Abstract
Study Objectives:
Clinical studies have investigated whether obstructive sleep apnea (OSA) can modulate bone metabolism but data are conflicting. Bone mineral density (BMD) measured by dual-energy x-ray absorptiometry is the standard technique for quantifying bone strength but has limitations in overweight patients (body mass index [BMI] ≥ 25 kg/m2). The aim of this study was to examine the association between OSA and BMD by examining CT images that allow true volumetric measurements of the bone regardless of BMI.
Methods:
Lumbar vertebrae BMD was evaluated in 234 persons (180 males and 54 females) by CT scan. The method was calibrated by a phantom containing a known concentration of hydroxyapatite.
Results:
BMD was lower in male patients with severe OSA (apnea-hypopnea index [AHI] ≥ 30/h) than non OSA (AHI < 5; p < 0.05), while OSA and BMD had no association in females. Linear and multiple regression analyses revealed that age (p < 0.0001, β = −0.52), hypertension (p = 0.0068, β = −0.17), and the alveolar-arterial oxygen pressure difference (A-aDO2) (p = 0.012, β = −0.15) in males were associated with BMD, while only age (p < 0.0001, β = −0.68) was associated with BMD in females.
Conclusion:
Males with severe OSA had a significantly lower BMD than non OSA participants. Age, hypertension, and elevation of A-aDO2 were significant factors for BMD by CT imaging. The usefulness of measuring BMD in OSA patients by CT scanning should be studied in future.
Citation:
Hamada S, Ikezoe K, Hirai T, Oguma T, Tanizawa K, Inouchi M, Handa T, Oga T, Mishima M, Chin K. Evaluation of bone mineral density by computed tomography in patients with obstructive sleep apnea. J Clin Sleep Med 2016;12(1):25–34.
Keywords: obstructive sleep apnea, bone mineral density, computed tomography, daytime oxygenation, hypertension
INTRODUCTION
Obstructive sleep apnea (OSA) is a disorder of growing health concern. Recent data have shown that the prevalence of moderate to severe OSA in adult males is 10–20% in Western and Asian countries.1,2 OSA is a disorder characterized by repetitive collapse and reopening of the upper airway during sleep, which can result in intermittent hypoxemia and sleep fragmentation.3 Intermittent hypoxemia is known to be associated with increased levels of many mediators involved in inflammatory processes, oxidative stress, and thrombotic activity.4 These adverse effects may have many pathological consequences, including cardiovascular diseases, neurocognitive deficits, and metabolic disorders.4
Hypoxemia is also known to directly stimulate the formation and activation of osteoclasts.5,6 Therefore, hypoxemia in OSA patients during sleep may become a risk factor for osteoporosis. Osteoporosis is a common skeletal disorder characterized by compromised bone strength and increased risk of fracture, and is associated with mortality, especially in males.7 Bone strength primarily reflects the integration of bone mineral density (BMD) and bone quality. Measurement of BMD by dual-energy x-ray absorptiometry (DEXA) is the standard technique for quantifying osteoporosis. Other techniques to analyze BMD include quantitative CT scanning, magnetic resonance spectroscopy and perfusion, and quantitative ultrasonography.8
BRIEF SUMMARY
Current Knowledge/Study Rationale: Reports on the relationships between obstructive sleep apnea (OSA) and bone metabolism are conflicting. Bone mineral density (BMD) is usually quantified by dual-energy x-ray absorptiometry, which has limitations in overweight persons. In the present study, we used quantitative CT to examine the relationships between OSA and BMD.
Study Impact: This study showed that BMD in male patients with severe OSA determined by CT images was lower than that in non OSA participants. Also, age, hypertension, and elevation of the alveolar-arterial oxygen pressure difference were significant factors for BMD by CT imaging.
There have been a few reports about the relationships between OSA and osteoporosis.9–13 It was reported that OSA could impair,9–11 not affect,12 or stimulate bone metabolism.13 Thus, reports on the associations between OSA and bone metabolism were conflicting. In these reports, DEXA was used for evaluating BMD. DEXA has a limitation in measuring BMD in over-weight patients (body mass index [BMI] ≥ 25 kg/m2) because superimposed soft tissue can cause inaccurate measurements of BMD owing to attenuation of the x-ray beams and a beam hardening artifact.8,14 Therefore, DEXA might not accurately evaluate BMD in patients with OSA, because the majority of persons with OSA are overweight or obese. Quantitative CT has important advantages over DEXA because it allows true volu-metric measurements of the lumbar spine regardless of BMI.8 Previously, we reported that BMD in thoracic and lumbar vertebral bones was assessed in patients with chronic obstructive pulmonary disease (COPD) by CT scan.15,16 We usually performed abdominal CT scans to measure abdominal fat accumulation.17 Therefore, these images could concurrently be used to evaluate BMD of lumbar vertebral bones. Vertebral fractures are among the most frequent complications of osteoporosis. Korean population data showed that mortality after vertebral fracture was significantly higher than that in the normal population.18 We hypothesized that there were close associations between OSA and BMD that might be confirmed by CT scan images. We here evaluated images obtained by CT of lumbar vertebral bones to examine the association between OSA and BMD.
METHODS
Study Participants
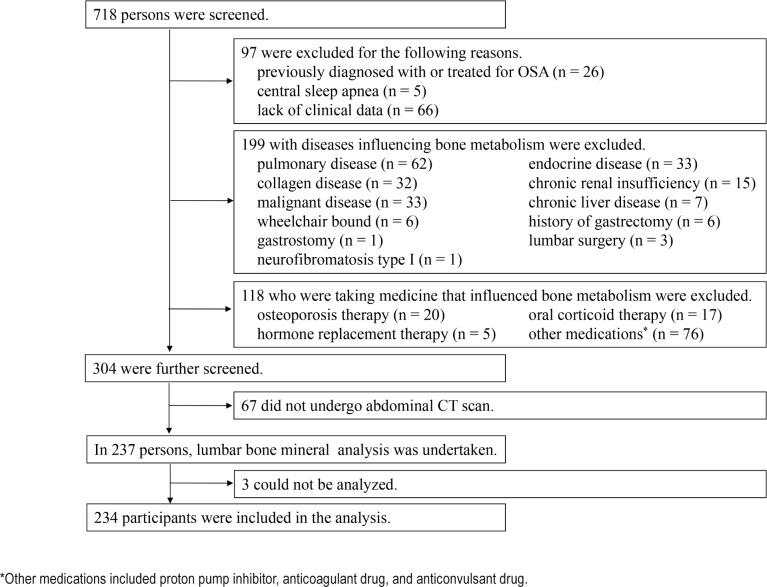
Studied were 718 adults (age 20–80 years) who were admitted to the Sleep Unit of Kyoto University Hospital for overnight polysomnographic (PSG) evaluation between December 2008 and July 2011. All had been referred to our sleep unit with symptoms such as snoring and/or daytime sleepiness. Figure 1 is a flow chart summarizing patient selection. Among 718 individuals, we excluded 97 for the following reasons: treatment for OSA (n = 26), central sleep apnea (n = 5), and lack of clinical data (n = 66). We excluded 199 patients who had the following diseases that can affect bone metabolism: pulmonary disease including asthma or COPD treated by inhaled corticosteroid treatment and interstitial lung disease (n = 62), endocrine disease (n = 33), collagen disease (n = 32), chronic renal insufficiency (serum creatinine > 2.0 mg/dL) (n = 15), occurrence of malignancy within the previous 5 years (n = 33), history of gastrectomy (n = 6) and history of lumbar surgery (n = 3), chronic liver disease (n = 7), being wheelchair bound (n = 6), neurofibromatosis type I (n = 1), and having a gastrostomy (n = 1). Also, we excluded 118 individuals who received medicine that could influence bone metabolism as follows: osteoporosis therapy (n = 20), oral corticoid therapy (n = 17), hormone replacement therapy (n = 5), and other medications (n = 76) including proton pump inhibitors, anticoagulant drugs, and anticonvulsant drugs. Of the remaining 304 patients, 67 did not undergo an abdominal CT scan. There was no difference between participants who did or did not have a CT scan (see Table S1 in supplemental material). Then, we analyzed lumbar BMD in 237 subjects. Three of these participants were ineligible for analysis because of a compression fracture and/or an osteophyte. Finally, the study consisted of 234 participants (180 males and 54 females).
