Abstract
Study Objectives:
The definition and the criteria for the successful treatment of obstructive sleep apnea vary, depending on the study. This study aimed to compare different success criteria of non-continuous positive airway pressure (non-CPAP) treatment for obstructive sleep apnea in terms of sleep quality by using cardiopulmonary coupling.
Methods:
We included 98 patients who had been treated with sleep surgery or with a mandibular advancement device at our sleep clinic from January 2011 to March 2013. The success and failure groups were divided by 6 criteria that have been used in the literature. The validity of each of the 6 criteria was evaluated by cardiopulmonary coupling-based sleep quality.
Results:
The parameters of cardiopulmonary coupling indicated that sleep quality improved with non-CPAP treatment: low-frequency coupling decreased from 57.4% ± 17.7% to 46.9% ± 16.5%, whereas high-frequency coupling increased from 30.2% ± 17.1% to 37.4% ± 16.7%. In multiple regression analysis, only the criterion of a reduction in the apnea-hypopnea index greater than 50% was significantly associated with sleep quality improvement (p = 0.016; 95% confidence interval, 1.008–1.076 in the high-frequency coupling increment; p = 0.001; 95% confidence interval, 1.025–1.099 in the low-frequency coupling decrement).
Conclusions:
Cardiopulmonary coupling analysis showed that a reduction in the apnea-hypopnea index of more than 50% might be the optimal criterion to determine the success or failure of non-CPAP treatment in terms of sleep quality.
Citation:
Lee WH, Hong SN, Kim HJ, Rhee CS, Lee CH, Yoon IY, Kim JW. A comparison of different success definitions in non-continuous positive airway pressure treatment for obstructive sleep apnea using cardiopulmonary coupling. J Clin Sleep Med 2016;12(1):35–41.
Keywords: cardiopulmonary coupling, non-continuous positive airway pressure, obstructive sleep apnea, sleep quality, success criteria
INTRODUCTION
Effectively managing obstructive sleep apnea (OSA) is important for preventing comorbidities such as hypertension, heart failure, arrhythmia, coronary artery disease, and stroke.1–5 Treatments for OSA have been aimed at decreasing the apneahypopnea index (AHI) to less than 5/h. This reduction can be achieved by using continuous positive airway pressure (CPAP); however, the success of CPAP treatment is limited because patient compliance and long-term acceptance remain low.6 Compliance is better for oral appliances such as the mandibular advancement device (MAD), but the treatment efficacy is lower for oral appliances than for CPAP.7–9 Surgical therapy is another therapeutic option. It can be curative without using any device during sleep. However, its efficacy is variable, depending on the surgical method. There is also an issue of recurrence.
BRIEF SUMMARY
Current Knowledge/Study Rationale: There is a need to find a standardized criterion to define the success of non-CPAP treatment for OSA. This study aimed to compare different success criteria of non-CPAP treatment for OSA in terms of sleep quality by using cardiopulmonary coupling.
Study Impact: We propose that a reduction in the AHI greater than 50% may be the optimum value for defining the success of non-CPAP treatment outcome in terms of CPC-based sleep quality. Well-designed studies should be performed in the future to prove that this criterion can be correlated with a satisfactory health outcome in patients with OSA.
To compare the efficacy of the various types of non-CPAP therapy, it is necessary to define success by each method. However, a standardized criterion to define the success of the treatment has not been available, until now. In general, the treatment outcome of non-CPAP therapy has been assessed by comparing the postoperative and preoperative AHI. The rate of successful treatment is calculated by the definition of the success criteria, which varies, depending on the study. We found six success criteria in the literature. Most criteria were based on the AHI value (Table 1).10–29 However, sleep clinicians encounter patients who state that their quality of sleep improved with non-CPAP treatment, even though their AHI had not improved. Therefore, we analyzed each criterion, based on sleep quality, as assessed by the cardiopulmonary coupling (CPC) technique. In CPC analyses, heart rate variability and electrocardiogram-derived respiration are coupled, and their coupling is correlated with objective sleep quality.30 It has a close relationship with the cyclic alternating pattern scoring system.31,32 Furthermore, in a comparison study using conventional polysomnography (PSG), CPC was better associated with the basic physiologic mechanisms of altered sympathetic and parasympathetic activity during sleep.33 Successful treatment of OSA has been associated with an increase in high-frequency coupling (HFC) and a decrease in low-frequency coupling (LFC).30
Table 1.
The criteria of “success,” based on the study definition.
