Abstract
Study Objectives:
Sleep restriction alters food intake, but less is known about how dietary patterns affect sleep. Current goals were to determine whether: (1) sleep is different after consumption of a controlled diet vs. an ad libitum diet, and (2) dietary intake during ad libitum feeding is related to nocturnal sleep.
Methods:
Twenty-six normal weight adults (30–45 y), habitually sleeping 7-9 h/night, participated in a randomized-crossover inpatient study with 2 phases of 5 nights: short (4 h in bed) or habitual (9 h in bed) sleep. Only data from the habitual sleep phase were used for the present analyses. During the first 4 days, participants consumed a controlled diet; on day 5, food intake was self-selected. Linear regression was used to determine relations between daytime food intake and nighttime sleep on day 5.
Results:
Sleep duration did not differ after 3 days of controlled feeding vs. a day of ad libitum intake. However, sleep after ad libitum eating had less slow wave sleep (SWS, P = 0.0430) and longer onset latency (P = 0.0085). Greater fiber intake predicted less stage 1 (P = 0.0198) and more SWS (P = 0.0286). Percent of energy from saturated fat predicted less SWS (P = 0.0422). Higher percent of energy from sugar and other carbohydrates not considered sugar or fiber was associated with arousals (P = 0.0320 and 0.0481, respectively).
Conclusions:
Low fiber and high saturated fat and sugar intake is associated with lighter, less restorative sleep with more arousals. Diet could be useful in the management of sleep disorders but this needs to be tested.
Clinical Trial Registration:
Citation:
St-Onge MP, Roberts A, Shechter A, Choudhury AR. Fiber and saturated fat are associated with sleep arousals and slow wave sleep. J Clin Sleep Med 2016;12(1):19–24.
Keywords: sleep duration, sleep architecture, food intake, diet
INTRODUCTION
It is now well established that short sleep duration is associated with obesity and risk of future weight gain. Cross-sectional and longitudinal studies alike have demonstrated this relationship in both adults and children.1,2 Moreover, Grandner et al. have shown that total sleep time (TST) was negatively associated with fat intake in women.3 These associations, however, do not equate causality, and studies assessing the effects of sleep restriction on energy balance have been undertaken to elucidate the causation of the relationship. Clinical studies have shown that sleep restriction leads to increased energy intake, energy intake from snacks, and intake of energy-dense foods.4–10 It seems, therefore, that altering sleep can affect food choice and macronutrient intake.
Interestingly, the reverse causation, whether food choice and dietary patterns affect sleep, has received much less attention. Severe energy restriction is known to disturb sleep.11 Karklin et al. reported that 4 weeks of an 800-kcal diet in 9 overweight women increased sleep onset latency (SOL) and decreased time spent in slow wave sleep (SWS).12 Two days of a high-carbohydrate, low-fat diet also decreased SWS and increased REM sleep in 8 normal-weight men compared to a 2-day diet low in carbohydrates, high in fat, and a balanced diet.13 More studies have assessed the effects of single meals, differing either in size or in macronutrient composition, on post-meal sleepiness or nightly sleep. Such studies have shown that a high-carbohydrate meal increases sleepiness in women relative to a low-carbohydrate meal14 but does not affect SOL to a post-meal nap.15 However, a high-glycemic index meal reduced SOL relative to a low-glycemic index meal, with no effect on TST or sleep architecture.16 Similarly, high-energy meals did not affect TST post-meal compared to low-energy meals.17,18
BRIEF SUMMARY
Current Knowledge/Study Rationale: Research has established a convincing link between short/disrupted sleep duration and food intake. Here we aimed to investigate the effects of dietary intake on subsequent sleep propensity, depth, and architecture.
Study Impact: Few studies have utilized controlled conditions to determine how food intake affects sleep. Current findings—that daytime fat and sugar/fiber content affect nocturnal sleep—imply that diet-based recommendations might be used to improve sleep in those with poor sleep quality.
