 3a). Immunohistochemically, it was demonstrated that aliskiren significantly reduced the nuclear staining of PPARγ in hepatocytes (Fig. 3b). Similarly, the nuclear protein expression of PPARγ1/2 was decreased significantly in the aliskiren-treated group (Fig. 3b). In addition, the down-signaling molecules of PPARγ such as free fatty acid uptake transporter (CD36), adipose differentiation-related protein (ADRP), fatty acid synthase (FAS) and acetyl-CoA carboxylase (ACC) were down-regulated by aliskiren (Fig. 3c,d). These findings suggest that aliskiren decreases hepatic lipogenesis by down-regulating a PPARγ signaling pathway.
Figure 3. Aliskiren reduced hepatic steatosis through inactivating PPARγ and upregulating CYP4A14 and CPT1a.
(a) RT-PCR of important genes involving in hepatic lipid metabolism. The results expressed were relative to the normal group, which was arbitrarily assigned a value of 1. Fatty acid transport protein 1/4 (FATP1/4), carbohydrate-responsive element-binding protein (ChREBP), liver X receptor alpha (LXRα), sterol regulatory element binding protein-1c (SREBP-1c), peroxisome proliferator-activated receptor alpha/gamma1/gamma2 (PPARα/γ1/γ2), cytochrome P450, family 4, subfamily a, polypeptide 14 (Cyp4a14), carnitine palmitoyltransferase 1a (CPT1a), acyl-CoA oxidase (AOX), microsomal triglyceride transfer protein (MTTP). N-V: mice fed with a normal diet (n = 6); N-Ali: mice fed with a normal diet and treated with aliskiren (n = 6). HFD-Ali (n = 7): mice fed with a high fat diet and treated with aliskiren (n = 7). (b) The immunohistochemical images of PPARγ and Western blot of nuclear PPARγ and lamin B1 in the HFD-V and HFD-Ali groups. Scale bar = 50 μm. Red arrows indicate positive nuclear stained cells. (c) The transcript expressions of fatty acid translocase (CD36) and adipose differentiation-related protein (ADRP) by RT-PCR in the HFD-V and HFD-Ali groups. The results expressed were relative to the HFD-V group, which was arbitrarily assigned a value of 1. (d) The immunohistochemical images of fatty acid synthase (FAS) and acetyl-CoA carboxylase (ACC) in HFD-V and HFD-Ali groups. Scale bar = 100 μm. #P < 0.05 vs. N-V; ##P < 0.01 vs. N-V; *P < 0.05 vs. HFD-V; **P < 0.01 vs. HFD-V groups.
On the other hand, aliskiren upregulated the transcript expression levels of CYP4A14 and CPT1a (Fig. 3a). These data suggest that on one hand, aliskiren may downregulate PPARγ to reduce de novo lipogenesis; on the other hand, it may stimulate CYP4A14 and CPT1a to promote fatty acid oxidation in liver.
Aliskiren attenuated Ang II-associated oxidative stress and downregulated phosphorylation of Akt and AMPK
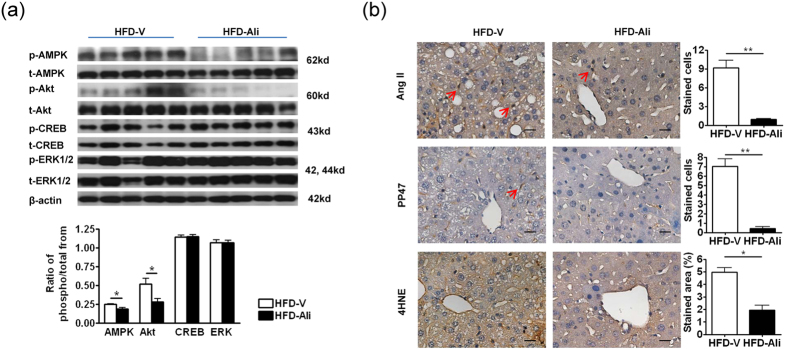
The hepatic expressions of upstream mediators of PPARγ, the phosphorylation of Akt, AMPK, CREB and ERK1/2 were measured in the HFD-V and HFD-Ali mice. After aliskiren treatment, the phosphorylation of Akt and AMPK, but not the phosphorylation of CREB and ERK1/2 decreased significantly in the HFD-fed liver (Fig. 4a). It suggests that downregulation of p-Akt may contribute to the lower PPARγ expression in the aliskiren-treated liver.
Figure 4. Aliskiren reduced phospho-Akt, phospho-AMPK and Ang II-induced oxidative stress.
(a) Western blots of total or phosphorylated AMP-activated protein kinase (t/p-AMPK), protein kinase B (t/p-Akt), cAMP response element-binding protein (t/p-CREB), extracellular signal-regulated kinases (t/p-ERK) and beta-actin (β-actin) in the HFD-V and HFD-Ali groups. (b) The immunohistochemical images of angiotensin II (Ang II), phosphorylated p47 (pp47), and 4-hydroxynonenal (4HNE) in livers of the HFD-V and HFD-Ali groups. Scale bar = 50 μm, *p < 0.05 vs. the HFD-V group; **p < 0.01 vs. the HFD-V group.