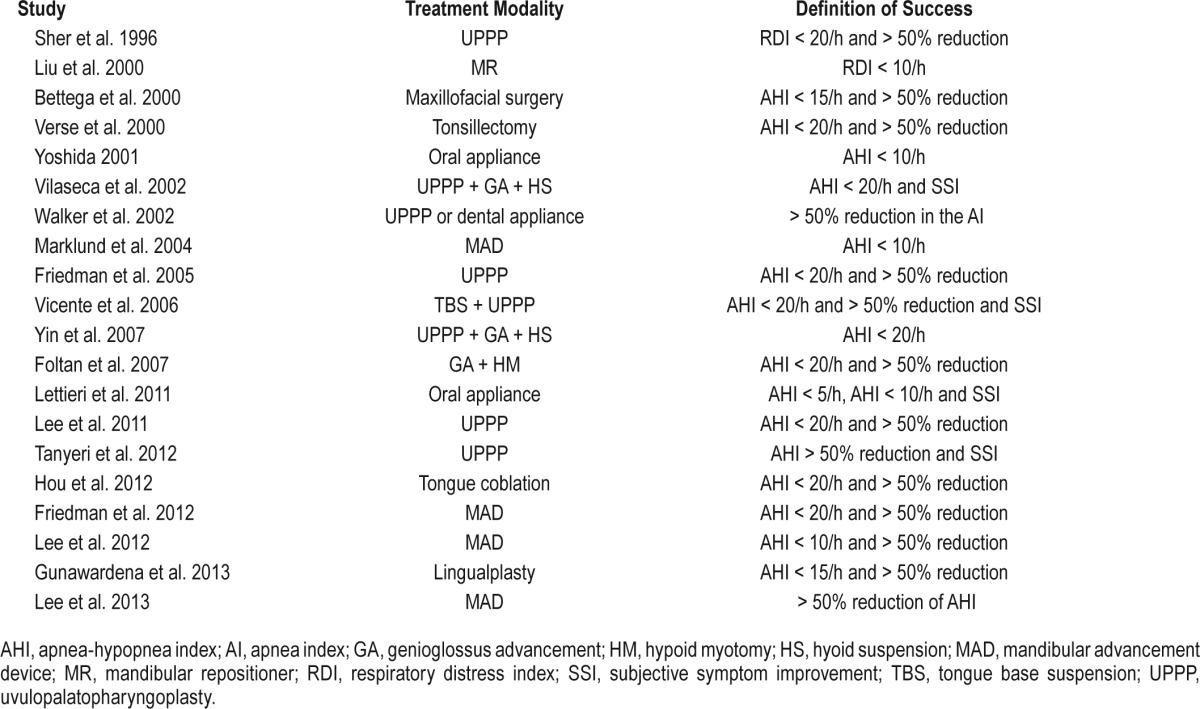
In the present study, we analyzed six success criteria with regard to CPC-based sleep quality, and we attempted to identify the criteria that are significantly associated with the parameters of CPC and identify the optimum criterion that can be used to define treatment success in terms of sleep quality.
METHODS
Patients
Patients were included who were diagnosed with OSA (AHI > 5/h) by an attended full-night PSG study and were treated by sleep surgery or by a MAD at our sleep center from January 2011 to March 2013. Nasal pressure was monitored to detect respiratory events during PSG. Apnea was defined as the complete cessation of airflow ≥ 10 s. Hypopnea was defined as a substantial reduction in airflow (≥ 50%) ≥ 10 s or a moderate reduction in airflow ≥ 10 s associated with electroencephalographic arousals or oxygen desaturation (≥ 4%). This study was approved by the Institutional Review Board of Seoul National University Bundang (IRB No. B-1308/214-111). All patients underwent 2 PSG studies: the first study was performed before treatment and the second study was performed ≥ 3 months after treatment. The exclusion criteria were the following: (1) arrhythmias, (2) artifact encompassing > 20% of the total sleep time of the PSG, (3) total sleep time < 4 h, and (4) sleep efficiency < 80%.