Figure 1. Flow chart of selection of study participants.
*Other medications included proton pump inhibitor, anticoagulant drug, and anticonvulsant drug.
This study was approved by the Kyoto University Graduate School and Faculty of Medicine Ethics Committee (E-2061), and informed consent was obtained from all subjects.
BMI was calculated as weight in kilograms divided by the square of height in meters. Waist circumference was measured midway between the lower costal margin and iliac crest.
Laboratory Analysis of Blood and Blood Pressure Measurements
Samples of peripheral venous blood were collected in the morning with the patient fasting after one-night PSG. Routine blood examinations were performed as follows: Fasting plasma glucose and hemoglobin A1c were measured as markers of glucose metabolism. Serum total cholesterol, serum triglycerides, and serum high-density lipoprotein cholesterol were measured as markers of lipid metabolism. The remaining blood samples were centrifuged immediately at 3,000 rpm at 4°C for 10 min. The separated samples were stored at −80°C until assay. Serum calcium, osteocalcin and 25-hydroxy vitamin D levels were measured by Chlorophosphonazo-III (Espa Ca, Nipro, Osaka, Japan), solid-phase sandwich enzyme-linked immunosorbent assay (BGP IRMA, LSI Medience, Tokyo, Japan) and competitive protein binding assay (LSI Medience), respectively.
Arterial blood for the analysis of gas under room air breathing was drawn just before PSG started at night with the participants in the supine position. pH, bicarbonate concentration (HCO3−), and arterial partial pressure of oxygen (PaO2) and carbon dioxide pressure (PaCO2) were measured in a blood gas analyzer (arterial blood gas analyzer RL 865; Chiron Corporation, Emeryville, CA). The alveolar-arterial oxygen pressure difference (A-aDO2) was obtained by subtracting the PaO2 from the alveolar partial pressure of oxygen (PAO2). PAO2 was calculated according to the simplified alveolar air equation as follows: PAO2 = 150 − PaCO2 / 0.8.
Blood pressure was measured 5 times at 1-min intervals in the sitting position after resting for at least 5 min. The average of the latter 2 recordings was calculated.
Definitions of Hypertension, Diabetes Mellitus, and Hyperlipidemia
Hypertension was defined by the use of antihypertensive medications or a systolic blood pressure ≥ 135 mm Hg or a diastolic blood pressure ≥ 85 mm Hg.19 Diabetes mellitus was defined by the use of antidiabetic medications or a fasting plasma glucose level ≥ 126 mg/dL or hemoglobin A1c level ≥ 6.5%.20 Hyperlipidemia was determined if the individuals used antihyperlipidemic medications or had a serum level of total cholesterol ≥ 220 mg/dL, high density lipoprotein cholesterol < 40 mg/dL, low density lipoprotein cholesterol ≥ 140 mg/dL or triglyceride ≥ 150 mg/dL.21
Physical Activity
We measured physical activity with a questionnaire on frequency of and time spent on three levels of physical activity (i.e., vigorous: jogging, swimming, strenuous sports, bicycling on hills; moderate: housework, ballroom dancing, golf, bicycling on level ground, lawn mowing, regular walking; and light: office work, strolling, personal care).22 We set the average intensity of vigorous activity at 2.15 metabolic equivalents (MET, kJ kg/h), and that of moderate and light activity were at 1.43 and 0.72, respectively, according to the compendium of physical activity.22 The amount of physical activity (kJ/week) was calculated by multiplying the corresponding MET values by frequency per week, hours spent at each session, and body weight, and summing up the values. This value was defined as the physical activity index (PAI).22
Epworth Sleepiness Scale
Daytime sleepiness was assessed by the Japanese version of the Epworth Sleepiness Scale (ESS).23 With the ESS, individuals score themselves on a scale of 0 (not at all likely to fall asleep) to 3 (very likely to fall asleep) according to how easily they would fall asleep in 8 different situations, with possible overall scores of 0 to 24. The higher the score, the sleepier is the individual.
PSG
All participants underwent PSG (SomnoStar pro, Cardinal Health, Dublin, OH, USA or Alice 5, Healthdyne, Atlanta, GA, USA), the details of which were described elsewhere.24 Ventilation was monitored by inductive plethysmography (Respitrace QDC, Viasys Healthcare, Palm Springs, CA, USA). Airflow was monitored by a nasal pressure transducer (PTAFlite, Pro-Tech Services Inc., Mukilteo, WA, USA) and supplemented by an oronasal thermal sensor (Sleepmate Technologies, Midlothian, VA, USA). Arterial oxygen saturation was measured transcutaneously at the fingertip by pulse oximetry (Adult Flex System, Nonin Medical, Plymouth, MN, USA). Apnea was indicated by the complete cessation of airflow ≥ 10 s and hypopnea was indicated by a decrease in airflow ≥ 30% lasting ≥ 10 s, accompanied by oxygen desaturation ≥ 4%.25 Obstructive and central sleep apnea/hypopnea were defined as the presence and absence of thoraco-abdominal effort, respectively. The apnea-hypopnea index (AHI) was calculated as the number of apnea and hypopnea episodes per hour of recording time in bed. OSA was defined as AHI ≥ 5 events/h, which were predominantly obstructive respiratory events (presence of thoraco-abdominal effort).25 Severity of OSA was also defined by the AHI as follows: non OSA (AHI < 5), mild OSA (5 ≥ AHI < 15), moderate OSA (15 ≥ AHI < 30), and severe OSA (AHI ≥ 30). The 4% oxygen desaturation index (4% ODI) indicated the number of oxygen desaturation events ≥ 4% below the baseline level per hour during sleep. The percentage of time with SpO2 < 90% (TST90) was the percentage of time that arterial O2 saturation was < 90% during sleep.