To observe the renin-angiotensin inhibitory effect of aliskiren on liver, IHC stains of Ang II were examined and showed that aliskiren decreased Ang II expression in aliskiren-treated liver (Fig. 4b). Similarly, the expression levels of Ang II down-signaling phosphorylated p47 and 4-hydroxynonenal (4HNE) product were subsequently reduced after aliskiren treatment.
Aliskiren reduced epididymal fat mass
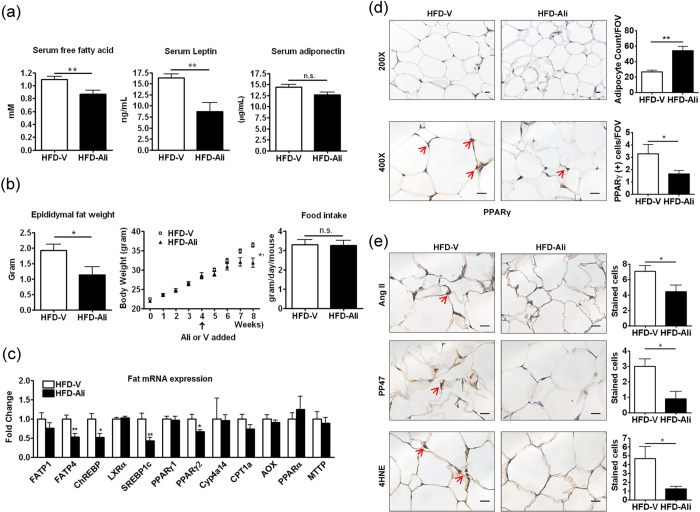
Because circulating free fatty acid also contributed to hepatic steatosis17, we investigated changes in its level. Aliskiren significantly decreased serum free fatty acid level in the HFD-Ali group (Fig. 5a). Peripheral fat is the most important source of serum free fatty acid17, and it was found that the HFD-Ali mice had significantly lower weights of epididymal fat tissue and the whole body without changes in the amount of oral intake (Fig. 5b). We then evaluated transcript expressions of genes regulating lipid metabolism in fat tissues. In contrast to the liver, the HFD-Ali epididymal fat tissues exhibited lower expression levels of FATP4, ChREBP, SREBP1c and PPARγ2 than the HFD-V group (Fig. 5c). The immunohistochemical images of the HFD-Ali fat tissues showed a reduction in the number of positive PPARγ-stained adipocytes and an increase in adipocyte counts which inversely correlated to the size of adipocytes (Fig. 5d). The expressions of angiotensin II, pp47, and 4HNE were decreased in the HFD-Ali epididymal fat tissues (Fig. 5e). Levels of the fat-secreted adipokines, leptin and adiponectin, were then evaluated. Aliskiren did not change the level of serum adiponectin but significantly reduced the level of leptin (Fig. 5a). These findings demonstrate that aliskiren inhibited expressions of lipogenic genes and Ang II in epididymal fat tissues, leading to the reduction in the fat mass, which contributed to the reduction in body weight, serum free fatty acid and leptin.
Figure 5. Aliskiren decreased weight, PPARgamma levels and Ang II-induced oxidative stress in epididymal fat.
(a) The serum free fatty acid, adiponectin and leptin, (b) epididymal fat weight, body weight, and oral intake, (c) transcript expressions of genes regulating lipid metabolism in epididymal fat (abbreviations of genes: see Fig. 3 legend; the results expressed were relative to the HFD-V group, which was arbitrarily assigned a value of 1.), (d) immunohistochemical images of PPARγ and adipocyte number per field of view (FOV) in the epididymal fat tissue (scale bar = 50 μm), and (e) immunohistochemical images of angiotensin II (Ang II), phosphorylated p47 (pp47), and 4-hydroxynonenal (4HNE) in the HFD-V and HFD-Ali groups. Scale bar = 50 μm, *p < 0.05 vs. the HFD-V group; **p < 0.01 vs. the HFD-V group. n.s.: non-significant.
Aliskiren improved insulin sensitivity in skeletal muscle
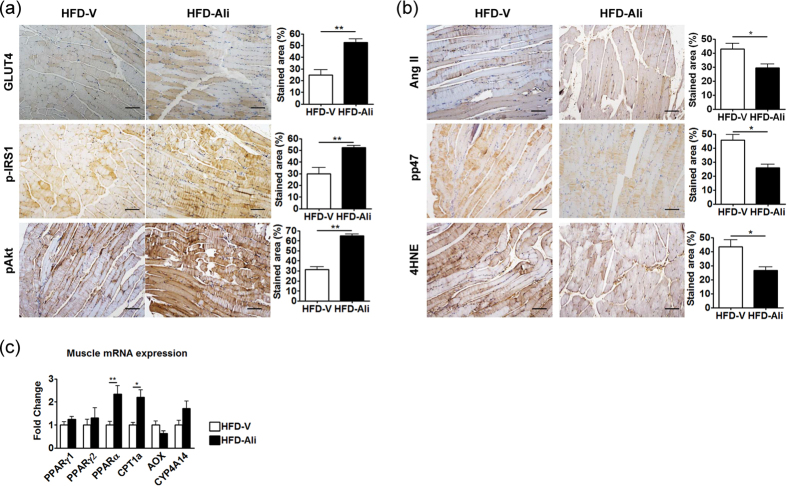
Skeletal muscle is the major insulin-sensitizing tissue and an important site for insulin-stimulated glucose utilization. Thus, the expression levels of type 4 glucose transporter (GLUT4), phosphor-insulin receptor substrate 1 (p-IRS-1), and phosphor-Akt were measured. Interestingly, aliskiren treatment significantly increased expression levels of GLUT4, p-IRS1 and p-Akt in the gastrocnemius muscle (Fig. 6a). It indicates that aliskiren may stimulate glucose utilization and increase insulin sensitivity in skeletal muscle, contributing to the improvement of systemic insulin resistance and reduction in serum glucose levels in HFD-fed mice.
