Abstract
Study Objectives:
Taking hypnotic agents 30 min before bedtime is the usual suggested administration time, but some patients report dissatisfaction with their sleeping pills. We investigated whether the timing of sleeping pill administration influences patient subjective satisfaction with these drugs.
Methods:
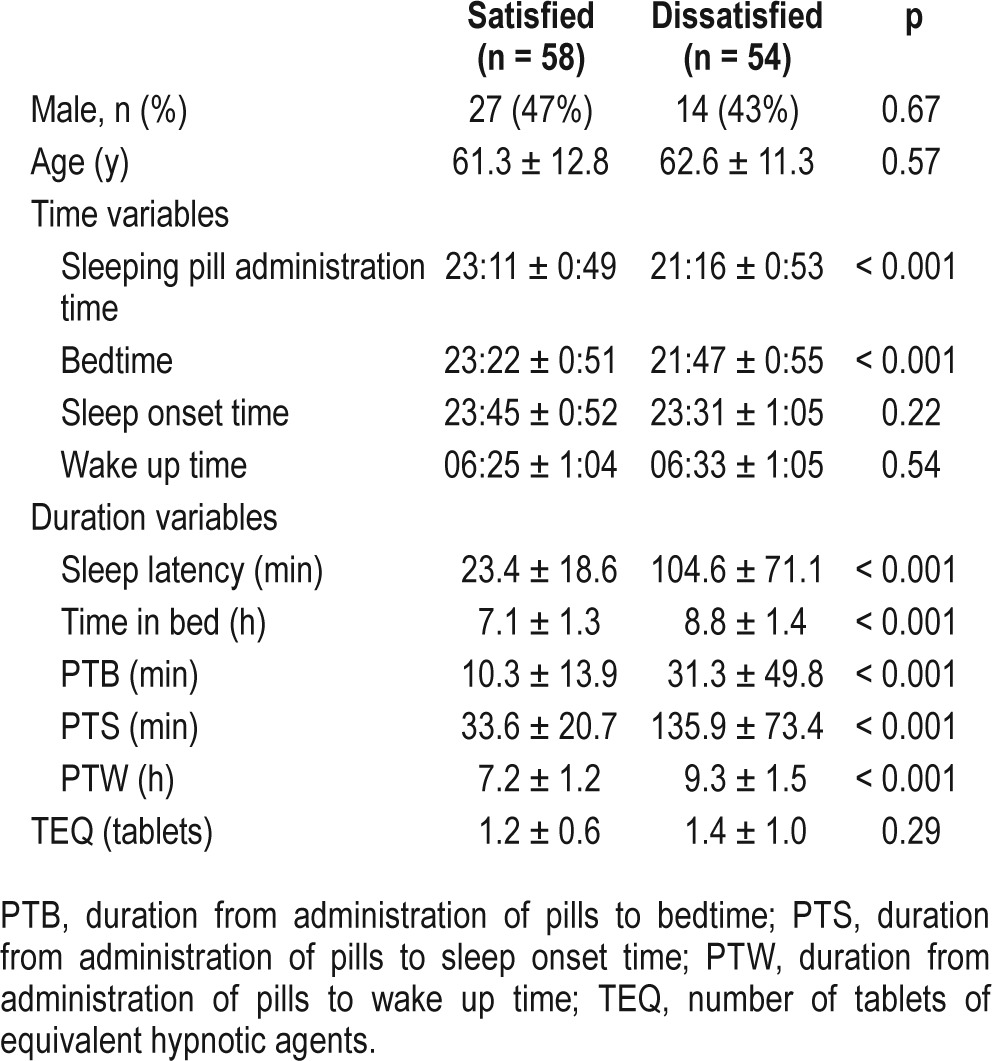
One hundred twelve patients with primary insomnia currently taking benzodiazepine or nonbenzodiazepine gamma-aminobutyric acid (GABA) agonists as sleeping pills were selected. The time of administration for their sleeping pills, bedtime, sleep onset time, and wake up time were obtained from their medical records. Subjects were also categorized into satisfied or dissatisfied groups.