There is thus very little information on the role of diet on sleep patterns. Studies have been small, of short duration, and with no clear focus on the nighttime sleep episode. Additionally, most work on the effects of diet on sleep has been based on epidemiological findings relying on self-report of food in-take or on the acute effects of a single meal. It is therefore important to have data based on direct observations of daily dietary intake to determine how this can affect nocturnal sleep. The purpose of this study, therefore, was two-fold: first, to assess whether sleep patterns differed after periods of controlled feeding and ad libitum intake; and second, whether intake on an ad libitum feeding day was related to sleep patterns at night. We aimed to answer these questions by comparing nocturnal sleep after a day of a strictly controlled and balanced weight-maintenance diet compared to a day wherein the participant could freely make their own food choices based on preference.
METHODS
This investigation is a secondary analysis of data from a previous study aimed at assessing the effects of sleep restriction on energy balance in normal sleeping adults. Details of the study and main results have been published.8,19,20 Briefly, 30- to 45-year-old men and women, with body mass index 22–26 kg/m2, and who reported sleeping 7–9 h/night with no daytime naps, were recruited for this study. Some exclusion criteria included shift work or any work that required frequent travel across time zones, metabolic disorders such as type 2 diabetes, cardiovascular disease, and hypertension, smoking, and eating disorders, sleeping disorders, or neurological disorders. Further exclusion criteria included the use of medication, including benzodiazepines, antidepressants, and other medications for insomnia. The presence of sleep disorders, excessive daytime sleepiness, and poor sleep quality were also exclusionary, and were assessed with the Sleep Disorders Inventory Questionnaire, Epworth Sleepiness Scale, and Pittsburgh Sleep Quality Index, respectively. Finally, recordings from the first night's polysomnographic (PSG) monitoring were analyzed to exclude participants with obstructive sleep apnea or periodic leg movement disorder. One participant was excluded for periodic limb movement disorder after the first phase of the study, and one participant was excluded for use of antidepressant medication prior to starting the study. Participants were screened by actigraphy over a 2-week period to ensure normal sleep duration of 7–9 h/night. To exclude participants with habitual short sleep, the mean sleep duration over 14 nights was required to fall between 7 and 9 h/night, with ≥ 10 nights of sleep of ≥ 7 h and < 4 nights with < 6 h of sleep. The study was approved by St. Luke's/Roosevelt Hospital Institutional Review Board and Columbia University Medical Center Institutional Review Board. All participants provided informed consent after being given information about the study and having the opportunity to ask questions.
Once enrolled, participants were randomly assigned to one of 2 intervention phases: restricted or habitual sleep. During the short sleep phase, participants were limited to 4 h time in bed, at 01:00–05:00; during the habitual sleep phase, participants spent 9 h in bed, from 22:00 to 07:00. Participants were tested under both conditions, in a crossover design, with a 3-week washout period separating each phase. Each intervention phase was 6 days in duration. During this time, participants were inpatients at Clinilabs, a sleep research facility in midtown Manhattan (New York, NY).
The first 4 days of each intervention were performed under controlled feeding conditions; the last 2 days were done under ad libitum feeding conditions. Meals for both phases were served at 08:00 (breakfast), noon (lunch), 16:30 (snack), and 19:00 (dinner). Meal times and eating occasions were not controlled during the ad libitum days. The controlled diet provided approximately 31% of energy from fat (approximately 7.5% from saturated fat), 53% of energy from carbohydrates, and 17% of energy from protein. Energy requirements were calculated for each participant using the Harris-Benedict equation.21 Each meal provided 30% of daily energy requirements and the snack provided the remaining 10%. Meals were assembled and prepared at the Bionutrition Unit of the Columbia University Irving Institute for Clinical and Translational Research (New York, NY). For the ad libitum feeding days, participants were given a monetary allowance ($25) to purchase foods and beverages of their choice to consume in the lab on days 5 and 6. Participants were told that they could purchase any item for which the nutrient content was readily available. Caffeine in-take throughout the study was limited to one coffee beverage per day, with breakfast. Food intake on days 5 and 6 was assessed by weighing foods pre- and post-consumption. Macronutrient in-take was determined using Diet Analysis Plus software, version 8.0 (Wadsworth, Florence, KY).8 Sleep was assessed every night in the lab using PSG, as previously described.22 Participants slept, on average, 3 h 46 min during the short sleep phase and 7 h 35 min in the habitual sleep phase. Because of the high sleep efficiency in the short sleep phase, only data from the habitual sleep phase were used for the present analyses. Sleep data from night 3, after 3 days of controlled feeding, and night 5, after one day of ad libitum food intake, were analyzed. Data from night 4 were not used because this was a night of nocturnal blood sampling, and the presence of a catheter may have disturbed sleep. In addition, food intake on day 4 was modified from previous days because an oral glucose tolerance test was performed in the morning, in lieu of the regular breakfast. Finally, participants were discharged on the evening of day 6, and no sleep data are available for that day. Therefore, day 5 is the only day when self-selected food intake was measured before a night of PSG sleep monitoring. Sleep scoring was conducted according to AASM 2007 criteria23 by a single certified benchmark scorer.