Figure 6. Aliskiren increased insulin sensitivity and upregulated expression levels of PPARα and CPT1a in muscle with reduction in Ang II-associated oxidative stress.
(a) immunohistochemical images of type4 glucose transporter (GLUT4), phosphor-insulin receptor substrate 1 (p-IRS-1), and phosphor-Akt in the gastrocnemius muscle (scale bar = 100 μm). (b) immunohistochemical images of angiotensin II (Ang II), phosphorylated p47 (pp47), and 4-hydroxynonenal (4HNE) (scale bar = 100 μm), and (c) RT-PCR of PPARα/γ1/γ2, CPT1a, AOX, and CYP4A14 in the HFD-V and HFD-Ali groups. The results expressed were relative to the HFD-V group, which was arbitrarily assigned a value of 1. *p < 0.05 vs. the HFD-V group; **p < 0.01 vs. the HFD-V group.
Moreover, aliskiren reduced the expression levels of Ang II, pp47 and 4HNE in the gastrocnemius muscle (Fig. 6b). However, the PPARγ level remained unchanged after aliskiren treatment (Fig. 6c). Interestingly, the transcript expression of PPARα and CPT1a increased significantly in the aliskiren-treated gastrocnemius muscle (Fig. 6c). It suggests that the fatty acid oxidation in skeletal muscle may be increased, contributing to the reduction in serum free fatty acids.
Discussion
This is the first study demonstrating that aliskiren attenuated hepatic steatosis in mice fed with a HFD. Aliskiren down-regulated lipogenic genes in the liver and epididymal fat, activated fatty acid oxidation genes in the liver and skeletal muscle and improved systemic insulin resistance though increasing glucose utilization in skeletal muscle. A few studies have investigated the effect of aliskiren on steatosis in different murine models and the results were inconsistent. Two of them found that aliskiren did not reduce hepatic steatosis in db/db mice14 and the C57BL/6 mice fed with a HFD18 (the routes and dosages of aliskiren were 25 mg/kg/day via mini-pumps subcutaneously and 50 mg/kg/day orally, respectively). However, in the current and other studies, aliskiren significantly decreased hepatic steatosis in C57BL/6 mice fed with a HFD or MCD10 diet and in fatty liver Shionogi-ob/ob mice9 (the routes and dosages of aliskiren were 50 mg/kg/day via mini-pumps subcutaneously and 100 mg/kg/day orally, respectively). In fact, the oral bioavailability of aliskiren is low (around 2%)19 and transdermal delivery of aliskiren increases its bioavailability by 4- to 54-fold compared to the oral route20. Therefore, it is suggested that sufficient bioavailability of aliskiren by an adequate dose or route is necessary to effectively reduce hepatic steatosis.
Kishina et al.9 reported that aliskiren reduced hepatic steatosis in Shionogi-ob/ob mice, however they only measured the hepatic mRNA expressions of SREBP1c, MTP and PPAR-α, and no differences were found between the aliskiren and control groups. It has been reported that genetic disruption of renin-angiotensin system components5,6,7 reduces hepatic steatosis in rodent models, implying that angiotensin II plays a role in fat accumulation in the liver. In the MCD mice of our previous study, angiotensin II levels in the liver were increased. Aliskiren treatment suppressed hepatic angiotensin II and stimulated fatty acid oxidation-related genes such as PPARα, CYP4A, CPT1a in the liver compared to the vehicle-treated MCD mice10. Similarly, in this study, the angiotensin II levels in the liver of the HFD mice were reduced after aliskiren treatment. Interestingly, aliskiren not only enhanced expression levels of fatty acid oxidation-related genes such as CYP4A and CPT1a but also down-regulated lipogenic gene, PPARγ. In fact, the underlying mechanisms of hepatic steatosis in these animal models are different; it seems that aliskerin may act on a particular pathway depending on the cause of hepatic steatosis development.