Results:
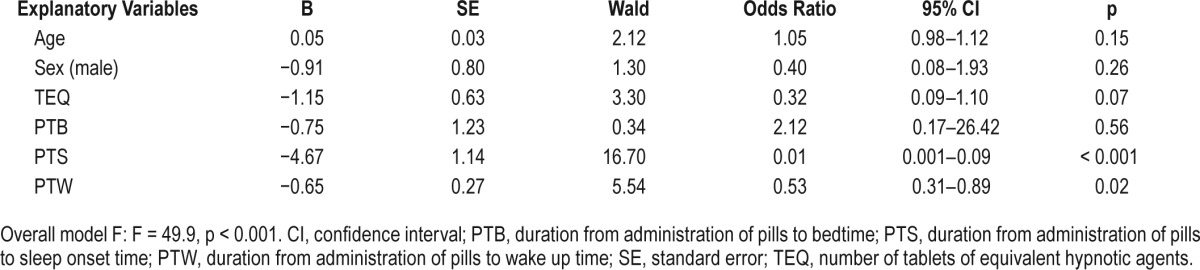
Hypnotic agents administration time (p < 0.001) and bedtime (p < 0.001), but not sleep onset or wake up time, occurred later in the night in the satisfied group. The durations from administration of pills to sleep onset (33.6 ± 20.7 min) and to wake up time (7.2 ± 1.2 h) were significantly shorter in the satisfied group when compared to the dissatisfied group (135.9 ± 73.4 min and 9.3 ± 1.5 h for time to sleep onset and wake up, respectively). Logistic regression analysis revealed that patient subjective satisfaction with hypnotic agents could be predicted by a short duration from administration of pills to sleep onset (odds ratio = 0.01; 95% confidence interval [0.001–0.09]) and a short duration from administration of pills to wake up time (0.53; [0.31–0.89], F = 49.9, p < 0.001).
Conclusions:
Taking sleeping pills at a later time and a shorter interval between pill administration and wake up time may increase patient subjective satisfaction with hypnotic agents. We propose that physicians advise patients to take sleeping pills approximately 7 h before their usual getting-out-of-bed time instead of the current standard of 30 min before bedtime.
Citation:
Chung S, Youn S, Yi K, Park B, Lee S. Sleeping pill administration time and patient subjective satisfaction. J Clin Sleep Med 2016;12(1):57–62.
Keywords: hypnotics, insomnia, patient satisfaction, sleep
INTRODUCTION
When physicians prescribe sleeping pills to patients with insomnia, they usually advise that these pills be taken 30 min before bedtime. Patients are also often generally aware of this guideline. Some studies have reported that administration of sleeping pills 30 min before bedtime shortens sleep latency and increases total sleep time.1–3 Numerous studies have defined normal sleep onset latency as less than 30 min,4–6 and clinical practice guidelines for insomnia also state that 30 min is an optimal length of time for sleep latency.7 In one study, a sleep latency of 31 min was found to be the most defensible quantitative criteria for insomnia.8
The typical 30-min guideline for administration of sleeping pills is generally helpful to patients with insomnia, but some patients complain that they do not fall asleep when using these drugs in this way. When hypnotics are not satisfactory, patients often want to increase the dosage to achieve better sleep quality. Physicians also tend to prescribe a higher dosage of hypnotics or switch to other medications when patients report dissatisfaction. It has been previously reported that 5% of the general population in South Korea were given at least one hypnotic tablet (zolpidem or triazolam) daily, and 0.6% took at least two tablets daily.9 If other sedative medications such as benzodiazepines, antidepressants, antihistamines, antipsychotics, or melatonin agonists are included, these percentages would most certainly increase. Higher dosages or multiple medications are likely to increase the risk of falls, daytime somnolence, sleepy driving, and drug abuse and dependency.10–12 Thus, it is important to understand other possible reasons for poor responses to these medications.
BRIEF SUMMARY
Current Knowledge/Study Rationale: Most insomnia patients usually take sleeping pills 30 min before the time when they desire to go to bed, not the time when they should go to bed based on their sleep-wake cycle. The exact time when patients take their sleeping pills may influence the efficacy of these hypnotics. This study was done to investigate the impact of timing on the effectiveness of sleeping pills and patient subjective satisfaction with these drugs.
Study Impact: Patients who were satisfied with their sleeping pills tended to take these hypnotic agents later in the evening than those who were not satisfied. Patients in the satisfied group also spent only 7.2 h until wake up in the morning after taking their sleeping pills, whereas patients in the dissatisfied group spent 9.3 h. We propose that physicians advise patients to take benzodiazepine or nonbenzodiazepine GABA agonists sleeping pills 7 h before their getting- out-of-bed time rather than 30 min before bedtime.
When physicians ask patients when they usually take their sleeping pills, most commonly respond 30 min before bedtime rather than give the precise time. Although most patients take hypnotics about 30 min prior to bedtime, this could be anywhere between 20:00 and 24:00. This means that patients with insomnia usually take sleeping pills 30 min before the time when they desire to go to bed, not the time when they should go to bed based on their sleep-wake cycle. We hypothesized that the exact time when patients take their sleeping pills may influence the efficacy of these hypnotics. The aim of this study was to investigate the effect of timing on the effectiveness of sleeping pills and patient subjective satisfaction with these drugs.