Criteria of Treatment Success
We conducted key word searches in the PubMed databases until December 2013. The words used to query the databases were “OSA,” “efficacy,” and “success.” The reference lists of relevant publications were manually searched for additional studies. The search was limited to studies published in English. Studies for CPAP therapy were excluded. Treatment success was defined by the following 6 criteria used in the literature: (1) posttreatment AHI < 10/h11,14,17; (2) posttreatment AHI < 20/h15,20; (3) reduction in the AHI > 50% and posttreatment AHI < 10/h27; (4) reduction in the AHI > 50% and post-treatment AHI < 15/h12,28; (5) reduction in the AHI > 50% and posttreatment AHI < 20/h10,13,18,21,23,25,26; and (6) a reduction in the AHI > 50%.24,29
Cardiopulmonary Coupling Analysis
The CPC was measured by exported single-lead electrocardiographic data using the commercially available PSG software RemLogic 2.0 CPC analyzer (Embla Systems, San Carlos, CA, USA). Technical details on how the data are processed have been described in a previous study.32 In brief, the software extracted the R-R interval series and electrocardiogram-derived respiration signal from a single-lead electrocardiogram. We then analyzed the R-R interval series and their associated electrocardiogram-derived respiration signal by using Fourier-based techniques to estimate the degree of CPC.32 The R-R interval time series and electrocardiogram-derived respiration signals were first decomposed into a set of sinusoidal oscillations with specific amplitudes and phases at each frequency. If both signals at a given frequency have relatively large oscillation amplitudes, then it is likely that the 2 signals are coupled with each other. This coupling effect can be measured by computing the cross-spectral power. If 2 oscillations at a given frequency are synchronized, the synchronizing effect can be measured by computing the coherence of the signals.32 The parameters of CPC analyses used in the present study were HFC, LFC, very low-frequency coupling (VLFC), and elevated low-frequency coupling (e-LFC).
Statistical Analysis
All values are expressed as mean ± the standard deviation, unless otherwise specified. The patients were divided into 2 outcome groups (“success” group and “failure” group), based on the 6 criteria of success. The paired t test was used to compare the CPC parameters before and after treatment in each outcome group. We also performed multiple regression analyses to determine the optimal criterion of success for non-CPAP treatment with regard to CPC-based sleep quality. A p value < 0.05 was considered statistically significant. Data analysis was performed using SPSS software, version 18 (SPSS Inc., Chicago, IL).
RESULTS