In this study, aliskiren decreased PPARγ1 and PPARγ2 in the liver of HFD-Ali mice. Both PPAR γ1 and PPARγ2 are expressed at low levels in normal liver, and exposure to the high fat diet in mice has been reported to up-regulate mainly PPARγ2 mRNA in steatotic liver21. Similarly, the HFD-V liver had a nearly 18-fold increase in PPARγ2 mRNA compared to the N-V liver in the current study. PPARγ2 is able to induce lipid accumulation in hepatocytes by selectively inducing several adipogenic and lipogenic genes such as ADRP, CD36, FAS, and ACC22,23,24, leading to increased hepatic de novo lipogenesis and hepatic fat accumulation. In the current study, the HFD-V liver exhibited higher expressions of ADRP, CD36, FAS and ACC. After aliskiren treatment, the expressions of ADRP, CD36, FAS and ACC were lowered with a concomitant reduction in PPARγ2 in the HFD-Ali group. Therefore, aliskiren reduced hepatic steatosis by inactivating a PPARγ2-mediated pathway.
Several upstream signaling molecules may mediate the expression of PPARγ. Activation of Akt and ERK has been reported to enhance PPARγ expression25,26 whereas CREB and AMPK have been reported to inhibit hepatic PPARγ expression to suppress lipid storage and synthesis27,28. In this study, the HFD-Ali liver had decreased phosphorylation levels of Akt and AMPK. The reduction of AMPK may be attributed to the decreased circulating leptin29 and the decreased circulatory insulin could contribute to the decreased phosphorylation of Akt in the liver of the HFD-Ali mice30. Briefly, the downregulation of PPARγ in liver may be the consequence of reduction in the insulin/Akt pathway, not the leptin/AMPK pathway.
Interestingly, aliskiren treatment reduced body weight, epididymal fat mass and serum leptin levels in this study without affecting diet intake of the HFD mice, which is similar to previous studies5,31. The reduction in circulating leptin level was probably due to the decreased adipose fat mass. In addition, because Ang II is involved in adipocyte differentiation, Stucchi et al.31 suggested that the reduction in the local expression of tissue angiotensin II in peripheral adipose tissues after aliskiren treatment might contribute to the reduction of local fat weight and mass. Similarly, in the current study, the Ang II level was reduced in epididymal fat. We further investigated the expressions of genes involving lipid metabolism. In contrast to the findings in the liver, aliskiren had no effect on fatty acid oxidation-related genes, but down-regulated several lipogenic genes such as SREBP1c, ChREBP and PPARγ2. The decreased expressions of lipogenic genes may be attributed to the decreased circulatory insulin. Furthermore, PPARγ promotes adipocyte differentiation and adipocyte hypertrophy which determine fat mass32,33 and increased fat mass with large adipocytes contributes to insulin resistance34. In addition, long-term activity of PPARγ in adipose tissue may lead to insulin resistance when being fed with a high caloric diet33,35. Kubota et al. reported that heterozygous PPARγ-deficient mice have lower insulin resistance, smaller fat mass and adipocyte size under a HFD compared to PPARγ+/+ mice33. Therefore, inactivation of insulin/PPARγ pathway in epididymal fat tissue by aliskiren may reduce fat mass and adipocyte hypertrophy, thereby contributing to a decrease in body weight and insulin resistance.
In this study, the action of aliskiren on the skeletal muscle played a critical role to improve systemic insulin resistance. Lastra et al. first showed that aliskrein-induced reduction in oxidative stress may improve insulin signaling in muscle13. Csibi et al. depicted that Ang II inhibited GLUT4 translocation and Akt activation in the skeletal muscle36. Thus, in this study, aliskiren-induced Ang II reduction may reverse the GLUT4 expression and attenuate oxidative stress to improve insulin resistance in muscle37, which manifested with increased phosphorylation of IRS-1 and Akt in the HFD-Ali group. These effects leaded to amelioration of systemic insulin resistance, subsequently reducing fat accumulation in liver and peripheral fat tissues.
There are limitations in this study. First, it has been reported that the renin knockout mice are lean, insulin sensitive, and resistant to diet-induced obesity without changes in food intake due to a higher metabolic rate and gastrointestinal loss of dietary fat5. However, we did not measure energy expenditure and fecal fat content because of lack of equipment. Second, why aliskiren had different mechanistic profiles in liver, fat and muscle remained unclear. Different expressive levels of Ang II in different tissues may lead to the discrepancy; however, further studies are needed to elucidate the underlying mechanisms.
In conclusion, aliskiren attenuated hepatic steatosis in mice fed with a HFD. Aliskiren increased insulin sensitivity and glucose transport activity in skeletal muscle of the HFD mice, contributing to amelioration of systemic insulin resistance. The reduced circulatory insulin inhibited expressions of lipogenic genes in the liver and epididymal fat, leading to decrease in hepatic steatosis and epididymal fat mass. Furthermore, aliskiren activated the fatty acid oxidation genes in the liver and skeletal muscle, which might play a role in lowering serum fatty acids and steatosis. Therefore, the clinical use of aliskiren treatment in patients with NAFLD may be promising.
Materials and Methods
Animals
Adult male C57BL/6 mice aged 6–8 weeks purchased from (BioLasco Taiwan Co., Ltd., Taipei, Taiwan) were used in all experiments. All of the mice were caged at 22 °C with a 12-hour light-dark cycle, and allowed free access to food (Laboratory Autoclavable Rodent Diet 5010) or a high-fat diet (HFD) (D12492; containing 34.9% fat (mostly saturated) by weight, which yields ∼60% calories from fat and has a caloric density of 5.24 kcal/g) and water. This study was approved by the Animal Experiment Committee of Taipei Veterans General Hospital and performed according to the “guides for the care and use of laboratory animals” prepared by the National Academy of Science, USA.
