Abstract
Background and objectives
Neurocognitive problems in CKD are well documented; time-efficient methods are needed to assess neurocognition in this population. We performed the first study of the efficient 1-hour Penn Computerized Neurocognitive Battery (CNB) in children and young adults with CKD.
Design, setting, participants, & measurements
We administered the Penn CNB cross-sectionally to individuals aged 8–25 years with stage 2–5 CKD (n=92, enrolled from three academic nephrology practices from 2011 to 2014) and matched healthy controls (n=69). We analyzed results from 12 tests in four domains: executive control, episodic memory, complex cognition, and social cognition. All tests measure accuracy and speed; we converted raw scores to age-specific z-scores on the basis of Philadelphia Neurodevelopmental Cohort (n=1790) norms. We analyzed each test in a linear regression with accuracy and speed z-scores as dependent variables and with (1) CKD versus control or (2) eGFR as explanatory variables, adjusted for race, sex, and maternal education.
Results
Patients with CKD (mean±SD eGFR, 48±25 ml/min per 1.73 m2; mean age, 16.3±3.9 years) and controls (mean eGFR, 98±20 ml/min per 1.73 m2; mean age, 16.0±4.0 years) were similar demographically. CKD participants had lower accuracy than controls in tests of complex cognition, with moderate to large effect sizes: −0.53 (95% confidence interval [95% CI], −0.87 to −0.19) for verbal reasoning, −0.52 (95% CI, −0.83 to −0.22) for nonverbal reasoning, and −0.64 (95% CI, −0.99 to −0.29) for spatial processing. For attention, patients with CKD had lower accuracy (effect size, −0.35 [95% CI, −0.67 to −0.03]) but faster response times (effect size, 0.44 [95% CI, 0.04 to 0.83]) than controls, perhaps reflecting greater impulsivity. Lower eGFR was associated with lower accuracy for complex cognition, facial and visual memory, and emotion identification tests.
Conclusions
CKD is associated with lower accuracy in tests of complex cognition, attention, memory, and emotion identification, which related to eGFR. These findings are consistent with traditional neurocognitive testing in previous studies.
Keywords: chronic kidney disease; psychological tests; attention; executive function; memory; neuropsychological; children, adolescents; cognition; humans
Introduction
Neurocognitive problems have been well documented in children and adults with CKD. Children with CKD show impairments in attention, memory, executive functioning, language, visual-spatial abilities, and academic achievement (1–4), while adults with CKD have increased risk of cognitive impairment and dementia (5–9). Impaired cognitive function has been linked to poor treatment adherence in multiple disease states, including kidney transplantation (10–13). Improved characterization of cognitive dysfunction in children and adults with CKD could therefore allow clinicians to implement targeted interventions (1,14,15) and provide more effective patient communication to promote adherence. A significant barrier to increased neurodevelopmental surveillance of patients with CKD, however, is the costly and time-intensive nature of traditional neurocognitive test batteries. Neuropsychological evaluations are often not completed in tandem with clinical care visits, which further limits the utility of cognitive assessment of changes related to disease status.
The Penn Computerized Neurocognitive Battery (CNB) was developed to provide an efficient method to assess performance in a range of cognitive domains linked to specific brain systems (16). The Penn CNB takes an average of 1 hour to complete and provides measures of accuracy and speed in tests spanning five areas of neurobehavioral function: executive control, episodic memory, complex cognition, social cognition, and sensorimotor speed. Results from the Penn CNB were similar to those of traditional neuropsychological testing in a healthy normative sample (17) and in adult patients with schizophrenia (18). The Penn CNB was also applied in the Philadelphia Neurodevelopmental Cohort (PNC), a large-scale community genomic study of brain development, which generated normative data in children and young adults aged 8–21 years (19).
To our knowledge, the current study is the first systematic investigation of the Penn CNB in children and young adults with CKD. The goal of this study was to use the Penn CNB to characterize neurocognitive function in children and young adults with CKD and in healthy controls. We also sought to examine whether findings in the Penn CNB are similar to those from previous studies that used traditional neurocognitive testing in children and young adults with CKD. We hypothesized that the Penn CNB would reveal deficits in domains similar to those identified in previous studies, including language, visual-spatial ability, memory, and executive functioning (1,4).