METHODS
Subjects and Assessments
Subjects were selected from patients who visited the sleep clinic in the Department of Psychiatry at the Asan Medical Center, Seoul, South Korea, between July and December 2014. The study protocol was approved by the Institutional Review Board of the Asan Medical Center. Subjects were included in this study if they received a diagnosis of primary insomnia and had been taking sleeping pills including benzodiazepine or nonbenzodiazepine gamma-aminobutyric acid (GABA) agonists (zolpidem, the only nonbenzodiazepine GABA agonist available in South Korea). All participants underwent a full history and psychiatric examination by a psychiatrist and a sleep specialist (SC), and received a new diagnosis of primary insomnia according to the diagnostic criteria of the International Criteria for Sleep Disorders, Second Edition.
Patients were excluded if they had (1) the presence of concurrent major psychiatric conditions such as major depressive disorder or anxiety disorder; (2) the presence of other concurrent sleep disorders such as circadian rhythm sleep disorder, restless legs syndrome, or periodic limb movements during sleep; (3) snoring, apneic symptoms, or severe daytime sleepiness suggestive of obstructive sleep apnea syndrome; (4) major medical or neurological diseases that can induce severe pain during the night or impair mobility and daily activities; (5) experienced cognitive-behavioral therapy (CBT) for insomnia prior to visiting our clinic; (6) usually spent over 30 min lying in bed during daytime; or (7) taking other psychotropic medication, such as trazodone, mirtazapine or antipsychotics, or melatonin agonist as sleeping pills.
When patients visit our clinic, we asked the following questions: “How many tablets of sleeping pills per day are you taking now?” “Are you satisfied with your sleeping pills to induce your sleep?” “What is the usual time to take sleeping pills?” “What is your usual bedtime?” “What is your usual time to fall asleep?” “What is your usual time to finally get out of bed in the morning?” We reviewed all study patient medical records retrospectively to acquire information that was routinely obtained in our sleep clinic. We categorized patients into satisfied or dissatisfied groups according to their answers regarding sleeping pills.
Calculating the Time and Duration Variables
We calculated the time and duration variables (Table 1) using this information. Patient time variables were obtained by averaging the usual times reported; (i.e., if a patient answered “I take sleep pills usually between 21:00 and 21:30”, then we calculated this time as 21:15). We transformed time variables into numeric variables because minutes vary from 0 to 60. Fifteen minutes (one quarter of 1 h) was transformed into 0.25 (one quarter) and 30 min (half of 1 h) was 0.50 (i.e., 22:15 was transformed into 10.25, and 23:30 into 11.50). Using these data, we defined new sleep indices such as duration from administration of pills to bedtime (PTB), duration from administration of pills to sleep onset time (PTS), and duration from administration of pills to wake up time (exactly, finally get-out-of-bed time) (PTW). Duration variables were calculated using numerically transformed time variables.
Table 1.
Clinical characteristics of patients who were satisfied or dissatisfied with their sleeping pills.
Calculating the Number of Tablets of Equivalent Hypnotic Agents
We defined the number of tablets of equivalent hypnotic agents (TEQ) in this study. Equivalent doses of each medication was calculated as follows13: alprazolam (0.25 mg), bromazepam (3 mg), clonazepam (0.25 mg), diazepam (5 mg), lorazepam (0.5 mg), triazolam (0.25 mg), and zolpidem (10 mg of immediate-release form and 12.5 mg of extended-release form). We calculated the TEQ by summing the number of prescribed tablets of sleeping pills per day. For example, if a subject was prescribed 0.125 mg of triazolam, 5 mg of zolpidem immediate-release form, or 6.25 mg of zolpidem extended-release form, the TEQ would be 0.5. If a subject was prescribed 0.25 mg of triazolam and 5 mg of zolpidem immediate-release form at the same time, the TEQ would be 1.5.
Statistical Analysis
Statistical analyses were performed with SPSS version 19.0 for Windows (IBM Corp., Armonk, NY, USA). Data are summarized as the means ± standard deviation (SD). Significance was defined as p < 0.05 in two-tailed tests for all analyses. A Student t-test for continuous variables and a chi-square test for categorical variables were performed for between group analyses. A Pearson correlation analysis was performed to explore the association among patients' clinical characteristics. A logistic regression model was used to explore what variables may predict patient subjective satisfaction with sleeping pills. A receiver operating characteristic (ROC) curve was plotted for patient satisfaction in accordance with the PTW.