Ninety-eight patients (33 with sleep surgery and 65 with a MAD) were included in this study. The surgical treatment procedures were tonsillectomy, uvulopalatopharyngoplasty, expansion sphincter pharyngoplasty, tongue base resection, and genioglossus advancement. There were 85 (86.7%) males and 13 (13.3%) females. Their mean age was 51.5 ± 9.9 years (range, 19–74 years) and their mean body mass index was 25.6 ± 2.6 kg/m2. Their mean AHI was 34.3 ± 18.0/h. There were no significant differences between the surgery group and the MAD group in sex, body mass index, and AHI. However, there was a significant difference in age (45.2 ± 6.4 years in the surgery group and 53.4 ± 10.0 years in the MAD group; p < 0.001).
Changes with Non-CPAP Treatment
Table 2 summarizes the PSG findings and CPC parameters of the non-CPAP treatment. After treatment, there was no significant improvement for total sleep time. However, there was significant improvement in wake after sleep onset, AHI, apnea index, hypopnea index, oxygen desaturation index, minimal oxygen saturation, average oxygen saturation, and snoring.
Table 2.
Changes in the sleep-related parameters before and after treatment in patients with obstructive sleep apnea.
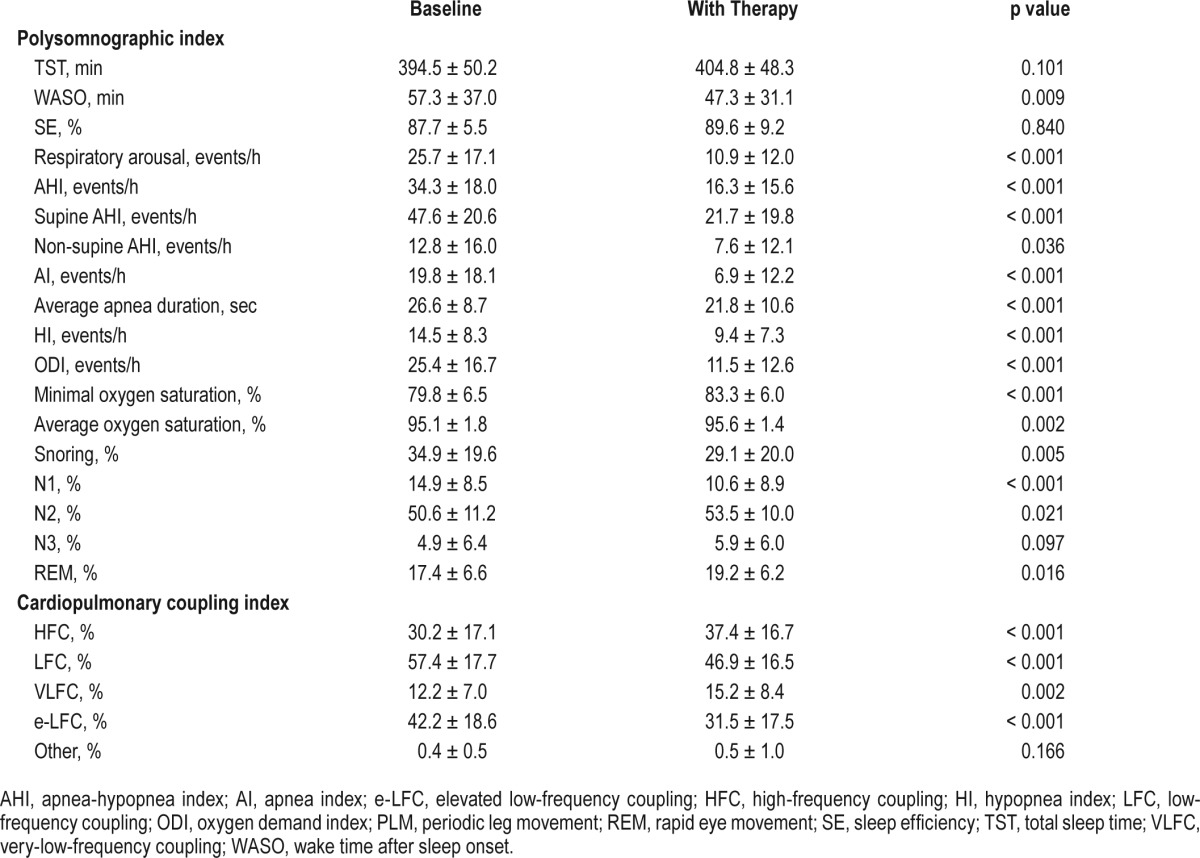
The parameters of CPC analyses also showed significant changes after treatment. High-frequency coupling and VLFC significantly increased (p < 0.001 and p = 0.002, respectively), whereas LFC and e-LFC significantly decreased after non-CPAP treatment (p < 0.001 for both). In the subgroup analyses, there was no difference in the pattern of improvement between the surgery group and the MAD group in the PSG and CPC parameters.
The rate of treatment success ranged from 44% to 70%, depending on success criteria (Table 3). The success rate was highest at 70.4% (69 of 98 patients) when using the criterion of posttreatment AHI < 20/h, and was lowest at 44.9% (44 of 98 patients) when using the criterion of a reduction in the AHI > 50% and posttreatment AHI < 10/h.
Table 3.
Percentage of patients reported as having a successful outcome, as defined by the different success criteria.

Changes in HFC and LFC in Success and Failure Groups Divided by Success Criteria
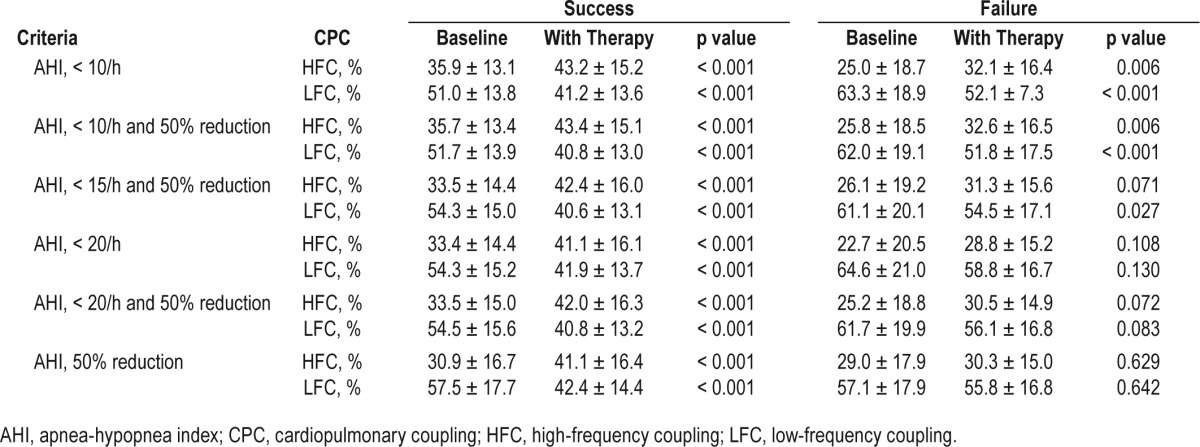
The pretreatment and posttreatment values of the CPC parameters (HFC and LFC) were compared between the success group and the failure group, based on the 6 criteria of treatment success (Table 4). In the success group, HFC significantly increased and LFC significantly decreased in all 6 criteria. In the failure group, we assumed that there would be neither a significant increase in HFC nor a significant decrease in LFC. However, even in the failure group, HFC significantly increased in 2 criteria: (1) posttreatment AHI < 10/h (p = 0.006) and (2) reduction in AHI > 50% and posttreatment AHI < 10/h (p = 0.06). Low-frequency coupling significantly decreased in 3 criteria: (1) posttreatment AHI < 10/h (p < 0.001); (2) reduction in AHI > 50% and posttreatment AHI < 10/h (p < 0.001); and (3) reduction in AHI > 50% and posttreatment AHI < 15/h (p = 0.027). Three criteria were eligible for further analysis because they showed significant improvements in sleep quality in the success group but remained unchanged in the failure group.