Statistical Analyses
Linear mixed-model repeated measure analysis (with an intercept/slope term, which was tested) was used to compare differences in the amounts of PSG sleep architecture parameters obtained on night 3 vs. night 5 during the habitual sleep duration condition. In particular, sleep parameters investigated included TST, SOL, number of arousals, and the amounts of stage 1 sleep, stage 2 sleep, SWS, and REM sleep, expressed in absolute minutes and as a percentage of TST. Night (5 vs. 3), sex, and phase order were included as independent variables, and participant ID as grouping variable. Linear model analysis was also used to assess the relationship between food intake parameters from day 5 and PSG-assessed sleep variables from night 5; dietary variables including percent of energy from protein, sugar, non-fiber/non-sugar carbohydrates, unsaturated fat, and saturated fat, and grams of fiber were designated as independent predictor variables, with TST, sleep stages (minutes and percentage of stage 1 sleep, stage 2 sleep, SWS, and REM sleep), and arousals from PSG analyses of night 5 designated as outcome variables. These models included sex and phase order as covariates. Data are expressed as mean ± SD. A p value < 0.05 was used to define statistical significance.
RESULTS
Data describing our research participants have been previously published.8 Briefly, 14 men and 13 women completed the study and one man was excluded from these analyses since he was considered an outlier based on his food intake data. A CONSORT diagram representing the flow of participants throughout the study was previously published.24 Participants were on average 35.1 ± 5.1 y of age and had a body mass index of 23.5 ± 1.3 kg/m2. All participants had at least some college education; 12 were white, 5 were black, 6 were Hispanic, and 3 had other or mixed racial background.
Sleep Differences between Night 3 and Night 5
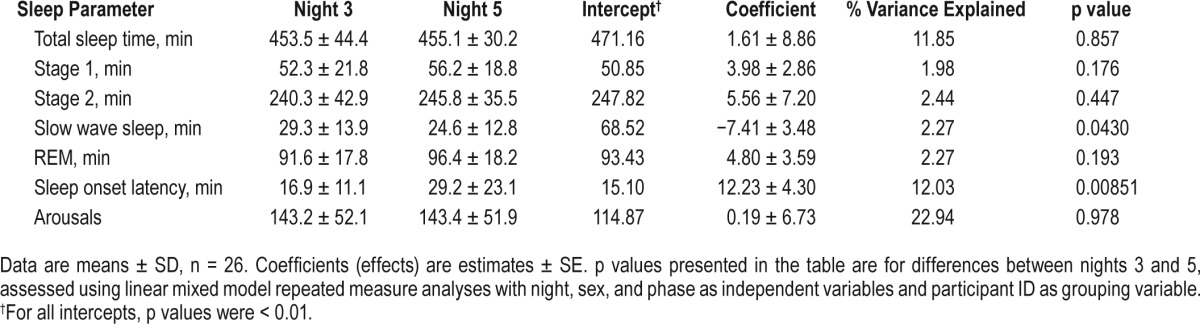
There were no differences in TST and absolute time spent in stage 1, stage 2, and REM sleep between nights 3 and 5 (Table 1). However, night 5 was associated with reduced absolute and percent time spent in SWS (p = 0.0430 and 0.0565, respectively) after adjusting for sex and phase order. Latency to the first 10 min of sleep was longer on night 5 than night 3 (p = 0.0085). Looking at individual data, a total of 9 participants (35% of sample) who initially had a SOL < 30 min on night 3 demonstrated an increase in SOL to > 30 min on night 5. No differences in sleep duration and architecture, except for arousals, were observed between men and women; men had more arousals than women (p = 0.011).