RESULTS
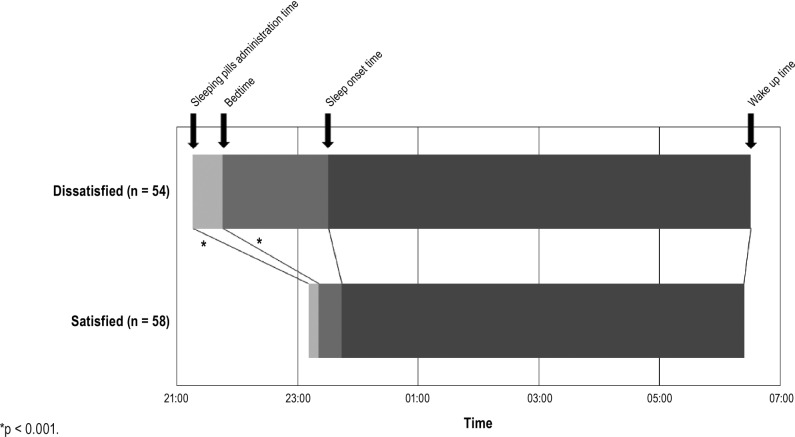
Among 180 patients with primary insomnia who visited our sleep disorder clinic, 68 patients were excluded due to insufficient records, using mirtazapine, trazodone, or antipsychotics, previously experiencing CBT for insomnia, or spending over 30 min lying in bed during daytime. A total of 112 subjects were included in this study. Among these cases, 58 patients (52%) reported satisfaction with their sleeping pills. There was no statistical differences in sex or age balance between the satisfied and dissatisfied subjects (Table 1). The mean administration time for sleeping pills was 23:11 in the satisfied group and 21:16 in the dissatisfied group, and the mean bedtime was 23:22 in the satisfied group and 21:47 in the dissatisfied group. Among the time variables, the pill administration time (p < 0.001) and bedtime (p < 0.001) occurred later in the evening in the satisfied group when compared to the dissatisfied group, but there were no significant differences in sleep onset time (p = 0.22) or wake up time (p = 0.54) between the two groups (Figure 1). Among the duration variables, patients in both group followed the general guidance of taking sleeping pills around 30 min before bedtime (PTB, 10.3 versus 31.3 min, p < 0.001), despite a significant difference. The duration from administration of pills to sleep onset (PTS, 33.6 versus 135.9 min, p < 0.001), the duration from administration of pills to wake up time (PTW, 7.2 versus 9.3 h, p < 0.001), and the total time in bed (7.1 versus 8.8 h, p < 0.001) were all significantly shorter in the satisfied group when compared to the dissatisfied group. The TEQ taken did not differ between the two groups (p = 0.29).
Figure 1. Time variables of sleeping pills administration and sleep pattern among satisfied and dissatisfied group.
*p < 0.001.
Correlation analyses revealed that age was positively correlated with sleep latency; however, the TEQ was negatively correlated with sleep onset time (Table 2). Logistic regression analyses (Table 3) revealed that the duration from administration of pills to sleep onset (odds ratio [OR] = 0.01; 95% confidence interval [CI] = 0.001–0.09) and the duration from administration of pills to wake up time (OR = 0.53; 95% CI = 0.31–0.89) were two significant variables that can predict a patient's subjective satisfaction with their sleeping pills (F = 49.9, p < 0.001).
Table 2.
Pearson correlation analysis of age and number of tablets of equivalent hypnotic drugs with the clinical characteristics of insomnia patients.