Materials and Methods
The Penn CNB was investigated within the Neurocognitive Assessment and Magnetic Resonance Imaging Analysis of Children and Young Adults with Chronic Kidney Disease (NiCK) Study. The NiCK study is a cross-sectional investigation of children and young adults, aged 8–25 years, with stage 2–5 CKD (eGFR<90 ml/min per 1.73 m2, including dialysis and post-transplant) compared with healthy controls matched on age, sex, and socioeconomic status (using insurance status as a proxy). Complete inclusion and exclusion criteria for the NiCK Study have been published elsewhere (20). Eligible patients with CKD were identified by manual review of medical records of patients who were scheduled to be seen in nephrology clinics at the Children’s Hospital of Philadelphia (CHOP), the Hospital of the University of Pennsylvania, and the Nemours Hospital for Children during the recruitment period (August 2011–October 2014); patients were invited to participate at their regular nephrology clinic visits. Healthy controls were identified from CHOP general practice locations and were invited to participate in the study via mail. Those who were interested in participating contacted the study coordinator at CHOP to be screened and enrolled. Patients with comorbidities that independently affect brain function (e.g., seizure disorder, traumatic brain injury, genetic syndromes) or the ability to complete test measures (profound developmental disabilities) were excluded from participating to ensure that study results reflect the effects of kidney disease. The institutional review board of the Children’s Hospital of Philadelphia approved the study, and the study adhered to the Declaration of Helsinki. All participants and/or guardians provided written informed consent.
The Penn CNB includes 14 tests assessing five neurobehavioral functions: executive control, episodic memory, complex cognition, social cognition, and praxis speed. Except for praxis speed (results of which were not analyzed for the current study), all tests measure both accuracy (number of correct responses) and speed (response time for correct items).
For the current analysis, raw scores for accuracy and speed for each test were converted to age-specific z-scores based on the performance of a cohort of 1790 typically developing healthy children and young adults selected from the larger PNC study (19). From this cohort, scores were collapsed in 2-year intervals to form the basis for the development of the z-scores. We selected 2-year intervals to ensure adequate numbers of participants within each age interval and to minimize the effect of normal variability in the rate of development of neurocognitive skills (21). For ease of interpretation, z-scores were calculated such that higher z-scores always reflect better performance (i.e., higher accuracy and shorter response times correspond to higher z-scores).
To assess the neurocognitive effects of CKD, we performed linear regression analysis, with z-scores for accuracy and speed for each test as the dependent variables and either (1) diagnostic group (CKD versus healthy control) or (2) eGFR as the explanatory variables. Analyses were adjusted for race (black versus nonblack race), sex, and maternal education (in years). To examine whether inclusion of transplant recipients in the CKD group affected our results, we also performed a sensitivity analysis comparing the CKD and control groups after excluding transplant recipients.
The performance of CKD participants was compared with that of a concurrently recruited cohort of healthy control participants in order to minimize confounding due to differences in test administration (e.g., environment, staff, and timing) between NiCK study participants and the historical PNC cohort.
Clinical and demographic characteristics were compared between the CKD and control groups using the Wilcoxon rank sum test for continuous variables, the Fisher exact test for categorical variables, and the Kruskal-Wallis test for ordinal variables.
As a secondary analysis, we also compared Penn CNB performance for CKD participants with that of the reference population of 1790 healthy individuals from the PNC study. Wilcoxon signed-rank tests were performed to determine whether observed median age-specific z-scores for accuracy and speed in each subtest for the CKD group significantly differed from the expected median z-scores of 0.
Data were analyzed using R software, version 3.1.0 (R Foundation for Statistical Computing, www.R-project.org). The significance level for all data analyses was set at P=0.05 (two-sided).
Results
Clinical and Demographic Characteristics
Penn CNB results were analyzed for 92 patients with CKD and 69 controls. Clinical and demographic characteristics of patients with CKD and controls are shown in Table 1. CKD and control participants were of similar age. Slightly higher proportions of patients with CKD were male, of Hispanic ethnicity, and from households with income <$30,000/year, but these differences were not statistically significant. A higher proportion of control participants was black, but this difference was also not statistically significant. Level of maternal education was slightly higher in the control group than in the CKD group (14.7 versus 13.6 years; P=0.03). As expected, patients with CKD had lower mean eGFR than controls (48 versus 98 ml/min per 1.73 m2; P<0.001). One quarter of patients with CKD had functioning transplants and 3% were receiving dialysis at the time of their study visit.
Table 1.