Protocol
The mice were divided into four groups. Hepatic steatosis, obesity and insulin resistance were induced by feeding with a HFD (n = 14). After 4 weeks of feeding, the HFD mice or mice fed with a normal chow diet were randomly divided to receive aliskiren (50 mg/kg/day for 4 weeks) via ALZET osmotic minipumps (1004 type, DURECT Corporation, Cupertino, CA) (N-Ali/HFD-Ali groups, n = 6/7) or vehicle (N-V/HFD-V groups, n = 6/7). Under zoletil anesthesia, minipumps filled with aliskiren (Novartis, Basel, Switzerland) or vehicle (double distilled water, 100 μL) were implanted subcutaneously into the right upper back of the mice. The dose of aliskiren we chose was according to a previous study38 which showed sufficient inhibition of renin activity in mice in vivo. In addition, we previously found that this dosage can decrease chronic liver injury in MCD mice10.
After feeding with a HFD for 8 weeks, all groups of mice were sacrificed. After an overnight fasting, blood samples were collected by submandibular venipuncture. Serum was separated by refrigerated centrifugation and stored at −80 °C until assay. The liver, epididymal fat, and gastrocnemius muscle were rapidly excised after phosphate-buffered saline perfusion, rinsed in ice-cold saline, and then weighed. Aliquots of tissues were snap frozen in liquid nitrogen and kept at −80 °C until being analyzed. A portion of each tissue was fixed in 10% formalin for histology.
Biochemistry measurements
Levels of serum glucose, aspartate aminotransferase, alanine aminotransferase, and triglyceride were measured using a standard auto SMAC analyzer (Roche Diagnostics GmbH, Mannheim, Germany). Serum free fatty acid was measured using a Free Fatty Acid Assay Kit (Zen-Bio, Inc., Durham, NC).
Measurement of serum insulin, leptin, and adiponectin
Serum insulin was measured using an Ultra-Sensitive Mouse Insulin ELISA Kit (Crystal Chem Inc., Downers Grove, IL), and serum leptin and adiponectin levels were measured using ELISA kits (Abcam, Cambridge, UK).
Glucose and insulin tolerance tests
Another set of HFD-V/HFD-Ali mice (n = 4/4) received glucose and insulin tolerance tests. After a 16-hour fast, glucose tolerance tests were performed by intraperitoneal injection of D-glucose (Sigma-Aldrich, Inc. St. Louis, MO) at a dose of 2.0 mg/g body weight. For insulin tolerance tests, mice were injected with regular human insulin (Santa Cruz Biotechnology, Inc., CA) at a dose of 0.75 U/kg body weight after a 6-hour fast. Blood glucose was measured using a portable blood glucose meter (one touch ultra2, life scan, Johnson&Johnson, USA).
Measurement of hepatic steatosis
Liver steatosis was measured by Oil Red O staining on frozen tissues (8 μm). Hepatic triglyceride levels were assessed using a commercially available kit (Cayman Chemical Company, Ann Arbor, Michigan).
Real-time quantitative reverse transcriptase-polymerase chain reaction (PCR)
The nucleotide sequences of the primers used in this study are shown in Table 1. Quantitative gene expressions were measured on an ABI PRISM 7900HT Sequence detection system (Applied Biosystems Inc. Foster City, CA) using SYBR Green. The specificity of each PCR product was evaluated by melting curve analysis, followed by agarose gel electrophoresis.
Table 1. Primer pairs used for quantitative real time PCR.
| Gene name | Primer | Sequence | Size |
|---|---|---|---|
| FATP1 | forward | 5′-CGC TTT CTG CGT ATC GTC TG -3′ | 120 |
| reverse | 5′-GAT GCA CGG GAT CGT GTC T -3′ | ||
| FATP4 | forward | 5′-GAT GGC CTC AGC TAT CTG TGA -3′ | 202 |
| reverse | 5′-GGT GCC CGA TGT GTA GAT GTA -3′ | ||
| ChREBP | forward | 5′-GGA CAA GAT CCG GCT GAA CA -3′ | 131 |
| reverse | 5′-CAG GTT TCC GGT GCT CAT CT -3′ | ||
| LXRα | forward | 5′- ATC GCC TTG CTG AAG ACC TCT G -3′ | 159 |
| reverse | 5′- GAT GGG GTT GAT GAA CTC CAC C -3′ | ||
| SREBP1c | forward | 5′- CTG GGG GTG AGA CAG GGG AC -3′ | 145 |
| reverse | 5′- GAT GGT GGA GGG GAC AAG GG -3′ | ||
| PPAR-γ1 | forward | 5′- GGACTGTGTGACAGACAAGATTTG -3′ | 52 |
| reverse | 5′- CTGAATATCAGTGGTTCACCGC -3′ | ||
| PPAR-γ2 | forward | 5′-TGG GTG AAA CTC TGG GAG AT -3′ | 454 |
| reverse | 5′-CCA TAG TGG AAG CCT GAT GC -3′ | ||