Measurement of BMD in Lumbar Vertebral Bone
To measure BMD, we used CT images obtained by Aquilion 64 (Toshiba, Tokyo, Japan; slice thickness, 7 mm) that were taken to measure visceral fat accumulation. Our protocol for lumbar bone mineral analysis used an axial slice in the midsection of the three vertebral bodies from L1 to L3, using a modified method reported previously.6,15,16 On the horizontal images of the selected slice, the elliptical region of interest (ROI) was encompassed manually as the largest possible area at the anterior portion of each vertebral body. Finally, the mean CT scan density of the ROI was measured. For the measurement of BMD, a CT scan calibration phantom (Kyoto Kagaku Co, Ltd; Kyoto, Japan) containing 8 tubes of known concentrations of hydroxyapatite (0, 50, 100, 150, 200, 250, 300, and 400 mg/ mL) was scanned with the same scanning parameters as those for the participants.15,16 BMD was expressed in milligrams per milliliter of hydroxyapatite using the following relationship: BMD (in milligrams per milliliter) = 0.68 × CT scan density (in HU) + 25.33 (correlation coefficient, 0.991). Measurements from all images were made twice by the same observer, who did not know the clinical status of the patient. The correlation coefficient for the measurements of CT scan density of each lumbar vertebral bone between two measurements was 0.975, 0.992, and 0.991 for vertebrae L1, L2, and L3, respectively. Final values were decided based on an average of 2 measurements.
Statistical Analysis
Data were expressed as the mean ± standard deviation (SD). Firstly, we compared differences based on the severity of OSA in both sexes by an analysis of variance. When a significant difference was observed, we used the Tukey-Kramer post hoc test to identify where the differences were significant. Categorical variables were compared by the χ2 test. Next, relationships between the average BMD of lumbar vertebrae from L1 to L3 and the other variables were analyzed by Pearson correlation coefficients or Spearman rank correlation coefficients. Then, to identify which variables could best determine the average BMD, stepwise linear regression analyses were performed. The variables entered in the multivariate analysis were those yielding a p value < 0.1 by univariate analysis. Also, when independent variables had very strong collinearity (r ≥ 0.7), the one that had the stronger correlation with the average BMD was selected. Cutoff values set in the stepwise analysis were p = 0.05 for both the forward and backward processes. A p value < 0.05 was considered to be significant. All statistical analyses were performed using a statistical software package (JMP 10 software; SAS Institute Inc; Cary, NC).
RESULTS
Baseline Characteristics
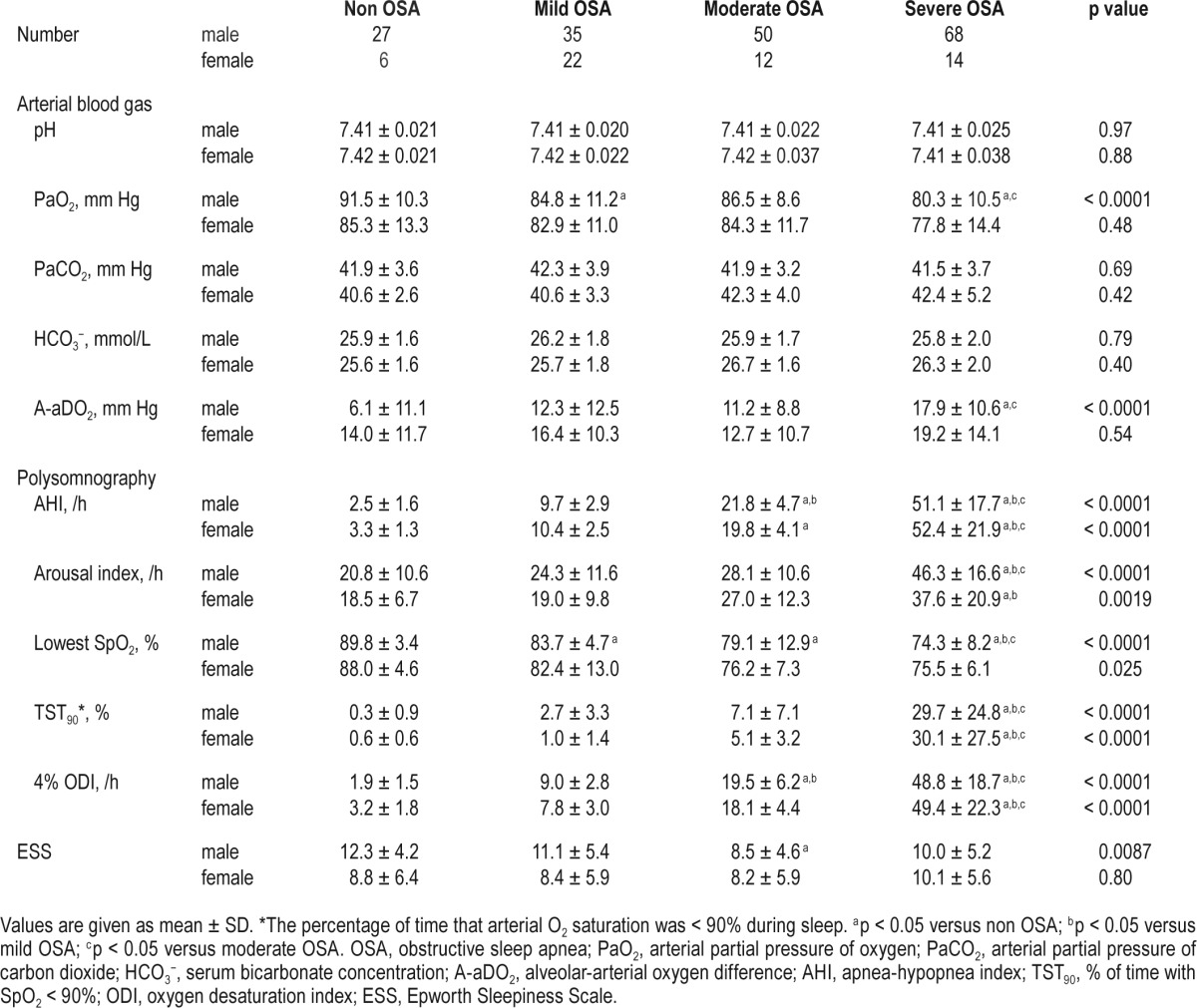
Clinical data on the study population are shown in Tables 1–3. Of the participants, 98, 74, and 40 patients took antihypertensive, antihyperlipidemic, and antidiabetic medications, respectively. Female patients with severe OSA were heavier (p < 0.05) and had a higher rate of diabetes mellitus (p < 0.05) than those with mild OSA. Male patients with severe OSA were the heaviest, as were female patients with severe OSA. Male participants with non OSA had higher ESS scores than those with moderate OSA (p < 0.05). Males with OSA regardless of severity had a higher rate of hypertension than those with non OSA (p < 0.05). Also, male patients with severe OSA had a lower PaO2 (p < 0.05) and a higher A-aDO2 (p < 0.05) than those with non OSA. The males with severe OSA had a significantly lower BMD level than those with non OSA (p < 0.05), while this difference was not noted in females. Data on PAI could be obtained from 218 of 234 participants (170 males and 48 females), and there was no difference in PAI scores between the non OSA and OSA groups.