Clinical and demographic characteristics of patients with CKD and controls
| Characteristic | CKD (n=92) | Control (n=69) | P Value |
|---|---|---|---|
| Age, yr | 16.3±3.9 | 16.0±4.0 | 0.64 |
| Male participants | 65 | 55 | 0.20 |
| Black | 27 | 38 | 0.17 |
| Hispanic | 10 | 7 | 0.78 |
| Income | 0.44 | ||
| <$30,000/yr | 35 | 26 | |
| >$75,000/yr | 37 | 39 | |
| Maternal education, yr | 13.6±2.7 | 14.7±2.9 | 0.03 |
| eGFR, ml/min per 1.73 m2 | 47.9±24.5 | 97.9±20.1 | <0.001 |
| Current dialysis | 3 | – | – |
| Functioning transplant | 26 | – | – |
Data shown as mean±SD or proportion (%). eGFR calculated with bedside Chronic Kidney Disease in Children equation (32) for participants aged <18 years or the Modification of Diet in Renal Disease equation (33) for those ≥18 years. eGFR for participants on dialysis was defined as 10 ml/min per 1.73 m2.
Group Comparisons of Patients with CKD versus Controls
As a group, patients with CKD showed significantly lower accuracy scores than controls in tests assessing complex cognition, with moderate to large effect sizes: −0.53 (95% confidence interval [95% CI], −0.87 to −0.19) for verbal reasoning (P=0.002), −0.52 (95% CI, −0.83 to −0.22) for nonverbal reasoning (P=0.001), and −0.64 (95% CI, −0.99 to −0.29) for spatial processing (P<0.001). Accuracy scores for attention were also lower in patients with CKD than controls (effect size, −0.35 [95% CI, −0.67 to −0.03]; P=0.03). There were no statistically significant differences in group comparisons between patients with CKD and controls in accuracy scores for tests assessing other domains of executive control (abstraction/mental flexibility and working memory). In addition, group comparisons between patients with CKD and controls did not detect any statistically significant differences in accuracy scores for tests assessing episodic memory or social cognition (Table 2).
Table 2.
Group differences between patients with CKD and control participants in z-scores for accuracy and speed in each Penn Computerized Neurocognitive Battery subtest, adjusted for race, sex, and maternal education
| Neurobehavioral Function and Domain | Test Name | Group Difference in Accuracy z-Scoresa (CKD−Control), Estimate (95% CI) | P Value | Group Difference in Speed z-Scoresa (CKD−Control), Estimate (95% CI) | P Value |
|---|---|---|---|---|---|
| Executive control | |||||
| Abstraction/mental flexibility | Penn Conditional Exclusion Test (PCET) | −0.20 (−0.51 to 0.12) | 0.22 | 0.27 (−0.12 to 0.66) | 0.17 |
| Attention | Penn Continuous Performance Test (PCPT) | −0.35 (−0.67 to −0.03) | 0.03 | 0.44 (0.04 to 0.83) | 0.03 |
| Working memory | Short Letter N-Back Test (SLNB) | −0.19 (−0.61 to 0.24) | 0.39 | 0.16 (−0.25 to 0.58) | 0.44 |
| Episodic memory | |||||
| Verbal memory | Penn Word Memory (CPW) | −0.25 (−0.65 to 0.15) | 0.23 | 0.13 (−0.25 to 0.50) | 0.52 |
| Facial memory | Penn Face Memory (CPF) | −0.32 (−0.68 to 0.04) | 0.08 | 0.28 (−0.14 to 0.69) | 0.20 |
| Spatial memory | Visual Object Learning Test (VOLT) | −0.35 (−0.72 to 0.01) | 0.06 | 0.37 (−0.02 to 0.76) | 0.06 |
| Complex cognition | |||||
| Verbal reasoning | Penn Verbal Reasoning Test (PVRT) | −0.53 (−0.87 to −0.19) | 0.002 | 0.08 (−0.29 to 0.45) | 0.66 |
| Nonverbal reasoning | Penn Matrix Reasoning Test (PMAT) | −0.52 (−0.83 to −0.22) | 0.001 | 0.27 (−0.07 to 0.61) | 0.12 |
| Spatial processing | Penn Line Orientation Test (PLOT) | −0.64 (−0.99 to −0.29) | <0.001 | −0.05 (−0.45 to 0.35) | 0.81 |
| Social cognition | |||||
| Emotion identification | Penn Emotion Identification Test (ER40) | −0.28 (−0.64 to 0.09) | 0.13 | 0.01 (−0.43 to 0.45) | 0.97 |
| Emotion differentiation | Penn Emotion Differentiation Test (MEDF) | −0.22 (−0.59 to 0.14) | 0.23 | 0.13 (−0.19 to 0.45) | 0.43 |
| Age differentiation | Penn Age Differentiation Test (ADT) | −0.24 (−0.61 to 0.14) | 0.21 | 0.16 (−0.18 to 0.50) | 0.35 |
95% CI, 95% confidence interval; PCET, Penn Conditional Exclusion Test; PCPT, Penn Continuous Performance Test; SLNB, Short Letter N-Back Test; CPW, Penn Word Memory; CPF, Penn Face Memory; VOLT, Visual Object Learning Test; PVRT, Penn Verbal Reasoning Test; PMAT, Penn Matrix Reasoning Test; PLOT, Penn Line Orientation Test; ER40, Penn Emotion Identification Test; MEDF, Penn Emotion Differentiation Test; ADT, Age Differentiation Test.