| CYP4A14 | forward | 5′-CCCTGCTCCGCTTTGAATTG -3′ | 135 |
| reverse | 5′-AGCTGCCCTGACTCCATCA -3′ | ||
| CPT1a | forward | 5′-CGC ACG GAA GGA AAA TGG -3′ | 211 |
| reverse | 5′-TGT GCC CAA TAT TCC TGG -3′ | ||
| AOX | forward | 5′- CTT GTT CGC GCA AGT GAG G -3′ | 213 |
| reverse | 5′- CAG GAT CCG ACT GTT TAC C-3′ | ||
| PPARα | forward | 5′- CCG AAC ATT GGT GTT CGC AG -3′ | 161 |
| reverse | 5′- AGA TAC GCC CAA ATG CAC CA -3′ | ||
| MTTP | forward reverse | 5′- CGT CCA CAT ACA GCC TTG AC -3′ 5′- CCA CCT GAC TAC CAT GAA GC -3′ | 111 |
| CD36 | forward reverse | 5′- CTTCCACATTTCCTACATGCAA -3′ 5′- ATCCAGTTATGGGTTCCACATC -3′ | 109 |
| ADRP | forward reverse | 5′- CTT GTG TCC TCC GCT TAT GTC AGT-3′ 5′- CTG CTC CTT TGG TCT TAT CCA CCA-3′ | 350 |
| GAPDH | forward | 5′- TGT TGA AGT CGC AGG AGA CAA CCT-3′ | 111 |
| reverse | 5′- AAC CTG CCA AGT ATG ATG ACA TCA -3′ |
Western blot analysis
Protein extraction was performed according to standard methods, and nuclear protein was extracted using NE-PER Nuclear extraction reagents (Thermo Scientific, Waltham, MA). The blots were incubated with the primary antibodies shown in Table 2. After washing, the membranes were incubated with horseradish peroxidase-conjugated goat anti rabbit (Jackson ImmunoResearch Laboratories Inc., West Grove, PA) for the rabbit primary antibodies for 1 hour. Subsequently, the blots were developed by enhanced chemiluminescence (ECL Western Blotting Analysis System, Amersham, UK). The intensities of the bands of interest were analyzed using Image J software (National Institutes of Health, Bethesda, MD).
Table 2. Antibody details and conditions used for Western blotting and immunohistochemistry.
| Antibody | Supplier | Catalog no. | Application | Dilution |
|---|---|---|---|---|
| β-actin | Novus | NB600-501 | WB | 1:5000 |
| Lamin B1 | Santa cruz | sc-6216 | WB | 1:1000 |
| PPARγ | Cell Signaling | #2435 | WB/IHC | 1:1000/1:400 |
| t-ERK | Cell Signaling | #9102 | WB | 1:1000 |
| p-ERK | Cell Signaling | #9101 | WB | 1:1000 |
| t-AMPK | Cell Signaling | #2532 | WB | 1:1000 |
| p-AMPK | Cell Signaling | #2535 | WB | 1:1000 |
| t-Akt | Cell Signaling | #9272 | WB | 1:1000 |
| p-Akt | Cell Signaling | #4058 | WB | 1:1000 |
| t-CREB | Cell Signaling | #9197 | WB | 1:1000 |
| p-CREB | Cell Signaling | #9198 | WB | 1:1000 |
| FAS | Cell Signaling | #3180 | IHC | 1:100 |
| ACC | Cell Signaling | #3676 | IHC | 1:200 |
| Ang II | phoenix pharmaceuticals | H-002-12 | IHC | 1:2000 |
| p-p47 | Assay biotech | A1171 | IHC | 1:2500 |
| 4HNE | Alpha diagnostic | HNE11-S | IHC | 1:2500 |
| GLUT4 | Santa cruz | sc-7938 | IHC | 1:200 |
| p-IRS1 | Santa cruz | sc-17200 | IHC | 1:200 |
| p-Akt | Epitomics | #2118-S | IHC | 1:100 |
Antibodies were purchased from Cell signaling (Beverley, MA), Novus biological (Littleton, CO), Santa cruz (Santa Cruz Biotechnology, Inc., CA), phoenix pharmaceuticals (Burlingame, CA), Assay biotech (Sunnyvale, CA), Alpha diagnostic (San Antonio, TX), Epitomics (Burlingame, CA). WB: western blotting; IHC: immunohistochemistry.
Histological studies
The tissues were fixed for 24 hours with 10% paraformaldehyde at room temperature, then dehydrated, embedded in paraffin, cut into 4-μm-thick slices, and stained with hematoxylin and eosin (H&E).
For immunohistochemical staining, the tissues were sliced into 4-μm-thick sections and transferred to a 10 mmol/L citrate buffer solution at pH 6.0 or proteinase K to retrieve antigens. After washing in phosphate-buffered saline, 3% H2O2 was applied to the slides for 10 minutes to block endogenous peroxidase activity. The slides were then incubated at room temperature with 5% heated bovine serum albumin for 30 minutes, followed by incubation at 4 °C overnight with the primary antibodies shown in Table 2. After overnight incubation, the slides were incubated with a secondary antibody for 30 minutes, and then colored using a super-sensitive polymer-HRP IHC detection system (BioGenex Laboratories Inc., Fremont, CA), followed by counterstaining with Mayer’s hematoxylin. Negative control sections were stained under identical conditions except that a buffer solution was substituted for the primary antibody.