Table 1.
Clinical characteristics of study population.
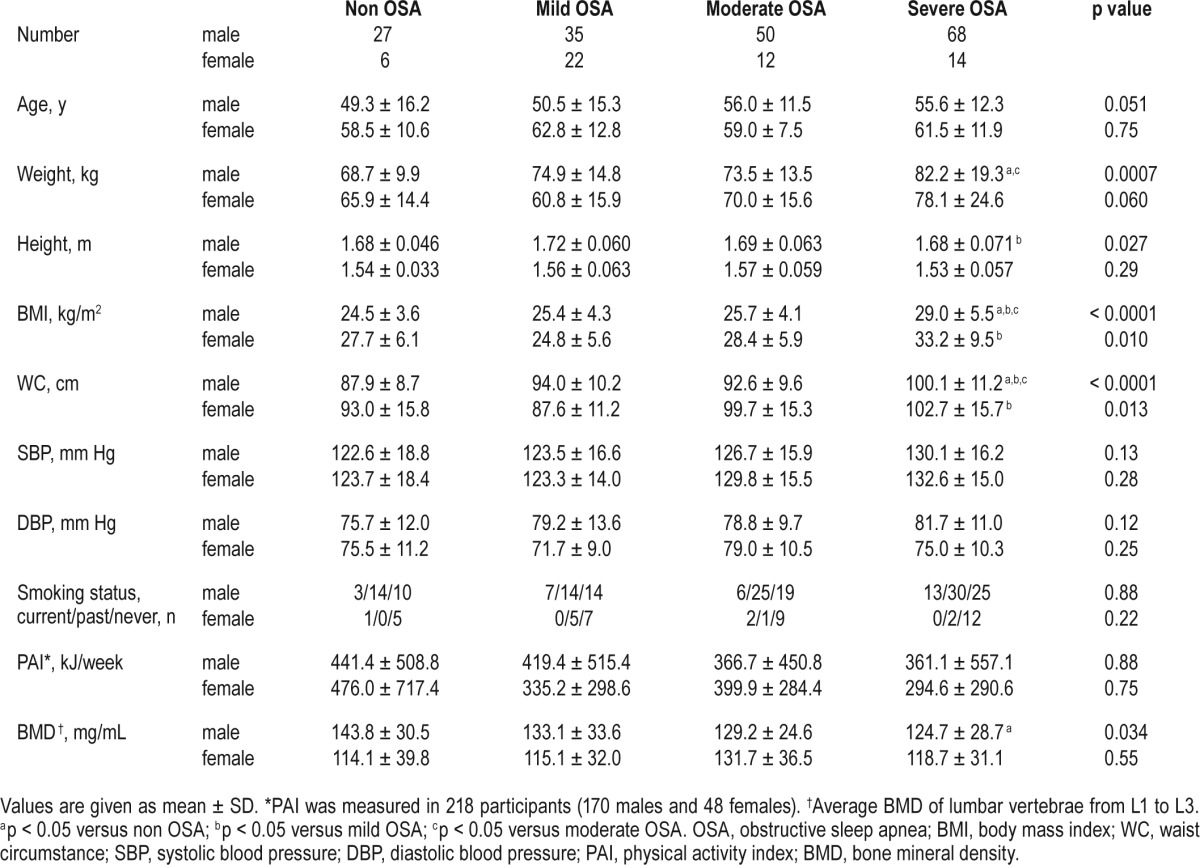
Table 2.
Metabolic data of study population.
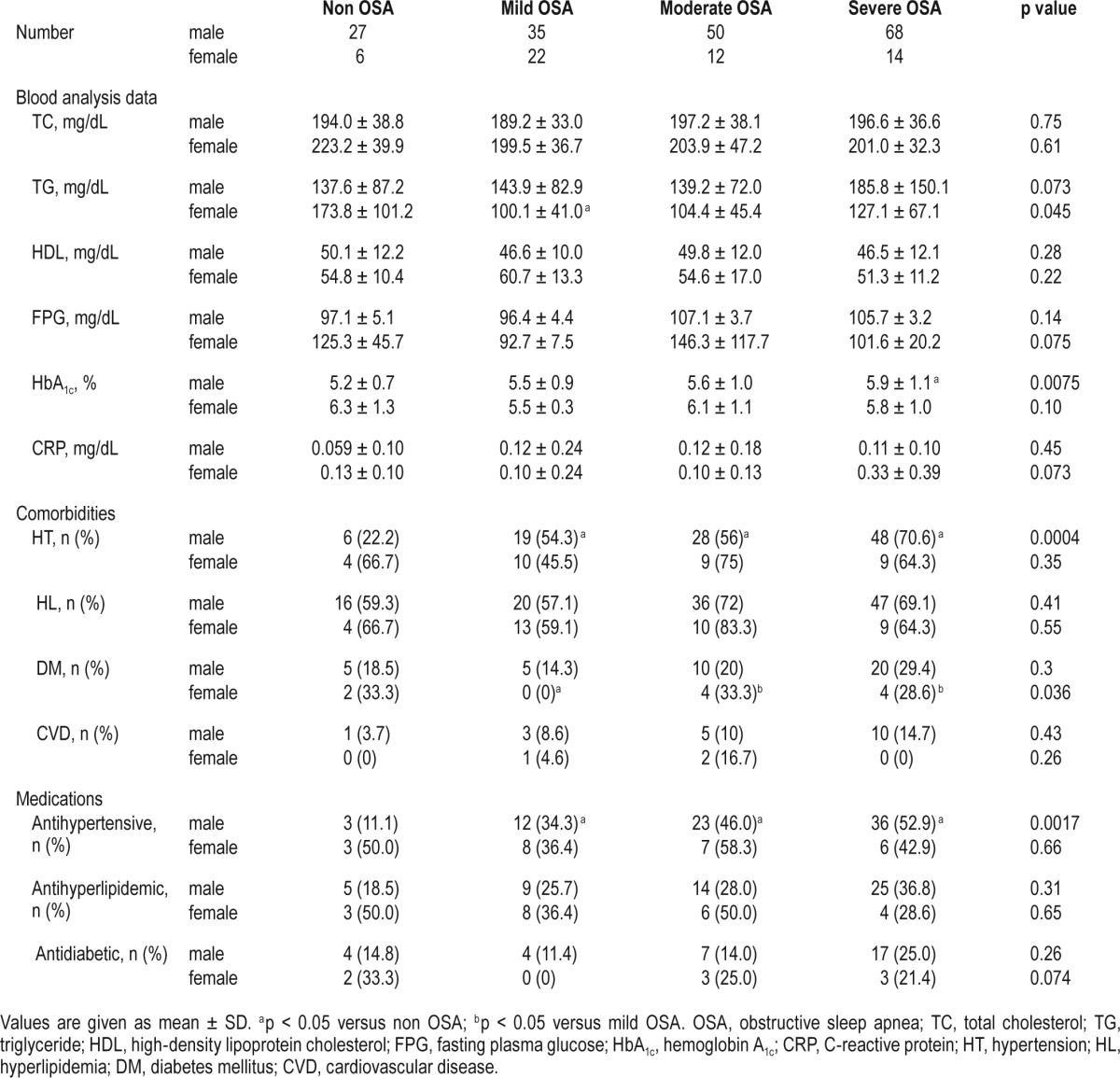
Table 3.