Adjusted for race, sex, and maternal education.
Comparison of speed scores between patients with CKD and controls revealed only one test with a statistically significant group difference in performance: Patients with CKD had higher speed scores (i.e., faster responses for correct items) than control participants for attention (effect size, 0.44 [95% CI, 0.04 to 0.83]; P=0.03) (Table 2).
Sensitivity analysis excluding kidney transplant recipients from the CKD group yielded similar results (results not shown).
Relationship of Penn CNB Results with eGFR
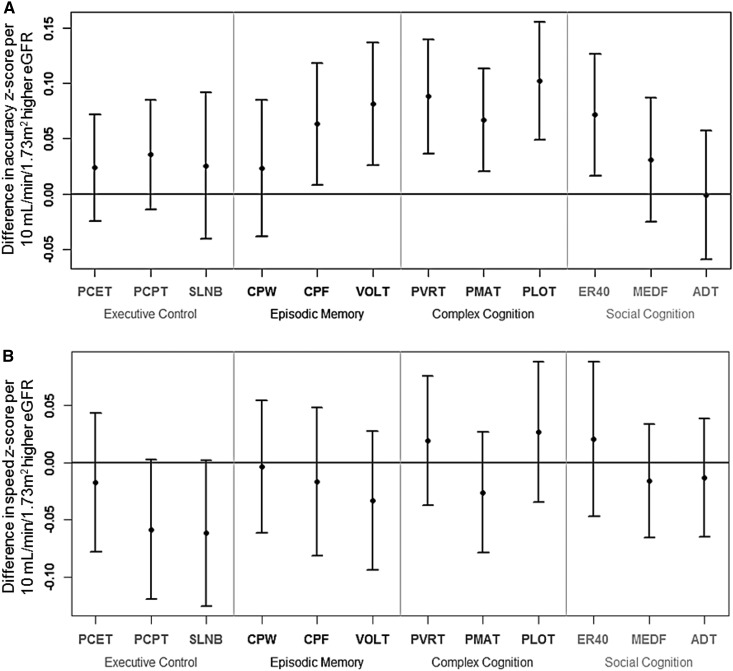
In analyses using eGFR as a continuous explanatory variable, higher eGFR was associated with higher accuracy performance on all three subtests assessing the domains of complex cognition: For every 10–ml/min per 1.73 m2 higher eGFR, accuracy z-score for verbal reasoning was higher by 0.09 (95% CI, 0.04 to 0.14; P=0.001 for regression); for nonverbal reasoning, it was higher by 0.06 (95% CI, 0.02 to 0.11; P=0.01); and for spatial processing, it was higher by 0.10 (95% CI, 0.05 to 0.16; P<0.001) (Figure 1A). In addition, higher eGFR was associated with higher accuracy in two of the three memory domains: For every 10–ml/min per 1.73 m2 higher eGFR, accuracy z-score for facial memory was higher by 0.06 (95% CI, 0.01–0.12; P=0.02) and for spatial memory was higher by 0.08 (95% CI, 0.03 to 0.14; P=0.004). Higher eGFR was also associated with higher accuracy performance in emotion identification, one of the three subtests comprising social cognition: For every 10–ml/min per 1.73 m2 higher eGFR, z-score was higher by 0.07 (95% CI, 0.02 to 0.13; P=0.01). There were no significant relationships between eGFR and accuracy performance in executive control, verbal memory, or the other domains of social cognition (Figure 1A). There were no statistically significant associations between eGFR and speed performance in any domains (Figure 1B).
Figure 1.