Statistical analysis
Data were analyzed using GraphPad Prism 4 (GraphPad Software, San Diego, CA) and expressed as means ± S.E.M. Statistical significance in each group was determined using one-way ANOVA with post hoc multiple comparisons performed using the Newman-Keuls test. When criteria for parametric testing were violated, the Mann-Whitney U-test was performed. Protein levels between the two paired groups were compared using the Student’s t-test. Significance was determined at a p-value less than 0.05.
Additional Information
How to cite this article: Lee, K.-C. et al. Aliskiren Reduces Hepatic steatosis and Epididymal Fat Mass and Increases Skeletal Muscle Insulin Sensitivity in High-Fat Diet-Fed Mice. Sci. Rep. 6, 18899; doi: 10.1038/srep18899 (2016).
Acknowledgments
The authors gratefully acknowledge Miss Chia-Li Chen for her technical assistance, Dr. Chen-Yu Wang for helpful discussion, and Novartis International for providing aliskiren powder. This work was supported by the Taipei Veterans General Hospital, Taipei, Taiwan (grants V103B-002 and V103C-012).
Footnotes
Author Contributions K.-C.L. research question, study design, data collection, data analysis and manuscript writing; C.-C.C. data collection; Y.-Y.Y. data interpretation; Y.-C.H. literature search, data collection; Y.-H.H. technical and material support; H.-C.L. study concept, data interpretation, obtained funding.
References
- Farrell G. C. & Larter C. Z. Nonalcoholic fatty liver disease: from steatosis to cirrhosis. Hepatology 43, S99–S112 (2006). [DOI] [PubMed] [Google Scholar]
- Fassio E., Alvarez E., Dominguez N., Landeira G. & Longo C. Natural history of nonalcoholic steatohepatitis: a longitudinal study of repeat liver biopsies. Hepatology 40, 820–826 (2004). [DOI] [PubMed] [Google Scholar]
- Ratziu V. & Poynard T. Assessing the outcome of nonalcoholic steatohepatitis? It’s time to get serious. Hepatology 44, 802–805 (2006). [DOI] [PubMed] [Google Scholar]
- Katan M. B. Weight-loss diets for the prevention and treatment of obesity. N Engl J Med 360, 923–925 (2009). [DOI] [PubMed] [Google Scholar]
- Takahashi N. et al. Increased energy expenditure, dietary fat wasting, and resistance to diet-induced obesity in mice lacking renin. Cell Metab 6, 506–512 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayasooriya A. P. et al. Mice lacking angiotensin-converting enzyme have increased energy expenditure, with reduced fat mass and improved glucose clearance. Proc Natl Acad Sci USA 105, 6531–6536 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabeshima Y., Tazuma S., Kanno K., Hyogo H. & Chayama K. Deletion of angiotensin II type I receptor reduces hepatic steatosis. J Hepatol 50, 1226–1235 (2009). [DOI] [PubMed] [Google Scholar]
- Paschos P. & Tziomalos K. Nonalcoholic fatty liver disease and the renin-angiotensin system: Implications for treatment. World J Hepatol 4, 327–331 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishina M. et al. Therapeutic effects of the direct renin inhibitor, aliskiren, on non-alcoholic steatohepatitis in fatty liver Shionogi ob/ob male mice. Hepatol Res 44, 888–896 (2014). [DOI] [PubMed] [Google Scholar]
- Lee K. C. et al. Aliskiren attenuates steatohepatitis and increases turnover of hepatic fat in mice fed with a methionine and choline deficient diet. PloS one 8, e77817 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rinella M. E. & Green R. M. The methionine-choline deficient dietary model of steatohepatitis does not exhibit insulin resistance. J Hepatol 40, 47–51 (2004). [DOI] [PubMed] [Google Scholar]
- Habibi J. et al. Renin inhibition attenuates insulin resistance, oxidative stress, and pancreatic remodeling in the transgenic Ren2 rat. Endocrinology 149, 5643–5653 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lastra G. et al. Direct renin inhibition improves systemic insulin resistance and skeletal muscle glucose transport in a transgenic rodent model of tissue renin overexpression. Endocrinology 150, 2561–2568 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang Y. S. et al. Aliskiren improves insulin resistance and ameliorates diabetic vascular complications in db/db mice. Nephrol Dial Transplant 26, 1194–1204 (2011). [DOI] [PubMed] [Google Scholar]
- Gandhi S., Srinivasan B. & Akarte A. S. Aliskiren improves insulin resistance and ameliorates diabetic renal vascular complications in STZ-induced diabetic rats. J Renin Angiotensin Aldosterone Syst 14, 3–13 (2013). [DOI] [PubMed] [Google Scholar]
- Marchionne E. M., Diamond-Stanic M. K., Prasonnarong M. & Henriksen E. J. Chronic renin inhibition with aliskiren improves glucose tolerance, insulin sensitivity, and skeletal muscle glucose transport activity in obese Zucker rats. Am J Physiol Regul Integr Comp Physiol 302, R137–142 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly K. L. et al. Sources of fatty acids stored in liver and secreted via lipoproteins in patients with nonalcoholic fatty liver disease. J Clin Invest 115, 1343–1351 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frantz E. D., Penna-de-Carvalho A., Batista Tde M., Aguila M. B. & Mandarim-de-Lacerda C. A. Comparative effects of the renin-angiotensin system blockers on nonalcoholic fatty liver disease and insulin resistance in C57BL/6 mice. Metabolic syndrome and related disorders 12, 191–201 (2014). [DOI] [PubMed] [Google Scholar]
- Vaidyanathan S., Jarugula V., Dieterich H. A., Howard D. & Dole W. P. Clinical pharmacokinetics and pharmacodynamics of aliskiren. Clin Pharmacokinet 47, 515–531 (2008). [DOI] [PubMed] [Google Scholar]
- Toulmond S., Kwong E. & Macdonald D. inventors; Merck Canada Inc., assignee. Transdermally administered aliskiren. European patent EP2373306 A1. 2011 Oct 12.