Laboratory data and sleep parameters of study population.
In this retrospective study, calcium, osteocalcin, and 25-hydroxy vitamin D levels were measured in 104 participants. There was no difference between subjects who did or did not undergo blood examinations (see Table S2 in supplemental material). In participants who underwent blood analyses, there was no difference between each OSA and non OSA group with regard to laboratory data of calcium, osteocalcin, and 25-hydroxy vitamin D levels (see Table S3 in supplemental material).
Factors of Predicting BMD
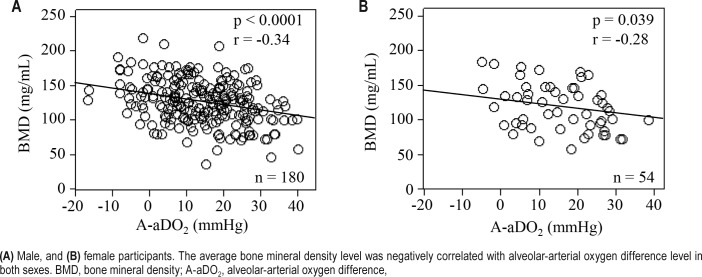
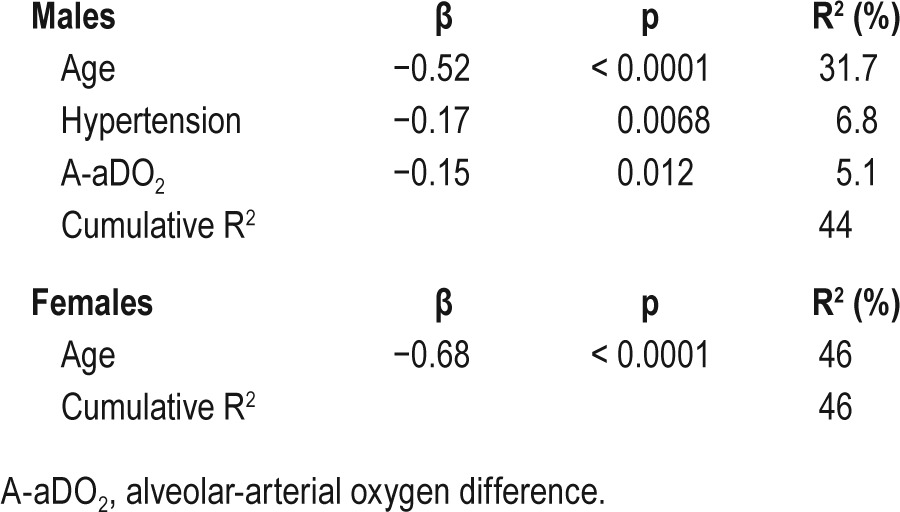
Correlations between the average BMD of lumbar vertebrae from L1 to L3 and clinical characteristics are shown in Table 4. In both sexes, the BMD was significantly correlated with age (male: r = −0.61, p < 0.0001; female: r = −0.68, p < 0.0001) and A-aDO2 (male: r = −0.34, p < 0.0001; female: r = −0.28, p = 0.039). Figure 2 shows the relationship between A-aDO2 and BMD. In males, the BMD was significantly correlated with hypertension (ρ = −0.39, p < 0.0001), pH (r = −0.25, p = 0.0009), PaO2 (r = 0.23, p = 0.0022), PaCO2 (r = 0.32, p < 0.0001), and arousal index (r = −0.27, p = 0.0003). Stepwise multiple regression analysis was performed to identify factors for predicting the average BMD of lumbar vertebrae from L1 to L3. In both sexes, PaO2 and A-aDO2 had strong collinearity (both sexes: r = −0.92). In males, PaCO 2 and HCO3− also had strong col-linearity (r = 0.70). A-aDO2 (r = −0.34) and PaCO2 (r = 0.32) had stronger correlations with the average BMD than PaO2 (r = 0.23) and HCO3− (r = 0.14), respectively. Therefore, we performed multiple regression analysis using A-aDO2 and PaCO2. In males, age (β = −0.52, p < 0.0001), hypertension (β = −0.17, p = 0.0068), and A-aDO2 (β = −0.15, p = 0.012) were independent explanatory variables of BMD (Table 5). In females, age was an independent explanatory variable of BMD (β = −0.68, p < 0.0001) (Table 5).
Table 4.
Linear regression analysis of average bone mineral density of lumbar vertebrae from L1 to L3.