Differences in z-scores for accuracy and speed per 10–ml/min per 1.73 m2 higher eGFR for each Penn Computerized Neurocognitive Battery subtest. Point estimates and 95% confidence intervals (error bars) for z-score differences are shown, adjusted for race, sex, and maternal education. (A) Accuracy. (B) Speed. ADT, Age Differentiation Test; CPF, Penn Face Memory; CPW, Penn Word Memory; ER40, Penn Emotion Identification Test; MEDF, Penn Emotion Differentiation Test; PCET, Penn Conditional Exclusion Test; PCPT, Penn Continuous Performance Test; PLOT, Penn Line Orientation Test; PMAT, Penn Matrix Reasoning Test; PVRT, Penn Verbal Reasoning Test; SLNB, Short Letter N-Back Test; VOLT, Visual Object Learning Test.
Comparison of Patients with CKD versus Reference Population from PNC Study
Median z-scores for accuracy and speed in each Penn CNB subtest for participants with CKD are shown in Supplemental Table 1. Similar to our findings in the group comparisons described in the preceding section, accuracy z-scores for patients with CKD were lowest in tests assessing complex cognition. These scores differed from the expected z-score of 0 from the PNC study reference population with a high degree of statistical significance. Median z-scores for patients with CKD were −0.49 for verbal reasoning (Wilcoxon 95% CI, −0.83 to −0.37 [P<0.001] for difference from PNC population), −0.54 for nonverbal reasoning (Wilcoxon 95% CI, −0.69 to −0.28; P<0.001), and −0.45 for spatial processing (Wilcoxon 95% CI, −0.60 to −0.16; P=0.001). Patients with CKD also had lower median accuracy z-scores than the reference population in several other subtests, albeit with somewhat smaller effect sizes. These included tests assessing episodic memory (−0.21 for verbal memory [Wilcoxon 95% CI, −0.62 to −0.04; P=0.01], −0.40 for facial memory [Wilcoxon 95% CI, −0.64 to −0.13; P=0.004], and −0.30 for spatial memory [Wilcoxon 95% CI, −0.53 to −0.05; P=0.02]), one subtest of social cognition (−0.34 for age differentiation [Wilcoxon 95% CI, −0.59 to −0.13; P=0.01]), and two subtests of executive control (−0.24 for abstraction/mental flexibility [Wilcoxon 95% CI, −0.48 to -−0.04; P=0.03] and −0.04 for working memory [Wilcoxon 95% CI, −0.52 to −0.09; P=0.01]) (Supplemental Table 1).
Comparison of speed z-scores for patients with CKD with the PNC study reference population showed that patients with CKD had lower median speed z-scores for attention (−0.33 [Wilcoxon 95% CI, −0.65 to −0.20; P<0.001]) and emotion identification (−0.17 [Wilcoxon 95% CI, −0.42 to −0.06; P=0.01]) but had higher speed z-scores for nonverbal reasoning (0.47 [Wilcoxon 95% CI, 0.18 to 0.50; P<0.001]) (Supplemental Table 1).
Discussion
Neurocognitive dysfunction is an important consequence of CKD and can have far-reaching social, economic, and health effects. On an individual level, neurocognitive impairment affects school performance (22,23), employment (24,25), and health care self-management (13), particularly when complex medical regimens are prescribed (26). From a societal perspective, this translates to increased burden on school and health systems and health insurance programs. Despite the importance of neurocognitive dysfunction in CKD, measuring these deficits in clinical research settings can be difficult given the cost and availability of traditional neurocognitive testing. We therefore sought to evaluate the use of a computerized testing method, the Penn CNB, in children and young adults with CKD.
In this study, we compared Penn CNB performance of children and youth with CKD with that of concurrently recruited health controls matched for age and socioeconomic status in the NiCK study. In addition, because of the wide range of eGFRs within our CKD cohort, we also examined the relationship between Penn CNB performance and eGFR. We found that patients with CKD as a group had significantly lower accuracy performance than controls in all three subtests of complex cognition (verbal and nonverbal reasoning and spatial processing). Group comparisons also revealed lower accuracy performance for attention in the patients with CKD. Notably, however, patients with CKD had faster response times than controls in the test for attention. This trade-off of accuracy for speed was found in a prior study of the Penn CNB evaluating age- and sex-related differences in performance (22). In that study of individuals aged 8–21 years, male participants were faster but less accurate than female participants in the test of attention, a finding attributed to greater impulsivity in males (22,27). Although our CKD sample did have a slightly higher proportion of male participants compared with the control group (Table 1), our analyses were adjusted for sex, suggesting a CKD-specific effect on impulsivity.
In a separate regression analysis, we found that lower eGFR was associated with lower accuracy performance in complex cognition, facial memory, spatial memory, and emotion identification.