Table 1.
Sleep architecture on night 3, after a period of controlled feeding, and night 5, after a day of ad libitum food intake.
Relationship between Diet and Sleep Parameters on Night 5
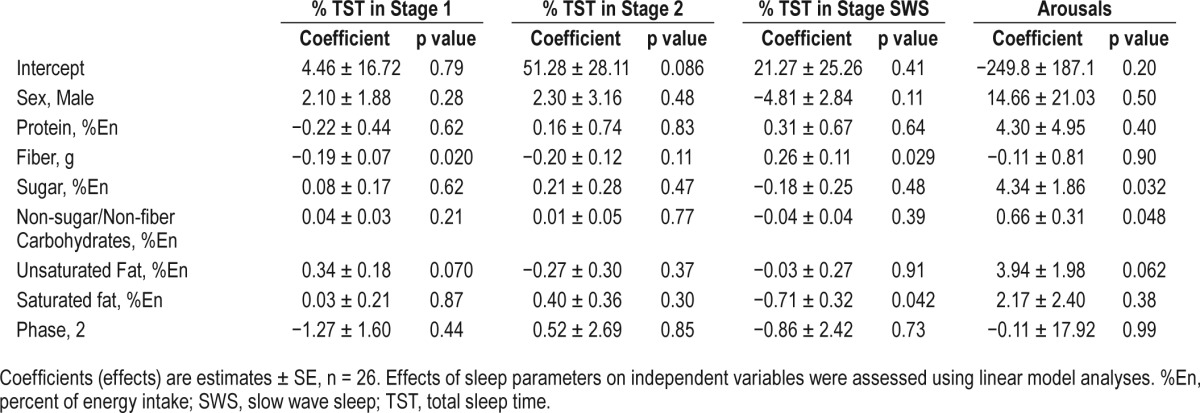
Energy intake on day 5 has been previously reported.8 In short, participants consumed significantly more energy on day 5 during the short vs. habitual sleep condition.24 Participants obtained approximately 14% of their energy intake from protein, 54.6% from carbohydrates, and 32.7% from fat (10% from saturated fat). Diet was not related to TST on night 5 (Table 2). However, fiber intake was associated with reduced time spent in stage 1 sleep (absolute time: p = 0.023; percent of TST: p = 0.020). Conversely, fiber intake was associated with greater time spent in SWS (absolute time: p = 0.039; percent of TST: p = 0.029). Percent energy consumed from saturated fat was associated with reduced time in SWS (absolute time: p = 0.031; percent of TST: p = 0.042). Arousals on night 5 were associated with male sex (p = 0.012) and percent of energy consumed from sugar (p = 0.032) and non-sugar/non-fiber carbohydrates (p = 0.048).
Table 2.
Results of the regression analysis for percent sleep time spent in stage 1, stage 2, and slow wave sleep after a day of ad libitum food intake in men and women.
DISCUSSION
This study shows that diet can influence nighttime sleep propensity, depth, and architecture. First, we report differences in nocturnal sleep after a 3-day controlled feeding period compared to one day of ad libitum, self-selected food consumption. Then, using regression analyses, we observed significant relationships between daytime intake of fiber and saturated fat on sleep depth. Ad libitum food intake was associated with a decrease in SWS and an increase in SOL. Indeed, over a third of participants increased their SOL to over 30 min after the ad libitum feeding day. This finding is clinically relevant since the 30-min SOL threshold is typically used as a cut-point to indicate sleep onset insomnia.25 A greater intake of saturated fat and lower intake of fiber were associated with a lighter, less deep sleep profile. Additionally, increased intake of both sugar and non-sugar/non-fiber carbohydrates was associated with more nocturnal arousals during sleep. These results are important since there is currently very little information on the role of diet on sleep, and dietary recommendations for lifestyle management of sleep disorders are lacking.