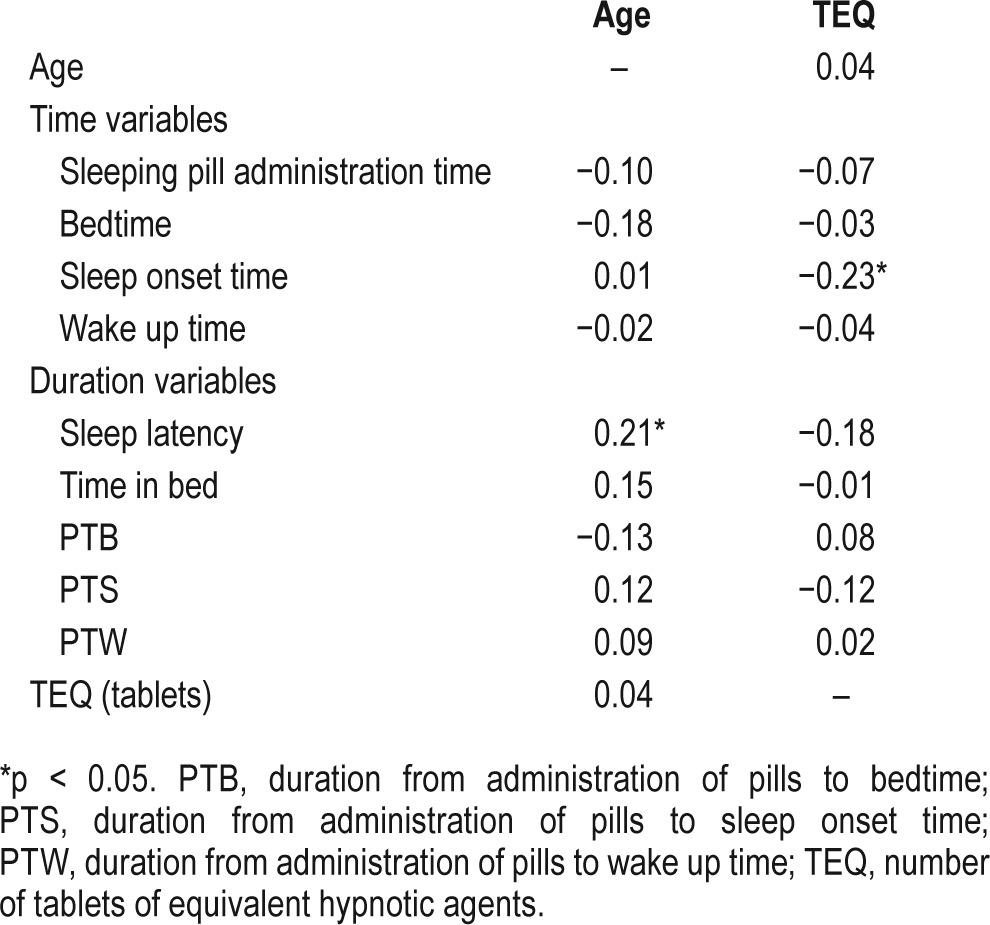
Table 3.
Results of logistic regression of clinical characteristics or duration variables on patient satisfaction with sleeping pills.
The ROC analysis was done to decide the appropriate PTW for patient satisfaction (Figure 2). When we defined the appropriate PTW ≤ 7 h, 86% of patients (32/37) were satisfied among subjects who had PTW ≤ 7 h, and 35% of patients (26/75) were satisfied among subjects with PTW > 7 h. When we defined the appropriate PTW ≤ 8, 85% of patients (47/56) were satisfied among subjects whose PTW ≤ 8, and just 20% of patients (11/56) were satisfied among subjects with PTW > 8. Based on PTB, 57 patients (62%) were satisfied with their sleeping pills among the 92 patients who took their sleeping pills within 30 min before their bedtime. However, among the 20 patients who took their sleeping pills more than 30 min before their bedtime, just one patient (5%) was satisfied with his or her sleeping pills.
Figure 2. Receiver operating characteristics curve for patients' satisfaction with their sleeping pills.
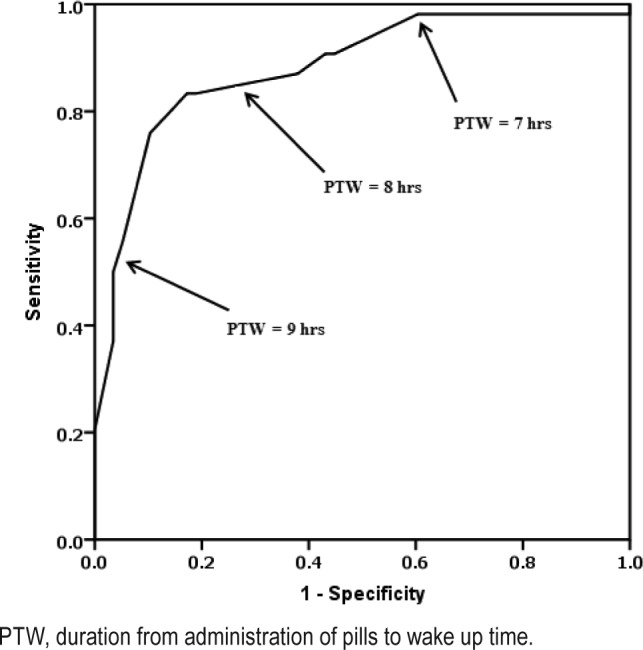
PTW, duration from administration of pills to wake up time.