As a secondary analysis, we also compared the performance of patients with CKD in the Penn CNB with that of a reference population of healthy children and young adults in the PNC study. Similar to our comparisons with the concurrent NiCK study control group, these analyses showed that patients with CKD had significantly lower than expected accuracy performance in tests of complex cognition. Patients with CKD also had lower than expected accuracy performance in tests of episodic memory, executive control, and social cognition.
Previous studies in children with CKD using traditional testing methods have demonstrated deficits in attention, memory, executive functioning, language, visual-spatial abilities, and academic achievement (1–4). The pattern of the findings uncovered using the Penn CNB in the current study show significant overlap with previous studies. However, the association between lower eGFR and lower accuracy in emotion identification is a relatively novel finding that adds to the knowledge base about the neurocognitive profile of CKD and may strengthen the utility of the Penn CNB. Measures of social cognition are not widely used in traditional neurocognitive testing, which may contribute to under-recognition of these deficits. One prior study in pediatric kidney transplant recipients demonstrated impairment in recognizing emotional states (14). A recent study in adult kidney transplant patients revealed that alexithymia (the inability to recognize and express emotions) is positively correlated with noncompliance (28), underscoring the importance of recognizing deficits in social cognition. These findings support further investigation and validation of the Penn CNB in CKD populations.
The Penn CNB is a cognitive neuroscience–based assessment that was developed on the basis of tasks shown experimentally to map to specific brain systems with functional neuroimaging studies (16) (available for download by investigators at http://www.med.upenn.edu/bbl). The domains showing decreased Penn CNB performance in CKD in our study are expected to map to several functional areas: bilateral temporo-parietal regions (complex cognition), frontal and bilateral anterior medial temporal lobes (episodic memory), frontal-parietal network (attention), and temporo-limbic regions (emotion identification) (16). The mechanisms by which CKD leads to neurocognitive alterations are poorly understood but are likely related to both metabolic derangements (29) and subclinical vascular disease (30). Ongoing analysis of findings from multimodal magnetic resonance imaging performed in the NiCK study will help to identify any regional changes in brain structure, functional connectivity, and cerebral blood flow associated with CKD and will help to shed further light on the neuropathology associated with CKD (20).
Strengths of this study include the direct comparison of neurocognitive performance of patients with CKD with that of a concurrently recruited cohort of healthy controls matched for age and socioeconomic status, rather than solely with historical controls or population-based normative samples. This decreases the potential for variability in neurocognitive performance arising from different testing conditions or other environmental factors. Although we consider our primary analysis to be the more rigorous one for this reason, our secondary analysis comparing our CKD group to the larger PNC study reference population strengthens our findings. In both analyses, participants with CKD had significantly lower accuracy in all three subtests of complex cognition (verbal and nonverbal reasoning and spatial processing).
Some demographic variables differed between the CKD and control groups, but our statistical analyses controlled for these by adjusting for important covariates, such as maternal education, race, and sex. However, we cannot exclude the effects of other unmeasured confounders on our results. Unmeasured confounders may also have affected our comparisons with the historical PNC reference population. Another potential limitation is the heterogeneity of our CKD group, which had a wide range of kidney function and also included post-transplant participants. However, sensitivity analysis excluding transplant recipients from the CKD group yielded similar results. Another potential limitation to our study is its cross-sectional nature. Although we found that lower eGFR was associated with lower performance in certain neurocognitive domains, a cross-sectional study does not allow us to determine whether this relationship is causal. Although we performed multiple analyses, here we report significant findings using P<0.05. However, the group differences between CKD and control participants in complex cognition performance in both our primary and secondary analyses were significant enough that they would have survived even the conservative Bonferroni correction (α=0.05/12=0.004).
The Penn CNB offers an efficient method for neurocognitive assessment of children and young adults with CKD. As a novel approach to assessing neurocognitive function, the Penn CNB demonstrates findings consistent with prior reports of poorer performance using traditional neurocognitive batteries in children with CKD. The Penn CNB’s ease of administration and the shortened time needed compare favorably to administration of a traditional neurocognitive battery. The Penn CNB may therefore facilitate increased collection of standardized neurocognitive data in a wider variety of clinical research settings and could allow further exploration of risk factors for cognitive dysfunction in CKD. A better understanding of neurocognitive functioning in dysfunction in patients with CKD may have important implications for how we communicate health care information, promote adherence, and tailor treatment plans as disease progresses.
Disclosures
None.