Our results show that higher saturated fat intake throughout the day was associated with a lesser amount of SWS at night. This is in contrast with a small study by Phillips et al.13 that showed less SWS after a 2-day consumption period of a high-carbohydrate/low-fat diet compared to a low-carbohydrate/high-fat diet in 8 healthy, normal-weight men. More recently, Crispim et al.26 reported that high fat intake, specifically at dinner and later in the evening, was related to lower sleep efficiency, greater time to the first REM episode, greater sleep time spent in stage 2 sleep with less time spent in REM sleep, and greater time spent awake after having fallen asleep (wake after sleep onset). Of note is that the study by Crispim and colleagues included a relatively large number of men and women (25 and 27, respectively), and food intake was assessed over a 3-day period via self-reported food diaries prior to the sleep assessment. Moreover, participants reported a macronutrient intake profile similar to that of the controlled diet in the present study. Participants were younger than those enrolled in our study but had similar body mass index. A limitation of those studies is their self-report nature, leading to potentially erroneous data,27 and failure to report on the type of fat consumed. Differences in fat type, saturated or unsaturated, may explain some of the discrepancies between studies. Our data, on the other hand, are based on direct observations of dietary intake.
Our data also revealed an association between percent of energy consumed from sugar and non-sugar/non-fiber carbohydrates throughout the day and nighttime arousals. A recent epidemiological study by Yamaguchi et al.28 showed greater odds of poor sleep-wake regularity in those with the highest reported intake of carbohydrates compared to moderate carbohydrate consumption (≥ 70.7% of energy vs. 61% to 66%, respectively). On the other hand, high carbohydrate intake was associated with reduced odds of having difficulty maintaining sleep in the 2007–2008 NHANES dataset.29 Spring et al.14 found that women reported feeling more sleepy, and men more calm, after a high carbohydrate meal (86% of energy from carbohydrates) compared to a high protein meal (85% of energy from protein), although subsequent nocturnal sleep was not reported. Along the same lines, Afaghi et al.16 found that participants tended to feel sleepier and less awake after a high glycemic index compared to a low glycemic index evening meal. In that study, sleep onset latency was 8.5 min shorter after the high glycemic index meal compared to the low glycemic index meal, but TST and sleep architecture and quality, including arousal index, were not different between conditions.
An effect on the circadian system may be one speculative explanation for the current finding of an association between carbohydrate intake and worsened nocturnal sleep. For example, a carbohydrate-rich meal in the evening was found to delay the circadian rhythm of core body temperature and reduce nocturnal melatonin secretion.30 This is relevant, since sleep propensity and quality are highest near the declining limb of the core body temperature curve when melatonin levels are increased.31,32 Although body temperature and melatonin were not recorded in the current study, these effects would be consistent with an increase in SOL on night 5 and the association with nocturnal arousals observed here. Conversely, fiber intake is associated with deeper, more restorative sleep. Therefore, it is possible that a diet rich in fiber, with reduced intake of sugars and other non-fiber carbohydrates, may be a useful tool to improve sleep depth and architecture in individuals with poor sleep. This hypothesis requires further investigation.
There is a large body of evidence showing a relationship between sleep and food intake. For example, Grandner et al.3 showed that sleep duration, assessed by actigraphy, was negatively correlated with fat intake, and subjective napping, positively correlated with fat intake, in women from the Women's Health Initiative. On the other hand, a report from the same group using National Health and Nutrition Examination Survey (NHANES) data showed lower intake of fats and carbohydrates among very short and long sleepers compared to normal sleepers.33 These results conflict with data from clinical intervention studies that report increased carbohydrate and fat intake when participants are forced to curtail their sleep by 2 to 4 h,5,7,9,24 and the findings of another epidemiological study which reported greater consumption of carbohydrates and lower consumption of fiber in short sleepers than individuals obtaining sufficient sleep duration.34 The NHANES study only used one survey round of data (2007–2008) rather than multiple survey years, which may cause unreliable statistical estimates.35 Additionally, the cross-sectional design, self-reported sleep, and one-day self-reported 24-h recall of dietary data from one cycle prevent conclusions on the direction of the relationship and the implications of causality. We and others have reported that alterations in sleep architecture may affect components of energy balance and hunger.22,36 There is likely a feed-forward mechanism whereby food intake patterns affect sleep architecture, which then further affects decision-making relative to food intake and leads to alterations in dietary consumption patterns. This, however, could not be tested using the data obtained in the present study since we only had one day of free-living energy intake and one night of PSG-assessed sleep under ad libitum feeding conditions.