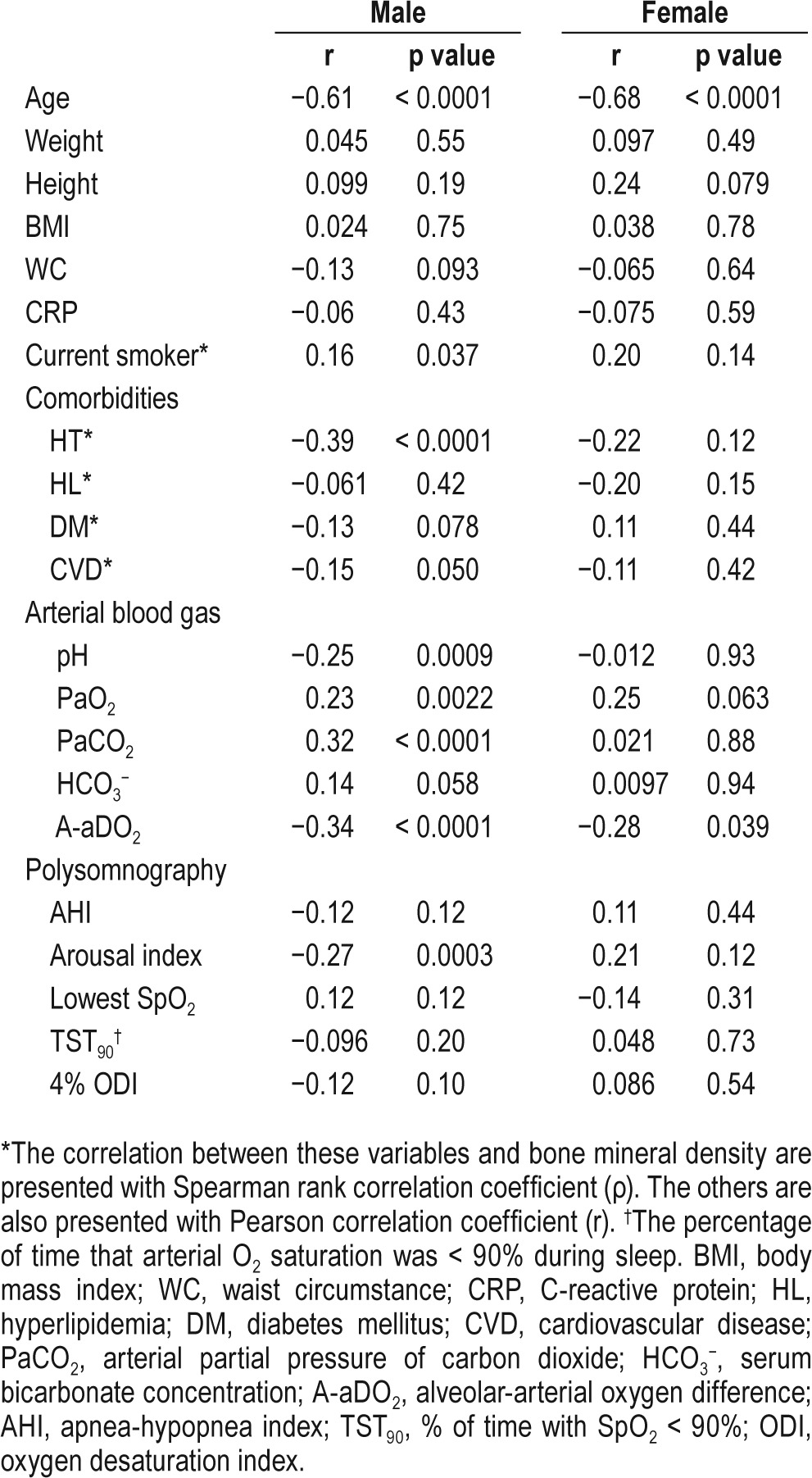
Figure 2. Scatterplot of average bone mineral density of lumbar vertebrae from L1 to L3 vs. alveolar-arterial oxygen difference.
(A) Male, and (B) female participants. The average bone mineral density level was negatively correlated with alveolar-arterial oxygen difference level in both sexes. BMD, bone mineral density; A-aDO2, alveolar-arterial oxygen difference,
Table 5.
Stepwise multiple regression analysis to predict the average bone mineral density of lumbar vertebral bones from L1 to 3.
DISCUSSION
The present study was designed to investigate the associations between OSA and BMD using abdominal CT scans. To our knowledge, this is the first report of a quantitative evaluation of BMD in individuals with OSA by this method. According to BMD determined by CT imaging, patients with severe OSA had lower BMD than those with non OSA. From the CT images obtained in one sleep center in Japan, there was a significant association between age, hypertension or elevation of A-aDO2 and BMD in males; however, age was the only determinant of BMD in females.
Although bone density is usually measured by DEXA methods, it is said that DEXA has a limitation in measuring BMD in overweight patients (BMI ≥ 25 kg/m2) because the superimposed soft tissue can cause inaccuracies measurements of BMD owing to attenuation of the x-ray beams and a beam hardening artifact. Indeed, the BMI of many participants in this study was over 25 kg/m2 (Table 1). Therefore, in this study, BMD was measured by CT, which was validated as our previous study by a calibration phantom containing eight tubes of known concentrations of hydroxyapatite.15,16 From CT imaging, it was determined that BMD in male OSA patients decreased according to the severities of OSA with a significant difference in BMD between severe OSA and non OSA participants. In addition to this study and previous studies,15,16 quantitative CT measurements also have been done to evaluate early changes in skeletal BMD.26 Furthermore, previous studies showed the availability of CT images obtained for various clinical indications to identify osteoporosis without additional radiation or cost as well as this study.27,28
Our data showed that male participants with severe OSA had low BMD status while AHI was not correlated with BMD. A few available data on the association between OSA and bone metabolism are conflicting.9–13 It was reported that OSA could impair,9–11 not affect,12 or stimulate bone metabolism.13 Tomiyama et al. reported an elevation of a bone absorption marker (urinary C-terminal telopeptide of type I collagen) in patients with OSA, which was reversed by continuous positive airway pressure therapy.10 Also, Uzkeser et al. revealed that BMD was decreased in patients with OSA.11 A population-based cohort study in Taiwan showed that patients with OSA were found to be at 2.74 times the risk of osteoporosis than patients without OSA.9 These data were consistent with our data. In contrast, Sforza et al. revealed that the presence of OSA was associated with higher BMD in large sample of elderly subjects.13 The patients with OSA had a greater daily energy expenditure than those without OSA, while in this report, there was no significant difference in physical activity assessed by PAI between OSA and non OSA group (Table 1). It is said that physical activity is the most important factor in the BMD score.29,30 Therefore, the difference in associations between BMD and OSA between Sforza's report and this report might be due to differences in physical activities. In addition, in the Sforza's study, the degrees of intermittent hypoxemia in patients with OSA were mild. The mean 4% ODI in Sforza's study was 9.4, while that in ours was 24.8; and TST90 was 2.0% in their study while ours was 12.8%. These differences might induce the opposite results. Recently, Cauley et al. showed that TST90 was associated with nonspinal fractures.31 In this study, BMD determined by CT scanning was only evaluated in the L1–3 vertebral bodies. Therefore, further prospective studies to understand the true effects of nocturnal/intermittent and/ or daytime/chronic hypoxemia on bone metabolism in various bone sites are needed.
In this study, an elevated A-aDO2 was correlated with reduced BMD. The A-aDO2 implies daytime oxygenation. Although previous studies showed that daytime hypoxemia decreased BMD and increased bone resorption markers in COPD patients,16,32 the significance of the elevated A-aDO2 found in the present work was not known because the daytime PaO2 was not low among our study participants.
Our results showed an association between hypertension and reduced BMD in male study participants. Bone metabolism and hypertension share a similar etiopathology, that is, low calcium intake and vitamin D values and high consumption of sodium salt.33,34 Recently, it has been recognized that OSA is a major factor in secondary hypertension. This is considered to be a result of high sympathetic activity induced by intermittent hypoxemia, sleep fragmentation and arousal.6 It is said that sympathetic stimulation could induce bone reabsorption by mediating osteoclast activation and osteoclastogenesis involving the receptor activator of the nuclear factor κ B ligand (RANKL)-RANK system.35 In this report, linear regression analysis of BMD showed that an elevation of the arousal index was correlated with reduced BMD (Table 4). Therefore, sympathetic stimulation (hypertension in this study) due to intermittent hypoxemia, arousals and sleep fragmentation have worsened bone metabolism in male patients in this study.