- Vidal-Puig A. et al. Regulation of PPAR gamma gene expression by nutrition and obesity in rodents. J Clin Invest 97, 2553–2561 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schadinger S. E., Bucher N. L., Schreiber B. M. & Farmer S. R. PPARgamma2 regulates lipogenesis and lipid accumulation in steatotic hepatocytes. Am J Physiol Endocrinol Metab 288, E1195–1205 (2005). [DOI] [PubMed] [Google Scholar]
- Zhang Y. L. et al. Aberrant hepatic expression of PPARgamma2 stimulates hepatic lipogenesis in a mouse model of obesity, insulin resistance, dyslipidemia, and hepatic steatosis. J Biol Chem 281, 37603–37615 (2006). [DOI] [PubMed] [Google Scholar]
- Zhou J. et al. Hepatic fatty acid transporter Cd36 is a common target of LXR, PXR, and PPARgamma in promoting steatosis. Gastroenterology 134, 556–567 (2008). [DOI] [PubMed] [Google Scholar]
- Kim S. P. et al. Transcriptional activation of peroxisome proliferator-activated receptor-gamma requires activation of both protein kinase A and Akt during adipocyte differentiation. Biochem Biophys Res Commun 399, 55–59 (2010). [DOI] [PubMed] [Google Scholar]
- Prusty D., Park B. H., Davis K. E. & Farmer S. R. Activation of MEK/ERK signaling promotes adipogenesis by enhancing peroxisome proliferator-activated receptor gamma (PPARgamma ) and C/EBPalpha gene expression during the differentiation of 3T3-L1 preadipocytes. J Biol Chem 277, 46226–46232 (2002). [DOI] [PubMed] [Google Scholar]
- Herzig S. et al. CREB controls hepatic lipid metabolism through nuclear hormone receptor PPAR-gamma. Nature 426, 190–193 (2003). [DOI] [PubMed] [Google Scholar]
- Sozio M. S., Lu C., Zeng Y., Liangpunsakul S. & Crabb D. W. Activated AMPK inhibits PPAR-{alpha} and PPAR-{gamma} transcriptional activity in hepatoma cells. Am J Physiol Gastrointest Liver Physiol 301, G739–747 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minokoshi Y. et al. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature 415, 339–343 (2002). [DOI] [PubMed] [Google Scholar]
- Pessin J. E. & Saltiel A. R. Signaling pathways in insulin action: molecular targets of insulin resistance. J Clin Invest 106, 165–169 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stucchi P., Cano V., Ruiz-Gayo M. & Fernandez-Alfonso M. S. Aliskiren reduces body-weight gain, adiposity and plasma leptin during diet-induced obesity. British journal of pharmacology 158, 771–778 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tontonoz P., Hu E. & Spiegelman B. M. Stimulation of adipogenesis in fibroblasts by PPAR gamma 2, a lipid-activated transcription factor. Cell 79, 1147–1156 (1994). [DOI] [PubMed] [Google Scholar]
- Kubota N. et al. PPAR gamma mediates high-fat diet-induced adipocyte hypertrophy and insulin resistance. Molecular cell 4, 597–609 (1999). [DOI] [PubMed] [Google Scholar]
- Guilherme A., Virbasius J. V., Puri V. & Czech M. P. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol 9, 367–377 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferre P. The biology of peroxisome proliferator-activated receptors: relationship with lipid metabolism and insulin sensitivity. Diabetes 53 Suppl 1, S43–50 (2004). [DOI] [PubMed] [Google Scholar]
- Csibi A., Communi D., Müller N. & Bottari S. P. Angiotensin II Inhibits Insulin-Stimulated GLUT4 Translocation and Akt Activation through Tyrosine Nitration-Dependent Mechanisms. PloS one 5, e10070 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenbit A. E. et al. GLUT4 heterozygous knockout mice develop muscle insulin resistance and diabetes. Nat Med 3, 1096–1101 (1997). [DOI] [PubMed] [Google Scholar]
- Lu H. et al. Renin inhibition reduces hypercholesterolemia-induced atherosclerosis in mice. J Clin Invest 118, 984–993 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]