Our study had several strengths, including the controlled nature of the study and therefore lack of bias due to self-report of dietary intake and sleep. On the other hand, data were obtained in the artificial setting of the laboratory. This limitation was somewhat mitigated by providing participants with a monetary allowance to purchase foods that they wanted to eat during the ad libitum feeding day. Another important aspect of this study is that ad libitum intake measurements were obtained after a 4-day period of controlled feeding, such that all participants had similar prior food exposure. However, our ad libitum measurement period was only for a single day. It is therefore unknown whether the associations observed in the current study represent transient acute effects of a change in dietary intake or whether they would persist with continued exposure and consumption of this dietary pattern. Moreover, the inpatient design of the study necessarily restricted physical activity and exercise opportunities in participants. This could have potentially affected some sleep parameters within the current report, as exercise is known to improve SWS and SOL in particular.37 However, the current reported differences in SWS and SOL occurred between night 3 and night 5 on the habitual sleep condition, when physical activity was relatively constant. This implies that changes in food intake, as opposed to changes in exercise or fatigue associated with the sleep intervention, are likely to be driving the results on SWS and SOL. Finally, the study would be strengthened by the inclusion of a morning sleep diary to evaluate subjective sleep quality of the preceding sleep episode. This could better contextualize the observations and ramifications of decreased SWS and increased SOL observed after ad libitum feeding compared to controlled feeding.
Future studies are needed to evaluate the role of diet on sleep. Emerging epidemiological evidence, along with the results of the present analysis, suggest that dietary patterns with differing fat and sugar/fiber content in particular, may affect nocturnal sleep depth, propensity, and architecture. However, further testing is needed to determine causality. If this is the case, then diet-based recommendations may be warranted for those who suffer from sleep disorders, including insomnia, short sleep duration, and poor overall sleep quality. Current findings also have clinical applications for patients undergoing dietary-based therapies. Specifically, a high-fat, low-carbohydrate ketogenic diet has been promoted as a therapeutic option for several neurological disorders including Alzheimer disease, Parkinson disease, and epilepsy.38 These dietary alterations may be associated with changes in nocturnal sleep, and indeed, insomnia has been reported in response to a ketogenic diet.39 Therefore, increasing our understanding of the impact of dietary intake on nocturnal sleep will have many important and practical ramifications for public health.
DISCLOSURE STATEMENT
This study was funded by the National Institutes of Health grants #1R01HL091352 (St-Onge) and P30 DK26687, and by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant Number UL1 TR000040, formerly the National Center for Research Resources, Grant Number UL1 RR024156. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The Almond Board of California provided almonds and Cabot Cheese provided cheese for the study. The funding agencies played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript. Dr. Roberts receives salary from and owns intellectual property rights with Healthy Bytes, Inc. The other authors have indicated no financial conflicts of interest.