DISCUSSION
We observed that patients who were satisfied with their sleeping pills tended to take these hypnotics later in the evening than those who were not satisfied. Patient subjective satisfaction with sleeping pills was found to be related to a shorter interval between taking pills to sleep onset and a shorter duration from administration of pills to wake up time, but not with the duration from administration of the pills to bedtime. From the viewpoint of CBT for insomnia,14 it has been shown to be useful to manage this condition by staying awake and out of bed until one can fall asleep.15 Some people believe that it is healthy to go to bed early,16 and to sleep well earlier if possible. However, patients with insomnia tend to go to bed early in the evening to increase the possibility of better sleep.17,18 This can lead to tension and anxiety that further impairs the ability to sleep.19,20
Patients in this study took sleeping pills approximately 30 min before bedtime (10.3 versus 31.3 min). Depending on the type (short or long acting) of benzodiazepine the patients took, the administration time for sleeping pills needs to be adjusted. However, patients with insomnia usually take sleeping pills “around” or “within” 30 min before their bedtime. In this study 82% of all patients (n = 92) took their sleeping pills “within” 30 min before their bedtime. However, satisfaction with sleeping pills varied. We found that patients who were not satisfied with sleeping pills tended to take sleeping pills at 21:16 and go to bed at 21:47, whereas patients who were satisfied tended to take sleeping pills at 23:11 and go to bed at 23:22. Considering that patients in both groups tended to fall asleep at similar times (23:31 versus 23:45), this indicated that patients in the dissatisfied group stayed awake in bed longer (104.6 min of sleep latency) when compared to patients in the satisfied group (23.4 min). From the viewpoint of CBT for insomnia, being awake for a long time in bed likely decreases sleep efficiency, i.e., the percentage of actual time sleeping versus time in bed. Therefore, reducing the total time in bed is recommended to increase sleep efficiency.18 Numerous studies have shown that 7 or 8 h of sleep is associated with a lower risk of cardiovascular complications.21–23 Patients in this study who were satisfied with their sleeping pills spent a shorter time in bed (7.1 h versus 8.8 h). Patients in the satisfied group also spent only 7.2 h until wake up in the morning after taking their sleeping pills, whereas patients in the dissatisfied group spent 9.3 h. We speculate that a longer time in bed may increase patient dissatisfaction with sleeping pills, although various factors including patient personality, sex, age, or the type of hypnotics taken may affect the degree of satisfaction with a sleeping pill.24 In our current study, there were no significant differences in age, sex, or the TEQ used between the two groups.
Regression analyses in our current study revealed that the duration from administration of pills to sleep onset and the duration from administration of pills to wake up time are significant variables contributing to a patient's subjective satisfaction with sleeping pills. If it takes a long time from taking the sleeping pill to falling asleep, patients may feel dissatisfied with their prescribed hypnotics. However, considering that we found no significant difference in the sleep onset time between our two study groups (23:31 versus 23:45), dissatisfaction with sleeping pills was associated with taking sleeping pills earlier in the evening. In our correlation analyses, older age was found to be significantly associated with longer sleep latency. This can be explained by elderly patients tending to take sleeping pills earlier. In this regard, it is widely known that the sleep phase can be somewhat advanced in elderly patients.25,26 The TEQ prescribed to the subjects in our current study was found to negatively correlate with sleep onset time. Although we cannot precisely determine the causal relationship, higher doses of medication may be related to more advanced sleep onset time.
Based on PTW, 85–86% of patients were satisfied with their sleeping pills when the appropriate PTW was defined as less than or equal to 7 and 8 h, respectively. However, the satisfaction rate was lower (62%) when we estimated it based on PTB ≤ 30 min. From the results, we can consider recommending patients take sleeping pills 7 or 8 h before their wake up time (exactly, finally getting-out-of-bed time) may be more effective when compared to the usual recommendation of 30 min before bedtime. The ROC analysis revealed that 7 or 8 h of PTW may be appropriate for patient satisfaction. If the aim of this study was to define the diagnostic cutoff, 8 h of PTW may be more appropriate. However, we think that a short PTW may be more helpful for patients with primary insomnia because they usually tend to spend their time in bed as much as they can. Therefore, we hypothesize that 7 h of PTW is more effective for patients with primary insomnia in clinical practice.