In this report, there was no association between factors other than OSA except for age and BMD in female study participants. Females experience a profound period of rapid bone resorption as they enter menopause, while males undergo a slow loss of bone with age.36 We did not investigate menopausal status, but we are aware that the menopausal status of the participants could have influenced our results. However, in our study, the mean age of the females was 61.5 years, and it was considered that the majority of females were postmenopausal. It is known that secondary causes for osteoporosis exist in 20% to 40% of females, while 65% of males who have osteoporosis have other contributory diseases.37 Therefore, there may be a difference in susceptibility to contributory factors influencing bone metabolism between the sexes. This background could have influenced our results.
This study has some limitations. First, our center is an expert sleep center so participants tend to have complaints about sleep and/or daytime sleepiness. Male participants with non OSA had high ESS scores (Table 3). Therefore, in this study, participants with non OSA might not be considered to be normal healthy controls, which could influence our results. Second, we can not know causality or the mechanism of our findings because this was a retrospective analysis. Third, there was a gender difference in the number of study participants. Indeed, the number of females was small, particularly those having non OSA. These differences in number could make a statistical bias. Future studies comprising a greater number of female patients are needed. Fourth, in this study, we could not analyze BMD in around half of the participants because of comorbidities and medications and did not perform all examinations in all participants. We measured biochemical markers of bone metabolism, including calcium, osteocalcin, and 25-hydroxy vitamin D levels in around half of the participants. Other biochemical markers, for example phosphate, CTX and bone-specific alkaline phosphatase were not investigated. Future research is needed to identify the effect of some of the factors that were not fully examined in this study. Fifth, we did not measure BMD by DEXA at the same time as the abdominal CT scans were performed. Therefore, we could not here detect the accuracy of quantitative CT in comparison with DEXA for evaluating BMD in overweight patients with OSA. In addition, we could not ascertain that the measurements of BMD in spinal bones were fully able to evaluate BMD or osteoporosis in the whole body. We need further investigations to clarify the differences between DEXA and quantitative CT in patients with OSA.
We concluded that severe OSA had lower BMD determined by CT imaging of the L1–3 vertebrae than non OSA participants. Also, age, hypertension, and elevation of A-aDO2 were significant factors for BMD in males. Despite the several limitations of this study, CT imaging to measure BMD in OSA patients as a marker of osteoporosis may be useful.
DISCLOSURE STATEMENT
This was not an industry supported study. This work was supported by grants from the Japanese Ministry of Education, Culture, Sports, Science and Technology, Respiratory Failure Research Group and Health Science Research Grants (Comprehensive Research on Life-Style Related Diseases including Cardiovascular Diseases and Diabetes Mellitus) from the Ministry of Health, Labor and Welfare of Japan, and the Japan Vascular Disease Research Foundation. There was no off-label use. The Department of Respiratory Care and Sleep Control Medicine is funded by endowments from Philips-Respironics, Teijin Pharma, Fukuda Denshi, and Fukuda Lifetec Keiji to Kyoto University. The authors have indicated no financial conflicts of interest.
ABBREVIATIONS
- A-aDO2
alveolar-arterial oxygen pressure difference
- AHI
apnea-hypopnea index
- BMD
bone mineral density
- BMI
body mass index
- COPD
chronic obstructive pulmonary disease
- DEXA
dual-energy x-ray absorptiometry
- ESS
Epworth Sleepiness Scale
- HCO3−
bicarbonate concentration
- MET
metabolic equivalents
- OSA
obstructive sleep apnea
- PaCO2
carbon dioxide pressure
- PAO2
alveolar partial pressure of oxygen
- PaO2
arterial partial pressure of oxygen
- PAI
physical activity index
- PSG
polysomnography
- RANKL
receptor activator of the nuclear factor κ B ligand
- ROI
region of interest
- SD
standard deviation
- TST90
percentage of time with SpO2 < 90%
REFERENCES
- 1.Peppard PE, Young T, Barnet JH, et al. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177:1006–14. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nakayama-Ashida Y, Takegami M, Chin K, et al. Sleep-disordered breathing in the usual lifestyle setting as detected with home monitoring in a population of working men in Japan. Sleep. 2008;31:419–25. doi: 10.1093/sleep/31.3.419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kapur VK. Obstructive sleep apnea: diagnosis, epidemiology, and economics. Respir Care. 2010;55:1155–67. [PubMed] [Google Scholar]
- 4.Arnardottir ES, Mackiewicz M, Gislason T, Teff KL, Pack AI. Molecular signatures of obstructive sleep apnea in adults: a review and perspective. Sleep. 2009;32:447–70. doi: 10.1093/sleep/32.4.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arnett TR, Gibbons DC, Utting JC, et al. Hypoxia is a major stimulator of osteoclast formation and bone resorption. J Cell Physiol. 2003;196:2–8. doi: 10.1002/jcp.10321. [DOI] [PubMed] [Google Scholar]
- 6.Muzylak M, Price JS, Horton MA. Hypoxia induces giant osteoclast formation and extensive bone resorption in the cat. Calcif Tissue Int. 2006;79:301–9. doi: 10.1007/s00223-006-0082-7. [DOI] [PubMed] [Google Scholar]
- 7.Center JR, Nguyen TV, Schneider D, Sambrook PN, Eisman JA. Mortality after all major types of osteoporotic fracture in men and women: an observational study. Lancet. 1999;353:878–82. doi: 10.1016/S0140-6736(98)09075-8. [DOI] [PubMed] [Google Scholar]
- 8.Link TM. Osteoporosis imaging: state of the art and advanced imaging. Radiology. 2012;263:3–17. doi: 10.1148/radiol.12110462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen YL, Weng SF, Shen YC, et al. Obstructive sleep apnea and risk of osteoporosis: a population-based cohort study in Taiwan. J Clin Endocrinol Metab. 2014;99:2441–7. doi: 10.1210/jc.2014-1718. [DOI] [PubMed] [Google Scholar]
- 10.Tomiyama H, Okazaki R, Inoue D, et al. Link between obstructive sleep apnea and increased bone resorption in men. Osteoporos Int. 2008;19:1185–92. doi: 10.1007/s00198-007-0556-0. [DOI] [PubMed] [Google Scholar]