Table 4.
Univariate analysis comparing pretreatment and posttreatment values of the cardiopulmonary coupling parameters in the success group and failure group, according to the six criteria.
The Optimum Criteria to Determine Success in CPC-Based Sleep Quality
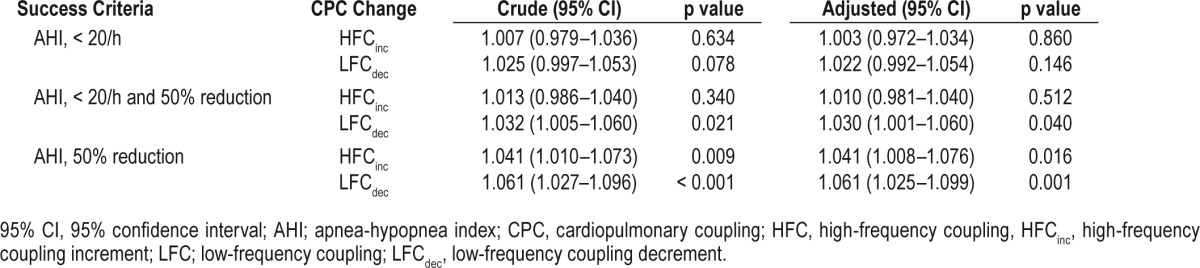
After adjusting for age, sex, body mass index, and treatment modality (surgery or MAD), multiple regression analysis was performed to identify the optimum criterion to differentiate the success group from the failure group with regard to CPC-based sleep quality (Table 5). When applying the criterion of reduction in the AHI > 50%, the probability of treatment success significantly increased by 4.1% with a 1% increase in HFC (adjusted odds ratio, 1.041; 95% confidence interval, 1.008–1.076; p = 0.016), and the probability of treatment success significantly increased by 6.1% with a 1% decrease in LFC (adjusted odds ratio, 1.061; 95% confidence interval, 1.025–1.099; p < 0.001). However, the success group was not differentiated from the failure group in CPC-based sleep quality when using the remaining 2 criteria: (1) posttreatment AHI < 20/h; and (2) reduction in the AHI > 50% and posttreatment AHI < 20/h.
Table 5.
Logistic regression analysis to identify the optimal criterion after adjusting for age, sex, body mass index, and treatment modality.
DISCUSSION
To the best of our knowledge, there have been no studies to validate whether previous criteria of success for non-CPAP treatments are appropriately defined in terms of sleep quality. Non-CPAP treatments such as sleep surgery and oral appliances are frequently applied to patients with OSA. However, there are no standardized criteria available to define treatment success for these non-CPAP treatments. Treatment success or failure may be evaluated from several aspects. From the perspective of comorbidities, success may be defined when the treatment prevents the occurrence of comorbidities such as cardiovascular and neurovascular diseases.34 However, improvement in the AHI during sleep is commonly used to define treatment success in practice. The criteria of success vary according to the study.10–29 Therefore, the treatment outcome cannot be directly compared on a common platform between studies. Our study also showed that the success rate ranged from 44.9% to 70.4%, according to the criterion applied. Furthermore, each criterion lacks justification to be used as the determinant for success or failure in treatment outcome.
In the present study, we analyzed six different criteria for success in terms of sleep quality, which was assessed by CPC analyses. The combination of heart rate variability and electrocardiogram-derived respiration produces HFC (0.1–0.4 Hz), LFC (0.01–0.1Hz), VLFC (0.001–0.01 Hz), and e-LFC.30,32 High-frequency coupling is associated with the breath-to-breath stability of the tidal volume and physiologic respiratory sinus arrhythmia. Therefore, it is a reliable biomarker of stable and consolidated sleep. On the other hand, LFC is correlated with the breath-to-breath cycle fluctuation of the tidal volume and cyclic variation in heart rate, and thus reflects unstable and fragmented sleep.32 Therefore, when sleep quality improves with successful treatment, HFC is increased and LFC is decreased.