ABBREVIATIONS
- SOL
sleep onset latency
- SWS
slow-wave sleep
- TST
total sleep time
REFERENCES
- 1.Chen X, Beydoun MA, Wang Y. Is sleep duration associated with childhood obesity? A systematic review and meta-analysis. Obesity (Silver Spring) 2008;16:265–74. doi: 10.1038/oby.2007.63. [DOI] [PubMed] [Google Scholar]
- 2.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008;16:643–53. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grandner MA, Kripke DF, Naidoo N, Langer RD. Relationships among dietary nutrients and subjective sleep, objective sleep, and napping in women. Sleep Med. 2010;11:180–4. doi: 10.1016/j.sleep.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bosy-Westphal A, Hinrichs S, Jauch-Chara K, et al. Influence of partial sleep deprivation on energy balance and insulin sensitivity in healthy women. Obes Facts. 2008;1:266–73. doi: 10.1159/000158874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brondel L, Romer MA, Nougues PM, Touyarou P, Davenne D. Acute partial sleep deprivation increases food intake in healthy men. Am J Clin Nutr. 2010;91:1550–9. doi: 10.3945/ajcn.2009.28523. [DOI] [PubMed] [Google Scholar]
- 6.Markwald RR, Melanson EL, Smith MR, et al. Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci U S A. 2013;110:5695–700. doi: 10.1073/pnas.1216951110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spaeth AM, Dinges DF, Goel N. Effects of experimental sleep restriction on weight gain, caloric intake and meal timing in healthy adults. Sleep. 2013;36:981–90. doi: 10.5665/sleep.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.St-Onge MP, Roberts AL, Chen J, et al. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr. 2011;94:410–6. doi: 10.3945/ajcn.111.013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009;89:126–33. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Spaeth AM, Dinges DF, Goel N. Sex and race differences in caloric intake during sleep restriction in healthy adults. Am J Clin Nutr. 2014;100:559–66. doi: 10.3945/ajcn.114.086579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lauer CJ, Krieg JC. Sleep in eating disorders. Sleep Med Rev. 2004;8:109–18. doi: 10.1016/S1087-0792(02)00122-3. [DOI] [PubMed] [Google Scholar]
- 12.Karklin A, Driver HS, Buffenstein R. Restricted energy intake affects nocturnal body temperature and sleep patterns. Am J Clin Nutr. 1994;59:346–9. doi: 10.1093/ajcn/59.2.346. [DOI] [PubMed] [Google Scholar]
- 13.Phillips F, Chen CN, Crisp AH, et al. Isocaloric diet changes and electroencephalographic sleep. Lancet. 1975;2:723–5. doi: 10.1016/s0140-6736(75)90718-7. [DOI] [PubMed] [Google Scholar]
- 14.Spring B, Maller O, Wurtman J, Digman L, Cozolino L. Effects of protein and carbohydrate meals on mood and performance: interactions with sex and age. J Psychiatr Res. 1982;17:155–67. doi: 10.1016/0022-3956(82)90017-6. [DOI] [PubMed] [Google Scholar]
- 15.Orr WC, Shadid G, Harnish MJ, Elsenbruch S. Meal composition and its effect on postprandial sleepiness. Physiol Behav. 1997;62:709–12. doi: 10.1016/s0031-9384(97)00012-7. [DOI] [PubMed] [Google Scholar]
- 16.Afaghi A, O'Connor H, Chow CM. High-glycemic-index carbohydrate meals shorten sleep onset. Am J Clin Nutr. 2007;85:426–30. doi: 10.1093/ajcn/85.2.426. [DOI] [PubMed] [Google Scholar]
- 17.Driver HS, Shulman I, Baker FC, Buffenstein R. Energy content of the evening meal alters nocturnal body temperature but not sleep. Physiol Behav. 1999;68:17–23. doi: 10.1016/s0031-9384(99)00145-6. [DOI] [PubMed] [Google Scholar]
- 18.Zammit GK, Ackerman SH, Shindledecker R, Fauci M, Smith GP. Postprandial sleep and thermogenesis in normal men. Physiol Behav. 1992;52:251–9. doi: 10.1016/0031-9384(92)90267-6. [DOI] [PubMed] [Google Scholar]
- 19.St-Onge MP, O'Keeffe M, Roberts AL, RoyChoudhury A, Laferrere B. Short sleep duration, glucose dysregulation and hormonal regulation of appetite in men and women. Sleep. 2012;35:1503–10. doi: 10.5665/sleep.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.St-Onge MP, McReynolds A, Trivedi ZB, Roberts AL, Sy M, Hirsch J. Sleep restriction leads to increased activation of brain regions sensitive to food stimuli. Am J Clin Nutr. 2012;95:818–24. doi: 10.3945/ajcn.111.027383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harris JA, Benedict FG. Washington, DC: Carnegie Institution of Washington; 1919. A biometric study of basal metabolism in man. [Google Scholar]