Supplementary Material
Acknowledgments
E.A.H is supported by the National Center for Advancing Translational Sciences (NCATS) of the National Institutes of Health (NIH) under award KL2TR000139. The NiCK study is funded, in part, under a Commonwealth Universal Research Enhancement grant with the Pennsylvania Department of Health, SAP 4100054843. The Clinical and Translational Research Center at the Children’s Hospital of Philadelphia is supported by the National Center for Research Resources and the NCATS, NIH, through grants UL1RR024134 and UL1TR000003.
Study data were collected and managed using REDCap electronic data capture tools hosted at the Children’s Hospital of Philadelphia. REDCap (Research Electronic Data Capture) (31) is a secure, web-based application designed to support data capture for research studies, providing (1) an intuitive interface for validated data entry, (2) audit trails for tracking data manipulation and export procedures, (3) automated export procedures for seamless data downloads to common statistical packages, and (4) procedures for importing data from external sources.
The data reported here were presented in abstract form at the American Society of Nephrology Kidney Week 2014.
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of NCATS or the NIH. The Pennsylvania Department of Health specifically disclaims responsibility for any analyses, interpretations or conclusions from this study.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
This article contains supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.02110215/-/DCSupplemental.
References
- 1.Gipson DS, Hooper SR, Duquette PJ, Wetherington CE, Stellwagen KK, Jenkins TL, Ferris ME: Memory and executive functions in pediatric chronic kidney disease. Child Neuropsychol 12: 391–405, 2006 [DOI] [PubMed] [Google Scholar]
- 2.Slickers J, Duquette P, Hooper S, Gipson D: Clinical predictors of neurocognitive deficits in children with chronic kidney disease. Pediatr Nephrol 22: 565–572, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gipson DS, Duquette PJ, Icard PF, Hooper SR: The central nervous system in childhood chronic kidney disease. Pediatr Nephrol 22: 1703–1710, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hooper SR, Gerson AC, Butler RW, Gipson DS, Mendley SR, Lande MB, Shinnar S, Wentz A, Matheson M, Cox C, Furth SL, Warady BA: Neurocognitive functioning of children and adolescents with mild-to-moderate chronic kidney disease. Clin J Am Soc Nephrol 6: 1824–1830, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elias MF, Elias PK, Seliger SL, Narsipur SS, Dore GA, Robbins MA: Chronic kidney disease, creatinine and cognitive functioning. Nephrol Dial Transplant 24: 2446–2452, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elias MF, Dore GA, Davey A: Kidney disease and cognitive function. Contrib Nephrol 179: 42–57, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kurella M, Chertow GM, Luan J, Yaffe K: Cognitive impairment in chronic kidney disease. J Am Geriatr Soc 52: 1863–1869, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Madan P, Kalra OP, Agarwal S, Tandon OP: Cognitive impairment in chronic kidney disease. Nephrol Dial Transplant 22: 440–444, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Kalirao P, Pederson S, Foley RN, Kolste A, Tupper D, Zaun D, Buot V, Murray AM: Cognitive impairment in peritoneal dialysis patients. Am J Kidney Dis 57: 612–620, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stilley CS, Bender CM, Dunbar-Jacob J, Sereika S, Ryan CM: The impact of cognitive function on medication management: Three studies. Health Psychol 29: 50–55, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alosco ML, Spitznagel MB, van Dulmen M, Raz N, Cohen R, Sweet LH, Colbert LH, Josephson R, Hughes J, Rosneck J, Gunstad J: Cognitive function and treatment adherence in older adults with heart failure. Psychosom Med 74: 965–973, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hinkin CH, Hardy DJ, Mason KI, Castellon SA, Durvasula RS, Lam MN, Stefaniak M: Medication adherence in HIV-infected adults: Effect of patient age, cognitive status, and substance abuse. AIDS 18[Suppl 1]: S19–S25, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gelb SR, Shapiro RJ, Thornton WJL: Predicting medication adherence and employment status following kidney transplant: The relative utility of traditional and everyday cognitive approaches. Neuropsychology 24: 514–526, 2010 [DOI] [PubMed] [Google Scholar]