Success and failure groups have been divided by the definition determined by the author of each criterion. Sher et al.10 defined the most commonly used criterion of surgical success as a reduction in the AHI greater than 50% and posttreatment AHI less than 20/h. Other investigators have recently proposed to narrow the criterion to a posttreatment AHI less than 5/h or 10/h.35 However, we found that a reduction in the AHI less than 50% was sufficient to indicate the success of non-CPAP therapy in terms of sleep quality. We hypothesized that HFC and LFC would both improve in the success group and remain unchanged in the failure group. Based on the CPC change, three of the six criteria (posttreatment AHI < 20/h; reduction in the AHI > 50% and posttreatment AHI < 20/h; and reduction in the AHI > 50%) supported our hypothesis, whereas the remaining criteria failed to meet our hypothesis. High-frequency coupling and LFC even improved in the failure group. On the other hand, after adjusting for age, sex, body mass index, and treatment modality, multivariate analyses showed that only one criterion—a reduction in the AHI greater than 50%—could significantly differentiate the success group from the failure group. When applying this criterion, the success rate significantly increased by the HFC increment, and it significantly increased by the LFC decrement. Therefore, we concluded that a reduction in the AHI less than 50% might be the optimum criterion when defining the success of treatment outcome in terms of CPC-based sleep quality.
Our study has some limitations. First, the criterion of AHI less than 50% is highly associated with hypoxemia after therapy. We do not believe that this criterion was ideal, but at least therapeutic approaches that fulfilled this criterion may be regarded as successful in terms of CPC-based sleep quality. A randomized control trial should be performed in the future to prove that this criterion can be correlated with a satisfactory health outcome in patients with OSA. Second, we included only sleep surgery and oral appliances as the non-CPAP treatments, although we were aware that there are other treatment modalities such as behavioral therapy, bariatric surgery, and pharmacological therapy. However, the two types of treatment we selected in our study are the most frequent treatment modalities besides CPAP. Third, the two treatment modalities were regarded together rather than separately. This study was not aimed to compare between sleep surgery and oral appliances, but to identify the optimum criterion that can be used objectively to differentiate success from failure by using CPC analyses. Furthermore, previous studies for both treatment modalities used similar criteria of success.10,18,23,26,27 However, further studies in a larger number of patients are needed to identify the optimum criterion of success that is specific to a treatment.
CONCLUSION
The present study is the first trial to identify the optimal criteria of success in the non-CPAP treatment of OSA. The CPC analyses were used as an objective method to assess sleep quality. Treatment success or failure was re-evaluated in terms of CPC-based sleep quality. Our study showed that the criterion of a reduction in the AHI greater than 50% was the most appropriate criterion to differentiate success from failure in terms of sleep quality. A uniform definition of treatment success should be established for non-CPAP therapy for OSA. Further studies should be performed to validate the criteria of success in terms of co-morbidities and in terms of sleep quality.
DISCLOSURE STATEMENT
This was not an industry supported study. This research was partly supported by a grant by the Seoul National University Bundang Hospital in Seongnam, Korea (grant #02-2013-011). The authors have indicated no financial conflicts of interest.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- AI
apnea index
- CPAP
continuous positive airway
- CPC
cardiopulmonary coupling
- e-LFC
elevated low-frequency coupling
- GA
genioglossus advancement
- HFC
high-frequency coupling
- HFCinc
high-frequency coupling increment
- HM
hypoid myotomy
- HS
hyoid suspension
- LFC
low-frequency coupling
- LFCdec
low-frequency coupling decrement
- MAD
mandibular advancement device
- MR
mandibular repositioner
- ODI
oxygen demand index
- OSA
obstructive sleep apnea
- PLM
periodic leg movement
- PSG
polysomnography