- 22.Shechter A, O'Keeffe M, Roberts AL, Zammit GK, RoyChoudhury A, St-Onge MP. Alterations in sleep architecture in response to experimental sleep curtailment are associated with signs of positive energy balance. Am J Physiol Regul Integr Comp Physiol. 2012;303:R883–9. doi: 10.1152/ajpregu.00222.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Iber C, Ancoli-Israel S, Chesson AL, Quan SF. The AASM manual for the scoring of sleep and assorted events. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 24.St-Onge M-P, Roberts A, Chen J, Kelleman M, O'Keeffe M, Jones P. Short sleep duration increases energy intakes but does not change expenditure in normal weight individuals. Am J Clin Nutr. 2011;94:410–6. doi: 10.3945/ajcn.111.013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4:487–504. [PMC free article] [PubMed] [Google Scholar]
- 26.Crispim CA, Zimberg IZ, dos Reis BG, Diniz RM, Tufik S, de Mello MT. Relationship between food intake and sleep pattern in healthy individuals. J Clin Sleep Med. 2011;7:659–64. doi: 10.5664/jcsm.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dhurandhar NV, Schoeller D, Brown AW, et al. Energy balance measurement: when something is not better than nothing. Int J Obes. 2015;39:1109–13. doi: 10.1038/ijo.2014.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yamaguchi M, Uemura H, Katsuura-Kamano S, et al. Relationship of dietary factors and habits with sleep-wake regularity. Asia Pac J Clin Nutr. 2013;22:457–65. doi: 10.6133/apjcn.2013.22.3.01. [DOI] [PubMed] [Google Scholar]
- 29.Grandner MA, Jackson N, Gerstner JR, Knutson KL. Sleep symptoms associated with intake of specific dietary nutrients. J Sleep Res. 2014;23:22–34. doi: 10.1111/jsr.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krauchi K, Cajochen C, Werth E, Wirz-Justice A. Alteration of internal circadian phase relationships after morning versus evening carbohydrate-rich meals in humans. J Biol Rhythms. 2002;17:364–76. doi: 10.1177/074873040201700409. [DOI] [PubMed] [Google Scholar]
- 31.Krauchi K. The human sleep-wake cycle reconsidered from a thermoregulatory point of view. Physiol Behav. 2007;90:236–45. doi: 10.1016/j.physbeh.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 32.Dijk DJ, Czeisler CA. Contribution of the circadian pacemaker and the sleep homeostat to sleep propensity, sleep structure, electroencephalographic slow waves, and sleep spindle activity in humans. J Neurosci. 1995;15:3526–38. doi: 10.1523/JNEUROSCI.15-05-03526.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grandner MA, Jackson N, Gerstner JR, Knutson KL. Dietary nutrients associated with short and long sleep duration. Data from a nationally representative sample. Appetite. 2013;64:71–80. doi: 10.1016/j.appet.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Haghighatdoost F, Karimi G, Esmaillzadeh A, Azadbakht L. Sleep deprivation is associated with lower diet quality indices and higher rate of general and central obesity among young female students in Iran. Nutrition. 2012;28:1146–50. doi: 10.1016/j.nut.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 35.Johnson CL, Paulose-Ram R, Ogden CL, et al. National health and nutrition examination survey: analytic guidelines, 1999-2010. Vital Health Stat 2. 2013;13:1–24. [PubMed] [Google Scholar]
- 36.Rutters F, Gonnissen HK, Hursel R, Lemmens SG, Martens EA, Westerterp-Plantenga MS. Distinct associations between energy balance and the sleep characteristics slow wave sleep and rapid eye movement sleep. Int J Obes. 2012;36:1346–52. doi: 10.1038/ijo.2011.250. [DOI] [PubMed] [Google Scholar]
- 37.Kredlow MA, Capozzoli MC, Hearon BA, Calkins AW, Otto MW. The effects of physical activity on sleep: a meta-analytic review. J Behav Med. 2015;38:427–49. doi: 10.1007/s10865-015-9617-6. [DOI] [PubMed] [Google Scholar]
- 38.Stafstrom CE, Rho JM. The ketogenic diet as a treatment paradigm for diverse neurological disorders. Front Pharmacol. 2012;3:59. doi: 10.3389/fphar.2012.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McClernon FJ, Yancy WS, Jr., Eberstein JA, Atkins RC, Westman EC. The effects of a low-carbohydrate ketogenic diet and a low-fat diet on mood, hunger, and other self-reported symptoms. Obesity (Silver Spring) 2007;15:182–7. doi: 10.1038/oby.2007.516. [DOI] [PubMed] [Google Scholar]