This study had several limitations. First, patients' subjective satisfaction with sleeping pills were assessed based on answers to our questions, rather than objective assessment tools. Satisfaction with the medication's ability to induce sleep may not be the same as satisfaction with sleep. Second, we did not assess the severity of insomnia in each patient. This study was a retrospective pilot study based on medical records in which the assessment of insomnia severity was not sufficient. More detailed assessments of sleep severity or quality will be included in our next prospective study. Third, objectively measured sleep efficiency or the incidence of frequent awakenings were also not analyzed in this study. These factors are reported to be associated with patient subjective satisfaction with sleeping pills.24,27 Occult obstructive sleep apnea and resultant frequent awakenings can significantly affect the degree of insomnia; nocturnal polysomnography is a useful tool for detecting comorbid obstructive sleep apnea. However, we did not perfrom routine polysomnography on our subjects because this test was not recommended for patients with insomnia according to care guidelines.28
In conclusion, taking sleeping pills at a later time may increase patient satisfaction with these drugs. The duration from administration of pills to bedtime (30 min before bedtime) may not engender patient satisfaction with sleeping pills if the physician does not educate patients about the concepts of a proper sleep schedule and better sleep efficiency. Patients who expressed dissatisfaction tended to stay in bed awake for a longer duration until falling asleep, and spent more time in bed until their normal wake up time. We propose that physicians advise patients to take benzodiazepeine or nonbenzodiazepine GABA agonists sleeping pills 7 hours before their getting out of bed time rather than 30 min before bedtime. Also, if we use CBT to treat insomnia, we can manage patient sleep more appropriately. However, it is not easy to compel most general practitioners to learn and practice CBT for insomnia, despite various types of CBT for insomnia being currently applied29,30 and shown to be helpful to facilitate physical health.31 Adopting a “7 h before getting-out-of-bed time” approach includes the concepts of CBT for insomnia and circadian rhythm regulation (delaying the advanced bedtime) because it incorporates the time when the patient takes sleeping pills and when they go to bed. We believe that taking 7 h before getting out of bed is an easier way for doctors to advise their patients with insomnia when they are not familiar with CBT concepts. We also propose to use new sleep indices such as PTB, PTS, or PTW to understand patients' sleeping pill administration patterns more precisely. We plan to investigate the real effectiveness of advising patients to take their sleeping pills “7 h before getting-outof-bed time” versus “30 min before bedtime” in a future study.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest.
ABBREVIATIONS
- CBT
cognitive-behavioral therapy
- PTB
duration from administration of pills to bedtime
- PTS
duration from administration of pills to sleep onset time
- PTW
duration from administration of pills to wake up time
- TEQ
number of tablets of equivalent hypnotic agents
REFERENCES
- 1.Kryger MH, Steljes D, Pouliot Z, Neufeld H, Odynski T. Subjective versus objective evaluation of hypnotic efficacy: experience with zolpidem. Sleep. 1991;14:399–407. doi: 10.1093/sleep/14.5.399. [DOI] [PubMed] [Google Scholar]
- 2.Mitler MM, Seidel WF, van den Hoed J, Greenblatt DJ, Dement WC. Comparative hypnotic effects of flurazepam, triazolam, and placebo: a long-term simultaneous nighttime and daytime study. J Clin Psychopharmacol. 1984;4:2–13. [PubMed] [Google Scholar]
- 3.Mayer G, Wang-Weigand S, Roth-Schechter B, Lehmann R, Staner C, Partinen M. Efficacy and safety of 6-month nightly ramelteon administration in adults with chronic primary insomnia. Sleep. 2009;32:351–60. doi: 10.1093/sleep/32.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dew MA, Hoch CC, Buysse DJ, et al. Healthy older adults' sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosom Med. 2003;65:63–73. doi: 10.1097/01.psy.0000039756.23250.7c. [DOI] [PubMed] [Google Scholar]
- 5.Edinger JD, Bonnet MH, Bootzin RR, et al. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27:1567–96. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 6.McCormac M. Managing hemorrhagic shock. Am J Nurs. 1990;90:22–7. [PubMed] [Google Scholar]
- 7.Schutte-Rodin S, Broch L, Buysse D, Dorsey C, Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4:487–504. [PMC free article] [PubMed] [Google Scholar]
- 8.Lichstein KL, Durrence HH, Taylor DJ, Bush AJ, Riedel BW. Quantitative criteria for insomnia. Behav Res Ther. 2003;41:427–45. doi: 10.1016/s0005-7967(02)00023-2. [DOI] [PubMed] [Google Scholar]
- 9.Chung S, Park B, Yi K, et al. Pattern of hypnotic drug prescription in South Korea: Health Insurance Review and Assessment Service - National Patients Sample. Sleep Med Res. 2013;4:51–5. [Google Scholar]
- 10.Victorri-Vigneau C, Gerardin M, Rousselet M, Guerlais M, Grall-Bronnec M, Jolliet P. An update on zolpidem abuse and dependence. J Addict Dis. 2014;33:15–23. doi: 10.1080/10550887.2014.882725. [DOI] [PubMed] [Google Scholar]
- 11.Obayashi K, Araki T, Nakamura K, et al. Risk of falling and hypnotic drugs: retrospective study of inpatients. Drugs R D. 2013;13:159–64. doi: 10.1007/s40268-013-0019-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vermeeren A, Vuurman EF, Leufkens TR, et al. Residual effects of low-dose sublingual zolpidem on highway driving performance the morning after middle-of-the-night use. Sleep. 2014;37:489–96. doi: 10.5665/sleep.3482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. http://www.benzo.org.uk/bzequiv.htm.