- 14.Haavisto A, Korkman M, Holmberg C, Jalanko H, Qvist E: Neuropsychological profile of children with kidney transplants. Nephrol Dial Transplant 27: 2594–2601, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Duquette PJ, Hooper SR, Icard PF, Hower SJ, Mamak EG, Wetherington CE, Gipson DS: Neurodevelopmental status and adaptive behaviors in preschool children with chronic kidney disease. J Spec Educ 43: 45–51, 2009 [Google Scholar]
- 16.Gur RC, Richard J, Hughett P, Calkins ME, Macy L, Bilker WB, Brensinger C, Gur RE: A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: Standardization and initial construct validation. J Neurosci Methods 187: 254–262, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gur RC, Ragland JD, Moberg PJ, Turner TH, Bilker WB, Kohler C, Siegel SJ, Gur RE: Computerized neurocognitive scanning: I. Methodology and validation in healthy people. Neuropsychopharmacology 25: 766–776, 2001 [DOI] [PubMed] [Google Scholar]
- 18.Gur RC, Ragland JD, Moberg PJ, Bilker WB, Kohler C, Siegel SJ, Gur RE: Computerized neurocognitive scanning: II. The profile of schizophrenia. Neuropsychopharmacology 25: 777–788, 2001 [DOI] [PubMed] [Google Scholar]
- 19.Gur RC, Richard J, Calkins ME, Chiavacci R, Hansen JA, Bilker WB, Loughead J, Connolly JJ, Qiu H, Mentch FD, Abou-Sleiman PM, Hakonarson H, Gur RE: Age group and sex differences in performance on a computerized neurocognitive battery in children age 8-21. Neuropsychology 26: 251–265, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartung EA, Laney N, Kim JY, Ruebner RL, Detre JA, Liu H-S, Davatzikos C, Erus G, Doshi JJ, Schultz RT, Herrington JD, Jawad AF, Moodalbail DG, Gur RC, Port AM, Radcliffe J, Hooper SR, Furth SL: Design and methods of the NiCK study: Neurocognitive assessment and magnetic resonance imaging analysis of children and young adults with chronic kidney disease. BMC Nephrol 16: 66, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Roalf DR, Gur RE, Ruparel K, Calkins ME, Satterthwaite TD, Bilker WB, Hakonarson H, Harris LJ, Gur RC: Within-individual variability in neurocognitive performance: Age- and sex-related differences in children and youths from ages 8 to 21. Neuropsychology 28: 506–518, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Broyer M, Le Bihan C, Charbit M, Guest G, Tete M-J, Gagnadoux MF, Niaudet P: Long-term social outcome of children after kidney transplantation. Transplantation 77: 1033–1037, 2004 [DOI] [PubMed] [Google Scholar]
- 23.Duquette PJ, Hooper SR, Wetherington CE, Icard PF, Gipson DS: Brief report: Intellectual and academic functioning in pediatric chronic kidney disease. J Pediatr Psychol 32: 1011–1017, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Kalechstein AD, Newton TF, van Gorp WG: Neurocognitive functioning is associated with employment status: A quantitative review. J Clin Exp Neuropsychol 25: 1186–1191, 2003 [DOI] [PubMed] [Google Scholar]
- 25.Groothoff JW, Grootenhuis MA, Offringa M, Stronks K, Hutten GJ, Heymans HSA: Social consequences in adult life of end-stage renal disease in childhood. J Pediatr 146: 512–517, 2005 [DOI] [PubMed] [Google Scholar]
- 26.Hinkin CH, Castellon SA, Durvasula RS, Hardy DJ, Lam MN, Mason KI, Thrasher D, Goetz MB, Stefaniak M: Medication adherence among HIV+ adults: Effects of cognitive dysfunction and regimen complexity. Neurology 59: 1944–1950, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cross CP, Copping LT, Campbell A: Sex differences in impulsivity: A meta-analysis. Psychol Bull 137: 97–130, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Calia R, Lai C, Aceto P, Luciani M, Camardese G, Lai S, Fantozzi C, Pietroni V, Salerno MP, Spagnoletti G, Pedroso JA, Romagnoli J, Citterio F: Emotional self-efficacy and alexithymia may affect compliance, renal function and quality of life in kidney transplant recipients: Results from a preliminary cross-sectional study. Physiol Behav 142: 152–154, 2015 [DOI] [PubMed] [Google Scholar]
- 29.Tryc AB, Alwan G, Bokemeyer M, Goldbecker A, Hecker H, Haubitz M, Weissenborn K: Cerebral metabolic alterations and cognitive dysfunction in chronic kidney disease. Nephrol Dial Transplant 26: 2635–2641, 2011 [DOI] [PubMed] [Google Scholar]
- 30.Bugnicourt J-M, Godefroy O, Chillon J-M, Choukroun G, Massy ZA: Cognitive disorders and dementia in CKD: The neglected kidney-brain axis. J Am Soc Nephrol 24: 353–363, 2013 [DOI] [PubMed] [Google Scholar]
- 31.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG: Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 42: 377–381, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL: New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20: 629–637, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F, Chronic Kidney Disease Epidemiology Collaboration : Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247–254, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.