- RDI
respiratory distress index
- REM
rapid eye movement
- SE
sleep efficiency
- SSI
subjective symptom improvement
- TBS
tongue base suspension
- TST
total sleep time
- UPPP
uvulopalatopharyngoplasty
- VLFC
very low-frequency coupling
- WASO
wake time after sleep onset
REFERENCES
- 1.Bazzano LA, Khan Z, Reynolds K, et al. Effect of nocturnal nasal continuous positive airway pressure on blood pressure in obstructive sleep apnea. Hypertension. 2007;50:417–23. doi: 10.1161/HYPERTENSIONAHA.106.085175. [DOI] [PubMed] [Google Scholar]
- 2.Kanagala R, Murali NS, Friedman PA, et al. Obstructive sleep apnea and the recurrence of atrial fibrillation. Circulation. 2003;107:2589–94. doi: 10.1161/01.CIR.0000068337.25994.21. [DOI] [PubMed] [Google Scholar]
- 3.Kaneko Y, Floras JS, Usui K, et al. Cardiovascular effects of continuous positive airway pressure in patients with heart failure and obstructive sleep apnea. N Engl J Med. 2003;348:1233–41. doi: 10.1056/NEJMoa022479. [DOI] [PubMed] [Google Scholar]
- 4.Marin JM, Carrizo SJ, Vicente E, et al. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: an observational study. Lancet. 2005;365:1046–53. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 5.Wessendorf TE, Wang YM, Thilmann AF, et al. Treatment of obstructive sleep apnoea with nasal continuous positive airway pressure in stroke. Eur Respir J. 2001;18:623–9. doi: 10.1183/09031936.01.00057201. [DOI] [PubMed] [Google Scholar]
- 6.Liu Y, Park YC, Lowe AA, et al. Supine cephalometric analyses of an adjustable oral appliance used in the treatment of obstructive sleep apnea. Sleep Breath. 2000;4:59–66. doi: 10.1007/BF03045025. [DOI] [PubMed] [Google Scholar]
- 7.Holley AB, Lettieri CJ, Shah AA. Efficacy of an adjustable oral appliance and comparison with continuous positive airway pressure for the treatment of obstructive sleep apnea syndrome. Chest. 2011;140:1511–6. doi: 10.1378/chest.10-2851. [DOI] [PubMed] [Google Scholar]
- 8.Lam B, Sam K, Mok WY, et al. Randomised study of three non-surgical treatments in mild to moderate obstructive sleep apnoea. Thorax. 2007;62:354–9. doi: 10.1136/thx.2006.063644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Phillips CL, Grunstein RR, Darendeliler MA, et al. Health outcomes of continuous positive airway pressure versus oral appliance treatment for obstructive sleep apnea: a randomized controlled trial. Am J Respir Crit Care. 2013;187:879–87. doi: 10.1164/rccm.201212-2223OC. [DOI] [PubMed] [Google Scholar]
- 10.Sher AE, Schechtman KB, Piccirillo JF. The efficacy of surgical modifications of the upper airway in adults with obstructive sleep apnea syndrome. Sleep. 1996;19:156–77. doi: 10.1093/sleep/19.2.156. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Zeng X, Fu M, et al. Effects of a mandibular repositioner on obstructive sleep apnea. Am J Orthod Dentofacial Othop. 2000;118:248–56. doi: 10.1067/mod.2000.104831. [DOI] [PubMed] [Google Scholar]
- 12.Bettega G, Pepin JL, Veale D, et al. Obstructive sleep apnea syndrome: fiftyone consecutive patients treated by maxillofacial surgery. Am J Respir Crit Care. 2000;162:641–9. doi: 10.1164/ajrccm.162.2.9904058. [DOI] [PubMed] [Google Scholar]
- 13.Verse T, Kroker BA, Pirsig W, et al. Tonsillectomy as a treatment of obstructive sleep apnea in adults with tonsillar hypertrophy. Laryngoscope. 2000;110:1556–9. doi: 10.1097/00005537-200009000-00029. [DOI] [PubMed] [Google Scholar]
- 14.Yoshida K. Influence of sleep posture on response to oral appliance therapy for sleep apnea syndrome. Sleep. 2001;24:538–44. doi: 10.1093/sleep/24.5.538. [DOI] [PubMed] [Google Scholar]
- 15.Vilaseca I, Morello A, Montserrat JM, et al. Usefulness of uvulopalatopharyngoplasty with genioglossus and hyoid advancement in the treatment of obstructive sleep apnea. Arch Otolaryngol. 2002;128:435–40. doi: 10.1001/archotol.128.4.435. [DOI] [PubMed] [Google Scholar]
- 16.Walker-Engstrom ML, Tegelberg A, Wilhelmsson B, et al. 4-year follow-up of treatment with dental appliance or uvulopalatopharyngoplasty in patients with obstructive sleep apnea: a randomized study. Chest. 2002;121:739–46. doi: 10.1378/chest.121.3.739. [DOI] [PubMed] [Google Scholar]
- 17.Marklund M, Stenlund H, Franklin KA. Mandibular advancement devices in 630 men and women with obstructive sleep apnea and snoring: tolerability and predictors of treatment success. Chest. 2004;125:1270–8. doi: 10.1378/chest.125.4.1270. [DOI] [PubMed] [Google Scholar]