- 11.Uzkeser H, Yildirim K, Aktan B, et al. Bone mineral density in patients with obstructive sleep apnea syndrome. Sleep Breath. 2013;17:339–42. doi: 10.1007/s11325-012-0698-y. [DOI] [PubMed] [Google Scholar]
- 12.Mariani S, Fiore D, Varone L, et al. Obstructive sleep apnea and bone mineral density in obese patients. Diabetes Metab Syndr Obes. 2012;5:395–401. doi: 10.2147/DMSO.S37761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sforza E, Thomas T, Barthélémy JC, Collet P, Roche F. Obstructive sleep apnea is associated with preserved bone mineral density in healthy elderly subjects. Sleep. 2013;36:1509–15. doi: 10.5665/sleep.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tothill P, Hannan WJ, Cowen S, Freeman CP. Anomalies in the measurement of changes in total-body bone mineral by dual-energy X-ray absorptiometry during weight change. J Bone Miner Res. 1997;12:1908–21. doi: 10.1359/jbmr.1997.12.11.1908. [DOI] [PubMed] [Google Scholar]
- 15.Ohara T, Hirai T, Muro S, et al. Relationship between pulmonary emphysema and osteoporosis assessed by CT in patients with COPD. Chest. 2008;134:1244–9. doi: 10.1378/chest.07-3054. [DOI] [PubMed] [Google Scholar]
- 16.Kiyokawa H, Muro S, Oguma T, et al. Impact of COPD exacerbations on osteoporosis assessed by chest CT scan. COPD. 2012;9:235–42. doi: 10.3109/15412555.2011.650243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Harada Y, Oga T, Chihara Y, et al. Differences in associations between visceral fat accumulation and obstructive sleep apnea by sex. Ann Am Thorac Soc. 2014;11:383–91. doi: 10.1513/AnnalsATS.201306-182OC. [DOI] [PubMed] [Google Scholar]
- 18.Lee YK, Jang S, Jang S, et al. Mortality after vertebral fracture in Korea: analysis of the National Claim Registry. Osteoporos Int. 2012;23:1859–65. doi: 10.1007/s00198-011-1833-5. [DOI] [PubMed] [Google Scholar]
- 19.Ogihara T, Kikuchi K, Matsuoka H, et al. The Japanese Society of Hypertension Guidelines for the Management of Hypertension (JSH 2009) Hypertens Res. 2009;32:3–107. [PubMed] [Google Scholar]
- 20.Kazuya T, Nakagawa S, Satoh J, et al. Report of the Committee on the classification and diagnostic criteria of diabetes mellitus. Diabetes Res Clin Pract. 2002;55:65–85. doi: 10.1016/s0168-8227(01)00365-5. [DOI] [PubMed] [Google Scholar]
- 21.Hata Y, Mabuchi H, Saito Y, et al. Report of the Japan Atherosclerosis Society (JAS) Guideline for Diagnosis and Treatment of Hyperlipidemia in Japanese adults. J Atheroscler Thromb. 2002;9:1–27. doi: 10.5551/jat.9.1. [DOI] [PubMed] [Google Scholar]
- 22.Morimoto T, Oguma Y, Yamazaki S, Sokejima S, Nakayama T, Fukuhara S. Gender differences in effects of physical activity on quality of life and resource utilization. Qual Life Res. 2006;15:537–46. doi: 10.1007/s11136-005-3033-2. [DOI] [PubMed] [Google Scholar]
- 23.Takegami M, Suzukamo Y, Wakita T, et al. Development of a Japanese version of the Epworth Sleepiness Scale (JESS) based on item response theory. Sleep Med. 2009;10:556–65. doi: 10.1016/j.sleep.2008.04.015. [DOI] [PubMed] [Google Scholar]
- 24.Aihara K, Oga T, Harada Y, et al. Comparison of biomarkers of subclinical lung injury in obstructive sleep apnea. Respir Med. 2011;105:939–45. doi: 10.1016/j.rmed.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 25.Ilber C, Ancoli-Israel S, Chesson A, Quan SF. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology, ad technical specifications. [Google Scholar]
- 26.Black DM, Greenspan SL, Ensrud KE, et al. The effects of parathyroid hormone and alendronate alone or in combination in postmenopausal osteoporosis. N Engl J Med. 2003;349:1207–15. doi: 10.1056/NEJMoa031975. [DOI] [PubMed] [Google Scholar]
- 27.Summers RM, Baecher N, Yao J, et al. Feasibility of simultaneous computed tomographic colonography and fully automated bone mineral densitometry in a single examination. J Comput Assist Tomogr. 2011;35:212–6. doi: 10.1097/RCT.0b013e3182032537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pickhardt PJ, Pooler BD, Lauder T, del Rio AM, Bruce RJ, Binkley N. Opportunistic screening for osteoporosis using abdominal computed tomography scans obtained for other indications. Ann Intern Med. 2013;158:588–95. doi: 10.7326/0003-4819-158-8-201304160-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Woolf AD, Akesson K. Preventing fractures in elderly people. BMJ. 2003;327:89–95. doi: 10.1136/bmj.327.7406.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nordström A, Karlsson C, Nyquist F, Olsson T, Nordström P, Karlsson M. Bone loss and fracture risk after reduced physical activity. J Bone Miner Res. 2005;20:202–7. doi: 10.1359/JBMR.041012. [DOI] [PubMed] [Google Scholar]
- 31.Cauley JA, Blackwell TL, Redline S, et al. Hypoxia during sleep and the risk of falls and fractures in older men: the Osteoporotic Fractures in Men Sleep Study. J Am Geriatr Soc. 2014;62:1853–9. doi: 10.1111/jgs.13069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stanojkovic I, Kotur-Stevuljevic J, Spasic S, et al. Relationship between bone resorption, oxidative stress and inflammation in severe COPD exacerbation. Clin Biochem. 2013;46:1678–82. doi: 10.1016/j.clinbiochem.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 33.Cappuccio FP, Meilahn E, Zmuda JM, Cauley JA. High blood pressure and bone-mineral loss in elderly white women: a prospective study. Study of Osteoporotic Fractures Research Group. Lancet. 1999;354:971–5. doi: 10.1016/s0140-6736(99)01437-3. [DOI] [PubMed] [Google Scholar]
- 34.Ilić K, Obradović N, Vujasinović-Stupar N. The relationship among hypertension, antihypertensive medications, and osteoporosis: a narrative review. Calcif Tissue Int. 2013;92:217–27. doi: 10.1007/s00223-012-9671-9. [DOI] [PubMed] [Google Scholar]
- 35.Togari A, Arai M. Pharmacological topics of bone metabolism: the physiological function of the sympathetic nervous system in modulating bone resorption. J Pharmacol Sci. 2008;106:542–6. doi: 10.1254/jphs.fm0070227. [DOI] [PubMed] [Google Scholar]
- 36.Seeman E. Clinical review 137: Sexual dimorphism in skeletal size, density, and strength. J Clin Endocrinol Metab. 2001;86:4576–84. doi: 10.1210/jcem.86.10.7960. [DOI] [PubMed] [Google Scholar]
- 37.Dy CJ, Lamont LE, Ton QV, Lane JM. Sex and gender considerations in male patients with osteoporosis. Clin Orthop Relat Res. 2011;469:1906–12. doi: 10.1007/s11999-011-1849-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.