- 14.Ong K, Suh S. Utilizing cognitive-behavioral therapy for insomnia to facilitate discontinuation of sleep medication in chronic insomnia patients. Sleep Med Res. 2012;3:1–6. [Google Scholar]
- 15.Bootzin RR, Perlis ML. Nonpharmacologic treatments of insomnia. J Clin Psychiatry. 1992;53:37–41. [PubMed] [Google Scholar]
- 16.Liu X, Liu L. Sleep habits and insomnia in a sample of elderly persons in China. Sleep. 2005;28:1579–87. [PubMed] [Google Scholar]
- 17.Espie CA. Insomnia: conceptual issues in the development, persistence, and treatment of sleep disorder in adults. Annu Rev Psychol. 2002;53:215–43. doi: 10.1146/annurev.psych.53.100901.135243. [DOI] [PubMed] [Google Scholar]
- 18.Morin CM. Cognitive-behavioral approaches to the treatment of insomnia. J Clin Psychiatry. 2004;65:33–40. [PubMed] [Google Scholar]
- 19.Ellis J, Hampson SE, Cropley M. The role of dysfunctional beliefs and attitudes in late-life insomnia. J Psychosom Res. 2007;62:81–4. doi: 10.1016/j.jpsychores.2006.06.007. [DOI] [PubMed] [Google Scholar]
- 20.Narisawa H. Anxiety and its related factors at bedtime are associated with difficulty in falling asleep. Tohoku J Exp Med. 2013;231:37–43. doi: 10.1620/tjem.231.37. [DOI] [PubMed] [Google Scholar]
- 21.Ford ES. Habitual sleep duration and predicted 10-year cardiovascular risk using the pooled cohort risk equations among US adults. J Am Heart Assoc. 2014;3:e001454. doi: 10.1161/JAHA.114.001454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Heslop P, Smith GD, Metcalfe C, Macleod J, Hart C. Sleep duration and mortality: the effect of short or long sleep duration on cardiovascular and all-cause mortality in working men and women. Sleep Med. 2002;3:305–14. doi: 10.1016/s1389-9457(02)00016-3. [DOI] [PubMed] [Google Scholar]
- 23.Sabanayagam C, Shankar A. Sleep duration and cardiovascular disease: results from the National Health Interview Survey. Sleep. 2010;33:1037–42. doi: 10.1093/sleep/33.8.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hosono T, Homma M, Satoh M, Kohda Y. Variables influencing patient satisfaction for hypnotics: difference between zolpidem and brotizolam. J Clin Pharm Ther. 2014;39:507–10. doi: 10.1111/jcpt.12174. [DOI] [PubMed] [Google Scholar]
- 25.Campbell SS, Dawson D. Aging young sleep: a test of the phase advance hypothesis of sleep disturbance in the elderly. J Sleep Res. 1992;1:205–10. doi: 10.1111/j.1365-2869.1992.tb00040.x. [DOI] [PubMed] [Google Scholar]
- 26.Edwards BA, O'Driscoll DM, Ali A, Jordan AS, Trinder J, Malhotra A. Aging and sleep: physiology and pathophysiology. Semin Respir Crit Care Med. 2010;31:618–33. doi: 10.1055/s-0030-1265902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamashiro T, Homma M, Kohda Y. Assessment of hypnotic effects and patient satisfaction in empirical use of sleep medicines. J Clin Pharm Ther. 2008;33:273–8. doi: 10.1111/j.1365-2710.2008.00917.x. [DOI] [PubMed] [Google Scholar]
- 28.Littner M, Hirshkowitz M, Kramer M, et al. Practice parameters for using polysomnography to evaluate insomnia: an update. Sleep. 2003;26:754–60. doi: 10.1093/sleep/26.6.754. [DOI] [PubMed] [Google Scholar]
- 29.Cheng SK, Dizon J. Computerised cognitive behavioural therapy for insomnia: a systematic review and meta-analysis. Psychother Psychosom. 2012;81:206–16. doi: 10.1159/000335379. [DOI] [PubMed] [Google Scholar]
- 30.Edinger JD, Sampson WS. A primary care “friendly” cognitive behavioral insomnia therapy. Sleep. 2003;26:177–82. doi: 10.1093/sleep/26.2.177. [DOI] [PubMed] [Google Scholar]
- 31.Chung S, An H, Park J, et al. The effect of non-pharmacological treatment for psychophysiological insomnia on cardiovascular autonomic regulation assessed using heart rate variability. Sleep Med Res. 2011;2:10–15. [Google Scholar]