- 18.Friedman M, Vidyasagar R, Bliznikas D, et al. Does severity of obstructive sleep apnea/hypopnea syndrome predict uvulopalatopharyngoplasty outcome? Laryngoscope. 2005;115:2109–13. doi: 10.1097/01.MLG.0000181505.11902.F7. [DOI] [PubMed] [Google Scholar]
- 19.Vicente E, Marin JM, Carrizo S, et al. Tongue-base suspension in conjunction with uvulopalatopharyngoplasty for treatment of severe obstructive sleep apnea: long-term follow-up results. Laryngoscope. 2006;116:1223–7. doi: 10.1097/01.mlg.0000224498.09015.d9. [DOI] [PubMed] [Google Scholar]
- 20.Yin SK, Yi HL, Lu WY, et al. Genioglossus advancement and hyoid suspension plus uvulopalatopharyngoplasty for severe OSAHS. Otolaryngol Head Neck. 2007;136:626–31. doi: 10.1016/j.otohns.2006.01.028. [DOI] [PubMed] [Google Scholar]
- 21.Foltan R, Hoffmannova J, Pretl M, et al. Genioglossus advancement and hyoid myotomy in treating obstructive sleep apnoea syndrome - a follow-up study. J Craniomaxillofac Surg. 2007;35:246–51. doi: 10.1016/j.jcms.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 22.Lettieri CJ, Paolino N, Eliasson AH, et al. Comparison of adjustable and fixed oral appliances for the treatment of obstructive sleep apnea. J Clin Sleep Med. 2011;7:439–45. doi: 10.5664/JCSM.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee CH, Kim SW, Han K, et al. Effect of uvulopalatopharyngoplasty on positional dependency in obstructive sleep apnea. Arch Otolaryngol. 2011;137:675–9. doi: 10.1001/archoto.2011.99. [DOI] [PubMed] [Google Scholar]
- 24.Tanyeri H, Polat S, Kirisoglu CE, et al. Long-term efficacy of submucosal uvulopalatopharyngoplasty for obstructive sleep apnea syndrome. Eur Arch Otorhinolaryngol. 2012;269:2069–74. doi: 10.1007/s00405-011-1919-x. [DOI] [PubMed] [Google Scholar]
- 25.Hou T, Hu S, Jiang X. Tongue coblation via the ventral approach for obstructive sleep apnea-hypopnea syndrome surgery. Laryngoscope. 2012;122:2582–6. doi: 10.1002/lary.23556. [DOI] [PubMed] [Google Scholar]
- 26.Friedman M, Hamilton C, Samuelson CG, et al. Compliance and efficacy of titratable thermoplastic versus custom mandibular advancement devices. Otolaryngol Head Neck Surg. 2012;147:379–86. doi: 10.1177/0194599812439683. [DOI] [PubMed] [Google Scholar]
- 27.Lee CH, Jung HJ, Lee WH, et al. The effect of positional dependency on outcomes of treatment with a mandibular advancement device. Arch Otolaryngol. 2012;138:479–83. doi: 10.1001/archoto.2012.452. [DOI] [PubMed] [Google Scholar]
- 28.Gunawardena I, Robinson S, MacKay S, et al. Submucosal lingualplasty for adult obstructive sleep apnea. Otolaryngol Head Neck Surg. 2013;148:157–65. doi: 10.1177/0194599812461750. [DOI] [PubMed] [Google Scholar]
- 29.Lee WH, Wee JH, Lee CH, et al. Comparison between mono-bloc and bi-bloc mandibular advancement devices for obstructive sleep apnea. Eur Arch Otorhinolaryndol. 2013;270:2909–13. doi: 10.1007/s00405-013-2417-0. [DOI] [PubMed] [Google Scholar]
- 30.Thomas RJ, Mietus JE, Peng CK, et al. An electrocardiogram-based technique to assess cardiopulmonary coupling during sleep. Sleep. 2005;28:1151–61. doi: 10.1093/sleep/28.9.1151. [DOI] [PubMed] [Google Scholar]
- 31.Ibrahim LH, Jacono FJ, Patel SR, et al. Heritability of abnormalities in cardiopulmonary coupling in sleep apnea: use of an electrocardiogram-based technique. Sleep. 2010;33:643–6. doi: 10.1093/sleep/33.5.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Thomas RJ, Mietus JE, Peng CK, et al. Differentiating obstructive from central and complex sleep apnea using an automated electrocardiogram-based method. Sleep. 2007;30:1756–69. doi: 10.1093/sleep/30.12.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu D, Yang X, Wang G, et al. HHT based cardiopulmonary coupling analysis for sleep apnea detection. Sleep Med. 2012;13:503–9. doi: 10.1016/j.sleep.2011.10.035. [DOI] [PubMed] [Google Scholar]
- 34.Becker HF, Jerrentrup A, Ploch T, et al. Effect of nasal continuous positive airway pressure treatment on blood pressure in patients with obstructive sleep apnea. Circulation. 2003;107:68–73. doi: 10.1161/01.cir.0000042706.47107.7a. [DOI] [PubMed] [Google Scholar]
- 35.Elshaug AG, Moss JR, Southcott AM, et al. Redefining success in airway surgery for obstructive sleep apnea: a meta-analysis and synthesis of the evidence. Sleep. 2007;30:461–7. doi: 10.1093/sleep/30.4.461. [DOI] [PubMed] [Google Scholar]


